Abstract
This review highlights some of the contributions that have appeared in the literature in the past decade on the pathogenesis and treatment of aplastic anemia (AA). This summary is brief because the field is vast, spaning from stem cell biology to stem cell disorders, from autoimmunity to transplantation, from graft-versus-host disease to late effects. The immune pathogenesis of AA is now based on several lines of evidence and will be discussed. Immunosuppressive therapy (IST) remains an important option for AA patients who are not candidates for transplantation. Favorable prognostic indicators for IST are young age and a short interval from diagnosis; the neutrophil count seems to have lost its predictive value with current antithymocyte globulin–cyclsoporin combination therapy. The outcome of allogeneic bone marrow transplantations has significantly improved in the past decade, particularly in the unrelated donor setting, to such an extent that treatment strategies may be affected. A short interval between diagnosis and treatment will also improve results for bone marrow transplantation; these rare patients should be referred to an experienced center immediately.
Diagnosis
Acquired aplastic anemia (AA) is characterized by peripheral blood cytopenia and reduced marrow cellularity. The diagnosis of acquired AA requires the exclusion of other conditions associated with pancytopenia: among these are congenital marrow failure, such as Fanconi anemia (FA), and myelodysplastic syndromes (MDS). FA can be suspected by examining the patient, especially if a child or a teenager, and can be exluded by a chromosomal breakage test using either peripheral blood lymphocytes or dermal fibroblasts exposed to di-epoxibutane (DEB) or mitomycin C (MMC). FA cells will show excessive chromosomal breakage. This test is not only of value in children with bone marrow failure, but young adults can also be found to have FA. Some rarer congenital marrow failures, without specific markers, can be more difficult to exclude. MDS can be ruled out by appropriate marrow cytology/histology and cytogenetic analysis. The distinction between FA, MDS and AA is important, because treatment may be different in these three conditions. In the presence of an empty marrow, pancytopenia, and transfusion dependence, the severity of the disease is based on neutrophil (PMN) count: nonsevere AA (nSAA; PMN > 0.5 × 109/L), severe AA (SAA; PMN 0.2–0.5 × 109/L), and very severe AA (vSAA; PMN < 0.2 × 109/L). We shall see that this may no longer be the case with current treatment strategies.
Pathogenesis of Acquired Aplastic Anemia
Normal hematopoiesis depends on a complex interaction of several cell types, including hemopoietic stem cells (HSC; the seed) and cells from the microenvironment (the soil). This being the case, is acquired AA a disease of seed or soil?1 In the past three decades we have accumulated evidence for abnormalities of the seed and also for abnormalities of the soil. The difficulty is deciding (1) which comes first and (2) whether there is a causal relationship between the two: an abnormal expansion of suppressor T cells may cause depletion and possibly also clonal abnormalities of HSC.2 Conversely, stem cell defects may be associated with secondary abnormalities of the microenvironment. Clinical observations have clarified some answers to these questions. We find that a significant proportion of patients with acquired AA, ranging from 30% to 80%, given immunosuppressive therapy (IST) exhibit long-lasting recovery of peripheral blood counts. Responders would have immune-mediated suppression of hematopoiesis; non-responders could either have marrow failure caused by a primary HSC defect or immune-mediated aplasia with complete exhaustion of the stem cell pool. A further argument in favor of a primary immune-mediated pathogenesis of acquired AA comes from a recent elegant study,3 suggesting that CD4+CD25+FOXP3+ regulatory T cells are deficient in these patients, similar to what is seen in other autoimmune conditions. Deficient regulation of T cells could then lead to an increase of T-bet protein levels in T cells,4 increased interferon (IFN)-γ,2 and stem cell destruction. Polymorphysims in cytokine genes associated with an increased immune response, including tumor necrosis factor -α, IFNγ, and interleukin-6, are also more prevalent in AA patients.2 The degree of progenitor cell depletion in these patients is in the order of 99%, as measured by long-term culture-initiating cells (LTC-IC) and is seen for decades after successful treatment with IST.5 Telomeres are short in one-third of AA patients, and this may not be exclusively due to stem cell exhaustion.2
Glycosyl-phosphatidyl-inositol
A number of surface proteins are linked to the cell through the glycosyl-phosphatidyl-inositol (GPI) anchor, a complex glycophospholipid. Normal hemopoietic cells are GPI+, but rare GPI− cells also exist in every individual;6 they arise in healthy individuals, just as they do in patients with paroxysmal nocturnal hemoglobinuria (PNH), from somatic mutations in an X-linked gene called PIG-A. One example of a GPI-linked protein is CD52, the target of alemtuzumab (Campath):7 when CD52+ cells are deleted with intravenous admninistration of Campath-1H in patients with lymphoma, T-cell recovery is initially CD52−; in other words it is accounted for by GPI− T-cell precursors.7 Thus, we have evidence that an antibody capable of deleting GPI+ cells “selects” for GPI− cells also in patients who do not have PNH. In patients with acquired AA, the emergence or the presence of small GPI− clone(s) has been described, and is a predictive marker for reponse to IS.8 It could be that in acquired AA, GPI− stem cells may be spared from autoimmune attack, suggesting that one or more of the putative “auto-antigens” could be GPI linked. This may explain the intriguing relation between acquired AA and PNH.9 Testing for expression of GPI-linked antigens on lymphocytes, monocytes, granulocytes and erythrocytes is currently part of routine diagnostic and follow-up procedures.
Treatment of acquired AA
Patients with acquired AA can be offered three different treatment strategies, based on the level of cytopenia. Patients with moderate cytopenia, not requiring transfusions, also referred to as hypoplastic anemia, can be offered supportive care or outpatient treatment with anabolic steroids and/or low-dose steroids or cyclosporine (CsA). Recently, androgens have been shown to increase telomerase activity in human CD34+ cells, which may provide an explanation for their effect, sometimes striking, in some patients with AA.2 Patients with cytopenia requiring transfusions should be treated as inpatients, with either IST or bone marrow transplantation (BMT), and the decision to begin treatment should not be delayed, as this may significantly decrease the chance of success.10 The choice between these two treatments is based on severity of the disease and patient age: young patients (<20 years) with vSAA are candidates for first-line transplantation. Older patients with a higher PMN count are generally offered IST as initial therapy.11
Immunosuppressive Treatment
Treatment with antithymocyte globulin (ATG) yields superior survival when compared with supportive care.2,12 Combinations of ATG with androgens13 or CsA14 improve the overall response rates, but not survival. The update of the German study comparing ATG plus CsA versus ATG alone shows a difference in event-free survival, indicating that patients receiving ATG alone required additional courses of IST compared with patients receiving ATG plus CsA; however, survival at 15 years was comparable.14 Event-free survival is an important outcome, because it indicates survival without transfusions and without additional courses of ATG, and this is relevant for quality of life.15 The median time to achieve a response is 120 days,16 and thus a second treatment should not be planned earlier than 4 months after the initial ATG treatment. Responses can be subdivided in complete (CR) and partial (PR): the former would require normal blood counts, although some reports will indicate CR as patients with a Hb greater than 10 G/ dL, a PMN count greater than 2 × 109/L and platelets greater than 100 × 109/L.16 Partial responses require at least transfusion indpendence. The probability of becoming transfusion independent may vary from 40% to 80% according to the IST regimen and the patient population.
Horse vs rabbit ATG
Both horse ATG and rabbit ATG have been used successfully in patients with acquired AA.2 The standard first-line IST is currently horse ATG plus CsA and second-line treatment is rabbit ATG plus CsA,17 although the latter has been successfully used also as first-line therapy.18 The infusion of ATG may cause allergic reactions, but with appropriate premedication with steroids/antihistamines and appropriately slow infusion (up to 24 hours for each dose), nearly all patients can complete the prescribed total course of ATG, usually lasting 5 days. Infections, hemorrhages, and fever are not an absolute contraindication for treatment with ATG; although these antibodies will make cytopenia worse in the first weeks, they should be considered as necessary therapy, just like chemotherapy is required for patients with leukemia presenting with cytopenia. In a recent analysis, patients treated beyond the median interval between diagnosis and IST of 23 days have significantly greater risk of failing in multivariate analysis.10
Cyclosporin dependence and relapse
Current IST regimens including CsA call for a full CsA dose (5 mg/kg orally per day) for 6 months; after thistime point, CsA is tapered, and it is unclear exactly (1) when and (2) how fast this should be done. A recent, as-yet-unpublished study of the Italian pediatric group has addressed these two questions. In this study, 42 children were divided into three groups: very slow tapering (<0.3 mg/kg/month), slow CsA tapering (0.4–0.7 mg/kg/month) and rapid tapering (≥0.8 mg/kg/month). The cumulative incidence of relapse was 8% in the slow/very slow taper group and 60% in the rapid taper group.19 Among patients who eventually discontinued CsA, the median duration of CsA treatment at full therapeutic dose (4–6 mg/kg) was 12 months (range 3–45 months), and tapering was completed in a median of 19 months (range: 4–64 months). In that study, the actuarial probability of discontinuing CsA was 21% at 5 years, 38% at 7 years, and 60% at 10 years, respectively.19 This study suggests that (1) it is safe to start taper CsA at 12 months of treatment (rather than 6 months) and (2) that taper should be very slow (less than 10% of the dose/month) for at least 1 year, to minimize the risk of relapse.
Relapse is defined as a patient requiring transfusions of red blood cells and/or platelets after having been independent from transfusion for at least 3 months.20 The risk of relapse is in the order of 30% and is not easily predicted;20 in a recent Japanese prospective study, the risk of relapse was significantly higher in patients receiving ATG plus CsA (42%) compared with that of patients receiving ATG plus CsA with the addition of granulocyte colony-stimulating factor (G-CSF; 15%) (P = .01),21 although 4-year survival was not significantly different (88% vs 94%).21 Relapse can be succesfully rescued by an additional course of ATG.20–22
Growth factors
The use of G-CSF has been described in conjunction with ATG and CsA as first-line treatment.16 The potential advantages of using G-CSF are faster neutrophil recovery23 and the opportunity to test for white blood cell (WBC) increments and therefore predict failures.16 We have recently confirmed that patients who do not achieve a WBC count of 5 × 109/L while receiving G-CSF in the first 3 months have a 72% chance of not responding, a 79% probability of failing primary therapy, and an 84% risk of death.24 In other words, the use of G-CSF allows early identification of non-responders and may allow early referral for BMT.
However, the use of daily G-CSF for 4 months has drawbacks: it is expensive and apparently does not improve survival at 3 years, as shown in two prospective randomized trials.21,23 Interestingly, both randomized trials showed no difference in the risk of late clonal disorders between patients who received G-CSF or not.21,23 This is in contrast to a retrospective European study showing a borderline increased risk for G-CSF patients,25 and the Japanese retrospective study also showing increased risk of clonal disorders.26 A correlation was also found between duration of exposure to G-CSF and clonal disease.26 However, the cause-effect relationship is unclear, because non-responders to IST are more likely to develop clonal disorders,16 and prolonged G-CSF would be given precisely to these patients. A large randomized study has been completed in the European Group for Blood and Marrow Transplantation (EBMT) and may definitively answer these questions. Finally, a further increase of G-CSF to 10 mg/kg/day failed to show any advantage over the conventional 5 mg/kg/day.24
Clonal evolution and second malignancies
There are several possible evolutions of the disease that may or may not be correlated with its etiology, pathogenesis and treatment. The appearance of a GPI− clone or the evolution towards frank PNH, with increasing levels of LDH and increasing spleen volume, may confirm the link between the two disorders.9 The appearence of cytogenetic clonal abnormalities (for example, trisomy 8) may suggest the pre-existence of this clone at diagnosis, or the emergence of a +8 clone during stressed hematopoiesis. Some patients with AA may proceed to frank acute leukemia. The overall risk of developing a clonal cytogenetic abnormality/MDS at 10 years is set between 5% and 20% and may depend on the degree of response to IST.16 An important contribution to this problem was a study published in 1993,27 that compared second malignancies in patients with AA treated with either IST or BMT. The risk of MDS/acute myeloid leukemia (AML) was significantly higher for patients who underwent IST as compared with those who underwent BMT, suggesting that MDS/AML follows IST rather than being present at diagnosis, because 200 mg/kg cyclophosphamide (CY), used in transplantation, would be unlikely to eradicate a neoplastic clone. Second tumors were frequent in patients receiving radiation before BMT, and radiation is currently not recommended in HLA-identical sibling transplantations. Very low dose (2 Gy) total body irradiation (TBI) is being explored in patients undergoing an alternative donor transplantation.28
Factors predicting survival after IST
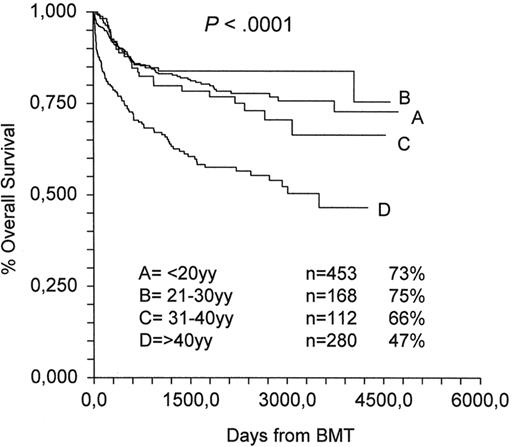
The importance of predicting response and survival is relevant for every disease, but in patients with AA, pancytopenia has acute consequences, with daily risk of bloodborne infections or cerebral hemorrhages, and this makes predictive factors very important. In a recent study on almost 1000 patients treated in Europe between 1991 and 2002, the strongest negative predictor was age (older than 16 years) (relative risk [RR], 1.76; P = .0009), followed by an IST protocol other than ATG plus CsA (RR, 1.29; P = .02) and interval between diagnosis and treatment over 23 days (RR, 1.32; P = .04).10 The year of IST (before or after 1997) had a borderline negative effect,10 suggesting little overall improvement in the last 5 years. Of interest is the fact that severity of the disease, as identified by PMN counts (<0.2, 02–0.5, and >0.5 × 109/L), had no impact on survival, in contrast to results from the original analysis of the EBMT showing that PMN count was the strongest predictor of survival.29 Indeed, results have improved dramatically in children with vSAA from 37% in the 1980s to 83% in the 1990s,10 but this has not been the case for SAA; actually, the non-severe patients showed a trend for worse outcome.10 Currently, survival can be predicted by age as shown in Figure 1: the 10-year actuarial survival is 73% for patients younger than 20 years, 75% for patients aged 21 to 30 years, 66% for patients aged 31 to 40, and 47% for patients older than 40 years.
IST for older patients
Also, patients with AA over the age of 70 years can be treated with ATG and CsA, although response rates and survival are lower compared with young patients:30 the actuarial 10-year survival is 45% for patients aged 51 to 70 years and 25% for patients over the age of 70 years.30 Nevertheless, the standardized mortality ratio (SMR), indicating the ratio between mortality of patients and of an age-matched population, is 33, 14, and 9, respectively, for the age groups younger than 50, 50–70, and older than 70 years.30 These data suggest that the corrected risk of death in AA is highest in young patients and becomes progressively lower with increasing age.
Pregnancies after IST
Can young women who have recovered their peripheral blood counts after IST become pregnant? In the one study that has addressed this issue, succesful pregnancy was found to be possible, but at the expense of a risk of relapse of the aplasia (approximately 20%); in this study, one of these relapses was fatal.31 Relapses were more frequent in partial responders compared with complete responders. So, if a young woman is in complete remission, off therapy, and aware of the risk of relapse of the disease, a pregnancy is a possibility.
Bone Marrow Transplantation
We have recently analyzed 1567 patients allografted in the period 1991 through 2002.10 Favorable predictors of survival were year of transplantation after 1997, matched sibling donor (MSD), age younger than 16 years, an interval between diagnosis and transplantation of less than 83 days, and a conditioning regimen without radiation.10
HLA-identical siblings
The current survival for patients with AA younger than 16 years who have received a BMT from an HLA-identical sibling after conditioning with 200 mg/kg CY is 91%:10 it will be difficult to design a better program for these patients, especially because 200 mg/kg CY does not cause infertility or second tumors. For older patients, results are less encouraging, and peripheral blood (PB) transplantations have been introduced with the aim of reducing rejection and infections. A recent EBMT/IBMTR study suggests that PB transplantations actually reduce survival compared with marrow transplantations, in patients with AA younger than 20 years, from 85% to 73%, and in patients older than 20 years, from 64% to 52%:32 the major cause of excess mortality in the PB arm is chronic graft-versus-host disease (GVHD). This study suggests that PB transplantations are not recommended in patients with acquired AA, possibly because increased chronic GVHD is not beneficial, as may be the case in leukemia. CY alone remains the best conditioning regimen for young patients, and ATG would seem to reduce the risk of graft failure:33 a recent randomized trial has shown a non-significant survival advantage for patients receiving ATG.34
GVHD prophylaxis should be the combination of CsA and methotrexate (MTX), since this has been shown to be superior to CsA alone.35 Therefore, standard cyclophopshamide 50 mg/kg/day for 4 days, with 3 days of ATG, followed by unmanipulated marrow and CsA plus MTX is still standard of care for patients with aquired AA undergoing an HLA-identical sibling transplantation. The use of radiation, PB, or other conditioning regimens should be tested in prospective trials because of their unproven benefit for the patient.
HLA identical BMT for patients older than 30 years
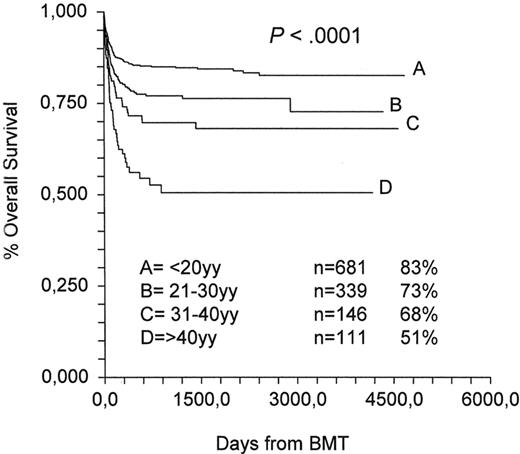
Notwithstanding the excellent results with a standard BMT in AA, the effect of age cannot be underestimated: overall survival in the last decade for HLA-identical sibling transplantations at 10 years is, respectively, 83%, 73%, 68% and 51% for patients age ranges 1–20, 21–30, 31–40 and ≥40 years Figure 2; EBMT data). The age effect has remained significant over time, with survival in the range of 50% for patients over the age of 40 years. The EBMT is exploring the use of low-dose CY (300 mg/m2 × 4) in combination with low-dose fludarabine (FLU; 30 mg/m2 × 4) and ATG in patients older than 30 years: the initial results are encouraging, with a transplantation mortality of 30%, rather than the expected 50%, but more patients need to be accrued (EBMT, unpublished data). Interestingly, a recent report has confirmed that the combination of FLU-CY and ATG may reduce the transplantation-related toxicity compared with CY alone.36
Unrelated donor transplants
Significant progress has been made in the past few years, and at least three studies have addressed the issue of the conditioning regimen.28,37,38 The first of these studies tested de-escalating doses of radiation, from 6 Gy down to 2 Gy, and concluded for best results in patients receiving 2 Gy, with 8 of 13 patients surviving.37 The Japanese study reported on 154 SAA patients undergoing an unrelated donor (UD) transplantation: 11% rejected, 20% experienced acute GVHD, 30% experienced chronic GVHD, and 64% survived.28 Unfavorable factors for survival were older age (>20 years), conditioning without ATG, and a long (>3 years) interval from diagnosis to transplantation. The Japanese study also included a large number of patients who received low-dose radiation (3 Gy). The EBMT study tested a non-radiation–based program:38 results were overall encouraging, with 70% surviving, although rejecton was high in young adults older than 14 years. EBMT is currently testing a conditioning regimen which is very similar to the Japanese regimen: FLU-CY-ATG and low-dose TBI (2 Gy) (unpublished).
As a consequence of improved donor/recipient matching, survival has almost doubled in the past decade10,39 from 38% in 1991–1996 to 65% in 1997–2002.10 Results of UD transplantations have improved to such an extent that treatment strategies may change. In children lacking a matched sibling donor, a UD search should be started at diagnosis, and transplantation seriously considered after one course of IST in the presence of a suitable donor. In young adults between 20 and 30 years old, the same may be true. Adults older than 30 years should be entered on a prospective trial because there are no data at present supporting the use of early UD transplantations.
Conclusions
Acquired SAA can be treated successfully with either IST or BMT: IST can be readily administered to all patients but is not curative. BMT produces rapid and long-lasting hematologic recovery without the long-term risk of MDS, but requires a suitable donor and appropriate financial resources, and may cause long-lasting chronic GVHD.
Significant improvement has been made in BMT, especially from alternative donors and in young patients. There has been less improvement with IST, with the exception of young children with vSAA: indeed, the neutrophil count seems to have lost its predictive value with combined IST. Age remains a major predictor and requires careful consideration when deciding the treatment strategy.
Two final important messages: a short interval between diagnosis and treatment will improve results and should call for immediate referral of these rare patients to an experienced center. In addition, because of the rarity of the disease, patients should be entered on well-designed prospective clinical trials, so that unanswered questions can be addressed, if necessary with international cooperation.
Immunosuppressive treatment 1991–2002 (Working Party Severe Aplastic Anemia).
HLA-identical sibling transplants 1991–2002 (Working Party Severe Aplastic anemia).
HLA-identical sibling transplants 1991–2002 (Working Party Severe Aplastic anemia).
Ospedale San Martino, Genova, Italy