Abstract
Recurrence of Hodgkin lymphoma (HL) occurs in about 50% of patients after autologous stem cell transplantation (ASCT), usually within the first year, and represents a significant therapeutic challenge. The natural history of recurrent HL in this setting may range from a rapidly progressive to a more indolent course. Patients in this setting are often young, without comorbidities and able to tolerate additional therapies: expectations are often still high. The approach to treatment depends on clinical variables (time to relapse, perceived sensitivity to additional cytotoxic therapy, disease stage), prior history of radiation therapy, the availability of an HLA-identical donor, and the availability of new agents via clinical trials. Although very few of these patients can be cured, results from reported series, albeit often small and sometimes with relatively short follow-up, document that excellent disease control can be achieved with radiation, single or multiagent chemotherapy, and reduced-intensity allogeneic transplantation. The results of these approaches will be reviewed, and a treatment algorithm incorporating the use of standard or investigational agents or approaches will be discussed.
Patients with Hodgkin lymphoma (HL) who experience disease progression during or within 3 months of doxorubicin-based chemotherapy (primary refractory HL) and those whose disease relapses after a complete response have a second chance at cure. Two randomized trials testing multi-agent chemotherapy compared to intensification with high-dose carmustine, etoposide, cytarabine, and melphalan (BEAM) show superior event-free survival for patients receiving the intensive chemotherapy supported by autologous stem cell transplantation (ASCT).1,2 The German Hodgkin Lymphoma Study Group/European Bone Marrow Transplant Group trial2 reported that freedom from treatment failure was significantly improved by ASCT both in patients with early relapse (< 1 yr after the end of primary therapy; 41% vs 12%; P = .08) and for those with later relapse (75% vs 44%; P = .025). A number of variables present at the time of disease relapse or immediately prior to ASCT have be evaluated with regard to their influence on risk of recurrence following ASCT. Higher risk of recurrence post ASCT is associated with less than a complete response to second-line therapy (either by CT scan criteria or by FDG-positron emission tomography [PET]), relapse in a prior radiation field or after combined modality therapy, duration of first remission less than 12 months and higher risk score as described by Hasenclever and Diehl at relapse.3–8
Efforts to improve on the outcome of ASCT include intensification of treatment by the use of tandem transplants,9,10 and, more recently, high-dose sequential treatment.11,12 Phase II trials of these strategies have addressed their feasibility and reported encouraging results, but randomized trials demonstrating an improvement in outcome for such patients have yet to be reported.
Published literature on the optimum therapy for the patient population with recurrence after ASCT is sparse, and is reviewed here.
Natural History of Relapsed Hodgkin Lymphoma Post-Autologous Stem Cell Transplant
Although it might be predicted that patients whose disease recurs after ASCT represent the worst end of the disease biology spectrum, in some cases at least the natural history of relapsed HL is reminiscent of other lymphomas more commonly regarded as indolent. Patients may achieve response to second, third, or even fourth line treatment, and approximately 20% of patients managed without intensive therapy and stem cell support are alive at 10 years.13 This difference in the natural history of HL compared to other aggressive lymphomas treated similarly with curative intent has an impact on decision making following relapse after intensive therapy. Who should be treated with single-agent or combination chemotherapy? Who should be referred for a reduced-intensity allogeneic SCT? What is the role of second autologous SCT? Who should be referred for investigational treatments?
The outcome of HL following relapse after ASCT was initially reported to be very poor, with a median survival of less than 1 year.14,15 More recent reports suggest that survival after relapse from ASCT may be longer, with a median in the range of 24 months;16–18 whether this difference is a reflection of therapy after transplantation, or of patients undergoing ASCT earlier in the course of their disease in more recent years is not clear.
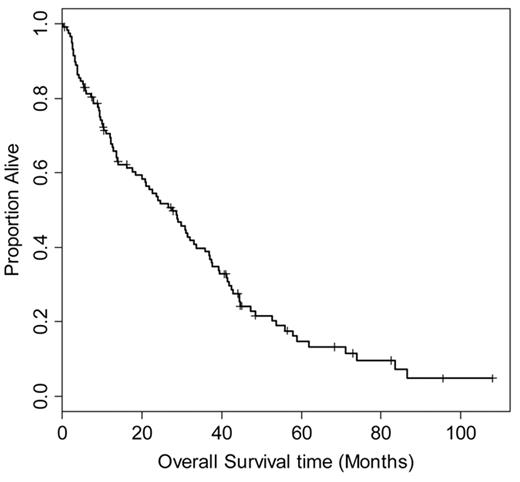
In order to better understand the natural history of relapsed (HL) following ASCT, we retrospectively reviewed the outcome of 330 patients treated at Toronto General Hospital and Princes Margaret Hospital between 1986 and 2006. One hundred thirty-nine patients experienced disease relapse, and 118 had adequate data on treatment and outcome for evaluation (Figure 1 ). The median time to relapse after transplant was 6.5 months (range 1–96 months). Initial treatment for relapse included involved or extended field radiation (39 patients), chemotherapy (60 patients), or combined modality treatment (6 patients); 13 patients had either surgical excision or a period of observation as initial management.
The treatment of patients at relapse was individualized and a number of chemotherapy treatments were employed. Nineteen patients received single agents and 47 received combination therapy. Response to chemotherapy was observed in 43 patients (CR 10, PR 33; overall response rate 72%). Median progression-free survival (PFS) for those receiving chemotherapy was 8 months and overall survival (OS) was 23 months; for complete responders PFS was 22 months and OS 37 months. Four patients received a second ASCT and 5 underwent allogeneic transplant; only 1 patient is in complete response 57 months after a second ASCT; 1 of 5 patients undergoing allotransplant is alive in relapse with extensive graft-versus-host disease (GVHD). Thirty-nine patients received radiotherapy alone as initial treatment; 20 had limited stage at relapse and 19 had advanced disease (stage III or IV). The median PFS for these patients was 6.9 months and OS 41 months, similar to those treated with systemic therapy (logrank P = .11).
We evaluated a number of variables for their effect on OS including age, gender, need for more than 2 cycles or an alternative chemotherapy regimen to achieve a response pre-transplant (“additional salvage”), disease status at the time of transplant, and time to relapse after transplant, in addition to stage, B-symptoms, disease bulk, extranodal disease, hemoglobin and lymphocyte count at relapse. In univariate analysis, predictors of OS were additional chemotherapy pre-ASCT, and hemoglobin less than 105 g/L. For PFS, additional salvage pre-transplant and extranodal disease at relapse were significant predictors. Before ASCT, time to relapse appears to be an important predictor of outcome; however, in our patients, the interval between ASCT and disease recurrence did not predict PFS or OS.
Using these variables (additional salvage and anemia for OS; additional salvage and extranodal relapse for PFS), it was possible to divide patients into prognostic groups according to the number of risk factors present. Patients who had 2 risk factors had an extremely poor OS (9 months), compared to those with no risk factors at time of recurrence (median survival 36 months, P < .001). In addition, Horning et al recently reported significant differences in OS depending on time from ASCT to relapse, highlighting the particularly poor survival for those whose disease relapses within 6 months of transplant.19 Patient and disease characteristics such as those described above can be used to guide decisions about “what’s next” and should also help us understand the results of the testing of novel agents or regimens, including reduced-intensity allogeneic transplantation.
The Role of Radiotherapy
A significant number of patients who relapse after SCT do so in previously involved sites20,21 and may present with disease that could be encompassed in a radiation field. Mature follow-up of patients receiving salvage radio-therapy after doxorubicin-based chemotherapy such as ABVD have been reported.22,23 Some of the patients in these reports have persistent disease following multiagent chemotherapy, but have shown rates of freedom from treatment failure (FFTF) following subsequent radiation as high as 30%. This strategy appears most beneficial in those who present with Ann Arbor stage I or II disease at relapse, without B symptoms, and no extranodal disease. Josting et al have reported 5-year FFTF of 28% in patients receiving either extended field or, in the majority of patients, involved field radiotherapy.23 Patients with limited-stage disease at progression and those without B symptoms appeared to have the best outcome; duration of response to initial chemotherapy did not influence FFTF.
What is the relevance of these observations to patients with disease recurrence following ASCT? Many centers currently include involved-field radiation to areas of bulky or residual disease, either before or after stem cell transplantation.21,24,25 Although there are no controlled trials of this approach, involved-field radiotherapy may improve disease control and long-term survival, especially for patients who have not previously received radiation.
In an earlier report by Vose et al14 of the outcome of 95 patients who relapsed after ASCT between 1983 and 1990, 22 patients received radiotherapy alone and 11 received additional chemotherapy followed by radiation; 6 patients treated with radiation alone and 3 with combined modality therapy were disease-free at the time of reporting. As mentioned, for the patients treated at Princess Margaret Hospital with initial radiotherapy, the median PFS was 6.9 months, and OS 41 months, suggesting that for at least some patients, the toxicity of systemic therapy can be deferred without compromising OS.
Based on the above data, patients with localized stage I or II disease at relapse, and without B symptoms, should be considered for local radiotherapy, either involved or extended field. Patients with disease in unirradiated lymph nodes should receive extended-field radiation, as a small percentage of such patients may have long-term disease control and may be cured. Involved-field radiation is an important option when recurrent disease extends beyond previously unirradiated lymph nodes. There is less information regarding the utility of the efficacy of radiation when relapse occurs in a prior radiation field, but this should be considered if tissue tolerance allows.
Chemotherapy for Relapsed HL after ASCT
Although a number of agents have been reported to have activity—as measured by rates of complete and partial response—in the setting of disease recurrence after ASCT, this clinical problem has not been evaluated systematically. There have been few reports of the activity or tolerability of combination chemotherapy regimens, and no comparisons of single agents to combinations. Reports of the activity of single-agent vinblastine,26 vinorelbine27 and gemcitabine28 in classical HL, and rituximab in classical30 and lymphocyte-predominant31 HL (Table 1 ) in this setting have provided clinicians and patients with relatively non-toxic treatment options. The duration of response reported for single-agent therapy is generally short, in the 6 to 8 month range, and may be more variable when applied in clinical practice. These small cohort studies do provide important information on toxicity in the post-ASCT setting—most notably myelosuppression—and may serve as a platform upon which new combinations may be tested. For example, Oki et al32 reported a response rate of 48% with the combination of gemcitabine (1250 mg/m2 intravenously day 1 and 8 every 3 weeks) plus rituximab (375 mg/m2/wk intravenously for 6 weeks), with responding patients continuing gemcitabine alone. The response rate of this combination appears to be better than with either alone,28,30 and encourages additional studies of targeting CD20 on both tumor cells and B cells in the microenvironment. Kuruvilla, et al;36 however, reported a small study adding thalidomide to vinblastine where the response rate did not appear to be better than would be expected with vinblastine alone, with significantly greater toxicity, despite the theoretical possibility of benefit of immunomodulatory agents in this disease.
The combination of vinorelbine and gemcitabine has been recently tested in a limited number of patients with HL post-ASCT, with an encouraging response rate (75%, 6 of 8 patients), and low rates of grade 4 thrombocytopenia and neutropenia;29 further evaluation of this combination is warranted. Bartlett et al reported the results of a novel combination of gemcitabine, vinorelbine and pegylated liposomal doxorubicin (GVD) in patients with recurrent HL, both prior to and for relapse after ASCT.37 Patients who had previously undergone ASCT had a lower maximum tolerated dose that those who were transplant naïve, and neutropenia and thrombocytopenia were the most common reasons for treatment delay or dose reduction: only 26% of patients received all treatment at full dose and on schedule. The response rate among 39 patients receiving GVD for relapse after prior ASCT was 75% (17% CR), and median event-free survival was 8.5 months. Combination therapy in this setting is feasible, but is associated with significantly more myelosuppression than observed in patients who have not previously undergone stem cell transplantation.
Second autologous transplantation may result in durable remission, but small patient numbers and the unknown effect of patient selection on outcome limit the interpretation of currently available data. TRM may be lower in more current series, but remains much higher than for first transplants.38 A recent report from the Center for International Blood and Marrow Transplant Research (CIBMTR) on 40 patients undergoing second transplants included 21 patients with HL: outcomes for patients relapsing within 12 months of the first transplant were very poor, but for those with relapse after more than 3 years, PFS and OS were 25% and 38%, respectively.39 Patients with HL refractory to additional chemotherapy did not benefit from a second ASCT.
Investigational Agents in Relapsed HL Post-ASCT
A number of new targeted therapies have been tested or are currently under study. Despite the sound preclinical rationale for the use of a proteosome inhibitor in relapsed HL,40 clinical results in the few small studies of bortezomib reported to date have been resoundingly negative.33–35 Similarly, response rates reported for monoclonal antibodies to the surface membrane antigen CD30 present on classical HL cells have been very low.41,42 However, very encouraging results have been reported with the use of the SGN30 antibody conjugated to monomethyl auristatin E (MMAE), an agent that binds to tubulin and causes cell-cycle arrest and apoptosis: 7 of 13 patients treated on an ongoing Phase I study at doses >1.2 mg/kg achieved a partial response.43 The deacetylase inhibitor LBH589,44 the isotype-selective histone deacetylase inhibitor MGCD0103,45 and the mTOR inhibitor RAD001 (everolimus)46 have also demonstrated activity in relapsed HL after ASCT and continue under active study. Testing of these agents and others in patients with a short duration of remission (< 6–12 months) after ASCT should be a priority.
Reduced Intensity Allogeneic Stem Cell Transplantation for HL Relapsing after ASCT
Myeloablative chemoradiotherapy and allogeneic transplantation for relapsed HL after autotransplant previously carried a prohibitively high treatment-related mortality, (TRM), and evidence of a graft-versus-tumor effect was sparse. For example, a report from the International Bone Marrow Transplant Registry of 114 patients with lymphoma undergoing myeloablative allogeneic transplants reported a rate of disease progression at 3 years of 52% and TRM of 22%.47 This translated to a relatively disappointing 3-year PFS of 25% and OS of 33%. With further follow-up it appeared that few patients were cured, with 5-year DFS reported to be only 5% and OS 24%. There was no difference in TRM, PFS or OS between patients with HL and other lymphoma subtypes. Multivariate analysis of factors predicting disease progression post-allotransplant identified the inclusion of total body irradiation in the preparative regimen and chemotherapy sensitivity or complete response to additional chemotherapy as significant factors. The authors of this multi-institutional study concluded that allogeneic transplant using myeloablative regimens was not curative for most patients with relapsed lymphoma post-autografting.
Significant progress has been made in the application of reduced- intensity conditioning regimens and planned donor leukocyte infusion, to facilitate use of this therapy in high-risk patient populations, in particular those with disease progression after an autologous transplant. Early case series were small, but suggested that such therapy was feasible even in heavily pre-treated recipients, with potentially lower early (day 100) transplant related mortality.48
Sorror et al. reported on 220 patients who had relapsed after ASCT who received myeloablative or non-ablative allogeneic stem cell transplants, including 27 patients with HL.49 A detailed assessment of comorbidities that may contribute to increased TRM was undertaken in this study. Three-year TRM for recipients of ablative transplants was 35% versus 25% for those receiving non-ablative treatment. Three-year OS rates were 45% and 53%, respectively. The two most important risk factors for TRM and OS were a hematopoietic cell transplant co-morbidity index49 (HCT-CI50) ≥ 1, and myeloablative conditioning. Age > 50, a diagnosis of HL and the use of peripheral blood stem cells all increased risk of TRM and reduced OS. Patients who had a comorbidity score of 0 fared much more favorably; TRM for non-ablative transplants was 18% versus 15% for ablative transplants and OS 68% versus 60%. Even adjusting for other risk factors, there was no difference in relapse rate, TRM or OS in this favorable patient group without significant co-morbidity. However, in patients with an HCT-CI of at least 1, TRM was significantly higher in patients receiving a myeloablative transplant (50% versus 28%, P = .009) and 3-year OS was worse (35% vs 47%, P = .04). Adjustment for histology and other variables did not change these results. Although the number of patients with HL in this series was small, it might be predicted that the patient population with relapsed HL, being on average younger than those with other lymphomas, would have a lower number of co-morbidities and be less likely to experience TRM with reduced-intensity transplantation (RIT), but this remains to be demonstrated. Further evaluation of factors contributing to toxicity and refinement of patient selection are needed.
Renewed interest in exploring RIT in relapsed HL has come from recent reports of graft-vs-tumor effects from related or unrelated donor transplants, as shown by disease response to donor leukocyte infusion for residual masses, mixed (incomplete) chimerism or documented disease regression post-transplant.51 This antitumor affect appears to be related to (and at the cost of) chronic GVHD in most but not all cases (Table 2 ). A number of patients who have had complete remission following donor leukocyte infusions have also had additional chemotherapy, making the true rate of response to this form of immunotherapy difficult to determine with confidence.
Not everyone has shown favorable disease control from graft-versus-HL effect. Baron et al56 recently reported a higher risk of relapse and inferior 3-year PFS (8%) for patients with HL compared to other hematologic malignancies, despite the fact that patients with extensive chronic GVHD had a significantly lower risk of relapse (hazard ratio 0.48). The only variable affecting overall survival in this analysis of 135 patients with a variety of lymphoma histologies was complete or partial remission at the time of transplant, indicating the importance of disease biology and chemotherapy sensitivity in outcome of allogeneic stem cell transplantation after ASCT.
The observations listed above—lower TRM with RITs in patients at lower risk for complications, and improved disease control in those with chronic GVHD—form the basis of the comparison of this strategy by Thomson et al to historical controls.57 These authors matched patients with recurrent HL after ASCT who had undergone RIT to those treated with standard strategies including chemotherapy and irradiation, and reported significantly better survival for the transplant cohort. This historical control comparison must be interpreted with caution: it generates a hypothesis, but does not provide an answer to the question of whether such patients should all be considered for RIT. In addition, a number of trials have emphasized the importance of continued sensitivity to chemotherapy prior to RIT, with disease status at transplant (CR or PR) being a very strong predictor of subsequent PFS and OS.48,56,58,59 Larger trials with uniform eligibility and treatment approaches are needed in order to more clearly define the benefits of allogeneic transplantation in this patient population.
Optimum Treatment Approach for Patients with HL with Disease Relapse Post-Autologous Transplantation
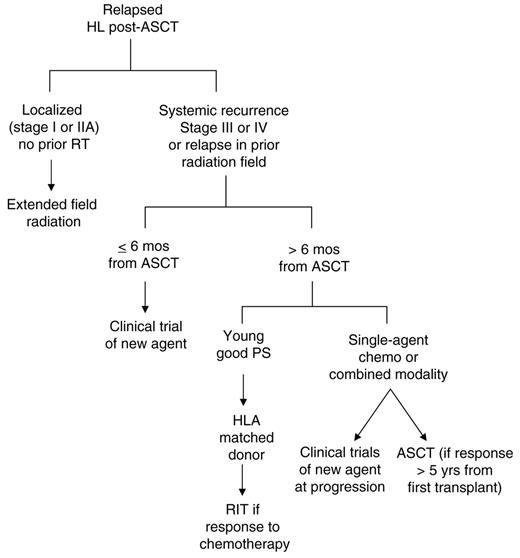
Patients who have recurred following autologous transplant have a number of treatment options available (Figure 2 ), although, in the majority of cases, treatment is ultimately palliative. Those with localized relapse in sites not previously irradiated, should be approached with involved or extended-field radiation at doses known to produce maximum disease control (30–40 Gy). Such patients can be treated in this fashion with disease control that appears at least comparable to that which is obtained with systemic therapy, with less toxicity and without loss of potential benefit from further therapies down the road. Careful follow-up of such patients allows appropriate application of systemic therapy when needed.
Patients who relapse early after autologous transplant or who present with unfavorable characteristics such as relative chemotherapy insensitivity (as suggested by multiple lines of salvage therapy prior to autologous therapy transplant), or the presence of anemia at relapse, should be considered candidates for participation in clinical trials testing new agents. A large number of novel agents are currently under investigation, with preliminary activity reported for histone deacetylase inhibitors, anti-CD30 antibody conjugates, and inhibitors of the mammalian target of rapamycin (mTOR). Such high-risk patients are unlikely to derive prolonged benefit from standard single-agent or combination chemotherapy regimens, and enrolment of these patients on clinical trials is a priority.
For patients with later relapse or who present with lower-risk features, therapeutic options may be broader. Such patients are more likely to respond to single-agent chemotherapy such as vinorelbine or gemcitibine, and may benefit from combination chemotherapy regimens (although, as stated above, the superiority of such regimens over sequential single agents has not been demonstrated). Selected patients with very long remissions (more than 5 years at our center) may undergo a second autograft, but the long-term benefits of this approach compared to other strategies is unclear,60 as is the probability of late toxicity and second malignancies.
Patients with relapse that is refractory to additional chemotherapy do poorly with RIT, and such patients should be considered for participation in clinical trials, and not allotransplant. Patients without co-morbidities, who have chemotherapy-sensitive HL and who have an HLA-identical sibling donor (or well-matched unrelated donor) might benefit from a reduced-intensity allogeneic transplant. However, this therapy still carries a substantial risk of TRM in the first year, in a group of patients whose median survival might otherwise exceed 3 years. RIT should only be undertaken in the context of well-designed clinical trials addressing fundamental aspects of stem cell transplantation, in particular those attempting to optimize the balance between non-relapsed mortality and long-term disease control. Although long-term disease-free survivors of RIT have been reported, this therapy in a post-ASCT setting remains experimental.
Chemotherapy and antibody therapy for relapsed Hodgkin lymphoma post-ASCT.
| Agent (reference) . | Number treated . | Prior ASCT . | Response . | PFS, mo . |
|---|---|---|---|---|
| Abbreviations: ASCT, autologous stem cell transplantation; PFS, progression-free survival; m, months; NS, not stated; CHL classical Hodgkin lymphoma; NLPHL, nodular lymphocyte predominant Hodgkin lymphoma. | ||||
| Vinblastine26 | 17 | 17 | 10/17 | 8 |
| Vinorelbine27 | 24 | NS | 11/24 | — |
| Gemcitabine28 | 27 | 18 | 6/27 | 6 |
| Vinorelbine + gemcitabine29 | 8 | NS | 6/8 | NS |
| Rituximab | ||||
| CHL30 | 22 | 18 | 5/22 | — |
| NLPHL31 | 15 | 2 | 14/15 | 33 |
| Rituximab + gemcitabine32 | 33 | 18 | 16/33 | 3 |
| Bortezomib33 | 14 | 13 | 1/14 | NS |
| Bortezomib34 | 30 | 19 | 0/30 | 1.4 |
| Bortezomib35 | 12 | NS | 0/12 | NS |
| Agent (reference) . | Number treated . | Prior ASCT . | Response . | PFS, mo . |
|---|---|---|---|---|
| Abbreviations: ASCT, autologous stem cell transplantation; PFS, progression-free survival; m, months; NS, not stated; CHL classical Hodgkin lymphoma; NLPHL, nodular lymphocyte predominant Hodgkin lymphoma. | ||||
| Vinblastine26 | 17 | 17 | 10/17 | 8 |
| Vinorelbine27 | 24 | NS | 11/24 | — |
| Gemcitabine28 | 27 | 18 | 6/27 | 6 |
| Vinorelbine + gemcitabine29 | 8 | NS | 6/8 | NS |
| Rituximab | ||||
| CHL30 | 22 | 18 | 5/22 | — |
| NLPHL31 | 15 | 2 | 14/15 | 33 |
| Rituximab + gemcitabine32 | 33 | 18 | 16/33 | 3 |
| Bortezomib33 | 14 | 13 | 1/14 | NS |
| Bortezomib34 | 30 | 19 | 0/30 | 1.4 |
| Bortezomib35 | 12 | NS | 0/12 | NS |
Recent results with reduced-intensity allogeneic transplantation for Hodkin lymphoma (HL).
| Author . | n . | Prior ASCT . | Prior regimens (median) . | TRM, % (time point) . | PFS, % (time point) . | OS, % (time point) . |
|---|---|---|---|---|---|---|
| Abbreviations: n, number of allogeneic transplants; ASCT, autologous stem cell transplant; TRM, transplant-related mortality; PFS, progression-free survival; OS, overall survival | ||||||
| Peggs51 | 49 | 44 | 5 | 16 (2 y) | 32 (4 y) | 56 (4 y) |
| Sureda52 | 89 | 55 | 85% ≥ 3 | 23 (1 y) | 18 (3 y) | 35 (3 y) |
| Alderlini53 | 40 | 30 | 5 | 22 (18 m) | 55 (18 m) | 61 (18 m) |
| Armand54 | 36 | 34 | 4 | 15 (3 y) | 22 (3 y) | 56 (3 y) |
| Alvarez55 | 40 | 29 | 55% ≥ 3 | 25 (1 y) | 32 (2 y) | 48 (2 y) |
| Author . | n . | Prior ASCT . | Prior regimens (median) . | TRM, % (time point) . | PFS, % (time point) . | OS, % (time point) . |
|---|---|---|---|---|---|---|
| Abbreviations: n, number of allogeneic transplants; ASCT, autologous stem cell transplant; TRM, transplant-related mortality; PFS, progression-free survival; OS, overall survival | ||||||
| Peggs51 | 49 | 44 | 5 | 16 (2 y) | 32 (4 y) | 56 (4 y) |
| Sureda52 | 89 | 55 | 85% ≥ 3 | 23 (1 y) | 18 (3 y) | 35 (3 y) |
| Alderlini53 | 40 | 30 | 5 | 22 (18 m) | 55 (18 m) | 61 (18 m) |
| Armand54 | 36 | 34 | 4 | 15 (3 y) | 22 (3 y) | 56 (3 y) |
| Alvarez55 | 40 | 29 | 55% ≥ 3 | 25 (1 y) | 32 (2 y) | 48 (2 y) |
Overall survival of 118 patients from date of relapse after autologous stem cell transplantation for relapsed or refractory Hodgkin lymphoma. Median survival is 27.6 months (95% confidence interval 20–36 months).
Overall survival of 118 patients from date of relapse after autologous stem cell transplantation for relapsed or refractory Hodgkin lymphoma. Median survival is 27.6 months (95% confidence interval 20–36 months).
Treatment algorithm for relapsed Hodkin lymphoma following autologous stem cell transplantation (ASCT)
Abbreviations: RIT, reduced-intensity allogeneic transplantation; PS, performance status; RT, radiation therapy
Treatment algorithm for relapsed Hodkin lymphoma following autologous stem cell transplantation (ASCT)
Abbreviations: RIT, reduced-intensity allogeneic transplantation; PS, performance status; RT, radiation therapy
Disclosures Conflict-of-interest declaration: The author declares no competing financial interests Off-label drug use: Gemcitabine, rituximab, lenalinomide, thalidomide, vinorelbine as components of treatment of advanced Hodgkin Lymphoma.
References
Author notes
Princess Margaret Hospital, University of Toronto, Toronto, Canada