Abstract
The aim of this review is to discuss current diagnostic approaches to, and classification of, patients presenting with thrombocytosis, in light of novel information derived from the discovery of specific molecular abnormalities in chronic myeloproliferative disorders (CMPD), which represent the most common cause of primary thrombocytosis. The JAK2V617F and the MPLW515L/K mutations have been found in patients with essential thrombocythemia, polycythemia vera, and primary myelofibrosis, and less frequently in other myeloproliferative disorders complicated by thrombocytosis. However, neither mutation is disease specific nor is it universally present in patients with elevated platelet counts due to a CMPD; therefore, distinguishing between reactive and primary forms of thrombocytosis, as well as among the different clinical entities that constitute the CMPD, still requires a multifaceted diagnostic approach that includes as a key step the accurate evaluation of bone marrow histology. The role of elevated platelet counts in thrombosis, which represent the predominant complication of CMPD,significantly affecting prognosis and quality of life as well as, paradoxically, in the pathogenesis of the hemorrhagic manifestations, will be discussed. Established and novel potential risk factors for thrombosis, including the clinical relevance of the JAK2V617F mutation, and current management strategies for thrombocytosis are also briefly discussed.
The incidental discovery of an elevated platelet count in an otherwise asymptomatic subject, in the absence of any other hematologic abnormalities, represents an important diagnostic challenge.1 In a steady state, platelet production is tightly regulated by the hormone thrombopoietin (TPO), which is primarily synthesized in the liver, with some contribution by the kidney.2 The receptor for TPO, c-Mpl, is expressed mainly on megakaryocytic and platelet membranes. The amount of TPO made available to megakaryocytes in the bone marrow for their proliferation and maturation is controlled by clearance of the hormone through binding to c-Mpl on circulating platelets. The lower the platelet mass, the greater the amount of TPO delivered to megakaryocytes; conversely, when the platelet count increases, more TPO is sequestered in the circulation and stimulation to megakaryocytopoiesis is reduced. Thrombocytosis may be driven by increased levels of TPO or be independent from it.
Most cases of thrombocytosis are not of strict hematologic pertinence, since they occur in the setting of a systemic disorder (secondary, or reactive, thrombocytosis); actually, less than 10% of isolated thrombocytosis reflect a hematologic disorder (primary thrombocytosis), and the prototype is represented by essential thrombocythemia (ET). Primary thrombocytosis occurs also in other hematologic diseases, most of which fall within the category of chronic myeloproliferative disorders (CMPD); they include, in addition to ET, polycythemia vera (PV) and primary myelofibrosis (PMF), plus chronic myelogenous leukemia (CML) and other less-frequent entities.3 Classification, pathogenetic mechanisms, and diagnostic approach to thrombocytosis have been the subject of recent comprehensive reviews, and for many details, the reader is referred to them.1,4 In this review, we will focus on the impact on classification and diagnosis of thrombocytosis of novel information derived from discovery of molecular abnormalities,3,5 such as the V617F mutation in JAK2 or the W515L/K mutations in MPL, which occur in typical CMPD and related disorders with elevated platelet count; in addition, data derived from large clinical studies, such as the European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP)6 and the United Kingdom Medical Research Council Primary Thrombocythemia 1 (MCR-PT1) studies,7 will be discussed for their relevance to patient risk classification and management.
Defining Diagnostic Levels of Thrombocytosis
In adults, platelet counts often fluctuate in the absence of any clinically apparent reason, while in childhood they follow an age-dependent pattern: platelet counts greater than 500 × 109/L have been found in 13% of neonates at birth, 36% during the first month with a prevalence of low-birth-weight infants, and 13% at 6 to 11 months of age; they then gradually decrease to normal levels in children aged 11 years or older.8 In a survey of 10,000 healthy volunteers, aged 18 to 65, Ruggeri et al found 99 incidental thrombocytosis (0.99%), considered as a platelet count greater than 400 × 109/L which corresponded to the 99th percentile in this population; in 92% of patients, the platelet count normalized at second examination accomplished within a median of 8 months.9 Interestingly, the likelihood of having confirmed thrombocytosis at second testing was 10-fold higher in patients with platelet counts greater than 600 × 109/L at discovery (50% vs 5%). However, the extent of thrombocytosis is not a criterion for discriminating a primary from a reactive process, since counts up to, or even greater than, 1000 × 109/L are not unusual among patients with solid neoplasia, in particular lung cancer, or with inflammatory bowel disease. Conversely, the JAK2V617F mutation has been detected in patients undergoing diagnostic procedures to exclude the possibility that they were bearing ET, even in the presence of platelet counts lower than 600 × 109/L, which is the threshold level indicated by both Polycythemia Vera Study Group (PVSG) and World Health Organization (WHO) diagnostic criteria (Table 1 ). In our own series of 421 patients with ET, we identified 49 who had platelet counts between 450 and 600 × 109/L; 27 of these (55%) harbored the mutation, and all 5 patients with platelet counts between 450 and 500 × 109/L had the JAK2V617F mutant. In the remaining 22 patients, the diagnosis of ET was made according to a combination of WHO criteria even if the platelet count was lower than 600 × 109/L (Table 1 ); incidentally, none of these patients became JAK2V617F positive during a median follow-up of 2.3 years (unpublished data).
Altogether, these observations suggested the need for a reappraisal of the currently set level of 600 × 109/L platelets for the diagnosis of ET, since this value might prevent detection of early phases of disease. Therefore, an international panel of pathologists and clinical investigators recently prepared a proposal document, endorsed by members of the Clinical Advisory Committee for the revision of WHO Classification of Myeloid Neoplasms, in which they suggested lowering the level of platelets considered diagnostic for ET to 450 × 109/L (Table 1 ).10 This should guarantee that virtually no case of ET is missed, although it will certainly result in a significant increase in the number of diagnostic procedures performed.
Causes of Thrombocytosis
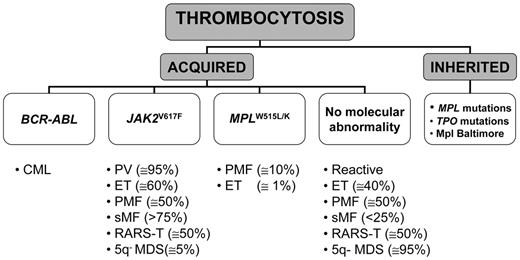
Elevation of platelet count occurring as an inherited familial disorder is exceedingly rare; most cases of thrombocytosis are acquired and represent either a secondary or a primary process. A working classification of thrombocytosis based on known recurrent molecular abnormalities is presented in Figure 1 .
Hereditary thrombocytosis (HT) is a genetically heterogeneous condition that may be due either to TPO mutations transmitted as autosomal dominant disease, which cause increased translational efficiency for mutant TPO mRNA resulting in markedly elevated serum TPO levels, or to a Ser505Asn-activating mutation in the transmembrane domain of TPO receptor, c-MPL.11 However, these mutations account for only a minority of HT, since in most cases the disease-causing gene remains unknown; furthermore, true prevalence of HT is likely underestimated because it is generally asymptomatic and often not adequately sought. Another form of HT is due to a lysine to asparagine substitution at amino acid 39 (G1238T) in MPL (Mpl Baltimore), which has been described exclusively in African-American descendants. This polymorphism, which is transmitted in an autosomal-dominant pattern with incomplete penetrance, is associated with mild to extreme elevation of platelet counts depending on the heterozygous or homozygous status, respectively.12 The molecular bases for thrombocytosis are unclear, but this allelic variant resulted in reduced Mpl expression due to abnormal post-translational processing of the protein.
Secondary, or reactive, thrombocytosis is observed in a variety of underlying conditions, which may cause either an acute and transient elevation of platelet count (trauma, major surgery, acute bleeding), or more sustained thrombocytosis (as is the case of iron deficiency, chronic infection, chronic inflammatory disease, or neoplasia), or even a lifelong increase of platelet count, such as in splenectomized or asplenic patients. In patients with lung cancer, thrombocytosis has prevalence as high as 30%, and it has been associated with extensive and/or metastatic disease and worse prognosis. Reactive thrombocytosis is generally accompanied by signs and symptoms of the underlying systemic disease, which provide a clue to diagnosis; however, especially in the case of hidden malignancy, getting a final diagnosis is difficult. Unlike in adults, from one-third to two-thirds of cases of reactive thrombocytosis in childhood are due to bacterial or viral infections involving the respiratory, gastrointestinal or urinary tract; less frequent causes are hemolytic or iron-deficiency anemia (6%–12%), autoimmune diseases (4%–11%), malignancy (1%–3%), or drugs.8
The most common cause of primary thrombocytosis is represented by one of the CMPD. CML sometimes presents with thrombocytosis, which may be even extreme; finding t(9;22)(q34–q11) by fluorescence in situ hybridization (FISH) analysis and/or BCR-ABL rearrangement with molecular techniques is diagnostic. On the other hand, a recurrent molecular abnormality among Philadelphia chromosome–negative CMPD is represented by a somatic mutation in JAK2, consisting of a G-to-T exchange at nucleotide 1849 in exon 14 and resulting in the substitution of valine to phenylalanine at codon 617 (V617F). The mutation is located in the JH2-negative regulatory pseudokinase domain and causes constitutive activation of the JAK2-STAT signaling pathway, which underlies the characteristic hypersensitivity of hematopoietic progenitors to cytokines.13–16 The mutation has been found in virtually all cases of PV (greater than 95% are positive), in 50% to 70% of ET and in 50% to 60% of PMF patients. As a result of mitotic recombination affecting chromosome 9p, a variable proportion of patients are homozygous for JAK2V617F. Other recurrent mutations are located in the transmembrane-juxtamembrane junction of MPL (tryptophan to either leucine [W515L] or lysine [W515K]);17 they have been reported in approximately 10% of PMF and 1% of ET, while they are absent in PV or other myeloid disorders.17–19 In some patients with PMF JAK2 and MPL mutations coexist,18,19 but no data are available yet for ET. Expression of the MPLW515L mutation in recipient mice resulted in a rapidly fatal myeloproliferative disorder with analogies to human myelofibrosis, associated with marked thrombocytosis.17 On the other hand, thrombocytosis did not develop after transplantation of cells transfected with mutant JAK2; recipient mice presented with very large increases of red cells and eventually progressed to a phenotype resembling postpolycythemic myelofibrosis. Therefore, the exact role of the V617F allele in the pathogenesis of thrombocytosis in CMPD remains unclear.20,21
The association of thrombocytosis with ringed sideroblasts is a rare condition that is referred as to refractory anemia with ringed sideroblasts and marked thrombocytosis (RARS-T) and is included in the WHO category of myelodysplastic/myeloproliferative disease, unclassifiable. The V617F mutation has been detected in about half of the patients reported in several small series. On the other hand, the JAK2V617F mutation has been found in only 6% of 97 patients with the 5q– syndrome, a myelodysplastic syndrome with isolated interstitial deletion of the long arm of chromosome 5(q31–q33), characterized by macrocytic anemia, variably elevated platelet count, hypolobated megakaryocytes in bone marrow biopsy, and a favorable course.22 In this scanty group of patients, the presence of the V617F mutation correlated with higher platelet count.
Differential diagnosis of thrombocytosis
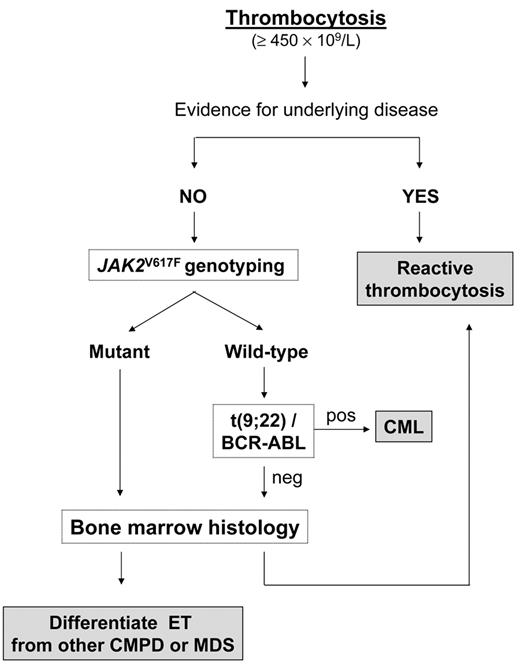
A diagnostic flowchart for thrombocytosis is presented in Figure 2 . Confirmation of incidentally discovered thrombocytosis by performing blood tests within a few weeks of each other, especially if the platelet count increase is borderline, is wise; if the platelet count is still greater than 450 × 109/L, the first step is to exclude any concomitant disease that might have caused a reactive increase. However, in the absence of any obvious clinical manifestations and laboratory abnormalities that might alert the physician towards a specific diagnosis, we suggest genotyping for JAK2V617F as a first intention diagnostic test. The allele-specific oligonucleotide (ASO) PCR assay, as originally described, is relatively simple, reproducible, and affordable, and if positive, will establish diagnosis of primary thrombocytosis; unfortunately, unlike in PV, the presence of mutation is not essential for a diagnosis, since nearly 40% of patients with ET according to WHO criteria are JAK2 wild-type. In addition, the median level of V617F allele burden in ET is in the low range, and only one-fourth of mutant patients may harbor more than 25% mutant alleles. Therefore, it is also possible that some patients with very low allele burden go undetected at the sensitivity level of the ASO-PCR, which is in the range of 2% to 3%, and will probably require more sensitive assays for their identification. The frequency of patients with ET who are JAK2V617F homozygous is only 2% to 4%, significantly lower than in PV and PMF (about 25% to 30%) or secondary (post-polycythemic/post-thrombocythemic) myelofibrosis (more than 60%). However, neither heterozygosity versus homozygosity nor findings of low V617F allele burden are useful criteria in favoring or excluding diagnosis of ET versus other CMPD. Frequency of the JAK2V617F mutation seems to be even lower in childhood ET. Randi et al found that only 4 of 20 children with ET harbored the mutation (20%),23 while 7 of 18 children (38%) were mutated in the study by Teofili et al.24 Finally, there are reports of a few patients with ET in which the mutation was apparently restricted to the megakaryocytic lineage.25
Therefore, while pathognomonic of a CMPD, presence of the JAK2V617F mutation in the setting of an isolated thrombocytosis lacks specificity in regards to diagnostic subtype; consequently, assessment of bone marrow morphology remains mandatory in the diagnostic work-up of ET10,26,27 (Table 1 and Figure 2 ). In this disorder, bone marrow megakaryocytes are increased in number and either dispersed throughout the section or in loose clusters; they have mature appearance, have deeply lobulated and hyperlobulated nuclei, and can be differentiated from the highly bizarre, tightly clustered, megakaryocytes found in typical PMF with reticulin and/or collagen fibrosis, or from the dysplastic, hypolobated and dispersed megakaryocytes, associated with signs of dysplastic erythropoiesis and/or granulocytopoiesis encountered in MDS.26,27 Furthermore, the stimulation of erythroid and granulocytic lineage that characterizes PV, or the granulocytic proliferation found in PMF, are typically absent in ET, and bone marrow cellularity is normal or minimally increased. On the other hand, distinction of ET from prefibrotic PMF is more cumbersome and requires accurate bone marrow evaluation to identify typical megakaryocyte morphologic changes accompanied by findings of increased cellularity with granulocyte proliferation and often decreased erythroid precursors.28 To enhance diagnostic accuracy, the revised WHO criteria require that at least two of the typical clinical features (leukoerythroblastosis, increased serum lactate dehydrogenase levels, anemia, and palpable splenomegaly) are present for a diagnosis of prefibrotic PMF when significant reticulin fibrosis is absent.10
In secondary thrombocytosis, serum levels of TPO are generally increased, either as a direct result of TPO overproduction or via the increased levels of acute-phase reactants, such as interleukin-6, which in turn induce the expression of TPO in liver cells.1,2 However, quantification of TPO levels is not clinically sound, because some patients with reactive thrombocytosis do not display an appreciable increase of plasma TPO, and conversely, TPO levels may be either elevated or inappropriately normal in patients with thrombocytosis due to CMPD. In this case, elevated TPO levels do not originate from increased TPO transcription, but from defective hormone clearance due to c-Mpl abnormalities. Raised levels of some inflammatory markers, such as C-reactive protein, ferritin or fibrinogen, and an elevated erythrocyte sedimentation rate, together with normal or minimally modified lactate dehydrogenase levels, are all typical of reactive thrombocytosis; however, an inflammatory process may be found in patients with ET as well, so these markers cannot be considered discriminatory at all.
Thrombocytosis and Thrombosis: An Uneven Relationship
Generally speaking, reactive thrombocytosis is not a risk factor for thromboembolic complications, notwithstanding that the rate of thrombosis, preferentially restricted to the venous system, is increased in patients with underlying malignancy, especially when additional risk factors are present. Erythromelalgia, a manifestation typical of patients with ET, has not been described in reactive thrombocytosis of various causes.29 Thrombotic complications have been reported infrequently in HT11 as well as in childhood reactive thrombocytosis, although some occurred in children with thalassemia or following splenectomy for different reasons. Conversely, thrombosis represents the second leading cause of mortality, after hematologic transformation, in patients with PV or ET. Reported figures ranged from 12% to 39% in PV and from 11% to 25% in ET; the retrospective, uncontrolled nature of the studies, together with the relatively small number of patients included and variable follow-up, may explain the wide range of values described.30,31 In childhood ET, thrombosis probably occurs at a rate similar to adults, affecting one-third of children at diagnosis and one-fifth during follow-up.8 Epidemiologic inference from two prospective studies, the ECLAP and the MCR-PT1, indicated that the cumulative rate of cardiovascular events ranged from 2.5% to 5.0% and from 1.9% to 3% per patient-year in PV and ET, respectively, depending on the low- or high-risk patient category.7,32 Arterial thrombosis accounts for 60% to 70% of the events, and includes ischemic stroke, acute myocardial infarction (AMI), and peripheral arterial occlusion. Events involving the venous system are preferentially represented by lower-extremities deep venous thrombosis, pulmonary embolism, and intra-abdominal (hepatic, portal, and mesenteric) vein thrombosis; the prevalence of the latter is unusually high among patients with PV or ET, and homozygosity for JAK2 mutation in the setting of ET may be a risk factor.33 Involvement of the microcirculatory system is typical, but not exclusive, of ET, and manifests as erythromelalgia, transient ischemic attacks (TIA), visual or hearing transitory defects, recurrent headache, and peripheral paresthesia; however, due to the lack of objective diagnostic criteria, true incidence is unknown.30
Pathogenesis of thrombosis in CMPD is multifactorial; rheologic abnormalities due to increased red cell mass in PV, abnormal function of platelets, and enhanced interaction with leukocytes and endothelial cells are all possible contributing factors. As for thrombocytosis per se, no obvious correlation with risk of major cardiovascular events has been demonstrated in a number of studies, although clinical improvement of microcirculatory disturbances and/ or improved platelet function after control of thrombocytosis have been reported.30,31 In the PVSG study, platelet count closest to thrombotic event did not predict for its occurrence. Also, in the ECLAP prospective study, which enrolled 1638 PV patients with a median follow-up of 2.8 years and registered 226 thrombotic events, platelet count was not associated with thrombosis; major thrombosis occurred in 8.3% and 9.3% of patients whose baseline platelet count was greater, or lower, than 400 × 109/L, respectively.34 Incidentally, in that analysis, hematocrit in the range of 40% to 55% was not associated with occurrence of thrombosis. Finally, against a direct relationship between increased platelet count and thrombosis stand the results of MRC-PT1 trial, which randomly allocated 809 patients with high-risk ET to receive either hydroxyurea (HU) or anagrelide, in addition to low-dose aspirin. Despite similar control of platelet count by either drug, patients receiving HU were less likely to reach the composite endpoint of arterial thrombosis (TIA, AMI, unstable angina, thrombotic stroke) or vascular death than patients receiving anagrelide; however, the frequency of venous thrombosis was significantly reduced to about 25% in the latter group.7 On the other hand, a correlation between thrombocytosis and thrombosis has been reported in PMF, a condition where cardiovascular events are definitely less frequent than in PV or ET, and in which thrombocytosis is found in only a proportion of the patients.35 In a series of 155 patients with 31 thrombotic events recorded in 18 of them (11.6%), multivariate analysis identified platelet count greater than 450 × 109/L as a predictor for major cardiovascular events occurring at diagnosis; other factors associated with thrombosis were presence of generic cardiovascular risk factor, hemoglobin greater than 110 g/L, and cellular phase of disease. Probability of thrombosis-free survival at 5 years was 80.6% and 96.2% in patients having more, or less, than 450 × 109/L platelets, respectively. Finally, uncertainties about the role of thrombocytosis in pathogenesis of thrombotic events are well reflected in the variable opinions about need, or opportunity, to control platelet counts in patients with PV that were manifested by expert hematologists from North America,36 and also in the wide range of platelet count observed in the ECLAP study as opposite to the relatively narrow levels at which hematocrit was maintained.34
Taken as a whole, these data weaken the intuitive role of increased platelet counts in the pathogenesis of thrombosis in CMPD, but they do not undermine the significance of several other lines of evidence for a contribution of platelets to thrombotic risk. In case of erythromelalgia in patients with ET, histopathology showed dermal arteriolar involvement, with arterial thrombi stained strongly for von Willebrand factor and weakly for fibrin. The prompt relief of symptoms with aspirin, the normalization of tests measuring in vivo platelet activation, and the inefficacy of warfarin and heparin were all highly suggestive for platelet-mediated microvessel occlusion.29 Furthermore, the ECLAP study has demonstrated the efficacy of low-dose aspirin in reducing the risk of cardiovascular events in PV (relative risk 0.72; 95% CI, 0.53–0.9)6,32; patients randomized to receive aspirin had a 60% reduction of combined endpoint of nonfatal AMI, nonfatal stroke, or death from cardiovascular causes. Unfortunately, notwithstanding a number of platelet functional abnormalities or defects of membrane and granuli proteins have been described in CMPD, no consistent correlation with cardiovascular events in either PV or ET has been demonstrated.5,31 However, increased formation of neutrophil-platelet aggregates, associated with enhanced expression of activation markers CD11b and CD62P, has been reported in patients with ET and PV,37 and possibly correlated with patient history of microvascular or major thrombotic events; interestingly, in vitro formation of leukocyte-platelet aggregates was reduced in patients who had been treated with aspirin and/or HU.37
A specific issue is represented by the occurrence of thrombocytosis following splenectomy in PMF. Thrombocytosis developed in 28.6% of 314 patients who underwent splenectomy, with 5.4% having platelet counts greater than 1000 × 109/L. No correlation was found between presence of postsplenectomy thrombocytosis and vascular events, which involved 10% of the patients; interestingly, thrombosis occurred in the portal vein or other close mesenteric/intestinal vessels in 84% of patients, suggesting a local, anatomic reason for thrombosis rather than a direct consequence of increased platelet count.38 However, although not evidence-based, there is general consensus on the opportunity to control thrombocytosis after splenectomy with cytotoxic therapy or platelet apheresis.
Paradoxically, the only clinical event that has been clearly associated with extreme thrombocytosis in patients with CMPD or CML is represented by hemorrhages, which are ascribed to increased clearance of the largest von Willebrand factor multimers from plasma mediated by platelet-dependent interactions. The same phenomenon, although generally asymptomatic, has been reported occasionally in secondary thrombocytosis, particularly in those arising after splenectomy.1 Reduction of platelet count proved effective in normalizing the von Willebrand multimer profile in plasma and in reducing bleeding tendency.1,30,31
Risk Factors for Thrombosis
Due to the considerations above, it is not surprising that thrombocytosis is not considered among the criteria for thrombosis risk assessment in CMPD (Table 2 ). Indeed, established risk factors for thrombosis in both ET and PV are older age (>60 years) and previous thrombosis history, and the presence of either of these define a “high-risk” category of candidates for myelosuppressive therapy.39 The role of conventional cardiovascular risk factors (smoking, hypertension, diabetes, dyslipidemia) is under debate and not currently considered in risk classifications; however, they should be actively corrected as much as possible, especially smoking habits. Also, the role of inherited or acquired thrombophilia conditions is unclear; the Italian guidelines for ET management recommended routine screening in ET patients with either personal or familial history of thrombosis,40 while indications from the British guidelines were the opposite.31
On the other hand, recent data indicate that a novel, powerful risk factor for thrombosis in both PV41 and ET42 is represented by leukocytosis. Particularly in ET, presence of leukocytosis allowed to identify a subgroup of “low-risk” patients who actually had a hazard ratio for thrombosis 3.1-fold greater than conventional “low-risk” category and similar to “high-risk” patients.42 These observations are in line with evidence of enhanced leukocyte activation and formation of platelet-neutrophil aggregates,37 and with the results of the PT-1 trial where HU (as a global myelosuppressive agent) was superior to anagrelide (a platelet-only reducing agent) in preventing arterial thrombosis. The effects of HU on leukocyte count and activation might also underlie the correlation between control of platelet count and reduction of thrombosis rate observed in the first prospective study in high-risk patients with ET by Cortelazzo et al.43
Whether the presence of the JAK2V617F mutation and/ or the burden of mutant allele affect on thrombosis occurrence is being investigated. An increased risk of thrombosis in ET patients with mutation has been reported in some studies44 but denied in others.45,46 In the U.K. study on 806 patients with ET, increased risk of venous thrombosis was found in patients with mutation;47 furthermore, in an Italian retrospective cooperative study on 639 patients with ET, the 14 patients harboring an homozygous mutation presented a significantly higher rate of both arterial and venous thrombosis compared with wild-type or heterozygous counterparts.33 In case of PV, evidence has been presented that thrombotic risk is significantly increased only in patients with 75% or greater mutant allele burden at diagnosis.48 However, it must be also considered that the JAK2 mutation is associated with raised leukocyte count and perhaps more pronounced activation of platelets and leukocytes, which might represent confounding factors. Therefore, available information concerning the prognostic relevance of JAK2 mutation as concerns thrombosis is still largely inconclusive.
How We Manage Thrombocytosis
It is out of the scope of this review to exhaustively address general treatment issues in CMPD, whether or not they have associated thrombocytosis; comprehensive reviews have recently been published.4,5,31,49–54
Improvement or resolution of underlying disease results in normalization of platelet count in reactive thrombocytosis; sometimes, particularly if generic cardiovascular risk factors are present, prolonged increase of platelet count after splenectomy for any cause is managed with aspirin for thrombosis prophylaxis, but no prospective trial supporting this practice exists. As concerns primary thrombocytosis, the lack of evidence-based correlation with occurrence of thrombotic events translates into the lack of a clear rationale for correcting platelet count to normal levels as an effective measure to prevent thrombosis. Conversely, the strongest recommendation for treating primary thrombocytosis is the prevention of hemorrhagic complications due to acquired von Willebrand disease, particularly when the platelet count is greater than 1500 × 106/L. Therefore, the decision whether to treat a patient with primary thrombocytosis relies on the identification of evidence-based clinical risk factors for thrombosis, with due attention to novel, potential, laboratory markers such as leukocytosis and JAK2V617F mutational status (Table 2 ), rather than on platelet count per se.
According to current guidelines, ET patients older than 60 and/or with a positive history of previous thrombotic or hemorrhagic events are candidates for cytoreductive treatment, with HU being considered as the drug of choice.40 The suggested platelet count target level varies; it should be 400 × 109/L in patients with history of thrombosis, while 600 × 109/L is considered acceptable in those at high risk because of older age but with no vascular episode.40 In cases of clinical resistance or intolerance to HU, defined according to criteria recently reported,52 anagrelide can be considered. Switching to alternative drugs such as alkylating agents is reported to be associated with an increased risk of developing acute leukemia; therefore, this choice should be confined to elderly people at very high risk of life-threatening vascular complications. The use of interferon-α may be worthwhile in younger patients, and it is the drug of choice in a pregnant woman with ET.53
There is clearly a rationale for the use of low-dose aspirin in patients with PV, according to the results of ECLAP study, regardless of the presence and extent of thrombocytosis6; in high-risk patients it is safely combined with HU, with no substantial increase of serious bleeding, while in low-risk patients it can be used as a single prophylactic agent. Although no formal randomized study demonstrates that aspirin effectively prevents thrombosis in ET, results of the PT-1 trial established low-dose aspirin plus HU as standard treatment for high-risk patients with ET.7 On the other hand, increased risk of bleeding was observed in patients receiving the combination of aspirin and anagrelide, likely due to combined inhibitory effect on platelet function by the two drugs. The use of aspirin as primary prophylaxis in asymptomatic, low-risk patients with ET is not supported by available evidence, and, indeed, Italian guidelines actually do not recommend it.40
Platelet apheresis is used in rare circumstances, due to short-term efficacy and the need for daily procedures; however, it may be recommended in emergencies when there is an urgent need to reduce extreme thrombocytosis, as is the case of acute and life-threatening, severe bleeding.
Finally, there is great interest and hope in the prospect of molecularly targeted therapy with inhibitors of the constitutively active mutant JAK2, or with novel drugs that might act at different points of the activated JAK-STAT signaling pathway.
Vis-a-vis comparison of the 2001 WHO diagnostic criteria for essential thrombocythemia (ET) and the 2007 expert panel proposal for revision.
| Current WHO criteria26 . | Proposals for revision10 . |
|---|---|
| * For details please refer to Vardiman et al26 and Tefferi et al10. | |
Positive criteria
|
|
| Current WHO criteria26 . | Proposals for revision10 . |
|---|---|
| * For details please refer to Vardiman et al26 and Tefferi et al10. | |
Positive criteria
|
|
Established and novel, potential risk factors for thrombosis in patients with polycythemia vera (PV) or essential thrombocythemia (ET).
| . | Established risk factors . | Potential risk factors . |
|---|---|---|
| PV | Age ≥ 60 y | Leukocytosis41 |
| Previous thrombosis | V617F allele ≥ 75%48 | |
| ET | Age ≥ 60 y | Leukocytosis42 |
| Previous thrombosis | JAK2V617F mutation44 | |
| Platelets ≥ 1500 x 109/L | JAK2V617F homozygosity33 |
A working, molecular-based, classification of thrombocytosis.
Abbreviations: CML, chronic myelogenous leukemia; PV, polycythemia vera; ET, essential thrombocythemia; PMF, primary myelofibrosis; sMF, secondary (post-polycythemic/post-thrombocythemic) myelofibrosis; RARS-T, refractory anemia with ringed sideroblasts and marked thrombocytosis; 5q– MDS, myelodysplastic syndrome with 5q–abnormality.
A working, molecular-based, classification of thrombocytosis.
Abbreviations: CML, chronic myelogenous leukemia; PV, polycythemia vera; ET, essential thrombocythemia; PMF, primary myelofibrosis; sMF, secondary (post-polycythemic/post-thrombocythemic) myelofibrosis; RARS-T, refractory anemia with ringed sideroblasts and marked thrombocytosis; 5q– MDS, myelodysplastic syndrome with 5q–abnormality.
Diagnostic algorithm for a patient with persistent thrombocytosis.
Abbreviations: CML, chronic myelogenous leukemia; CMPD, chronic myeloproliferative disorders; MDS, myelodysplastic syndromes.
Diagnostic algorithm for a patient with persistent thrombocytosis.
Abbreviations: CML, chronic myelogenous leukemia; CMPD, chronic myeloproliferative disorders; MDS, myelodysplastic syndromes.
University of Florence, Hematology Department, Florence; Ospedali Riuniti di Bergamo, Divisione di Ematologia, Bergamo, Italy
Acknowledgments
Supported in part by Associazione Italiana per la Ricerca sul Cancro, Milano, and the MPD-RC, Myeloproliferative Disorders- Research Consortium.