Abstract
Chronic graft-versus-host disease (GVHD) is an immune-mediated disorder that occurs frequently after allogeneic hematopoietic cell transplantation (HCT). Most cases are diagnosed within the first year at a median of 4 to 6 months after HCT, but 5–10% of cases are initially diagnosed beyond the first post-transplant year. Chronic GVHD most often involves the skin and mouth, but almost any other organ system can be involved. Correct diagnosis is critical so that appropriate therapy can be started promptly to minimize symptoms and prevent irreversible organ damage. Initial treatment should be with cortico-steroid-based therapy. Optimal secondary treatment as not been established, although a large number of agents may provide benefits. A 2004 NIH conference focused on development of consensus criteria for chronic GVHD. Six papers published in 2005 and 2006 propose consensus definitions for chronic GVHD diagnosis and scoring, pathology, biomarkers, response criteria, supportive care and design of clinical trials.
This review will focus on common clinical presentations and principles for managing chronic GVHD. The most frequently used secondary therapies and ongoing trials are summarized. New concepts from the NIH consensus conference are discussed.
Introduction
Chronic graft-versus-host disease (GVHD) is the most serious and common long-term complication of allogeneic HCT, occurring in 30% to 70% of adults and children surviving more than 100 days.1,2 The median time to onset is 4 to 6 months after HCT, but 5% to 10% of cases are diagnosed beyond one year. Approximately half of affected people have 3 or more involved organs, and treatment typically requires immunosuppressive medications for a median of 2 to 3 years. In a subset of patients, treatment is prolonged, with 15% still receiving immune suppressive therapies 7 or more years after the initial diagnosis of chronic GVHD. Because of higher treatment-related (non-relapse) mortality, chronic GVHD remains a major cause of late death despite its association with a lower relapse rate.3,4 The morbidity and mortality associated with chronic GVHD is caused both by chronic GVHD-associated immunodeficiency and organ dysfunction and by the immune suppressive medications used treat it. The effectiveness of management strategies beyond systemic corticosteroids is controversial,5 and practicing physicians are unenthusiastic about the effectiveness of most agents.6 This review will focus on common clinical presentations of chronic GVHD and principles for managing patients with this late complication.
Common Clinical Presentations
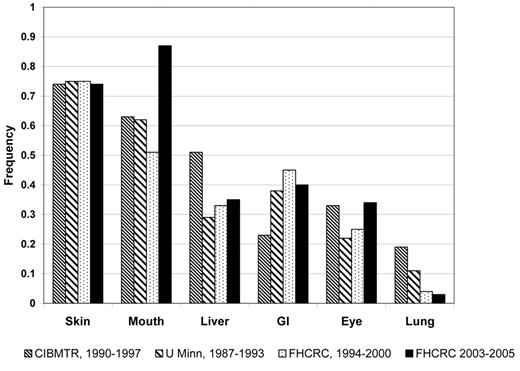
Although chronic GVHD manifestations at onset are heterogeneous, the frequency of specific organ manifestations appears to be similar after peripheral blood and bone marrow grafts, related and unrelated donors, myeloablative and reduced-intensity conditioning, and in adults and children. “Diagnostic” features sufficient to establish the diagnosis of chronic GVHD include sclerosis, lichen-planus–like lesions, poikiloderma, esophageal webs, fasciitis and bronchiolitis obliterans. In contrast, “distinctive” features are highly suggestive of chronic GVHD but are not sufficient to establish the diagnosis by themselves. Distinctive features include oral ulcers and atrophy, onchodystrophy, and sicca syndrome. For the purpose of clinical trials, distinctive features need to be confirmed as chronic GVHD by a biopsy or other diagnostic test.7 The most common sites involved at the initial diagnosis of chronic GVHD are skin (75%), mouth (51%–63%), liver (29%–51%), and eye (22%–33%). Other less frequently involved organs include gastrointestinal tract/weight loss (23%–45%), lung (4%–19%), esophagus (7%), female genital tract (1%), and joints (6%)4,8,9 (Figure 1 and Table 1 ).
Recognizing chronic GVHD and distinguishing it from other problems can be challenging. A high level of suspicion for chronic GVHD should be maintained for any perturbation in laboratory tests, symptoms or signs in a patient within the first post-transplant year. Conversely, every problem after allogeneic HCT is not chronic GVHD. For instance, other conditions caused by eczema, iron overload, hypothyroidism, adrenal insufficiency, infections or medications can be mislabeled as chronic GVHD.
The skin and mouth are most commonly involved, and are judged on the basis of physical examination and biopsy results. A variety of clinical atlases provide guidance on skin and mouth physical findings10,11(http://marrow.org/Physicians/Post-Transplant_Care/Post-Transplant_Care.aspx#graft). Signs of skin chronic GVHD which are present earlier in the course are lichen-planus–like lesions (violaceous, flat-topped and usually less confluent than the rash of acute GVHD) or dry papulosquamous lesions (resembling eczema or actinic keratoses). Later involvement includes sclerotic changes (morphea, sclerosis, fasciitis, panniculitis) and poikiloderma (thinning of the epidermis and dermis, telangectasias, and mottled pigmentation). Both sclerosis and poikiloderma may be associated with poorly healing skin ulcers.
Early oral involvement includes lichen-planus–like lacy buccal involvement, xerostomia from salivary gland dysfunction and food sensitivity. Oral pain, erythema and non-healing ulcers can occur. Fibrosis involving buccal tissues occurs late and causes decreased jaw range of motion.
Many organ manifestations are less common but are associated with disabling symptoms. Eye xerophthalmia and keratoconjunctivitis sicca cause irritation and pain. Bronchiolitis obliterans causes dyspnea, cough, wheezing and an obstructive pattern on pulmonary function testing with air trapping on high-resolution computerized tomography of the chest. Vulvo-vaginal involvement results in ulcers, web formation, and strictures causing discomfort. Arthralgias, myalgias, serositis and neurologic manifestations may occur. Thrombocytopenia, eosinophilia, autoimmune neutropenia and hemolytic anemia are also observed.
Until recently, acute and chronic GVHD were distinguished based on whether immune-mediated organ dysfunction occurred before 100 days or more than 100 days after HCT. Acute GVHD was clinically diagnosed when diffuse maculopapular rash, erythroderma, nausea, vomiting, anorexia, profuse diarrhea, ileus or cholestatic hepatitis occurred before day 100 after HCT. However, the NIH consensus conference clarified that when these manifestations occur after day 100, they should still be categorized as “acute” GVHD. Specifically, a patient with hepatic (transaminitis, cholestasis) or GI involvement (stomach, intestinal or colon dysfunction causing nausea, vomiting, diarrhea or weight loss) without concomitant diagnostic or distinctive manifestations of chronic GVHD is now categorized as “persistent, recurrent or late-onset” acute GVHD. Table 2 shows the distinction between the types of GVHD following HCT. Flowers and colleagues at Fred Hutchinson Cancer Research Center (FHCRC) and Jagasia and colleagues at Vanderbilt reviewed medical records to see how many patients previously considered to have chronic GVHD would no longer qualify per the NIH consensus criteria. Approximately 40% were reclassified into persistent, recurrent or late-onset acute only without features of chronic GVHD12 (P. Martin, personal communication, May 2008).
Management Principles
Initial therapy
Local treatment alone may ameliorate some bothersome chronic GVHD manifestations.13 Examples include dexamethasone oral rinses for mouth sensitivity; eyedrops, punctal plugs and Boston scleral lenses14 for dry eyes; and topical steroids or topical tacrolimus for localized epidermal skin involvement. However, systemic immune suppressive therapy should be started if symptoms are more bothersome or organ involvement more widespread. The NIH guidelines suggest consideration of systemic treatment if 3 or more organs are involved or any single organ has a severity score of more than 2 (e.g., 19% to 50% BSA involvement, moderate oral symptoms with disease signs with partial limitation or intake, joint tightness).7 Systemic treatment may also be considered for patients with mild overall chronic GVHD severity if they have high-risk characteristics associated with chronic GVHD-related mortality (i.e., platelets below 100,000/μL, progressive onset, corticosteroid dose greater than 0.5 mg/kg/day at the time of initial chronic GVHD diagnosis). Initial therapy usually includes corticosteroids at a dose of 1 mg/kg/day actual body weight unless contraindicated by co-morbid disease. Some clinicians cap the maximum daily steroid dose at 80 or 100 mg/day even if patients weigh more. At Fred Hutchinson Cancer Research Center, full-dose steroids are given for approximately 2 weeks, followed by a taper schedule to reach an alternative day dose regimen as soon as allowed by signs and symptoms. There is no evidence that the threshold for initiating systemic chronic GVHD therapy or choice of initial agent should be different for patients deemed at higher risk for recurrent malignancy.
Calcineurin inhibitor therapy is often increased to therapeutic levels or started concurrently, but there is little evidence that combination therapy is required for control of chronic GVHD. Koc et al reported results of a randomized study comparing prednisone alone to prednisone plus cyclosporine in patients with extensive chronic GVHD without thrombocytopenia.15 The cumulative incidence of transplant-related mortality, survival, relapse, need for secondary chronic GVHD therapy and discontinuation of immunosuppressive medications were not significantly different between the two study arms, although the rate of avascular necrosis was lower in the combination treatment arm. Thus, this study did not confirm that initial combination therapy including cyclosporine improved control of chronic GVHD although steroid complications were reduced with combination therapy. A post hoc analysis found that survival without recurrent malignancy was better in the prednisone-only arm (P = .03) if patients had high-risk chronic GVHD.
Nevertheless, the quest to improve initial therapy for chronic GVHD continues. Randomized trials have evaluated thalidomide and hydroxychloroquine, but not documented benefit.16,17 Initial combination therapy with mycophenolate mofetil is being tested in Phase III studies in both the United States and Europe. The U.S. trial was closed in June 2008 when an interim analysis after 150 of 230 patients were enrolled concluded that the primary endpoint was unlikely to differ between the two arms. Accrual on the European trial continues.
Vogelsang has estimated that about 90% of patients who are going to respond to treatment do so within 3 months and will be able to begin a steroid taper.18,19 One approach to steroid tapering at FHCRC is as follows: If chronic GVHD manifestations are stable or improving after two weeks, corticosteroids are tapered by 25% per week to a target dose of 1 mg/kg every other day over the next 6 to 8 weeks. If organ manifestations are severe, the patient has high-risk chronic GVHD features or the patient has less than a complete response, the dose may be held at 1 mg/kg every other day for another 2 to 3 months, then tapered by 10% to 20% per month for a total corticosteroids treatment course of 9 months. Another approach is to skip the plateau phase of 1 mg/kg every other day and continue tapering by 10% to 20% per month but slow down the taper once a dose of 0.5 mg/kg every other day is reached. If chronic GVHD flares during the corticosteroid taper, increasing the dose slightly may bring manifestations under control again. Pediatricians may treat with higher doses of steroids for a longer period than physicians treating adults.20 After successful completion of the steroid taper, the other immune suppressive medications are tapered off sequentially with dose reductions every 2 to 4 weeks. Review of 330 patients transplanted in Seattle from 1994–2000 and diagnosed with chronic GVHD showed that approximately a third of patients respond to initial therapy and never receive secondary agents.21
Supportive care
As infection is the primary cause of death in patients with chronic GVHD, patient education, infection prophylaxis and supportive care are very important components of chronic GVHD management.13,22 Approaches aimed at symptomatic relief rather than chronic GVHD resolution can provide great benefits by improving patients’ functional status and quality of life.13
Chronic GVHD is associated with a higher rate and earlier onset of viral, fungal and bacterial infections,23,24 and most physicians prescribe prophylactic anti-infective agents in hopes of preventing infections. Prophylaxis against Pneumocystis jiroveci should be administered to all patients undergoing treatment of chronic GVHD. Splenic dysfunction occurs with chronic GVHD, and prophylaxis against encapsulated bacteria is recommended with daily trimethoprim-sulfamethoxazole, penicillin VK or equivalent antibiotics.13 Patients should receive prophylactic antiviral agents for prevention of varicella zoster virus reactivation while they are still on immune suppressive medications. At FHCRC, we continue antibiotic prophylaxis for 6 months after discontinuation of all immune suppressive agents for two reasons: prevention of infections that may be associated with chronic GVHD flares, and because this seems to be the period of risk for recurrent chronic GVHD activity requiring reinitiation of GVHD treatment and infectious prophylaxis. Patients at risk for late cytomegalo-virus (CMV) disease, such as those receiving high-dose systemic corticosteroids, should have CMV activity monitored closely, and treatment initiated if indicated. Post-transplant vaccination guidelines are available on the Centers for Disease Control and Prevention web site (http://www.cdc.gov/mmwr/mmwr_rr/rr_cvol.html).22 Live virus vaccinations such as measles, mumps, rubella (MMR) are contraindicated until patients are free of chronic GVHD and off immune suppression for 1 year or after 2 years after HCT, whichever is longer.
A higher incidence of secondary malignancy of the skin, buccal cavity, liver, brain/central nervous system, thyroid, bone and connective tissue is seen after allogeneic HCT.25 Chronic GVHD has been associated with increased risks for basal cell carcinoma, squamous cell carcinoma, thyroid cancer and oral cancers.25–28 Thus, periodic physical examination of the skin, mouth and thyroid seems prudent. All patients with chronic GVHD should be advised to use adequate sun protection and report suspicious lesions.
Secondary therapy
If chronic GVHD fails to respond or progresses through corticosteroid-based therapy, then additional therapy is indicated. Additional therapy may also be necessary if chronic GVHD improvement plateaus after 4 to 8 weeks of sustained therapy or if a patient develops treatment-related toxicity from initial therapy.29 “Steroid-refractory” chronic GVHD is generally defined as either failure to improve after at least 2 months or progression after 1 month of standard corticosteroid-based immunosuppressive therapy. Care must be taken to establish the trajectory of signs and symptoms since chronic GVHD manifestations can fluctuate.
A number of Phase II trials of secondary agents have been published, and most report encouraging complete plus partial success rates. However, most trials contain relatively few patients with heterogeneous organ involvement and advanced phases of organ manifestations. Reported response rates are usually based on four categories: complete (resolution of all reversible chronic GVHD manifestations), partial (> 50% but less than complete organ responses), no response (< 50% response), and progression (worsening while on therapy). The NIH consensus conference has suggested some criteria to help standardize the response definitions with objective measurement tools.29,30
Choice of secondary therapy is currently based on the clinician’s expertise and sense of what appears to represent the most effective intervention for the patient’s particular manifestations of chronic GVHD. Also considered are any comorbidities, anticipated toxicities and logistical issues (need for therapeutic level monitoring, need for intravenous access, insurance coverage, and available clinical trials). Initial therapies are usually continued, unless unacceptable toxicity has occurred. Table 3 summarizes the published organ specific response rates for the best studied secondary agents (mycophenolate mofetil, high-dose corticosteroids, extracorporeal photopheresis, sirolimus, 2-deoxycoformycin, calcineurin inhibitors, rituximab, thalidomide). The final two columns in Table 3 show the percentage of adult and pediatric physicians selecting each agent to treat steroid-refractory multiorgan chronic GVHD.20
Smaller phase II studies or case reports note efficacy for acitretin, alemtuzumab, antithymocyte globulin, azathioprine, bortezomib, clofazimine, daclizumab, etanercept, halofuginone, hydroxychloroquine, infliximab, imatinib, lidocaine, mesenchymal stem cells, methotrexate, monteleukast, pravastin, psoralen and UVA, thoraco-abdominal radiation, ursodeoxycholic acid, and UVB.
Tertiary therapy and beyond
A subset of patients requires additional treatment approaches because of poorly controlled or progressive chronic GVHD. As with secondary therapy, choice of tertiary agents is often made based on clinical or research considerations, balancing the risks of ongoing chronic GVHD organ damage versus the risks of increased susceptibility to infections. Patients who require tertiary therapy tend to require protracted immune suppressive treatment. Discontinuation of secondary therapy after the new agent is added should be considered since progression or persistent chronic GVHD occurred despite current therapy. Minimizing ineffective immune suppressive treatments may decrease infectious risks and other undesirable adverse treatment effects.
Treatment duration
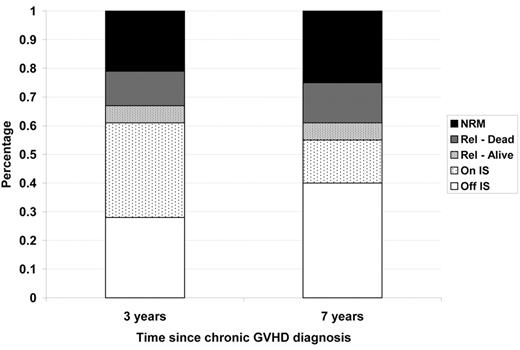
The median time until discontinuation of systemic immunosuppression is 2 to 3 years depending on a variety of patient, donor and graft source variables. Although chronic GVHD is associated with a graft-versus-malignancy effect and lower risk of recurrent disease, relapse and non-relapse mortality still occurs in approximately 39% of patients treated for chronic GVHD by 3 years and 45% by 7 years (Figure 2 ). The nature of the graft-versus-malignancy effect is poorly understood,4 and it is not known whether the protective effect relies on continued chronic GVHD activity or is durable once chronic GVHD resolves. The impact of the type or duration of immune suppressive treatments for control of chronic GVHD on the graft-versus-malignancy effect is also unknown.
Once chronic GVHD is controlled, corticosteroids are generally tapered first as they are the most toxic long-term medication. A schedule is devised so that one agent at a time is tapered and discontinued over a 3 to 9 month period, depending upon the toxicity, the severity and difficulty in controlling active manifestations and the time needed to detect progression if it were to occur. After discontinuation of all systemic treatment, approximately 10% to 25% of patients experience increased activity of chronic GVHD and require reinstitution of systemic treatment.31 A common trajectory is one of waxing and waning persistent manifestations that can be satisfactorily controlled with mid-dose medications but that flare if immune suppression is tapered too low. In these cases, the goal is to find the lowest tolerated dose of immune suppression that can control symptoms without toxicity.
Ongoing clinical trials
Review of the NIH Clinical Trials database (accessed May 2008) showed 21 ongoing trials related to chronic GVHD attempting to accrue 1231 patients. The analysis was limited to trials which were actively recruiting participants and excluded those designated as “completed,” “active, not recruiting,” “not yet recruiting,” or “terminated.” Also excluded were protocols where the conditioning regimen and/or GVHD prophylaxis agents were being tested, even if the primary endpoint involved chronic GVHD. Approximately 2 to 6 chronic GVHD trials have been registered annually since 2004. Several Phase II and Phase I/II studies are testing a variety of therapeutic agents including alefacept, alemtuzumab, extracorporeal photopheresis, IL-2, monteleukast, mesenchymal stem cells, pentostatin, revlimid, rituximab, and sirolimus. Sirolimus and rituximab are being tested in Phase II prevention studies. Phase II studies of topical oral agents include dexamethasone rinses, thalidomide and UV-B. Two randomized Phase III trials, targeting a total of 430 patients, are testing whether addition of mycophenolate mofetil to initial therapy improves outcome.
Summary
Chronic GVHD will continue to be a major complication of allogeneic HCT for the foreseeable future. A better understanding of the human immune system and the complex cellular and cytokine interactions that regulate it will allow targeted treatments aimed at preventing end-organ damage without compromising the graft-versus-malignancy effect. Clinical trials for treatment of chronic GVHD currently represent the most appropriate intervention, and efforts to enroll eligible patients are necessary to improve chronic GVHD-related outcomes. The goal of allogeneic HCT should be disease-free survival without chronic GVHD.
Other clinical manifestations at initial diagnosis of chronic graft-versus-host disease (GVHD) and ever noted, based on 324 patients diagnosed with chronic GVHD according to NIH consensus criteria, transplanted at FHCRC 2003–2005.(M. Flowers, unpublished data)
| . | At onset, % . | Ever, % . |
|---|---|---|
| Sites involved | ||
| Skin | 74 | 81 |
| Finger/toenail/mouth | 8 | 19 |
| Mouth | 87 | 89 |
| Eyes | 34 | 47 |
| G.I. tract | 40 | 48 |
| Esophagus | 1 | 5 |
| Liver | 35 | 47 |
| Vagina/penis | 4 | 10 |
| Lungs | 3 | 14 |
| Joints | 7 | 15 |
| Muscle | 2 | 2 |
| Serosa | 2 | 2 |
| Myofascial | 6 | 16 |
| Clinical manifestations/complications | ||
| Weight loss | 22 | 31 |
| Joint contracture | 2 | 7 |
| Malabsorption | <1 | 1 |
| Keratoconjunctivitis | 3 | 8 |
| Cutaneous sclerosis | 4 | 13 |
| Esophageal stricture | <1 | 1 |
| Bronchiolitis obliterans syndrome | <1 | 7 |
| Eosinophilia | 14 | 20 |
| Bronchiolitis obliterans organizing pneumonia | 2 | 4 |
| Other characteristics | ||
| Progressive onset | 13 | |
| Quiescent onset | 60 | |
| De novo onset | 27 | |
| On steroids at diagnosis | 34 | |
| Platelets < 100,000 at diagnosis | 31 | |
| . | At onset, % . | Ever, % . |
|---|---|---|
| Sites involved | ||
| Skin | 74 | 81 |
| Finger/toenail/mouth | 8 | 19 |
| Mouth | 87 | 89 |
| Eyes | 34 | 47 |
| G.I. tract | 40 | 48 |
| Esophagus | 1 | 5 |
| Liver | 35 | 47 |
| Vagina/penis | 4 | 10 |
| Lungs | 3 | 14 |
| Joints | 7 | 15 |
| Muscle | 2 | 2 |
| Serosa | 2 | 2 |
| Myofascial | 6 | 16 |
| Clinical manifestations/complications | ||
| Weight loss | 22 | 31 |
| Joint contracture | 2 | 7 |
| Malabsorption | <1 | 1 |
| Keratoconjunctivitis | 3 | 8 |
| Cutaneous sclerosis | 4 | 13 |
| Esophageal stricture | <1 | 1 |
| Bronchiolitis obliterans syndrome | <1 | 7 |
| Eosinophilia | 14 | 20 |
| Bronchiolitis obliterans organizing pneumonia | 2 | 4 |
| Other characteristics | ||
| Progressive onset | 13 | |
| Quiescent onset | 60 | |
| De novo onset | 27 | |
| On steroids at diagnosis | 34 | |
| Platelets < 100,000 at diagnosis | 31 | |
Categories of acute and chronic graft-versus-host disease (GVHD).Reprinted from Filipovich et al.7
| Category . | Time of symptoms after HCT or DLI . | Presence of acute GVHD features . | Presence of chronic GVHD features . |
|---|---|---|---|
| Acute GVHD | |||
| Classic acute | < 100 days | Yes | No |
| Persistent, recurrent or late-onset acute | > 100 days | Yes | No |
| Chronic GVHD | |||
| Classic chronic | No time limit | No | Yes |
| Overlap syndrome | No time limit | Yes | Yes |
| Category . | Time of symptoms after HCT or DLI . | Presence of acute GVHD features . | Presence of chronic GVHD features . |
|---|---|---|---|
| Acute GVHD | |||
| Classic acute | < 100 days | Yes | No |
| Persistent, recurrent or late-onset acute | > 100 days | Yes | No |
| Chronic GVHD | |||
| Classic chronic | No time limit | No | Yes |
| Overlap syndrome | No time limit | Yes | Yes |
Secondary agents (updated from Lee et al33).
| Agent . | Published overall response rates . | Organ specific response rates (CR+PR) . | Hypothesized mechanism of action . | Side effects . | Survival . | Adult use, %* . | Pediatric use, %* . |
|---|---|---|---|---|---|---|---|
| * Percentages of adult and pediatric use from survey of 526 transplant physicians asked to choose an agent to treat steroid-refractory chronic GVHD.20 | |||||||
| Common agents | |||||||
| Mycophenolate mofetil34–36 (Cellcept) | 46–75% response rate (N = 21–26) | Skin (53%, n = 15), mouth (67%, n = 15), liver (54%, n = 13) | Prodrug of mycophenolic acid interferes with purine synthesis in lymphocytes | Nausea, vomiting, diarrhea, abdominal cramps, neutropenia | 85% survival at 2 years | 52 | 50 |
| High-dose corticosteroids37 | 48% major response rate (N = 56) | Lympholytic at a dose of 10 mg/kg/d for 4 days | Infection, glucose intolerance, psychological effects including psychosis, insomnia | 88% survival at 1 year | 6 | 16 | |
| Extracorporeal 38,39 photopheresis | 61% response rate (N = 71) | Skin (40%, n = 48),40 sclerotic skin (67%, n = 21), mouth (77%, n = 7), eye (67%, n = 4) and lung (54%, n = 6). | Induces apoptosis in 5–10% T cells, increases regulatory T cells | Anemia, potential need for central IV access | 53% survival at 1 year | 10 | 4 |
| Sirolimus/rapamycin41,42 (Rapamune) | 63–94% clinical response (n = 16–35) | Skin (65%, n = 29), mouth (75%, n = 8), liver (33%, n = 6), eye (64%, n = 11), GI (67%, n = 6) | Binds to FKBP-12 and mTOR (mammalian target of rapamycin) to inhibit cytokine-driven T cell proliferation | Hypertriglyceridemia, renal insufficiency, cytopenias, infection | 41% survival at 2 years; 89% survival at 3 years | 7 | 4 |
| 2-deoxycoformycin43 (Pentostatin) | 53% major response rates, 2% minor response (N = 58) | Lichenoid skin (69%, n = 39), sclerotic skin, fascial, mouth (52–57%, n = 39) | Nucleoside analog that inhibits adenosine deaminase (ADA) | Nausea, vomiting, infection, renal dysfunction, rash, headache | 78% survival at 1 year | 2 | 7 |
| Tacrolimus (Prograf)44,45 | 35% response rate (N = 39) | Binds to FKBP-12 (FK binding protein) and inhibits T lymphocyte activation, concentrates in liver | Renal dysfunction, thrombotic microangiopathy, neurotoxicity, hypertension | Survival not reported | 12 | 7 | |
| Less common agents | |||||||
| Rituximab (Rituxan)46,47 | 65–70% response rate (N = 21–38) | Skin (63%, n = 28), mouth (48%, n = 21), eyes (43%, n = 14), liver (25%, n = 12), lung (38%, n = 8) | Chimeric anti-CD20 monoclonal antibody | Allergic reactions, infections, hepatitis reactivation | 76% survival at 2 years | 2 | 1 |
| Thalidomide48–50 | 20–38% response rate (N = 23–80) | Skin (46%, n = 30), mouth (22%, n = 14); joint (78%, n = 14), lung (0%, n = 6) | Anti-inflammatory and immunosuppressive properties | Neuropathy, somnolence, constipation, neutropenia | 41% survival at 2 years | 2 | 0 |
| Agent . | Published overall response rates . | Organ specific response rates (CR+PR) . | Hypothesized mechanism of action . | Side effects . | Survival . | Adult use, %* . | Pediatric use, %* . |
|---|---|---|---|---|---|---|---|
| * Percentages of adult and pediatric use from survey of 526 transplant physicians asked to choose an agent to treat steroid-refractory chronic GVHD.20 | |||||||
| Common agents | |||||||
| Mycophenolate mofetil34–36 (Cellcept) | 46–75% response rate (N = 21–26) | Skin (53%, n = 15), mouth (67%, n = 15), liver (54%, n = 13) | Prodrug of mycophenolic acid interferes with purine synthesis in lymphocytes | Nausea, vomiting, diarrhea, abdominal cramps, neutropenia | 85% survival at 2 years | 52 | 50 |
| High-dose corticosteroids37 | 48% major response rate (N = 56) | Lympholytic at a dose of 10 mg/kg/d for 4 days | Infection, glucose intolerance, psychological effects including psychosis, insomnia | 88% survival at 1 year | 6 | 16 | |
| Extracorporeal 38,39 photopheresis | 61% response rate (N = 71) | Skin (40%, n = 48),40 sclerotic skin (67%, n = 21), mouth (77%, n = 7), eye (67%, n = 4) and lung (54%, n = 6). | Induces apoptosis in 5–10% T cells, increases regulatory T cells | Anemia, potential need for central IV access | 53% survival at 1 year | 10 | 4 |
| Sirolimus/rapamycin41,42 (Rapamune) | 63–94% clinical response (n = 16–35) | Skin (65%, n = 29), mouth (75%, n = 8), liver (33%, n = 6), eye (64%, n = 11), GI (67%, n = 6) | Binds to FKBP-12 and mTOR (mammalian target of rapamycin) to inhibit cytokine-driven T cell proliferation | Hypertriglyceridemia, renal insufficiency, cytopenias, infection | 41% survival at 2 years; 89% survival at 3 years | 7 | 4 |
| 2-deoxycoformycin43 (Pentostatin) | 53% major response rates, 2% minor response (N = 58) | Lichenoid skin (69%, n = 39), sclerotic skin, fascial, mouth (52–57%, n = 39) | Nucleoside analog that inhibits adenosine deaminase (ADA) | Nausea, vomiting, infection, renal dysfunction, rash, headache | 78% survival at 1 year | 2 | 7 |
| Tacrolimus (Prograf)44,45 | 35% response rate (N = 39) | Binds to FKBP-12 (FK binding protein) and inhibits T lymphocyte activation, concentrates in liver | Renal dysfunction, thrombotic microangiopathy, neurotoxicity, hypertension | Survival not reported | 12 | 7 | |
| Less common agents | |||||||
| Rituximab (Rituxan)46,47 | 65–70% response rate (N = 21–38) | Skin (63%, n = 28), mouth (48%, n = 21), eyes (43%, n = 14), liver (25%, n = 12), lung (38%, n = 8) | Chimeric anti-CD20 monoclonal antibody | Allergic reactions, infections, hepatitis reactivation | 76% survival at 2 years | 2 | 1 |
| Thalidomide48–50 | 20–38% response rate (N = 23–80) | Skin (46%, n = 30), mouth (22%, n = 14); joint (78%, n = 14), lung (0%, n = 6) | Anti-inflammatory and immunosuppressive properties | Neuropathy, somnolence, constipation, neutropenia | 41% survival at 2 years | 2 | 0 |
Initial presentation of chronic graft-versus-host disease (GVHD) in four cohorts.
The FHCRC 2003–2005 cohort is limited to patients diagnosed with chronic GVHD according to NIH consensus criteria. (M. Flowers, unpublished data) Abbreviations: CIBMTR, Center for International Blood and Marrow Transplant Research;32 U Minn, University of Minnesota;8 FHCRC, Fred Hutchinson Cancer Research Center.9
Initial presentation of chronic graft-versus-host disease (GVHD) in four cohorts.
The FHCRC 2003–2005 cohort is limited to patients diagnosed with chronic GVHD according to NIH consensus criteria. (M. Flowers, unpublished data) Abbreviations: CIBMTR, Center for International Blood and Marrow Transplant Research;32 U Minn, University of Minnesota;8 FHCRC, Fred Hutchinson Cancer Research Center.9
Outcomes among 743 patients with chronic GVHD transplanted at FHCRC between 1994 and 2000.
Abbreviations: NRM, non-relapse mortality; Rel, relapse; IS, immune suppressive therapy.
Outcomes among 743 patients with chronic GVHD transplanted at FHCRC between 1994 and 2000.
Abbreviations: NRM, non-relapse mortality; Rel, relapse; IS, immune suppressive therapy.
Disclosures Conflict-of-interest disclosure: S.L. has received honoraria from Therakos (speaker) and Millennium (DSMB). M.E.D.F. declares no competing financial interests. Off-label drug use: No drugs are approved for chronic GVHD treatment, so all discussion about therapeutics is off-label.
Acknowledgments
The authors wish to thank Barry Storer, PhD, for data analysis.
References
Author notes
Fred Hutchinson Cancer Research Center, Seattle, WA, USA