Abstract
This review will begin with a detail of the revision of the WHO classification, and pathological definitions of Burkitt lymphoma. Over the past several years, molecular understanding of Burkitt lymphoma has improved significantly. Using gene expression profiling, a genomic “signature” of Burkitt lymphoma may be identified, that has fidelity beyond c-myc expression, and the presence of the classical t(8;14). Then, evaluation and therapy of the adult patient with Burkitt lymphoma will be reviewed. Relatively few data exist on optimal therapy of the adult patient with Burkitt lymphoma. Principles of therapy should include high doses of alkylating agents, frequent administration of chemotherapy, and attention to central nervous system (CNS) prophylaxis with high doses of systemic chemotherapy, intrathecal therapy, or both. The outcome of adult patients with Burkitt lymphoma, particularly those over 40 years of age, is inferior to the outcome of younger patients, but may be improving over the past few years. Results from an international collaborative effort, which are helpful in evaluating results of Burkitt lymphoma therapy in adults, will be presented. HIV-associated Burkitt lymphoma, and elderly patients with Burkitt lymphoma, comprise special clinical situations that will be also covered in this review.
Introduction: Burkitt Lymphoma
Burkitt lymphoma is an uncommon form of non-Hodgkin lymphoma in adults, with an incidence of approximately 1200 patients per year in the United States. Although most literature suggests the disease is most common in males during childhood and young adult life, the 2007 National Cancer Institute Surveillance, Epidemiology and End Results (SEER) database suggests that “older” adult patients (age > 40 years) account for roughly 59% of all adult Burkitt lymphoma cases in the United States. The syndrome of “endemic” Burkitt lymphoma that presents as a jaw tumor with eventual spread to extranodal sites, particularly the bone marrow and leptomeninges, does not occur in adult patients in the United States and will not be discussed in this review.
Denis Burkitt first described the disease in children in 1958.1 Standard therapy for Burkitt lymphoma in children now consists of either an acute lymphocytic leukemia (ALL)-like approach or shorter duration, dose-intensive, multiagent chemotherapy with central nervous system (CNS) prophylaxis. With these treatments, most pediatric patients are cured of their disease, with long-term survival of 60% to 90%. Results in adults are more variable and depend largely on patient populations enrolled in the few studies that have an appreciable number of adult patients. There are no randomized trials in this disease in adults, and, unlike other non-Hodgkin lymphomas, few novel therapies have been introduced into the treatment paradigm over the past two decades. Moreover, the definition of Burkitt lymphoma has evolved significantly during this period, largely due to improvements in immunohistochemical, cytogenetic, and molecular diagnostic techniques and to an increased understanding of the molecular basis of this disease. Many of the older clinical trials thus enrolled heterogeneous patient populations, which potentially included large numbers of patients with non-Burkitt diagnoses according to contemporary criteria.
In this review, the modern diagnostic criteria of Burkitt lymphoma will be discussed, including recent data from gene expression profiling experiments. Evaluation and therapy of the adult patient will then be covered and discussed in light of the limitations of the current literature.
Pathologic Evaluation of Burkitt Lymphoma
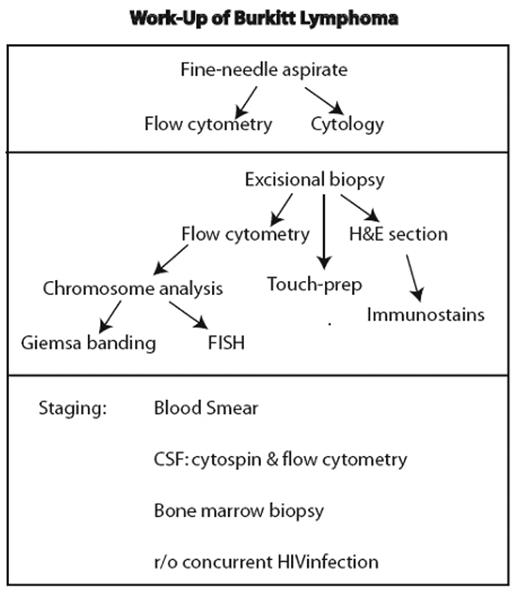
The work-up of a lesion suspicious for Burkitt lymphoma initially involves fairly standardized assessment that is part of the analysis of any lesion considered to be lymphoma (Figure 1 ). However, it is essential that studies for c-MYC translocation be performed, as detailed below.
Histology and cytology
Histologically, Burkitt lymphoma has a diffuse pattern of growth comprised of intermediate-sized B cells (12 μ) with high nuclear-to-cytoplasmic ratio. Nuclear contours are round to oval without cleaves or folds, a key feature in the distinction from diffuse large cell lymphoma. Nucleoli are typically multiple, small-to-intermediate in size, and the nuclear chromatin is relatively immature, being finely granular. The rate of proliferation, as determined with Ki-67 staining, is at or above 95%. Also high is the rate of cell death or apoptosis, with the dead cells being taken up by pale histiocytic cells within the tumor, which punctuate the low-power view giving a “starry sky” appearance (Figure 2A; see Color Figures, page 504). Characteristic features of Burkitt lymphoma on cytology are the strongly basophilic cytoplasm (due to the abundant polyribosomes) and the presence of lipid-filled cytoplasmic vesicles, some of which overlie the nucleus (Figure 2B; see Color Figures, page 504), and tingible body macrophages.
Immunohistochemistry
Burkitt lymphoma cells are mature B cells, positive for CD19, CD20, CD22, and CD79a, and have monotypic surface IgM. Burkitt lymphoma cells also show immunologic similarity to germinal center cells of B cell follicles rather than activated B cells,2 –4 being positive for Bcl-6, CD10, Tcl1, and CD38, and negative for Mum-1, CD44, CD138, and Bcl-2. However, germinal center (GC) markers are not specific for Burkitt lymphoma, since a significant proportion of diffuse large cell B cell lymphomas (DLBCL) also have this GC signature.
While Epstein-Barr virus (EBV) is associated with 98% of endemic Burkitt lymphoma, it is also seen in 20% of sporadic cases, and 30% to 40% of HIV-associated cases.2 This can be detected using in situ hybridization for EBV-encoded RNAs (EBER). While EBV likely plays a key role in B-cell stimulation during a pre-lymphoma stage, the role for EBV after lymphoma development is unclear, as is whether EBV positivity is clinically meaningful. HIV is associated with Burkitt lymphoma3 with 30% to 40% of these cases having EBV+ lymphoma cells. In contrast to primary effusion lymphomas and DLBCL, EBV+ HIV-associated Burkitt lymphoma does not express LMP1 nor EBNA2. Peripheral blood involvement is less common in HIV+ than in HIV– cases. Furthermore, a subset of Burkitt lymphoma may show plasmacytoid differentiation. This subtype is unique to AIDS patients. Interestingly, Burkitt lymphoma is associated with HIV infection but not other forms of immunosuppression.
Flow cytometry
Flow cytometric analysis provides independent confirmation of the immunophenotype and, given its rapidity, serves as an important ancillary tool. Since the tumor expresses clonal IgM, surface expression of kappa and lambda should be evaluated. B cell markers (CD19, CD20, and CD22) are positive, as are GC markers (CD10). The lack of staining with cytoplasmic terminal deoxynucleotidyl transferase (TdT) is important to rule out acute lymphoblastic leukemia/lymphoma (ALL). The immunophenotypic features of Burkitt lymphoma, together with the finding that they have undergone immunoglobulin variable region hypermutation and harbor chromosomal translocations mediated through the immunoglobulin switch region, effectively rules out ALL.4
Cytogenetics
Routine analysis of lesions suspected of being Burkitt lymphoma should include both routine Giemsa banding and fluorescence in situ hybridization (FISH) (Figure 2C; see Color Figures, page 504). A defining feature of Burkitt lymphoma is activation of the MYC gene at 8q24 through translocation with one of three immunoglobulin loci, which introduces a transcriptional enhancer element. In 80% of cases, this involves the immunoglobulin heavy chain locus at 14q32, with the breakpoint in the class switch region. In 15%, the gene encoding the kappa light chain at 2p11 is involved, while in 5% the lambda light chain gene at 22q11 is translocated. In most cases of sporadic Burkitt lymphoma in adults, the breakpoint in the MYC gene is between the first exon, which is noncoding, and the second exon, which harbors the start of the open reading frame. This leads to enhancer-driven activation of a cryptic promoter termed P3 within intron 1 resulting in overproduction of MYC protein proper. MYC is a transcriptional regulator that plays a master regulatory role in cell growth, division, metabolism, and apoptosis. MYC protein levels are critically regulated, and even relatively small increases can destabilize cell growth control.
The translocations involving MYC can be easily detected by FISH using so-called MYC “break apart” probes (Figure 2C; see Color Figures, page 504): a set of two fluorescently tagged DNA probes of two different colors that hybridize to the upstream and downstream side of the gene. In an unperturbed gene, they hybridize within inter-phase cells, giving a composite color, while with translocation, the two fluors are separated. This test is often much faster than Giemsa banding, and therefore should be performed whenever Burkitt lymphoma is suspected.
A key feature of Burkitt lymphoma is the relative simplicity of its karyotype: in a good proportion of cases, the MYC translocation is the sole abnormality. This distinguishes it from DLBCL. However, in one third of Burkitt lymphoma cases, alterations at the p53 gene at 17p can be found. These likely contribute to tumorigenesis by resulting in loss of p53-mediated apoptosis, which high levels of MYC are known to activate. Whether this portends a distinct prognosis relative to cases with normal 17p is not known.
Diagnostic challenges: “atypical Burkitt”
The majority of adult Burkitt lymphoma cases possess all of the criteria for the diagnosis: high mitotic rate in an appropriate morphologic and immunophenotypic setting, together with an Ig-positive MYC translocation. However, a minority of cases, particularly in adults, do not fit neatly into the diagnosis of either Burkitt lymphoma or DLBCL. Typically, this arises due to the absence of key morphologic features of Burkitt lymphoma in a lesion that otherwise resembles Burkitt lymphoma: a non-monomorphic nuclear morphology; fewer tingible body macrophages than is typical; or an abnormal immunophenotype. In comparison to the pediatric age group, this diagnostic (and concomitant treatment) conundrum is more frequent in the adult patient. To date, the diagnostic “touchstone” for this group of lesions, if such exists, has been pathology, particularly the cellular morphology and rate of Ki-67 positivity as discussed above.5
Gene expression analysis
Two recent studies have applied gene expression analysis to the diagnosis of Burkitt lymphoma, specifically to help refine the diagnosis of cases within the Burkitt lymphoma/ DLBCL overlap zone. Hummel and colleagues used a core group of 8 cases of pediatric Burkitt lymphoma who fulfilled all World Health Organization (WHO) criteria for Burkitt lymphoma.6 Tumors that matched this expression pattern were termed “molecular Burkitt lymphoma” (mBL); further analysis was performed on a set of cases diagnosed DLBCL. The analysis parsed this set into three groups: mBL (which matched nearly all molecular criteria), non-mBL (which lacked nearly all molecular criteria), and an intermediate group (Figure 2D; see Color Figures, page 504). Key characteristics of the mBL group were lower cytogenetic complexity and MYC translocations involving immunoglobulin genes rather than non-Ig partners, as well as lack of expression of genes in the NF-κB pathway. A study by Dave et al also identified a Burkitt lymphoma signature that included highly expressed target genes of MYC, as well as markers of germinal center B cells.7 They also found a lower expression target genes of the NF-κB pathway. Importantly, based on molecular expression, they identified a set of high-grade B-cell neoplasms with the Burkitt lymphoma signature that had been treated with a CHOP-like regimen rather than more aggressive therapy. These patients fared poorly, suggesting that the molecular expression diagnosis may more accurately predict outcome than conventional techniques.
However, one of the disturbing conclusions of these papers was that considerable disagreement exists among experts on cases within the diagnostic overlap between Burkitt lymphoma and DLBCL. Furthermore, the molecular analysis led to reclassification of diagnoses originally based on pathology, both into the “Burkitt lymphoma” group and out of this group. It remains unclear which analysis is “correct”—one based on morphology or one based on molecular analysis. Therapy (detailed below) is quite different for Burkitt lymphoma compared with DLBCL; undertreatment of Burkitt lymphoma can lead to premature relapse and death from disease; likewise, overtreatment of DLBCL, particularly in the older patient population, can have considerable morbidity. No clinical trial to date has utilized the gene expression diagnostic criteria for eligibility; all published trials have utilized morphologic criteria.
What new tools exist to allow the identification of lymphomas that warrant the diagnosis of Burkitt lymphoma and the high-intensity Burkitt-type therapies? One possible metric revealed by the recent gene array studies and follow-up papers is high-level activation of MYC. The molecular markers identified by the gene array studies were in large part surrogate markers for high levels of MYC expression. This is illustrated by a follow-up to the microarray analyses: Rodig et al showed that with three immunostains that were highlighted as differentially expressed by gene expression studies—Tcl1, CD38, and CD44—they could effectively identify tumors with a translocation at MYC: Tcl1+/CD38+/CD44– was 100% specific and about 80% sensitive in their study.8 While most cases in their study with this immunophenotype were Burkitt lymphoma, these markers were not effective at distinguishing MYC+ DLBCL from Burkitt lymphoma.
Further help may be offered by considering the partners of the MYC gene: in straightforward Burkitt lymphoma, MYC translocates to IgH or IgL, while DLBCL is associated with MYC translocation to non-IgH loci. The identification of non-Ig partners for MYC may be helpful in future analysis. As a group, these tend to have lower levels of MYC expression relative to translocations involving IgH or IgL.9 Additional refinement in the diagnosis might come from consideration of the overall karyotype: Burkitt lymphoma is found in the context of an overall more simple karyotype, while DLBCL is often accompanied by karyotypic complexity involving BCL2 (e.g., t(14;18)) or BCL6 (at chromosome 3q27). It is likely that in cases of DLBCL, the MYC translocation is acquired as a secondary event after the establishment of the tumor, the origins of which lie in deregulation of BCL2 and BCL6. Thus, the overall phenotype is that of a DLBCL due to the initial genetic event; the subsequent MYC translocation gives it features of Burkitt lymphoma, such as a high growth rate, but does not supplant the underlying established phenotype. DLBCL with both BCL2 and/or BCL6 abnormalities plus MYC rearrangement appears to have a worse prognosis than other DLBCL in most series.10 –12
As mentioned, karyotypic analysis is relatively time consuming, and given the tempo of disease in patients with high-grade lymphoma, a need for rapid diagnosis is real. Unfortunately, the microarray studies did not reveal specific immunomarkers that can accurately distinguish Burkitt lymphoma from DLBCL. If gene expression analysis becomes a routine diagnostic procedure that can be performed from paraffin blocks,13 it is anticipated this will define a molecular entity of Burkitt lymphoma. At the present time, diagnosis of the atypical cases continues to rely on expert opinion, synthesizing morphologic, karyotypic and molecular information.
Clinical Evaluation of Burkitt Lymphoma
Most adult patients with Burkitt lymphoma in the United States present with a bulky abdominal mass, B symptoms, and laboratory evidence of tumor lysis. Bone marrow involvement is present up to 70% of the time, and leptomeningeal involvement may be present in up to 40% of adults at diagnosis. Extranodal involvement in other sites also may occur, and Burkitt lymphoma should be considered over DLBCL in cases when numerous extranodal sites are involved with disease. A recent retrospective review of consecutive Asian patients suggested that the majority of those patients presented with early-stage disease and low-risk International Prognostic Index (IPI) scores, with less bone marrow and CNS involvement compared to Western series of patients.14 It is essential that Burkitt lymphoma be evaluated as quickly as possible, and in our institution, we aim to initiate therapy within 48 hours of suspecting Burkitt lymphoma. As previously stated, adequate diagnostic tissue is a mandatory first step. Additional clinical evaluation should include computed tomographic (CT) imaging of chest, abdomen and pelvis, bone marrow aspiration and biopsy, renal and liver function evaluation, and testing for HIV disease. There are no studies evaluating the role of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) imaging in Burkitt lymphoma. Although Burkitt lymphoma is almost certainly FDG-avid,15 it is unlikely that findings on FDG-PET would alter therapy for a newly diagnosed patient with Burkitt lymphoma, and we generally do not perform PET scans on these patients. If anthracycline therapy is to be utilized, in older adult patients a cardiac evaluation with radionucleotide ventriculography or echocardiogram is warranted. In the absence of symptoms, we generally reserve cerebrospinal fluid evaluation until initiation of therapy, as intrathecal therapy is a component of most regimens.
In the adult literature, both the St. Jude/Murphy staging system (Table 1 ) and the Ann Arbor staging system have been utilized. As surgery is no longer employed for this disease, we favor the Ann Arbor staging system; however certain therapeutic regimens are risk-adaptive by Murphy staging, so it is important to be familiar with both staging systems when treating adult patients and when interpreting the literature.
Therapy of Burkitt Lymphoma in Adults
Principles of therapy include high doses of alkylating agents, frequent administration of chemotherapy, and attention to CNS prophylaxis with high doses of systemic chemotherapy, intrathecal therapy, or both. Standard doses of chemotherapy utilized for DLBCL such as CHOP are inadequate for treating Burkitt lymphoma.16 There is no role for radiation therapy in the modern treatment of Burkitt lymphoma, even for localized disease or paraspinal presentations, which respond very quickly to chemotherapy. Tumor lysis is common with this disease, and prophylaxis with aggressive bicarbonate hydration, and allopurinol or rasburicase17 is required during the first cycle of chemotherapy. There are three major categories of therapy that adhere to these principles and are currently utilized as treatment for this disease: 1) intensive, short-duration chemotherapy; 2) ALL-like therapy; and 3) therapy that includes consolidation with high-dose therapy and autologous stem cell transplantation (ASCT).
The specific regimens that have been developed to treat Burkitt lymphoma in adults are generally adapted from pediatric literature, and representative trial results are depicted in Table 2 . There are no comparative studies evaluating these specific regimens, and age distributions within these trials are often not detailed. Patients enrolled in these various single-arm trials do not have uniform diagnostic criteria, staging, or other risk factors, rendering it impossible to compare results between trials. Early studies of these regimens were also often limited to younger, highly selected patient populations, limiting the ability to extend these results in older patient populations.
For example, the CODOX-M/IVAC regimen (cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate alternating with ifosfamide, etoposide and high dose cytarabine, along with intrathecal methotrexate and cytarabine) developed at the National Cancer Institute by Magrath and colleagues was initially published in 1996, and suggested similar “excellent” outcome of adults and children with a short duration, highly intensive chemotherapeutic regimen, with cure rates approaching 90%.18 This older series included patients with Burkitt-like lymphoma and did not use modern WHO classification system. Moreover, the median age of the “adult” patients in this study was 24, and there are very limited data on this regimen for patients older than 40 years of age. Despite these limitations, CODOX-M/IVAC remains perhaps the most commonly utilized regimen in the United States for adults with Burkitt lymphoma. The regimen stratifies patients by risk, defining an uncommon (in adults) low-risk group of a single extra-abdominal mass or completely resected abdominal disease, and a normal LDH; all other patients are approached as high-risk disease. Two subsequent small Phase II trials have utilized this regimen with minor modifications of drug dosing and intrathecal schedules, and have successfully enrolled greater numbers of older patients, demonstrating cure rates of approximately 64%.19,20 These cure rates are substantially less than the initial Magrath publication, but still better than historical data with standard-dose regimens, endorsing this approach as a reasonable one for the majority of adult patients.
The Cancer and Leukemia Group B (CALGB) has published a trial of intensive, short-duration chemotherapy that enrolled patients with a median age of 47, perhaps the highest median age of any trial incorporating an intensive regimen for this disease.21 In this study, only approximately half of the patients were cured of disease, and tolerability improved after adjustments were made to the CNS prophylactic regimen following observations of transverse myelitis, neuropathies, and blindness.
Other treatment approaches have included an approach similar to a regimen utilized in childhood ALL. Hoelzer and colleagues published results of 68 patients with L3 ALL (Burkitt lymphoma) treated on 3 successive protocols, demonstrating a leukemia-free survival rate of 71% in the most recent regimen, with a median age of enrolled patients of 36 years.22 The HOVON group has demonstrated the feasibility and efficacy of consolidating responses to aggressive, intensive chemotherapy with high dose BEAM (carmustine, etoposide, cytarabine and melphalan) and autologous stem cell support.23 In their study, which enrolled patients with a median age of 36 years, the 5-year event-free survival estimate was 73%.
It is clear from these series that there are limited published data including “older” adult patients. To better define optimal therapy of this group of patients with Burkitt lymphoma, we coordinated an international effort focused on the group of patients with Burkitt lymphoma older than age 40.24 Authors of 12 large treatment series (10 prospective; 2 retrospective) provided detailed outcome information of patients enrolled on their clinical trials who were older than age 40. In this pooled analysis, 470 total adult patients with Burkitt lymphoma ages 15 to 79 were included, and 183 (39%) were greater than 40 years of age. Patients older than age 40 had significantly inferior outcomes in 10 of the 12 series. The median overall survival (OS) at 2 years for all patients treated with short-duration therapy was 71%, and for patients greater than age 40 was 39%. The OS at 2 years for all patients treated with ALL-like therapy was 51%, and for patients older than age 40 was 40%. Patients older than age 40 treated with ASCT as part of induction regimens had somewhat better outcomes (median OS at 2 years of 62%); however, this represented a small number of patients who may have been subject to more rigorous selection criteria. It was clear from this analysis that patients older than age 40 with Burkitt lymphoma were significantly under-represented in published clinical trials over the past 15 years, and had inferior OS compared to the frequently cited, aforementioned published outcomes in younger patients.
Recently, however, a few published trials have included more substantial numbers of older patients with promising results. The M.D. Anderson Cancer Center has used Hyper-CVAD (cyclophosphamide, vincristine, doxorubicin and dexamethasone) alternating with methotrexate and cytarabine for adult patients with Burkitt lymphoma, a regimen designed initially to treat ALL. In the initial publication of this program for patients with Burkitt lymphoma, the complete response rate was 81%, there were 5 induction deaths, and 57% of patients remained in continuous CR. The survival rate was only 17% for patients older than age 60 in this initial series.25 More recently, the group added rituximab to this program, and treated a larger number of older patients. Results were significantly better, with OS at 3 years 89%. Of these patients, 29% were older than age 60, and this group had an equivalent OS to younger patients.26 Whether the observed improved outcomes are directly related to the addition of rituximab, refined diagnostic criteria, increased experience with the regimen, or enhanced supportive care is unknown.
Kujawski and colleagues published a series of 11 patients older than 33 years of age (median age 51) with Burkitt lymphoma, treated with escalated-dose CHOP, high-dose methotrexate, and intrathecal therapy.27 Ten patients achieved a complete response, with a 3-year OS of 72%. Song and colleagues demonstrated that an approach including ASCT consolidation cured approximately 50% of patients, with a median age of 33; many patients in this series were unable to get to transplantation due to disease progression.28 There have been no major recent prospective studies evaluating the role of allogeneic transplantation for patients with Burkitt lymphoma. Registry studies suggest the technique has been utilized in both first remission and in the setting of relapsed disease. The median survival of 71 patients with Burkitt lymphoma treated with allogeneic transplantation in Europe was only 4.7 months.29 Given the excellent results in Burkitt lymphoma of intensive regimens without allogeneic transplantation, and the high morbidity and mortality associated with this procedure, we reserve allogeneic transplantation for the setting of relapsed disease in the context of a clinical trial.
In summary, the outcome of adult patients with Burkitt lymphoma, particularly those over 40 years of age, is inferior to the outcome of younger patients but may have been improving over the last few years. Options remain aggressive short-duration chemotherapy regimens, ALL-like regimens, or consolidation with ASCT. The incorporation of rituximab looks promising in a single published clinical trial and additional preliminary results published only in abstract form.30,31 Because combining rituximab with other chemotherapy combinations has been shown to be safe, the drug is now often included as part of therapy for Burkitt lymphoma by many physicians. We generally use the CODOX-M/IVAC regimen as originally published by Magrath,18 with or without rituximab for our patients. If rituximab is to be used, we recommend omitting this agent from the first cycle of therapy to minimize the risk of tumor lysis.
Special Situations
HIV-positive Burkitt lymphoma in adults
Until recently, the immunocompromised state of patients with concomitant HIV/AIDS and Burkitt lymphoma was thought to limit the ability to administer intensive chemotherapeutic regimens due to infection rate. However, the advent of highly active antiretroviral therapy (HAART) and evidence in DLBCL that HIV-positive patients can tolerate standard chemotherapeutic regimens with improved outcomes have led investigators to treat HIV-positive patients with the same intensive chemotherapy regimens used to treat immunocompetent patients. Data suggest that these current approaches, along with supportive care, may result in improved patient outcomes, similar to those in the immunocompetent patient population.32
For example, the LMB86 regimen (escalated CHOP-based therapy, with consolidation cytarabine and etoposide) has recently been evaluated in 63 HIV-infected patients with Murphy stage IV (bone marrow and/or CNS involvement) Burkitt lymphoma and a median age of 40.33 At diagnosis of Burkitt lymphoma, the median CD4 count was 239. Forty-four patients (70%) achieved complete response, and only 7 treatment-related deaths occurred despite significant cytopenias observed in all patients during therapy. The 2-year OS (estimated) was 47%. Low CD4 count and poor performance status predicted for inferior survival. This study supports therapy with aggressive chemotherapy regimens for HIV-positive patients, particularly those with CD4 counts higher than 200 and favorable performance status.
Short, intensive approaches have also been demonstrated to be efficacious in HIV-positive patients with Burkitt lymphoma. The rate of treatment failure was significantly lower with the German GMALL protocol compared with patients treated with CHOP chemotherapy, and patients tolerated the aggressive protocol reasonably well, particularly when HAART therapy was incorporated into the regimen.34 Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, with rituximab and intrathecal methotrexate) has recently been presented as an option with a very high response rate for both HIV-positive and -negative patients in short follow-up.35
Given some data in DLBCL suggesting increased infectious deaths when rituximab is incorporated into standard chemotherapeutic regimens,36 we do not recommend including rituximab in treatment for these patients, particularly if they present with low CD4 counts. A recent series from Germany suggests a non-statistically significant increased infection risk, with excellent overall outcome, when rituximab is incorporated into an ALL-like regimen for patients with HIV-associated Burkitt lymphoma.37 Based upon these recent studies, patients with HIV and Burkitt lymphoma should therefore be approached as immunocompetent patients are approached, with the addition of HAART therapy.
Elderly patients with Burkitt lymphoma
Very few patients with Burkitt lymphoma older than age 60 have been included on prospective therapeutic trials. According to data from SEER, up to 30% of Burkitt lymphoma diagnosis in the United States includes this group of patients. Although the relatively small number of patients older than age 60 treated with the HyperCVAD-rituximab regimen had favorable outcome,26 studies of HyperCVAD in other histologies have demonstrated the inability of the majority of elderly patients to tolerate the regimen.38 Older patients may not be able to tolerate the high doses of methotrexate and cytarabine included in many short-duration protocols and may not be candidates for ASCT. For elderly patients deemed too infirm for these intensive protocols, new therapeutic options are clearly required. We generally approach these patients with standard CHOP chemotherapy with rituximab and intrathecal methotrexate, with a palliative, not curative, intent.
Conclusions
Burkitt lymphoma in adults remains a highly curable condition, and it appears that the outcomes of older adult patients have improved over recent years. There remains a paucity of data on treating patients older than age 40; a pooled analysis has suggested inferior outcomes compared to younger patients, but at least half of patients are cured. Recent gene expression analyses have demonstrated that a subset of patients with “atypical Burkitt lymphoma” actually have DLBCL. It is likely that the majority of literature on our current treatment protocols included a substantial number of these patients. Future trials in Burkitt lymphoma should focus on the older patient population and must incorporate modern pathologic diagnostic techniques, including gene expression analysis, to rigorously define the patient population. Alternatively, trials of c-MYC–positive aggressive lymphomas may be appropriate. Treating elderly patients with Burkitt lymphoma remains a difficult therapeutic challenge, with significant need for novel therapeutic approaches.
Murphy staging system for Burkitt lymphoma.
| Notes: | |
| 1) Favorable disease for Magrath regimen is stage I or stage IIR AND normal LDH. | |
| 2) > 25% bone marrow involvement is defined as L3 ALL and is not included in this staging system. | |
| Stage I: | Single nodal or extranodal site excluding mediastinum or abdomen |
| Stage II: | Single extranodal tumor with regional nodal involvement Two extranodal tumors on one side of diaphragm Primary gastrointestinal tumor with or without associated mesenteric nodes Two or more nodal areas on one side of diaphragm |
| Stage IIR: | Completely resected intra-abdominal disease |
| Stage III: | Two single extranodal tumors on opposite sides of diaphragm All primary intrathoracic tumors All paraspinal or epidural tumors All extensive primary intra-abdominal disease Two or more nodal areas on opposite sides of diaphragm |
| Stage IIIA: | Localized, non-resectable abdominal disease |
| Stage IIIB: | Widespread multiorgan abdominal disease |
| Stage IV: | Initial CNS or bone marrow involvement (< 25%) |
| Notes: | |
| 1) Favorable disease for Magrath regimen is stage I or stage IIR AND normal LDH. | |
| 2) > 25% bone marrow involvement is defined as L3 ALL and is not included in this staging system. | |
| Stage I: | Single nodal or extranodal site excluding mediastinum or abdomen |
| Stage II: | Single extranodal tumor with regional nodal involvement Two extranodal tumors on one side of diaphragm Primary gastrointestinal tumor with or without associated mesenteric nodes Two or more nodal areas on one side of diaphragm |
| Stage IIR: | Completely resected intra-abdominal disease |
| Stage III: | Two single extranodal tumors on opposite sides of diaphragm All primary intrathoracic tumors All paraspinal or epidural tumors All extensive primary intra-abdominal disease Two or more nodal areas on opposite sides of diaphragm |
| Stage IIIA: | Localized, non-resectable abdominal disease |
| Stage IIIB: | Widespread multiorgan abdominal disease |
| Stage IV: | Initial CNS or bone marrow involvement (< 25%) |
Selected prospective studies of Burkitt lymphoma in adults.
| Citation . | Regimen . | N . | Median age* . | 2-year survival . |
|---|---|---|---|---|
| Hoelzer B-NHL83 (1996)22 | Short duration/dose intensive; pediatric NHL based | 24 | 33 | 49% (estimated) |
| Hoelzer B-NHL86 (1996)22 | Short duration/dose intensive; pediatric NHL based | 35 | 36 | 51% (estimated) |
| Magrath (1996)18 | CODOX-M/IVAC | 54 | 24 | 89% (actual) |
| Thomas (1999)25 | Hyper-CVAD | 48 | 58 | 39% (estimated) |
| Mead (2002)20 | Modified CODOX-M/IVAC | 52 | 26.5 | 70% (estimated) |
| Rizzieri (2004)21 | Short duration/dose intensive | 92 | 47 | Cohort 1: 54% (estimated); Cohort 2: 50% (estimated) |
| Lacasce (2004)19 | Modified CODOX-M/IVAC | 14 | 47 | 71% (estimated) |
| Van Imhoff (2005)23 | Short duration/dose intensive; ASCT | 27 | 36 | 81% (estimated) |
| Thomas (2006)26 | Hyper-CVAD with rituximab | 31 | 46 | 89% (estimated) |
| Kujawski (2007)27 | Short duration/dose intensive | 10 | 51 | 72% (estimated) |
| Citation . | Regimen . | N . | Median age* . | 2-year survival . |
|---|---|---|---|---|
| Hoelzer B-NHL83 (1996)22 | Short duration/dose intensive; pediatric NHL based | 24 | 33 | 49% (estimated) |
| Hoelzer B-NHL86 (1996)22 | Short duration/dose intensive; pediatric NHL based | 35 | 36 | 51% (estimated) |
| Magrath (1996)18 | CODOX-M/IVAC | 54 | 24 | 89% (actual) |
| Thomas (1999)25 | Hyper-CVAD | 48 | 58 | 39% (estimated) |
| Mead (2002)20 | Modified CODOX-M/IVAC | 52 | 26.5 | 70% (estimated) |
| Rizzieri (2004)21 | Short duration/dose intensive | 92 | 47 | Cohort 1: 54% (estimated); Cohort 2: 50% (estimated) |
| Lacasce (2004)19 | Modified CODOX-M/IVAC | 14 | 47 | 71% (estimated) |
| Van Imhoff (2005)23 | Short duration/dose intensive; ASCT | 27 | 36 | 81% (estimated) |
| Thomas (2006)26 | Hyper-CVAD with rituximab | 31 | 46 | 89% (estimated) |
| Kujawski (2007)27 | Short duration/dose intensive | 10 | 51 | 72% (estimated) |
Standard work-up for lesion considered lymphoma. If fine-needle aspirate is performed, it should not delay an excisional biopsy necessary for definitive diagnosis.
Standard work-up for lesion considered lymphoma. If fine-needle aspirate is performed, it should not delay an excisional biopsy necessary for definitive diagnosis.
Disclosures Conflict-of-interest disclosure: J.W.F. is a member of an advisory committee for Genentech. A.S.P. declares no competing financial interests. Off-label drug use: Chemotherapy and antibody therapy for Burkitt lymphoma.
References
Author notes
Lymphoma Program, James P. Wilmot Cancer Center, University of Rochester, Rochester, NY
Scholar in Clinical Research of the Leukemia & Lymphoma Society