Abstract
It is clear that the clinical heterogeneity of multiple myeloma (MM) is dictated, in large part, by disease biology, predominantly genetics.1 As novel therapeutics have emerged, and augmented our treatment armamentarium against the disease, it is increasingly important to introduce a risk-adapted approach for the optimal management of patients.2 The selection of ideal candidates for high-dose chemotherapy with stem cell support (HDT) and maintenance will undoubtedly have to include baseline knowledge of the genetic nature of the individual. The limited duration of responses after HDT for patients with t(4;14)(p16;q32), t(14;16)(q32;q23) and 17p13 deletions highlight the need to develop a risk-adapted treatment strategy.3,–5 Novel ways of determining outcome such as the use of gene expression profiling have demonstrated differentiating capabilities not previously observed.6 Likewise, the order of introduction of novel therapeutic agents (during induction and in the relapsing patient) will be potentially directed by similar information. As we have previously stated, MM is not only multiple but also “many.”7 Accordingly, treatment strategies will be tailored based on risk determination, genetic composition and host features.
Introduction
The field of multiple myeloma (MM) has seen a revolution in the availability of novel agents for a disease for which therapeutic agents had been scarce. These agents have dramatically improved the possibility of having a disease response, and better quality and duration of responses. Although response to therapy traditionally has not been a good predictor of long-term outcome, most recent studies suggest that response is an adequate surrogate indicator of improvement in survival. For instance, a recent phase 3 study by the Intergoupe Francophone du Myelome (IFM) showed clear improvements in response and survival with the addition of thalidomide to a melphalan regimen compared with both standard melphalan and prednisone and high-dose therapy with stem cell transplantation.8 This is also suggested in recent phase 2 data regarding the use of lenalidomide in combination with dexamethasone used for the upfront treatment of patients.9 All of these regimens, while clearly adding alternative, and potentially better, treatment modalities to our therapeutic armamentarium, can carry added toxicity and cost. But even when comparing the different regimens, it is impossible to make definitive therapy recommendations, since so many of these regimens have a high level of efficacy. Until there are additional data from ongoing clinical trials, provisional recommendations can be made for treatment. In this review, we hope to provide a logical algorithm based on current knowledge.
Definition of “High-Risk” Disease
The correct identification of patients at higher risk of early death is paramount to establishing proper treatment and closer surveillance.1,–5 In our studies, we had originally set the goal of being able to identify a group of 25% of patients who had a median survival of 2 years or less as an arbitrary definition of high-risk MM. In contrast, the identification of all other patients (standard-risk MM) allows for the optimization of their treatments with minimization of toxicity or excessive treatment. To this effect, many classifications have been published. The most extensive and validated is the International Staging System (ISS) proposed by Greipp and colleagues10 (Table 1 ). This classification can identify patients with dissimilar survival and is based solely on the level of the β2-microglobulin and serum albumin. Patients with elevated β2-microglobulin (ISS Stage III) have a much shorter survival irrespective of treatment modality, age or geographic location. A potential limitation of the ISS classification is that it reflects a constellation of host features. For example, the β2-microglobulin is a reflection of tumor burden but also renal function. Thus, while patients with a high ISS are at higher risk of early death, this is may be due to the aforementioned reasons, where providing more intensive or novel therapy has proven problematic (and required dose reduction).
More recently, genetic markers have been examined to determine prognosis.6 The results of many studies have shown that several genetic categories exist, defining subsets of the disease with dissimilar outcome, unique clinco-pathologic features and response to therapy.4,5 These genetic categories cannot be said to explain all subtypes of MM, but can define up to 80% of patients.1 At the very top level, two subtypes of the disease are evident: hyperdiploid MM (H-MM), with lower frequency of IgH translocations; and nonhyperdiploid MM (NH-MM) with a higher prevalence of IgH translocations.11,12 The NH-MM can be further subdivided by the specific chromosome translocations, primarily including the t(11;14)(q13;q32), t(4;14)(p16;q32), t(14;16)(q32;q23) and other less common translocations. Taken together, these categories account for at least 85% of patients. Other subtypes have not been fully elucidated but include patients who show elevated expression of cyclin D2 or low levels of Rb by gene expression profiling (GEP).1 The exact mechanisms for these observations remain to be elucidated.
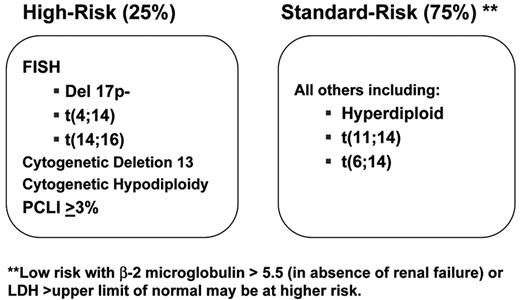
At our center, the consistency in the prognostic value of these categories has helped us incorporate their determination into routine clinical practice. This information helps form the basis for the discussion of patients’ management strategies. To simplify this, we have identified that patients with t(4;14)(p16;q32), t(14;16)(q32;q23) or deletion 17p13 constitute a high-risk genetic category2,4,5 (Figure 1 ). Also, because of the known negative prognostic impact of specific abnormalities detected by karyotype (hypodiploidy and chromosome 13 deletion/monosomy) or a high proliferative rate (a plasma cell labeling index >3%), we consider that patients having any one of these markers belong to the high-risk category.2 With this information, we have then developed a dynamic treatment algorithm that takes into consideration two main factors: (1) Is the patient an optimal high-dose chemotherapy with stem cell support (HDT) candidate? (2) Is the patient in the high-risk genetic category? The information is available at www.msmart.org. This a preliminary but relatively simple attempt at providing a more individualized approach for our treatment recommendations will likely change as more information becomes available.
Can we do better in determining prognosis? The answer is probably yes, and new studies have shown that by harnessing the power of GEP, we can potentially identify patients who are “higher risk” and for whom novel strategies are needed.6 A provocative example is the recently published GEP signature for high risk proposed by the University of Arkansas. They were able to identify and validate a signature indicative of high-risk disease that takes into account 70 genes, but that can be further reduced to 17 genes.6 Importantly, their GEP signatures of risk combine a number of clonal features beyond those implicated in the primary biology of the disease, such as cell-cycle–associated genes, apoptosis implicated genes, and so forth, which may further enhance the clinical validity of those profiles.6
The major challenge continues to be the practical application of these signatures in routine and community-based clinical practices. The challenge is even greater in MM, where the tumor cells coexist with normal myeloid elements, requiring cell selection prior to performing GEP. Even at experienced reference laboratories this may be challenging, since one needs enough cells to be purified for the GEP assay.13 In our experience, in unbiased collection of samples, adequate RNA is obtained from 50% of “mailed-in” patients, with the IFM reporting a similar experience (H. Avet-Loiseau, personal communication). Another GEP-derived signature that looks only at cell-cycle–associated genes has been published.1 We have also found a high prognostic determination for centrosome amplification–associated genes, which we believe is not just a surrogate of cell cycle.14 The prognostic strength of these signatures is such that performing GEP in association with phase 3 clinical trials is going to be vital. This will be especially important to help clarify recommendations for emerging new therapies. Unfortunately, the routine clinical application of these signatures is difficult and will likely come from transformations of these assays into standard clinical laboratory tests such as flow cytometry, immunohistochemistry, or (less likely) RT-PCR. The many other proposed prognostic markers available in the literature are likely to fade with the advent of genome-based risk determination.
Role of HDT in High-Risk Disease
The major application of genetic stratification is currently centered on the selection of candidates for HDT. Several large studies have shown that patients defined as high-risk genetically (determined by either karyotype, fluorescent in situ hybridization [FISH] or GEP) do not derive durable responses to HDT strategies as currently practiced, and many relapse within 1 year of undergoing treatment.3,4,15 This should not be taken to mean that it is an ineffective treatment modality; rather, that the therapeutic benefit is limited by the short duration of responses, and may be improved with the integration of novel therpaies. HDT has become widely used and safer; it can be done with a mortality of less than 1% to 2%, can be completed even as an outpatient procedure, but remains expensive and can be toxic. Proponents of this treatment modality have argued that while patients with high-risk disease have shorter remissions, HDT is still an effective therapy, and nothing has been shown to be better than HDT for these patients. While it is true also that delayed HDT is an equivalent treatment strategy (as opposed to up front),16 the relevance for patients with high-risk disease remains uncertain, and most large prospective studies have suggested inferior outcome.
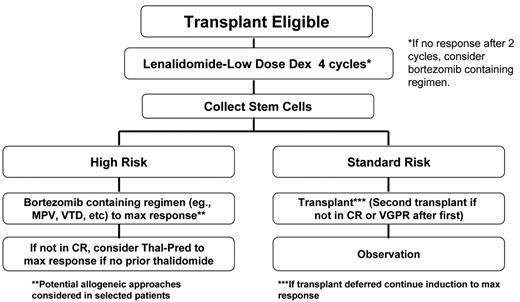
In sum, therefore, better management strategies are needed for high-risk disease. Many emerging studies have now shown that early introduction of bortezomib results in better-quality of response and prolonged survival for high-risk patients (see below). Given the aforementioned limitations of HDT for high-risk disease and the emerging promise of bortezomib, our group has recommended that for patients with high-risk disease contemplating HDT, stem cells are collected after induction and that bortezomib is introduced early in the course of treatment (Figure 2 ).2 Although performing HDT up front for these patients is a consideration, the duration of disease control makes this option worthy of caution.
One caveat is worth mentioning. The IFM has also shown in the largest dataset published to date that for patients with t(4;14)(p16;q32) and low β2-microglobulin, HDT is still associated with durable responses.4 Limited studies at our center have failed to confirm this positive effect on prognosis for the β2-microglobulin in patients treated with conventional doses of melphalan, suggesting that a prognostic dichotomy using β2-microglobulin in patients with t(4;14)(p16;q32) is not apparent with conventional-dose melphalan, but may occur with HDT.
Induction Therapy
It is now expected that all MM will have responsive disease with initial therapy and that only a small minority will remain refractory. In one phase 2 study we conducted at Mayo Clinic, we showed that the combination of lenalidomide with dexamethasone had an overall response rate of 91%.9 Similarly high response rates have been achieved with bortezomib in combination with dexamethasone.17 This appears superior to VAD chemotherapy, high-dose single-agent dexamethasone, and even thalidomide and dexamethasone in combination.18 Current studies are looking at the quality, depth, and duration of responses as well as safety. The incorporation of genetic markers is likely to be increasingly relevant in selecting the best agents, as illustrated below.
Given that quality of response is becoming a benchmark for the upfront clinical trials, the logical corollary question is does this matter? The evidence to date would suggest it does. A recent study by the IFM showed that attaining a better quality response with a bortezomib-containing regimen resulted in more favorable outcomes after HDT.19 Importantly, far fewer patients receiving bortezomib had to undergo the second of two (tandem) HDT, since they had attained maximal response with the first one.19
The implications of these findings are considered not least from the standpoint of improving patient oucomes with less toxicity but also in terms of health economics. Furthermore, in the Eastern Cooperative Oncology Group (ECOG) study E4A03 patients receiving lenalidomide with low-dose dexamethasone had a one year probability of survival of 96% (National Cancer Institute [NCI] and ECOG press release, April 2007, available at http://www.newratings.com/analyst_news/article_1506504.html). In this study, numerous patients opted to, despite the original plans for upfront HDT, continue lenalidomide therapy with the hope of further improvements in quality of response over time. Indeed, Lacy et al showed that, with extended duration of therapy, lenalidomide in combination with dexamethasone earlier in our phase 2 studyhad an overall rate of very good partial response or better in 65% of patients.20 As greater depth in the quality of responses is attained, new, validated and practical tools that provide amplitude in the measurement of complete response (CR) will hopefully further improve our ability to differentiate the effects of therapy.
Toxicity and ease of administration are also factors in the selection of novel induction regimens. The recent developments in E4A03 highlight the importance of addressing these issues. In this study, the use of lower-dose dexamethasone (120 mg/cycle as opposed to the classic 12-day schedule resulting in 480 mg/cycle) is associated with an improved survival, reduced rate of infections, and lower incidence of venous thrombosis resulting in lower mortality. Response data from this study are not yet available and should be presented soon. It is clear that a large fraction of the improvement in survival with low-dose dexamethasone relates to reduced mortality secondary to infections. While this mortality figure approaches a historic 10% with high-dose dexamethasone (a dose originally chosen on empirical basis only), the mortality in the low-dose E4A03 trial was less than 2%. At this point, it is clear that the lower doses with this combination are better overall, and the reduced toxicity documented with lower-dose dexamethasone confirms concerns that have existed for some time in the field regarding the side-effect profile. Other studies have shown an excellent response with low-dose combinations in conjunction with other agents such as bortezomib. Efforts are now under way to eliminate dexamethasone altogether from novel combinations, and so provide patients with true steroid-sparing approaches.
Presuming that quality of response remains an important goal of induction, the role of lenalidomide and/or bortezomib (with or without low-dose dexamethasone), has become a new focus since with thalidomide-based regimens and high-dose dexamethasone alone, CR is in fact rare. Will it matter later if we use these agents up front, or will there be any loss of effectiveness for future use? Current studies suggest not, but a careful analysis of trials will be warranted, since it is not yet known how risk categories will dictate the choice for induction therapy, as it is possible that CR rates will differ according to risk, which may in turn impact both on the sequence and combination of agents.
Consolidation
Consolidation of the initial gains in disease control with induction have traditionally used HDT, but its limitations, especially in high-risk disease, are evident. Based on several recent studies, the early introduction of bortezomib is important in poor-prognosis disease. Specifically, Mateos and colleagues (albeit in patients older than 65 years) found that adding bortezomib to melphalan and prednisone (MP) resulted in similar response rates irrespective of deletions of chromosome 13 by FISH or IgH translocation status21 (Table 1 ). The combination of these two factors is enriched for cases with high-risk MM (mostly NH-MM), and comparing their data with historic matched cases, they found a clear improvement in survival as compared with MP. A large, international phase 3 study (VISTA) has recently been completed, and its results should be available soon to elucidate this further. Importantly, several other large studies had shown that with bortezomib, the likelihood of response and associated clinical benefit was the same with or without chromosome 13 deletion.22 At our center, we now recommend that all patients who have high-risk disease and are HDT candidates consider early collection followed by consolidation with a bortezomib-containing regimen (Figure 2 ).
If indeed bortezomib-based regimens result in better long-term outcome for high-risk disease, this will be a major step forward, allowing the HDT to be used for recurrent disease. Unfortunately, a study by Stewart and colleagues showed that recurring t(4;14)(p16;q32) may be less sensitive to alkylators, and this may potentially diminish the efficacy of HDT as salvage therapy.23 In any case, if the outcome is still improved compared with what was previously achieved with HDT, this approach may still be better, especially as in our experience relapse-free survival after HDT is limited and was only 8 months for patients with t(4;14)(p16;q32) disease.3,4,15
Allogeneic Strategies for High-Risk MM
Allogeneic HDT strategies for the treatment of MM continue to be tested. Given the complexities of this modality, along with the significant risk of graft-versus-host disease, this treatment strategy is perhaps best reserved for individuals with high-risk MM. In the first reported clinical trial, the IFM tested the hypothesis that nonmyeloablative allogeneic stem cell transplantation (NM-SCT) would be superior to tandem HDT for patients with high-risk disease.24 They used an elevated β2-microglobulin and chromosome 13 deletion by FISH to identify these patients25,26 but failed to show superiority of the allogeneic strategy. More recently, another randomized trial with a similar design except that it enrolled patients irrespective of risk, showed superiority of NM-SCT over tandem HDT.27 This later study was limited in that the number of patients studied was very small. In addition, while not reaching statistical significance (because of the low numbers), there were twice as many complete responders after the first HDT in the NM-SCT arm. This potentially challenges the specific contribution of NM-SCT for upfront disease control. Data from the US intergroup study (BMT CTN 0102) and a large EBMT study assessing the role of NM-SCT in MM are eagerly awaited.
Maintenance
The role of maintenance therapy for MM is an area of active investigation. While intuitively it would seem reasonable to engage in a maintenance strategy for individuals at high risk or with persistent disease, the data derived from large clinical trials are still elusive. In one study done by the University of Arkansas (Total Therapy II), patients were randomized to receive maintenance with thalidomide or none at all.28 Introduction of thalidomide in this study is one element of a complex therapy strategy. The study did show improvement in the event-free survival but no effect on overall survival. A large randomized study by the IFM did show that maintenance with thalidomide resulted in improved overall survival. Interestingly, this effect was most pronounced for patients who lacked chromosome 13 deletion and in patients with less than very good partial response after HDT). The role of new agents such as lenalidomide and bortezomib as maintenance after HDT in high-risk disease are being explored further .
Elderly Patients
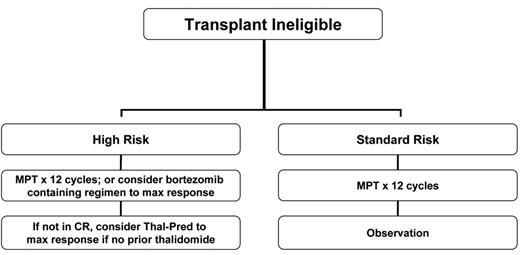
The advent of novel therapies has also made a remarkable difference in the outcome of elderly patients with MM. These were individuals who are typically no candidates for HDT, had limited treatment options and for whom relapse after MP was followed by less effective second-line treatments. Two major combinations have emerged as treatment options for the elderly: MP in combination with immunomodulatory drugs and MP in combination with bortezomib. Both of these approaches have resulted in superior outcomes compared with the prior use of MP alone.
Two major studies are worth noting. Based on the combination development by Palumbo et al29 and others, the IFM pioneered a phase 3 study that compared MP, versus MP plus thalidomide, versus high-dose intravenous melphalan.8 The intravenous melphalan arm received induction therapy with VAD and stem cell collection with cyclophosphamide, followed by HDT tandem (melphalan at 100 mg/m2). The study demonstrated the superiority of MP plus thalidomide over both MP and HDT such that this combination has become a new standard of care in this population.8 Detailed analysis regarding the best candidates based on genetic stratification has not yet been reported. The study of bortezomib in combination with MP by Mateos and colleagues reported heretofore-unprecedented response rates of CR: 43% CR and near-CR.21 As previously mentioned, this effect was observed even for individuals with high-risk genetic categories.
To address the value of MP plus lenalidomide over MP plus thalidomide, a phase 3 study will soon be launched in the U.S. that will compare both regimens (ECOG study E1A06). A preliminary report by the IFM shows that the benefits of MP plus thalidomide over those of MP alone are also evident for individuals over the age of 75 years.30 The formal comparison of MP plus bortezomib versus MP alone (VISTA trial) has already been completed and results are eagerly awaited. The winner of E1A06 will have to be tested against the winner of the VISTA trial (most likely MP plus bortezomib). Given that the addition of bortezomib has been shown in many trials added efficacy against the high-risk disease, it is possible that for the poor-prognosis patient, MP plus bortezomib will become a new standard, while for the patient with standard-risk disease, using MP plus one of the immunomodulatory agents will be sufficient (Figure 3 ).
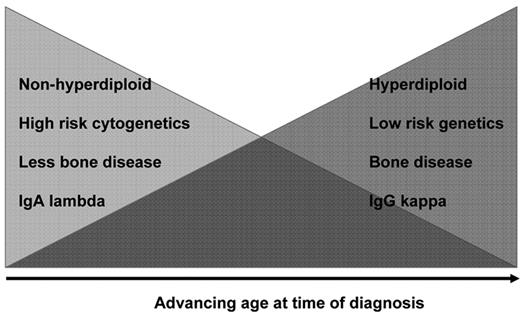
Because of the high activity of some of these combinations in the elderly, we and others have asked whether they should be considered in younger patients as sole primary therapy, and so obviate the need for HDT. It is important to remember that the genetic nature of MM in the elderly is potentially different from that observed in younger individuals; elderly patients may have more indolent disease (H-MM and t(11;14)(q13;q32)), IgGκ MM, more bone disease and less enrichment for chromosome 13 deletion (Figure 4 ).31,32 In contrast, younger patients are more likely to be enriched for high-risk features, including NHMM, multiple IgH translocations, IgAλ isotype, renal failure, bone disease, and relatively modest but more extramedullary MM.
Relapsed and Refractory Disease
Limited information is available regarding the role of genetic stratification for patients with relapsed and refractory MM. In fact, until recently were few data regarding the natural history of patients with relapsing and refractory MM.33 Furthermore, those individuals with the most aggressive variants of MM will likely not have lived long enough to be significantly represented in some clinical trials. Mulligan and colleagues have done one of the first comprehensive pharmacogenomic efforts to define factors predictive of benefit from bortezomib in the relapsed and refractory MM setting.13 They derived the gene expression signature by studying the CREST34 and SUMMIT35 studies and validated these genomes with material derived from the APEX36 study; these studies all used bortezomib in the relapsed and refractory setting, and the authors were able to predict the likelihood of response with reasonable accuracy and identified factors responsible for sensitivity.13 Specifically, they were able to find evidence of NF-κB activation in patients who had stable disease or better, as opposed to those who did not derive benefit from treatment. While this remains an early effort, it is reflective of a promising future pathway for disease analysis and putative drug selection.
Several other studies have shown that, in the relapsed and refractory setting, chromosome 13 deletion on a gene does not seem to confer the negative prognostic effect it imparts at the time of new diagnosis.22 Conversely, several studies have recently shown that bortezomib is able to overcome all of the negative prognostic effect of chromosome 13 (as a surrogate marker of the high-risk genetic categories, mostly NH-MM).22
Conclusions
Given the impressive improvements in the therapeutic options for MM, it is increasingly likely that some patients will enjoy long-term disease control. It is still sobering to recall that we are not there yet, and for almost all patients, MM is lethal. Garnering the power of genetics and genomics to provide a risk-based management of the disease has become an increasingly important part of care. How to best incorporate selected markers in the process of treatment decisions is still evolving but will ultimately involve a more personalized approach. At this point, alternative pathways are needed for individuals who derived lesser benefit from upfront HDT (without detriment to the potential of HDT as a rescue option in younger patients). There is, however, a need to simplify the diagnostic tools needed to ascertain this risk. The use of large-scale genomic strategies is complex and unlikely to have widespread application. As of now, molecular cytogenetic approaches using interphase FISH provide important information. Development and validation of other diagnostic tools such as flow cytometry, immunohistochemistry or other genetic tests (e.g., RT-PCR) has the potential to further disseminate the application of this platform towards patient care.
International Staging System for multiple myeloma (MM).
| Stage . | Parameters . | Median survival . |
|---|---|---|
| Adapted with permission from Greipp et al.10 | ||
| 1 | β2-microglobulin <3.5 mg/L and albumin >3.5 g/dL | 62 months |
| 2 | β2-microglobulin <3.5 mg/L and albumin <3.5 g/dL, or β2-microglobulin 3.5–5.5 mg/L | 44 months 29 months |
| 3 | β2-microglobulin >5.5 mg/L | |
| Stage . | Parameters . | Median survival . |
|---|---|---|
| Adapted with permission from Greipp et al.10 | ||
| 1 | β2-microglobulin <3.5 mg/L and albumin >3.5 g/dL | 62 months |
| 2 | β2-microglobulin <3.5 mg/L and albumin <3.5 g/dL, or β2-microglobulin 3.5–5.5 mg/L | 44 months 29 months |
| 3 | β2-microglobulin >5.5 mg/L | |
Mayo Clinic Arizona Cancer Center; Professor of Medicine; Scottsdale, AZ