Abstract
Systemic iron homeostasis depends on the regulated expression of hepcidin, a peptide hormone that negatively regulates iron egress from intestinal cells and macrophages by altering the expression of the cellular iron exporter ferroportin. In doing so, hepcidin can control both the total body iron by modulating intestinal iron absorption as well as promote iron available for erythropoiesis by affecting the efficiency with which macrophages recycle iron from effete red blood cells. This review focuses on the systemic and cellular physiology of hepcidin regulation in relation to iron stores, erythropoiesis, inflammation, and hypoxia and how hepcidin regulation and dysregulation contributes to normal iron homeostasis and iron metabolism disorders.
Systemic Iron Metabolism and Erythropoiesis
In the adult human, the daily production of more than 200 billion erythrocytes requires more than 20 mg of elemental iron. The vast majority of this iron comes from the recycling of senescent erythrocytes by macrophages of the reticuloendothelial system; only 1 to 2 mg of the daily iron supply derives from intestinal absorption, which, at a steady state is sufficient only to replace the iron lost through insensible means, such as epithelial cell sloughing and functional and dysfunctional bleeding (reviewed in Andrews1). Because of their singularly large requirement for iron, erythroid progenitors are uniquely dependent upon the transferrin (Tf) cycle, which provides both a high affinity and high avidity mechanism to satisfy their iron needs. In the absence of sufficient Tf-bound iron, erythropoiesis rapidly becomes iron-limited, leading to anemia, as occurs in iron-deficiency or chronic inflammation (see below). Within the last 7 years, it has become increasingly evident that the major physiological regulator of body iron stores and the availability of serum iron is the peptide hormone hepcidin.
The Iron Regulatory Hormone Hepcidin and Its Effector, the Iron Exporter Ferroportin
Hepcidin is a 25–amino acid peptide that is produced primarily by the liver in response to a variety of stimuli known to modulate tissue iron stores and serum iron availability (see below).2–4 It circulates in the plasma, is filtered by the kidney, and accumulates in urine.3,4 The presence of hepcidin in the plasma negatively regulates the egress of iron from cells, such as intestinal epithelial cells and macrophages, involved in transport of iron into extracellular spaces.5–8 For example, iron taken up from the diet across the apical membrane of duodenal enterocytes—the site of intestinal iron absorption—must be transported across the basolateral membrane of the cell in order to enter the extracellular fluid within the intestinal villus. Similarly, reticuloendothelial macrophages engulf senescent red blood cells (RBCs) and the iron recovered from heme is transported to the plasma or extracellular fluid where it can bind Tf, whereupon it becomes the primary source of iron-Tf available for erythropoiesis.
The iron exporter required for iron egress from enterocytes, macrophages, as well as all other iron exporting cells including placental syncytiotrophoblasts and hepatocytes, is ferroportin 1 (FPN1, SLC40A1, IREG1, or MTP1).9–12 FPN1 is a 12 transmembrane domain protein present within intracellular compartments as well as on the basolateral surface of epithelia and on the surface of non-polarized cells. It transports ferrous iron (Fe2+), which must be oxidized to ferric iron (Fe3+) by one of several ferroxidases that also serve to stabilize FPN1,13–15 before iron can bind to Tf. FPN1 is remarkable insofar as it is not only the effector of cellular iron export, but also the receptor for hepcidin, its primary regulator.16 Hepcidin binds to FPN1 present on the cell surface, induces the phosphorylation of amino acids located on an intracellular loop of FPN1 triggering the internalization of the hepcidin-FPN1 complex, leading to the ubiquitinization of FPN1 and lysosomal degradation of both proteins.17 Commensurate with this mechanism, hepcidin deficiency leads to persistence of FPN1 on the cell membrane and iron overload,6,7,18 whereas administration of synthetic hepcidin leads to a rapid, sustained decrease in serum iron (i.e., hypoferremia),19,20 due to internalization of macrophage expressed ferroportin,21 as well as inhibition of intestinal iron absorption.22 Thus, overall, hepcidin expression both restricts iron absorption and macrophage iron release, eventuating in reduced body iron stores and limiting iron available for erythropoiesis.
Hepcidin Regulation in Response to Iron Stores: the BMP Signaling Pathway
Since there is no known regulated physiological pathway for excreting excess iron from the body, homeostatic mechanisms that control total body iron stores are deftly tuned to regulating intestinal iron absorption. The constellation of regulatory systems that contribute to this effect have been referred to as the “stores regulator.”23 Until recently, the identity of the stores regulator has been elusive; however, the rapid progress in understanding hepcidin regulation as it relates to the pathophysiology of the primary, inherited iron overload disorders, collectively called “hereditary hemochromatosis” (HH), has clarified many of the cellular and molecular mechanisms of regulating body iron stores. We now recognize at least four autosomal recessive and one dominant, genetically distinct forms of hereditary hemochromatosis. Each shares the common pathophysiology of a chronic inappropriately high rate of intestinal iron absorption for the degree of systemic iron stores. In the four autosomal recessive forms—due to mutations in the genes encoding HFE (HFE),24 hepcidin (HAMP),25 hemojuvelin (HJV),26 and transferrin receptor-2 (TFR2)27—the level of plasma or urinary hepcidin is inappropriately low for the degree of systemic iron stores.26,28–34 Furthermore, the extent of this relative hepcidin deficiency appears to correlate roughly with the clinical severity of the phenotype. In addition to parenchymal (liver, heart, pancreas) iron overload, all recessive forms of HH are accompanied by a deficiency of iron within macrophages; parenchymal iron overload and relative macrophage iron deficiency are the cardinal cellular phenotypes of hepcidin deficiency and ferroportin excess. The autosomal dominant form of HH—due to mutations in the FPN1 gene itself—has a somewhat variable clinical phenotype, however, those patients with a clinical-pathological presentation similar to the autosomal recessive forms of HH have mutations that render FPN1 insensitive to down-regulation by hepcidin.35–38 Thus, it would appear that dysregulation of the hepcidin-ferroportin axis can account for many, if not all, aspects of the systemic and cellular HH phenotypes. It is not surprising, then, that work to determine the cellular functions of the autosomal recessive HH proteins has provided abundant insight into how hepcidin is regulated at the molecular and cellular levels in response to iron stores.
Although it had been know that in vivo hepatocytes responded to iron overload by up-regulating hepcidin,2 the true significance of hepcidin in iron metabolism was not appreciated until the targeted disruption of the adjacent Usf2 locus in mice led to the fortuitous discovery that profound down-regulation of hepcidin expression led to rapid, severe iron overload.7 This observation was further substantiated by targeted disruption of the murine Hamp gene itself,6 as well as by the observation that a small number of individuals with a severe, early onset form of HH, termed juvenile hemochromatosis (JH), have recessive loss-of-function mutations in hepcidin.25
Regulation of hepcidin mediated by BMP signaling
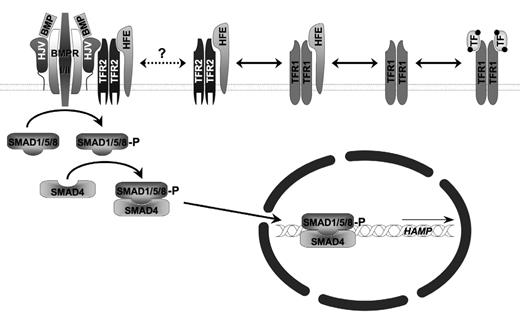
While the pathogenesis of JH due to hepcidin mutations is easily understood—complete loss of hepcidin leads to unabated cell surface expression of FPN causing unregulated intestinal iron uptake—the pathogenesis of JH due to mutations of hemojuvelin (HJV), which constitute ~95% of JH cases,26,39 was initially more obscure, but eventually led to some of the greatest insights into the molecular control of cellular hepcidin expression (Figure 1 ).
HJV is a glycosylphosphoinositide (GPI)-linked cell surface protein expressed by skeletal muscle and cardiac muscle, as well as by hepatocytes.26 It is homologous to several proteins expressed in the central nervous system termed repulsive guidance molecules (RGMs), which exert an effect on neuronal growth by acting as co-receptors for bone morphogenetic proteins (BMPs). BMPs are soluble autocrine or paracrine factors that signal through hetero-multimeric cell surface receptors. BMP binding to a co-receptor facilitates the interaction with a type I BMP receptor with a constitutively active serine/threonine kinase BMP type II receptor. Phosphorylation of the type I receptor initiates an intracellular signaling cascade involving phosphorylation of stimulatory SMAD (sons of mothers against decapentaplegic homologue) proteins (SMADs 1/ 5/8) that associate with a common co-SMAD, SMAD4, which together then translocate to the nucleus to stimulate gene transcription by binding to BMP-responsive DNA elements (BREs).
Substantial recent data support the notion that HJV is a BMP co-receptor and can facilitate signaling and induce hepcidin expression by many different BMPs in primary hepatocytes and hepatocyte cell lines.40–43 The observation that infusion of BMP-2 into mice stimulates hepcidin mRNA expression and induces hypoferremia demonstrates the in vivo relevance of this pathway.42 Furthermore, HJV also exists in a soluble form, sHJV, in plasma that appears to originate from proteolytic processing by furin or another pro-protein convertase with similar specificity.44,45 In model systems, sHJV can antagonize BMP signaling, presumably by binding BMPs such that cell surface-dependent association of the heteromeric BMP type I/II receptor does not occur.42,46 The concomitant loss of HJV from the cell surface may equally contribute to a dampened BMP response. Initial reports indicate that HJV-dependent, liver-specific BMP signaling can utilize a number of different BMP receptors, including the BMP type II receptors activin receptor IIA (ActRIIA) and BMP receptor II (BMPRII) and the BMP type I receptors anaplastic lymphoma kinase (ALK)-2 and -3.47 The evolving data also suggest that BMPs 2, 4 and 6 may be most relevant for HJV-mediated hepcidin regulation in hepatocytes, as they are each expressed there and the effect of BMP-2 and BMP-4 can be inhibited by soluble HJV.42,48,49 A liver-specific knockout of SMAD4 leads to a severe systemic iron overload phenotype, further substantiating the central role of this pathway in regulating hepcidin expression in the hepatocyte.50 As yet undefined DNA sequences located within the region 1.6 kb to 2.0 kb upstream of the hepcidin transcriptional initiation site appear to mediate the response to BMPs.51
Iron-dependent regulation of hepcidin expression
In vitro exposure of hepatocytes to medium with increasingly more saturated transferrin, as well as chronic iron overload and acute iron administration in vivo, promotes HJV-dependent BMP signaling and hepcidin gene transcription, suggesting that the BMP signaling pathway in hepatocytes is iron regulated.41,49,52 As none of the BMP-related mechanisms described above depends upon iron, how then is iron-dependent regulation of hepcidin achieved? The key link may be that holo-Tf (diferric-Tf) appears to regulate BMP signaling through a mechanism that involves a series of multi-protein complexes and competitive cell surface regulatory interactions that include the transferrin receptor 1 (TFR1) and the other HH proteins, HFE and TFR2.
Mutations in the atypical class I major histocompatibility complex (MHC)-like molecule HFE underlie the great majority of cases of HH, so-called HFE-associated, Type I, HLA-linked, or “classical” HH.53 HFE-associated hemochromatosis has a substantially reduced penetrance and concordantly not as severe a relative deficiency in hepcidin expression as other forms of HH.30,54 HFE associates with TFR1 and has a partially overlapping binding site on TFR1 with holo-transferrin.55–60 Consequently, HFE can compete with TFR1 for holo-Tf binding. Nonetheless, the binding site on TFR1 for HFE and holo-Tf are structurally distinct and mutants that selectively permit the one, but not the other, interaction have demonstrated that hepcidin expression is related to the amount of HFE not complexed with TFR1 and available to bind TFR2.61 Likewise, HFE can associate with TFR2 in a manner distinct from TFR1, can bind holo-Tf, and is stabilized by this interaction.62–68 Preliminary results have been presented suggesting that TFR2 can associate with HJV, linking iron sensing with the BMP pathway.69 Thus, it would appear that the link between iron and HJV-dependent BMP signaling lies in competitive interactions between HFE, TFR1, TFR2 and holo-transferrin, stabilization of TFR2 by holo-transferrin, and a provisionally reported positive regulatory effect of interactions between TFR2 and HJV (Figure 1 ). In this schema, disruption of HFE, TFR2, or HJV would shift the equilibrium toward diminished hepcidin transcription by desensitizing the BMP-signaling cascade to the regulatory influences of holo-transferrin; whereas an increase in the holo-Tf concentration (nominally the Tf saturation) that typically accompanies an iron replete or overloaded status would favor enhanced hepcidin mRNA production, in the absence of HFE, TFR2, or HJV this regulatory effect is decoupled from its effector signaling pathway.
In sum, although some of the molecular mechanisms that link the sensing of iron stores to the BMP signaling pathway in hepatocytes have not been fully elucidated, it is abundantly clear that this pathway likely constitutes the predominant mechanism of regulating hepcidin in response to body iron stores.
Hepcidin Expression in Response to Inflammation: IL-6- and STAT3-Dependent Pathways
Hepcidin was originally described as a cationic urinary antimicrobial peptide,3,4 but its actual role in bacterial and fungal cytotoxicity is debatable, at best. Nevertheless, the hepcidin gene remains robustly responsive to inflammation, whereupon it could be seen as a mediator of a primitive innate immune pathway designed to withhold iron from invasive organisms. While this response might be an appropriate reaction to infection, in other contexts, such as non-infectious inflammatory conditions (e.g., autoimmunity), the induction of hepcidin by inflammation could be viewed as maladaptive.
The anemia of inflammation
Nowhere is this regulatory maladaptation more relevant than in the pathophysiology of the anemia of inflammation (AOI), also known as the anemia of chronic disease. AOI is characterized by a normochromic, normocytic anemia, associated with abnormal iron utilization, erythropoietin hyporesponsiveness, and decreased RBC survival. The increased serum ferritin—indicative of increased macrophage iron stores—and decreased serum iron/transferrin saturation—indicative of decreased macrophage iron recycling—typical of this disorder suggest a condition of hepcidin excess, as is seen in several murine models of hepcidin overexpression.5,70,71 Indeed, hepcidin is markedly increased in patients with AOI,72 and administration of inflammatory mediators such as interleukin-6 (IL-6) or bacterial lipopolysaccharide (LPS) induces hepcidin production in mice and/or humans.73–75 Furthermore, in animal models, chronic sterile or septic abcesses induce hepcidin expression and lead to serum hypoferremia.75,76 Similarly patients with hepatic tumors that over-express hepcidin77 or mutations in a protein, trans-membrane protease serine 6 (TMPRSS6)78–82 that leads to constitutive hepcidin over-expression develop a microcytic anemia that resembles AOI insofar they are characterized by functional or actual iron deficiency associated with resistance to iron therapy. Consequently, it is not difficult to imagine how some patients with AOI can become truly iron deficient and develop microcytic RBCs.
A substantial body of evidence indicates that the predominant inflammatory mediator of hepcidin expression is IL-6. In addition to in vivo studies cited above, primary hepatocytes or hepatocyte cell lines exposed to IL-6 respond by up-regulating hepcidin mRNA.42,43,50,72,75 The extent to which hepcidin may be induced by other inflammatory agents, such as LPS, interleukin-1 (IL-1) or tumor necrosis factor-α (TNFα), likely depends up their ability to secondarily promote IL-6 expression.72,75,83
Similar to many other cytokines, IL-6 binding to its receptor stimulates a signaling pathway that leads to the activation of a signal transduction and activator of transcription (STAT) family member. Several research groups have independently reported that STAT3—the STAT involved in IL-6–dependent signal transduction—activates hepcidin gene transcription by binding to a conserved DNA element in the proximal promoter of the hepcidin gene.84–86 While this observation could sufficiently explain the inflammation-hepcidin link, it is increasingly evident that the hepcidin response to IL-6 may require cooperative activity of the BMP pathway. Specifically, soluble HJV, chemical inhibitors of BMP signaling, and hepatocyte-specific loss of SMAD4 all negatively modify the transcriptional response of the hepcidin gene to IL-6.42,43,50
Regulation of Hepcidin in Response to Anemia, Erythropoiesis, and Hypoxia
The erythroid regulator
A wealth of historical data indicates that the stereotypical physiological response to anemia, accelerated erythropoiesis, and hypoxia is to stimulate iron absorption. This is particularly the case in anemias predominantly characterized by ineffective erythropoiesis—lysis of immature nucleated erythroid cells within the bone marrow—as is seen in the thalassemias, megaloblastic anemias, and sideroblastic anemias. By contrast, the iron absorption pathway is not substantially activated in anemias associated with hemolysis—peripheral destruction of mature enucleated erythroid cells—such as hereditary spherocytosis. The means by which the bone marrow communicates intra-medullary iron usage and turnover with the iron absorptive machinery has been termed the “erythroid regulator.”23
Hepcidin response to anemia and erythropoiesis
Whether or not the iron modulatory signals in response to anemia, accelerated erythropoiesis, and hypoxia are identical, it is clear that they, like the stores regulator and the reaction to inflammation, each ultimately alters hepcidin expression as a final common pathway. For example, in mice, anemia due to acute phlebotomy- or phenylhydrazine-induced acute hemolysis suppresses hepcidin production.76,87–89 This effect of anemia is not dependent on a pathway involving HJV,90 but does require ongoing erythropoietic activity, as chemotherapy- or radiation-induced marrow aplasia abrogates this effect and actually stimulates hepcidin expression above baseline.87,88 Furthermore, as shown by the demonstration that animals pre-loaded with iron dextran down-regulate hepcidin in response to anemia,76 it would appear that the erythroid regulator can supervene in situations where anemia and iron overload coexist. In practical terms, this observation can account for the relatively low hepcidin levels seen in patients91–93 and mice94–96 with β-thalassemia intermedia, who have coexisting highly active erythropoiesis and iron overload. By comparison, β-thalassemia major patients on chronic transfusion therapy, which suppresses erythropoiesis and contributes substantially to iron stores, have more appropriately elevated hepcidin levels.91–93
The signal that communicates the level of erythropoiesis to the liver to signal hepcidin suppression is an area of active research. Historically, serum transferrin receptor (sTFR1), which correlates well with erythroid mass and is responsive to iron deficiency, was considered a strong candidate for this activity, but over-expression of sTFR1 does not appear to alter iron metabolism in mice.97 Nevertheless, several research groups have provisionally identified activities induced in thalassemic serum that suppress hepcidin expression by hepatocytes or hepatocyte cell lines.98,99 Using a transcriptional profiling approach to identify factors up-regulated during erythropoiesis, Tanno and colleagues recently demonstrated that the transforming growth factor-β (TGFβ) superfamily member growth and differentiation factor 15 (GDF15) is up-regulated in thalassemic serum and can suppress hepcidin expression in vitro.99 More recently it has been shown that in vitro erythropoietin signaling down-regulates hepcidin expression through a mechanism involving the transcription factor core element binding protein α (C/EBPα) and a cognate DNA binding site present in the hepcidin promoter.100,101 The extent to which these and other humoral factors produced by erythropoietic cells or elsewhere in response to anemia or hypoxia directly contribute to hepcidin suppression in vivo remain to be seen.
Hepcidin response to hypoxia
While any response of the liver to erythropoietic mass necessitates a circulating factor, it is clear that hypoxia can inhibit hepcidin expression in hepatocytes directly, without an intermediary, as hepatocyte cell lines or non-anemic animals exposed to hypoxic conditions down-regulate hepcidin production.76 It is highly likely that the hypoxia inducible factor/von Hippel-Lindau (HIF/vHL) pathway, which mediates responses to hypoxia, among other cellular stressors, serves to regulate hepcidin in response to ambient oxygen tension. In normoxic, iron-sufficient conditions, an oxygen and iron-dependent prolyl hydroxylase modifies a HIF regulatory subunit, such as HIF-1α, that then associates with the VHL protein that promotes HIF α-subunit degradation. In hypoxia or iron deficiency, the hydroxylases are inactive, allowing the regulatory subunit to accumulate, translocate to the nucleus, associate with a constitutively expressed HIF subunit, HIF-1β and bind to promoter elements to modulate gene transcription.102 Peyssonnaux and colleagues recently demonstrated that mice with liver-specific, conditional inactivation of HIF1α maintained on an iron-deficient diet develop inappropriately high levels of hepcidin.103 This would have the consequence of further limiting iron availability despite the nutritional deficiency. Although, they did not examine the effect of hypoxia on hepcidin production in these animals, one might infer that a similar paradoxical effect on hepcidin expression might also be observed. In vitro, inhibition of the prolyl hydroxylases that promote HIF α-subunit degradation negatively regulates hepcidin transcription.104 There is, however, no consensus as to whether or not this regulation is effected through a hypoxia response element (HRE) typical of HIF/vHL pathway-dependent genes.102,104 Nevertheless, it is apparent that HIF/vHL can directly participate in hepcidin gene regulation by both iron and hypoxia.
Summary
Although the regulatory pathways that control hepcidin gene transcription are diverse, the role of hepcidin as a final common mediator of systemic and cellular iron transport and storage in response to iron stores, inflammation, erythropoiesis, and hypoxia is now well established. Insights gained into the pathogenesis of common disorders such as hereditary hemochromatosis and the anemia of chronic disease have not only contributed to our knowledge of hepcidin regulation, but have also sowed the seeds for a new era in hepcidin biology—one that is predicated on the notion that measuring and manipulating hepcidin levels will, in the future, have a role in diagnosing and treating any number of iron related disorders.
Iron-dependent BMP signaling. Transferrin receptor 1 (TFR1), the autosomal recessive hereditary hemochromatosis proteins HFE, transferrin receptor 2 (TFR2), and hemojuvelin (HJV) as well as the bone morphogenetic protein receptor type I and II (BMPR I/II) complex are associated with the cell surface. HFE associates with TFR1 and TFR2 in an equilibrium modified by the concentration of holotransferrin (TF). It has been suggested that HFE and TFR2 can further associate with the HJV-BMPR I/II signaling complex to modify BMPR signaling mediated by phosphorylation upon sons of mothers against decapentaplegic homologues (SMADs) 1, 5, and 8. Association of phosphorylated SMAD1/5/8 with a common co-SMAD, SMAD4, leads to translocation of the complex into the nucleus and stimulation of hepcidin (HAMP) gene expression.
Iron-dependent BMP signaling. Transferrin receptor 1 (TFR1), the autosomal recessive hereditary hemochromatosis proteins HFE, transferrin receptor 2 (TFR2), and hemojuvelin (HJV) as well as the bone morphogenetic protein receptor type I and II (BMPR I/II) complex are associated with the cell surface. HFE associates with TFR1 and TFR2 in an equilibrium modified by the concentration of holotransferrin (TF). It has been suggested that HFE and TFR2 can further associate with the HJV-BMPR I/II signaling complex to modify BMPR signaling mediated by phosphorylation upon sons of mothers against decapentaplegic homologues (SMADs) 1, 5, and 8. Association of phosphorylated SMAD1/5/8 with a common co-SMAD, SMAD4, leads to translocation of the complex into the nucleus and stimulation of hepcidin (HAMP) gene expression.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Associate Professor of Pathology, Children’s Hospital Boston; Harvard Medical School, Boston, MA