Abstract
Coagulation abnormalities are frequently reported in hemolytic anemias (HA). Several pathophysiologic mechanisms are common to different HA. In this review three different hemolytic disorders will be discussed.
In sickle cell disease and in β-thalassemia, a thrombophilic status has been well documented as multifactorial involving hemostatic changes and activation of the coagulation cascade. Moreover, in such disorders, elevated levels of endothelial adhesion protein (ICAM-1, ELAM-1, VCAM-1, von Willebrand factor, and thrombomodulin) are often increased, suggesting that endothelial activation may be involved in vascular occlusion. As an additional mechanism of hypercoagulability in thalassemia, a procoagulant status of thalassemic red cells was recognized.
The main clinical manifestation of paroxysmal nocturnal hemoglobinuria (PNH) is HA, and the most common complications are thrombosis, pancytopenia, and myelodysplastic syndrome or acute leukemia. The intravascular hemolysis is explained by a deficiency of glycosil phosphatidylinositol (GPI)-anchored complement regulatory proteins such as CD59 and CD55 on the membrane of red blood cells (RBCs), but the mechanism responsible for the increased incidence of thrombotic events in PNH remains unclear.
Recent advances have been made in understanding the coagulation involvement in a heterogeneous group of diseases, thrombotic microangiopathies (TMA) characterized by microangiopathic hemolytic anemia and thrombocytopenia due to platelet clumping in the microcirculation, leading to ischemic organ dysfunction with neurologic symptoms and renal impairment.
Introduction
An increased incidence of thrombosis has been reported in different hemolytic anemias (HA), particularly in sickle cell disease (SCD),1–3 thalassemia4,5 and paroxysmal nocturnal hemoglobinuria (PNH).6 Although HA have different patho-physiologies, hemolysis per se, whatever the cause, seems to be a procoagulant condition. Several mechanisms can be involved, including abnormal red blood cell (RBC) properties, increased plasma concentrations of microparticles, release of cell-free hemoglobin and RBC arginase resulting in impaired nitric oxide (NO) bioavailability, increased blood concentration of oxidants, and endothelial dysfunction. The major clinical consequence is an increased tendency to develop venous thrombosis, although the clinical sequelae of hemolysis may include a variety of symptoms caused by NO depletion as a consequence of increased cell-free plasma hemoglobin.7 Hemoglobinemia, which is a feature shared by most of the HA, has recently also been suggested as a possible contributing factor to explain the clinical manifestations in thrombotic microangiopathies (TMAs), characterized by microangiopathic HA and thrombocytopenia.8Table 1 summarizes conditions characterized by intravascular hemolysis that, although having specific symptoms, often share cell-free plasma hemoglobin sequalae. Among such conditions, SCD and thalassemia syndromes, PNH, and TMAs will be discussed.
Sickle Cell Disease and Thalassemia
SCD and thalassemia represent the most common genetic disorders worldwide. Although they have different patho-physiologies, patients with both diseases share many clinical manifestations, including thrombotic complications.3 Stroke, caused by large-vessel obstruction with superimposed thrombosis, is one of the major complications in SCD.9 Pulmonary hypertension (PAH) is a life-threatening complication in SCD, which was documented by echo-cardiography (pulmonary artery pressure [PAP] > 30%) in approximately 30% of adult patients with SCD who were screened.10 Autopsy series showed evidence of new and old thrombi in the pulmonary vasculature in up to 75% of SCD patients at the time of death.11,12 A recent report based on data from the National Hospital Discharge Survey in the U.S. showed that patients with SCD who were younger than 40 years old had a higher discharge diagnosis of pulmonary embolism than African Americans without SCD (0.44% vs 0.12%), although the prevalence of deep venous thrombosis (DVT) was similar.13 Furthermore, the Nationwide In-patient Sample from the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality, from 2000 to 2001, reported that SCD is a significant risk factor for pregnancy-related venous thromboembolism, with an odds ratio of 6.7 (95% confidence interval: 4.4–10.1).14 The thrombophilic status in SCD has been well documented as multifactorial, involving hemostatic changes, activation of the coagulation cascade and endothelial activation as shown by elevated levels of endothelial adhesion proteins (ICAM-1. ELAM-1,VCAM-1, von Willebrand factor [VWF] and thrombomodulin). Moreover, intimal hyperplasia was found in a variety of vascular beds, including pulmonary, renal, splenic, and placental vessels,15 and in arterioles adjacent to leg ulcers of patients with SCD. Most SCD-related complications are thought to be caused by microvascular occlusion and ischemic tissue necrosis following the adhesion of erythrocytes (RBCs) and other cellular elements to vascular endothelium.
Several clinical series describe the occurrence of DVT, pulmonary embolism and portal vein thrombosis in patients with β-thalassemia major and β-thalassemia intermedia.16 In a recent survey of 8860 patients with β-thalassemia in the Mediterranean region and Iran, the overall prevalence of various thrombotic events was 1.65%, precisely 0.9% in thalassemia major and 4.0% in thalassemia intermedia.17 While less common in thalassemia than in SCD, stroke has also been reported in patients with β-thalassemia major from Greece and Italy, with an incidence of 20% and 2%, respectively,18 and in patients with β-thalassemia/hemoglobin E (HbE) disease.19 This complication occurs more commonly in patients who are not regularly transfused. Evidence for asymptomatic brain damage has also been reported by magnetic resonance imaging in patients with β-thalassemia intermedia and is inversely correlated with Hb level and increasing age. PAH is increasingly recognized as part of the clinical spectrum for adult β-thalassemia patients, with a frequency ranging from 10% to 74%; the overall higher rate is in thalassemia intermedia.20 Regular transfusions were thought to reduce the risk of thrombosis in thalassemia. In a recent study, Singer et al21 found PAH among transfused patients with thalassemia major, most of whom were splenectomized, suggesting that regular transfusions do not prevent PAH or other thrombotic complications. In thalassemia, several factors have been implicated in the pathogenesis of the hypercoagulable state, such as the specific changes in the lipid membrane composition of the abnormal RBCs with increased expression of negatively charged phosphatidylserine at the outer surface, postsplenectomy thrombocytosis, cardiac dysfunction, and liver dysfunction leading to protein C and protein S reduction. Recently, it has been suggested that absence of the spleen in a variety of hematologic hemolytic diseases may contribute to an increased propensity to thromboembolic complications.22 Both β-thalassemia and sickle cell anemia, have a high proportion of patients who have no splenic function, either due to surgical splenectomy or due to functional hyposplenism. In a series of 83 patients with β-thalassemia intermedia followed for 10 years, 29% developed pulmonary embolism, DVT of the lower extremities and portal vein thrombosis, and all but 1 patient had been sple-nectomized.16 The development of these complications has been attributed to the presence of high platelet counts following splenectomy.23 Moderate thrombocytosis has been reported in older children and adults with SCA, a likely consequence of the loss of splenic sequestration that follows autosplenectomy in these patients. However, the literature on the relationship between splenectomy (or autosplenectomy) and thrombosis in SCA is scant
Although specific features, several thrombotic complications in patients with SCD and thalassemia may share a similar pathogenesis. Intravascular hemolysis is a common feature of both SCD and thalassemia. Recent evidence associates chronic intravascular hemolysis with a state of endothelial disfunction that is characterized by reduced NO bioavailability.24
Paroxysmal Nocturnal Hemoglobinuria
PNH is an acquired genetic X-linked disease clinically characterized by a severe HA, venous thrombosis and blood cytopenias.25 The molecular basis of PNH is a somatic mutation of the X-chromosome gene PIGA, which encodes a subunit of N-acetylglucosamine phosphatidylinositol transferase, essential for synthesis of the glycosil phosphatidylinositol (GPI) that serves as the membrane anchor for different cellular proteins.26 The somatic mutation takes place in one or several hematopoietic stem cells that undergo clonal expansion, and as a consequence, all GPI-anchored proteins (GPI-AP) are deficient on affected stem cells and their progeny. Intravascular hemolysis is the primary clinical manifestation of the classic PNH form, due to deficiency of CD55 and CD59 proteins responsible for controlling the activity of plasma complement. Although a thromboembolic event as the presenting manifestation of PNH is uncommon (approximately 5%),27 thrombosis remains the leading cause of death, particularly among patients from the U.S.28 and Europe.29 A recent Medline database review on thrombosis and PNH provided data on 363 patients with PNH with thrombosis and outcome in 339.30 A total of 25% of thrombotic events have proven fatal; 20.5% involved more than 1 site (range, 2 to 5 sites). Hepatic vein thrombosis, pulmonary embolism, mesenteric vein thrombosis and venous stroke were significant predictors of thrombosis-related mortality. Hepatic vein thrombosis leading to Budd-Chiari syndrome appears as the most frequent (40.7%) thrombotic complication in PNH, accounting for the majority of deaths. Cerebral vein and sinus thrombosis was the second most common type of thrombosis. Recent clinical studies support the hypothesis that the probability of a thromboembolic event is directly related to the size of PNH clone. Hall et al30 proved that the 10-year risk of thrombosis in patients with more than 50% GPI-AP–deficient granulocytes was 44% compared with 5.8% for patients with less than 50%. Moyo et al,31 using a logistic regression model, calculated that the odd ratio for thrombosis was 1.64 for each 10% increase in the percentage of GPI-AP-deficient granulocytes. According to that study, a patient with more than 70% GPI-AP–deficient granulocytes had an 11.8-fold greater risk of thrombosis than a patient with a PNH clone of 20%. An increase in procoagulant and fibrinolytic activity, resulting in increased fibrin generation and turnover, has been found. Apart from hemolysis itself, several failures of the fibrinolytic system have been highlighted, including a deficiency of urokinase-type plasminogen activator receptor on leukocytes presenting the PNH phenotype and increased plasma levels of soluble urokinase-type plasminogen activator receptor. In PNH platelet stimulation is accompanied by the loss of membrane phospho-lipid asymmetry, resulting in phosphatidylserine externalization and microvesicle generation, as shown in an in vitro study. This process is similar to that observed in thalassemia and SCD. Membrane-derived microparticles have been shown to provide the catalytic surface necessary for the assembly of the procoagulant enzyme complexes, prothrombinase and tenase. In the blood flow, the presence of high levels of procoagulant microparticles, stemming from lysed RBCs, apoptotic cells, or activated platelets, could therefore be responsible for the dissemination of prothrombotic status.27
However, although the pathogenesis of thrombophilia in PNH remains speculative, the chronic intravascular hemolysis that is the primary clinical manifestation probably remains the most important underlying factor. Episodes of severe intravascular hemolysis as indicated by significant increase of lactate dehydrogenase (LDH) and hemoglobinuria are frequently associated with thrombotic events as well as with dysphagia, abdominal pain, erectile disfunction and disabling fatigue. All these symptoms are consistent with smooth muscle perturbation through the cell-free plasma hemoglobin and NO scavenging.7 The relationship between intravascular hemolysis and plasma hemoglobin/NO-dependent symptoms in PNH was recently evaluated with a specific drug intervention using a monoclonal antibody that blocks cleavage of the complement component C5, preventing complement-mediated RBC lysis.32 Furthermore, NO plays an important role in the maintenance of normal platelet functions through the down-regulation of platelet aggregation and adhesion. The lack of the complement regulatory proteins CD55 and CD59 on the surface of PNH platelets, which renders these cells more sensitive to complement-mediated activation, may also contribute to thrombotic tendency in patients with PNH.26 Thus PNH is a prototypic disease of intravascular hemolysis with all the clinical sequelae of NO reduction, including thrombophilia.
Thrombotic Microangiopathies
Recent advances have been made in understanding the coagulation involvement in a heterogeneous group of diseases (TMAs) characterized by microangiopathic HA and thrombocytopenia due to platelet clumping in the micro-circulation leading to ischemic organ dysfunction with neurologic symptoms and renal impairment. The two principal forms of TMA are the hemolyticuremic syndrome (HUS), in which severe renal failure prevails, and thrombotic thrombocytopenic purpura (TTP), characterized by systemic occlusion of the microcirculation that frequently affects the central nervous system. In such disorders, the fragmentation of erythrocytes is a hallmark and it occurs, presumably, as blood flows through turbulent areas of the microcirculation partially occluded by platelet aggregates. Schistocytes, or fragmented red cells, appear on the peripheral blood smear (>1% of total RBCs) as indicators of “microangiopathic HA.” Serum levels of LDH are extremely elevated as a consequence of hemolysis and leakage of LDH from ischemic or necrotic tissue cells. It has been recognized for several years that VWF has a role in the pathogenesis of TMAs. VWF is a large multimeric glycoprotein contained in plasma, platelets and vascular endothelial cells. It mediates the adhesion of platelets to sites of vascular lesions and, as the carrier protein for coagulation factor VIII, is required for normal factor VIII survival in the circulation. Ultralarge multimers of VWF (ULVWF) are the most biologically active forms in platelet-vessel wall interactions and directly induce platelet aggregation under condition of high shear stress.33 The link between ULVWF multimers and TMAs was elucidated in the late 1990s when Furlan et al34 and Tsai and Lian35 observed in patients with TTP a deficiency of the plasma metalloprotease that physiologically cleaves VWF at the peptide bond between amino acid residues 842 Thr and 843 Met in the A2 domain of the VWF subunit (ADAMTS13). A severe deficiency of ADAMTS13 was reported to be a specific finding of acute TTP. In hereditary TTP (Upshaw-Schulman syndrome), a constitutional deficiency of ADAMTS13 seems due to compound heterozygous or homozygous mutations in the ADAMTS13 gene, whereas the acquired form is often caused by circulating autoantibodies (inhibitors) that neutralize ADAMTS13 activity. However, low plasma levels of ADAMTS13 have been reported in many physiologic (age > 65 years; last 2 trimesters of pregnancy) and pathologic conditions (cirrhosis, uremia, postoperative period). It is not completely clear whether low plasma levels in such conditions are due to decreased synthesis, increased turnover, or other mechanisms. The interpretation of the low plasma levels of ADAMTS13 is currently uncertain; however, it seems that they are not pathognomonic of TMAs. Certainly the presence of ULVWF is responsible for the formation of platelet thrombi: ULVWF multimeric strings are anchored to the endothelial cells via P-selectin molecules. Passing platelets adhere via their GPIbα receptors to the long ULVWF multimeric strings anchored to P-selectin, favoring further platelet aggregation under flow conditions to form large, potentially occlusive platelet thrombi. The platelet-ULVWF complex may detach from endothelial cells in the absence of ADAMTS13 activity and embolize to microvessels and contribute to organ ischemia. However, it is difficult to explain all the different clinical features of TMA only as consequences of ADAMTS13 deficiency or inhibition. Other factors could be involved. The fragmentation of erythrocytes responsible for “microangiopathic HA” releases free Hb into the plasma, which overwhelm the protective Hb-scavenging mechanisms, and causes NO reduction similar to what has been suggested for other hemolytic conditions.8 This hypothesis could be supported by the effectiveness of plasma exchange in patients with TTP, which has been attributed to the removal of ADAMTS13 autoantibodies and replacement of normal protease activity. However, since plasma exchange also seems to be effective for patients who do not have a severe deficiency of ADAMTS13 activity, it is possible that this procedure cleans hemolytic products from the plasma and restores the NO. Intense hemolysis and NO consumption, in addition to platelet aggregation and ADAMTS13 deficiency, link TMA to other hemolytic conditions and could explain the wide spectrum of clinical manifestations.
Hemolysis and Coagulation Abnormalities in HA
Hemolysis contributes to coagulation abnormalities in HA in three ways.
Red blood cell membrane alterations and micro-particles. Several HA are characterized by RBC membrane abnormalities, either genetic or acquired, that alter the membrane phospholipid asymmetry observed in normal RBCs. An increased exposure of anionic phospholipids such as phosphatidylserine (PS) confer procoagulant properties to thalassemic RBC and sickle cells. It has been shown that erythroid cells in such conditions may act as activated platelets and enhance conversion of prothrombin to thrombin, especially in splenectomized patients.22 Similarly, microparticles produced by erythrocyte fragmentation during hemolysis have polynegative niches that activate the intrinsic phase of blood coagulation and thrombin generation has been shown in vivo and in ex-vivo systems.36,37 In addition, a striking correlation has been found between PS-positive sickle RBCs and adhesion to vascular endothelium, suggesting an important contribution of these PS-positive cells to endothelial adhesion.
Erythrocyte/endothelium interaction. It has been recently shown that plasma hemoglobin and heme as products of erythrocyte destruction during hemolysis stimulate the expression of the adhesion molecules ICAM-1 (intracellular adhesion molecule 1), VCAM-1 (vascular cell adhesion molecule 1), and E-selectin on endothelial cells, leading to vessel obstruction, tissue hypoxia and ultimately tissue death. Red blood cells from patients with β-thalassemias and SCD show enhanced adhesion to the vascular endothelium that induces a state of oxidative stress with consequent transendothelial migration of monocytes. Tissue factor (TF), the principal initiator of coagulation, is abnormally expressed on circulating endothelial cells in patients with SCA and increases further during pain episodes. Microparticles (MP) released during hemolysis are TF positive. Several potential mechanisms for increased TF expression in SCA include a role for ischemia-reperfusion injury, increased levels of sCD40 ligand, and possibly increased hemolysis of type II PS-positive cells. There is a correlation between the high levels of sCD40L observed in patients with SCD and increased TF antigen, suggesting a contribution of sCD40L to the hypercoagulable state observed in these patients.38
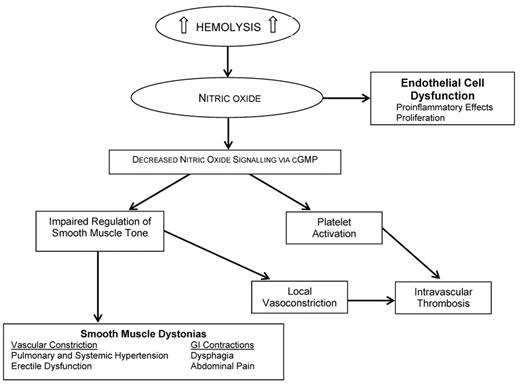
NO deficiency. During hemolysis, hemoglobin in the plasma dimerizes and is rapidly bound by the serum protein haptoglobin, forming a haptoglobin/hemoglobin complex that is recognized by the hemoglobin scavenger receptor (CD163) exposed on the surface of monocytes/ macrophages. In the presence of continuous or severe hemolysis, haptoglobin is rapidly depleted since this protein is not recycled; in fact, in severe hemolytic diseases, serum haptoglobin is typically undetectable. As a consequence, levels of hemoglobin and heme increase in the plasma (hemoglobinemia) and urine (hemoglobinuria). Free plasma hemoglobin scavenges NO, to which it has high affinity, and depletes it, while heme has multiple proinflammatory and pro-oxidant properties. In addition, hemolysis also releases erythrocyte arginase, an enzyme that converts L-arginine, the substrate for NO synthesis, to ornithine; thus, it causes a further reduction of NO. NO is a regulator of smooth muscle tone, and platelet activation and reduction in NO plasma levels lead to smooth muscle dystonias, including gastrointestinal contractions, hypertension, erectile dysfunction as well as clot formation.7 Interestingly, patients with SCD, PNH and TM during hemolytic crises exhibit symptoms that are consistent with smooth-muscle perturbation related to the release of hemoglobin and NO scavenging. In conclusion, cell-free plasma hemoglobin characterizes several diseases with a hemolytic component, and NO consumption seems to be the key contributing factor to thrombotic tendencies in hemolytic disorders (Figure 1 ).7
Hemolytic conditions with substantial intravascular hemolysis.
|
|
Consequences of nitric oxide depletion during intravascular hemolysis.
Adapted from Rother, et al.7
Consequences of nitric oxide depletion during intravascular hemolysis.
Adapted from Rother, et al.7
Policlinico, Mangiagalli, Regina Elena Foundation IRCCS, University of Milan, Milan, Italy