Abstract
Although for many patients with acute myeloid leukemia (AML) allogeneic hematopoietic cell transplantation (HCT) from a matched related donor provides the best, and sometimes the sole chance for cure, only about 30% of individuals have HLA-matched family members. Fortunately, recent advances on a number of fronts have expanded the acceptable donor pool. With the use of high-resolution typing, HCT outcomes using unrelated donors matched at HLA-A, -B, -C and -DRB1 give results very similar to those expected with matched related donors. A single mismatch, as determined either by low- or high-resolution testing, results in modestly worse outcomes, with mismatches at B or C better tolerated than mismatches at A or DRB1. Initial results of umbilical cord blood transplantation for adults showed a clear association of cell dose and outcome, limiting the procedure to a minority of adults where cord bloods with at least 2.5 or 3 × 107 total nucleated cells/kg could be found. More recently, the use of double cord transplants has shown considerable promise, lowering the risk of graft rejection and possibly the risk of relapse as well. Haploidentical transplantation using T-cell–replete marrow and post-transplant high-dose cyclophosphamide, or T-cell–depleted peripheral blood and marrow containing high doses of CD34+ cells is under investigation. Together, these various approaches are broadening the transplant options for patients with AML.
Introduction
For many patients with acute myeloid leukemia (AML), allogeneic hematopoietic cell transplantation (HCT) from a matched related donor provides the best, and sometimes the only, chance for long-term survival. However, only approximately 30% of individuals have an HLA-matched family member. A considerable amount of new information about the use of unrelated volunteer donors, mismatched family member donors and unrelated umbilical cord blood has recently become available that helps address two major questions: which patients with AML are candidates for alternative donor transplants, and which donors should be chosen.
HCT from Matched Related Donors for AML
The indications for allogeneic transplantation have been defined most clearly for patients with matched related donors. For patients who fail to achieve remission with initial induction therapy, allogeneic HCT is the only form of treatment that offers a chance for cure.1 Similarly, except for the rare patient with a very long first remission, few patients who relapse after achieving a first remission can be cured with further chemotherapy, and thus allogeneic HCT is generally indicated for patients with relapsed AML.2
Numerous prospective trials have been conducted attempting to define appropriate therapy for patients with AML in first complete remission (CR1). In most of these studies, patients were entered at diagnosis, and those who achieved CR and had an HLA-matched family member donor were allocated to allogeneic HCT, while the others were treated with consolidation chemotherapy. A recent meta-analysis of all such trials conducted between 1995 and 2003 showed superior survival with allogeneic HCT.3 This advantage was most obvious in patients with unfavorable-risk cytogenetics, was less impressive in those with intermediate-risk cytogenetics, and was not apparent among patients with favorable-risk cytogenetics. There is considerable heterogeneity within the three major AML cytogenetic risk groups. Thus, although the meta-analysis would argue against transplantation in first remission for AML with favorable-risk disease, adults with favorable-risk AML but with mutations in c-kit appear to do as poorly with standard chemotherapy as patients with unfavorable-risk cytogenetics.4 Thus, such otherwise good-risk patients might be considered for matched sibling allogeneic HCT in CR1. Similarly, patients with cytogenetically normal AML who are generally considered to have intermediate-risk disease can be divided into groups with better or worse prognosis based on the mutational status of NPM1 and FLT3. Specifically those with mutations in NPM1 but without FLT3-ITD have an outcome with conventional chemotherapy that approaches those with favorable-risk cytogenetics, while those with any other combination do almost as badly as patients with unfavorable-risk cytogenetics.5 Thus, allogeneic HCT from a matched sibling for AML in CR1 should be considered for younger patients (variably defined as less than age 50, 55 or 60) with unfavorable-risk cytogenetics, intermediate-risk cytogenetics except the NPM1+/FLT-ITD3− subgroup, and favorable-risk cytogenetics with mutations in c-kit.
Unrelated Donor Transplantation for AML
Indications
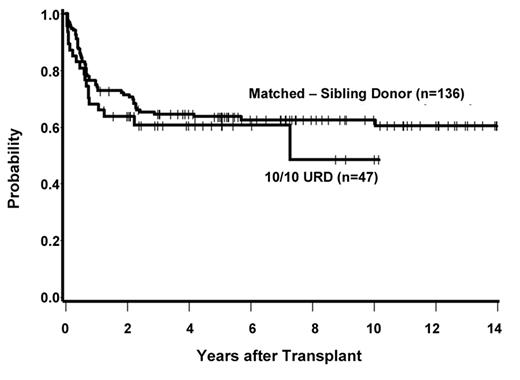
The indications for transplantation in AML using unrelated donors are less well defined. Almost no prospective trials have been performed, and retrospective comparisons are hampered by numerous issues, including patient selection bias and changes in HLA-typing technologies. The German AML 01/99 trial is the sole study attempting to prospectively address the value of unrelated HCT for AML in CR1.6 In this trial, patients with high-risk disease, defined as having unfavorable cytogenetics or >5% blasts on the day 15 post-induction marrow, were treated with allogeneic HCT from a matched sibling if such a donor was available, for those without matched siblings HCT from a matched unrelated donor if such a donor could be found, or autologous transplantation. Among 234 patients entering the trial, 58% achieved a complete remission and were allocated to matched sibling HCT (n = 34), unrelated HCT (n = 29), or autologous transplantation (n = 26). Survival at 4 years was 68% with sibling HCT, 56% with unrelated HCT and 23% with autografting (P = .01). A recent retrospective analysis from the Fred Hutchinson Cancer Research Center (FHCRC) examined the outcome of 183 patients with AML in CR1 transplanted from matched sibling donors or unrelated donors matched at 10 of 10 antigens.7 Survival at 5 years was 63% with matched related donors versus 61% for matched unrelated donors (see Figure 1 ). Others have also reported similar outcomes for patients with AML in first remission given matched-related donor and matched unrelated donor grafts.8 Based on these data, it would seem reasonable to accept similar indications for HCT in AML using matched related or 10 of 10 matched unrelated donors. Broad application of this strategy requires appropriate risk assessment of the patient at diagnosis and early HLA typing in order to initiate an unrelated donor search in a timely fashion.9
Whether to use unmanipulated bone marrow (BM) or growth factor–mobilized peripheral blood (PB) as the source of hematopoietic cells for transplantation in the setting of matched related donor transplantation has been the subject of numerous trials. In aggregate, these trials demonstrate that use of PB results in more rapid engraftment, an increased risk of chronic graft-versus-host disease (GVHD), diminished relapse rates, and a trend toward improved survival.10 The comparative outcomes of BM versus PB as the source of hematopoietic cells for matched unrelated transplantation is the subject of an ongoing Bone Marrow Transplant Clinical Trials Network prospective randomized trial.
Impact of HLA matching
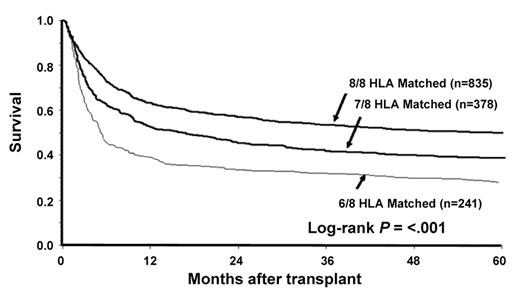
Prior to 1990, HLA-typing for unrelated donors relied on low-resolution (antigen) typing using serologic methodology. More recently, high-resolution (allele) typing using molecular methodologies has become increasingly used. A large retrospective study of the impact of high-resolution typing on the outcome of unrelated donor HCT was recently reported by Lee et al.11 In this study, the results of 3857 transplants performed in the United States between 1988 and 2003 were analyzed. The best outcomes were seen when donor-recipient pairs were matched at HLA-A, -B, -C and -DRB1 (8/8 match). A single mismatch, as determined either by low- or high-resolution testing at HLA-A, -B, -C or -DRB1, was associated with higher treatment-related mortality, more acute GVHD and lower survival at 1 year compared with 8/8 matching. Mismatches at A or DRB1 were less well tolerated than mismatches at B or C. There was no statistically significant effect of mismatching on the incidence of disease relapse. Mismatching at 2 or more loci was associated with even greater risk (Figure 2 ). Mismatching at HLA-DP or –DQ was not associated with altered survival. In this analysis, HLA mismatching exerted a similar effect regardless of patient age, disease status, use of T-cell depletion or other factors.
Other studies of the impact of high resolution typing have been conducted and have reached similar, but not identical conclusions.12,13 The minor differences between the conclusions of these prior studies and those of Lee et al probably reflect the larger number of donor-recipient pairs in Lee’s study and thus greater power to detect effects.
Effect of haplotype matching
Even with molecular typing, use of unrelated donors is associated with more GVHD than seen with HLA-matched related donors. An explanation for this observation has been posited by Petersdorf et al, who suggest that HLA haplotypes may contain within them previously unrecognized histocompatibility antigens. Using a novel technique that allows for the typing of individual DNA strands, they were able to show that approximately a third of 6/6 matched unrelated donor-recipient pairs do not have the same physical linkage of HLA-A, -B and -DRB1 (Figure 3; see Color Figures, page 504).14 Among 6/6 matched donor-recipient pairs, the likelihood of severe GVHD was significantly greater in those who were haplotype mismatched compared to those who were haplotype matched, a finding that is consistent with the hypothesis that HLA haplotypes contain within them previously unrecognized histocompatibility antigens and that there is a far greater likelihood of matching for these antigens between similar haplotypes. This effect appears to be quite strong. Although the results are preliminary, the incidence of severe GVHD was less in 5/6 matched pairs with one matched haplotype compared to 6/6 matches without a matched haplotype.
KIR genes in unrelated HCT
Human natural killer (NK) cells participate in the innate immune response. NK receptors with inhibitory function were the first to be defined and were given the name killer immunoglobulin-like receptor (KIR). Subsequently, the family of NK receptors has been more extensively characterized and is now known to also include receptors with activating function; the term KIR has been retained to signify receptors with either inhibitory or activating potential. The best-characterized receptors are the inhibitory KIRs, which have specificity for HLA class I antigens and have functional significance in allogeneic HCT. KIR genes are named according to their protein structure. KIRs have either two (2) or three (3) extracellular immunoglobulin domains (D) and either a long (L) or short (S) cytoplasmic tail. The number of genes encoding the protein completes the name. Hence, KIR2DL1, KIR 2DL2, and KIR 2DL3 are inhibitory KIRs, each with two extracellular immunoglobulin domains and a long tail, but representing 3 different genes. KIR genes are highly polymorphic, and new alleles continue to be discovered. While the ligands for many KIR receptors are unknown, the inhibitory receptors KIR2DL1, KIR2DL2 and KIR3DL1 bind epitopes of HLA- C (“HLA-C2, -C1” groups) and HLA-B and some HLA-A molecules (“Bw4”), respectively. Upon encountering self-class HLA antigen, NK cell–mediated lysis and cytokine release are inhibited.
Investigators have considered several possible ways to take advantage of KIR biology in the selection of related or unrelated donors. Leukemias that express ligands for activating KIR receptors appear to be more responsive to NK cell killing, but changing the ligand expressed by the leukemia is not possible. However, it is possible to select related or unrelated donors based on their KIR receptor status compared with the patients KIR-ligand status. In particular, it may be possible to select donor-recipient pairs where the donor has a KIR receptor and the ligand is either mismatched or missing on recipient cells. The latter situation can occur in either the HLA-mismatched or HLA-matched setting. In the HLA-mismatched setting, the recipient may lack the appropriate ligand due to an HLA class I mismatch. In the HLA-matched setting, the recipient may simply lack ligand because of, for example, homozygosity for the ligand (“missing ligand”). Both situations should favor NK cell–mediated lysis of the target and cytokine release since the appropriate ligand for the NK receptor is absent. The initial report that stimulated interest in this possibility came from the Perugia group in the specific setting of haploidentical transplantation using T-cell–depleted bone marrow and no post-grafting immuno-suppression.15 The Perugia group reported markedly diminished relapse rates and improved 5-year survival following transplantation for myeloid malignancies when there was a predicted KIR mismatch.16 This effect did not apply to lymphoid malignancies and had its greatest impact if patients were transplanted in remission. Alloreactive NK clones could be isolated from patients transplanted from HLA haploidentical related donors in those circumstances where the recipient did not have the epitope present in the donor.
The Perugia experience has led to numerous studies of the effect of NK ligand mismatching in settings other than haploidentical HCT with T-cell depletion and no post-transplant immunosuppression. Results to date have been mixed. In a recent study of T-cell–replete transplantation, genotyping of HLA and KIR genes was performed and outcomes examined among patients who were HLA class 1 and KIR-ligand matched, class I antigen-mismatched but KIR-ligand matched, or HLA class I and KIR-ligand mismatched.17 Outcomes were best in the group who were HLA class I and KIR-ligand matched. Thus, in the setting of T-replete HCT, KIR mismatching did not appear to be of benefit and, if anything, was associated with greater risk of transplant-related mortality. It is possible that the impact of KIR-ligand mismatching is lost when there are alloreactive T cells in the graft and when post-transplant immunosuppression is required. In the specific setting of KIR reactivity on the basis of “missing ligand,” the impact of KIR reactivity may have a more favorable impact.18 Given our current understanding of KIR biology, it is probably premature to select donors on the basis of KIR reactivity outside of the specific setting of haploidentical transplantation using T-cell depletion and no post-transplant immunosuppression.
HCT from HLA Partially Matched Related Donors
Definition of HLA disparity
Family members may possess one haplotype that is identical with the patient and a second that is variably mismatched for 0, 1, 2 or 3 HLA-A, -B, and –DR loci on the unshared haplotypes. A more precise description of the degree of mismatching also takes into consideration the vector of incompatibility. For example, if the donor but not the recipient is homozygous at one loci, this results in a one antigen mismatch in the direction of GVHD but not in the direction of graft rejection. The likelihood of finding a relative who is partially matched depends on both the search strategy employed as well as the social/ethnic background of the patient. One report from Germany found that a matched sibling was found in 40% of searches, a one HLA-A, -B or –DR locus mismatched sibling in 3.1% of cases and a one-locus mismatched relative in 10.4% of cases.19 Current policies at most United States transplant centers would be to first search for an HLA-matched or one-locus mismatched sibling, and if one is not found, to then conduct an unrelated donor search, which will yield an acceptable match in approximately 75% of cases. Only if the initial unrelated search is unsuccessful would it be justified to conduct an extended family search beyond siblings. Computer programs have been developed that help define the best search strategy.20
Impact of HLA disparity in related donor HCT
The Center for International Bone Marrow Transplant Research (CIBMTR) compared outcomes of HCT from HLA-identical siblings, HLA partially matched related donors and unrelated donors for the treatment of leukemia.21 The best results were seen with the use of HLA-identical siblings. Use of one-antigen mismatched related donors and matched unrelated donors gave similar results, both of which were worse than seen with matched siblings. Use of two-antigen mismatched family members and one-antigen mismatched unrelated donors were associated with greater risk.22 These results are similar to an earlier study from Seattle examining the same basic question. In both the Seattle and the CIBMTR report, increasing HLA disparity was associated with an increase in graft rejection, GVHD and transplant-related mortality. Both of the retrospective reports suffer from issues of patient selection and heterogeneity; furthermore, the CIBMTR study relied on outdated HLA-typing methodologies compared to the current standards. Nonetheless, these studies, together with reports from Japan and elsewhere, support the position that limited HLA disparity between donor and recipient can be tolerated with T-replete marrow transplants. If one excludes from the conversation the use of cord blood or experimental haplotypes mismatched transplants, these studies also support the position that the general priority for allogeneic donor selection should be HLA-matched siblings, one-locus mismatched siblings, matched unrelated donors, followed by single-antigen–mismatched unrelated donors.
Haploidentical HCT
Because virtually all patients will have available to them a haploidentical family member donor, there has been great interest in developing transplant approaches that permit this degree of HLA disparity. Two general strategies are under study. The groups from Johns Hopkins and Seattle have evaluated the safety and efficacy of a regimen that relies on a reduced-intensity conditioning regimen, transplantation of T-replete bone marrow and high-dose post-transplant cyclophosphamide.23,24 In the initial studies of this approach, graft failure, usually non-fatal, was seen in 18%, with the greatest risk in patients who had not previously been treated with intensive chemotherapy. The cumulative incidences of grades II–IV and III–IV acute GVHD were 35% and 10% respectively. A broader Phase II trial of this approach is being conducted by the Bone Marrow Transplant Clinical Trials Network (BMT/CTN).
As noted earlier, a great deal of interest in the possibility of haploidentical transplantation was generated by the initial 1994 report from Perugia of the use of intensive conditioning followed by transplantation of high doses of CD34+ T-cell–depleted cells harvested from both PB and marrow.25 The initial protocol has since been modified, and a more recent summary involves the use of a conditioning regimen of total body irradiation, fludarabine, thiotepa and antithymocyte globulin followed by the transplantation of CD34-selected cells from the peripheral blood.26 Primary engraftment was achieved in 94 of 101 patients. Acute GVHD developed in 8 patients and chronic GVHD in 5. Non-relapse mortality was seen in 38 cases, mostly due to infectious complications including aspergillus, cytomegalovirus and other pathogens. Event-free survival at 1 year was 48% and 46% for 42 AML and 24 ALL patients transplanted in remission, respectively, and only 4% for the 38 patients with AML or ALL transplanted in relapse.
Umbilical Cord Blood Transplantation
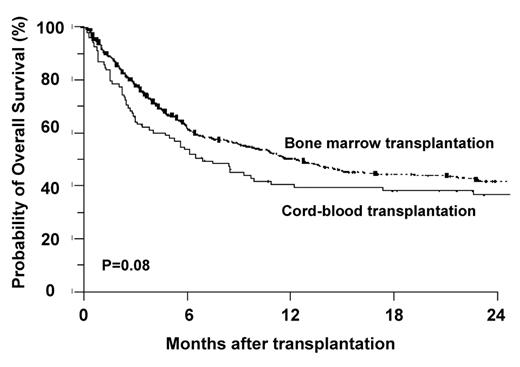
Use of umbilical cord blood (UCB) for HCT has the theoretical advantages of quicker availability and an apparent tolerance for greater HLA disparity than use of T-replete marrow of PB from related or unrelated donors. Initial studies of UCB HCT were performed predominantly in children where a clear association between cell dose, measured as total nucleated cells (or CD34+ cells)/kg recipient body weight and survival was found.27 Limited cell dose was, in retrospect, probably the major contributor to the poor survival seen in initial studies of UCB HCT for adults.28 More recently, transplant centers and cord blood banks have been limiting adult UCB HCT using a single cord to settings where a cord with a least 2 × 107 TNC/kg can be found. Actually, Eurocord recommends a minimum of 3 × 107, but this excludes most adult Americans given their higher average weight. Two retrospective reports have compared outcomes of unrelated donor and UCB HCT for adults with acute leukemia.29,30 In the report from the IBMTR, the rates of treatment-related mortality and overall mortality were lowest with the use of matched unrelated marrow transplantation, and similar with mismatched unrelated marrow and UCB transplantation. A report from Eurocord found a similar trend (P = .08) towards improved survival with the use of matched unrelated marrow over UCB. In both studies, there was a significantly higher incidence of treatment-related deaths with the use of cord blood compared to the use of matched unrelated marrow, likely related to increases in graft rejection, slow engraftment and increased early infectious complications. However, in both studies, the differences in long-term survival were sufficiently small (Figure 4 ) to make cord blood transplant a potential option for appropriate adults.
The cell dose requirement for cord blood transplantation significantly limits the applicability of this approach. In an effort to overcome this limitation, investigators have explored the use of double cord transplantation, in which two different cords are transplanted at the same time. Although only limited Phase II trials have been published, this approach does appear to lessen the risk of graft rejection and to improve early survival.31 Why this works is less clear; the time to initial engraftment is not particularly faster and only one of the units ultimately becomes responsible for sustained donor engraftment. Nonetheless, this approach makes cord blood transplantation an option for adults without a single large cord available. The lower limit of cell dose for each cord in the setting of double cord transplants has not been determined, but most centers would require a cell dose of at least 1.7 × 107 total nucleated cells/kg for each unit.
Conclusion
The option of allogeneic HCT for adults with AML is no longer limited to those patients with matched siblings. With advances in typing technology and supportive care, survival for patients with matched unrelated donors is essentially the same as seen with matched related donors. We now understand much more about the impact of mismatching in the related and unrelated setting, and particularly about what degree of HLA disparity is permissible. Outcomes with cord blood transplantation in adults have clearly improved. The American Cancer Society estimates that over 13,000 cases of AML were diagnosed in the United States last year, the majority of whom will die of their disease. The CIBMTR estimates that there were approximately 2000 allogeneic transplants performed for AML, a figure that seems quite a bit below what would be appropriate if we took advantage of current knowledge of the use of alternative donors.
Survival following transplantation for AML in CR1 in 183 patients with either matched sibling donors or matched unrelated donors.7
Survival following transplantation for AML in CR1 in 183 patients with either matched sibling donors or matched unrelated donors.7
Survival following unrelated donor transplantation for patients with favorable-risk disease according to degree of HLA disparity.11
Survival following unrelated donor transplantation for patients with favorable-risk disease according to degree of HLA disparity.11
Retrospective comparison of outcome of cord blood versus matched unrelated donor transplantation.30
Retrospective comparison of outcome of cord blood versus matched unrelated donor transplantation.30
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Fred Hutchinson Cancer Research Center, Seattle, WA; University of Washington, Seattle, WA