Abstract
Transfused platelets (plts) are either pooled random-donor platelet (plt) concentrates or single-donor apheresis plts. When stored for 5 days, all of these products are equally efficacious.
A 10,000/μL prophylactic plt transfusion trigger has been documented to be both hemostatically efficacious and cost effective in reducing plt transfusion requirements. The optimal plt dose/transfusion is being evaluated in an ongoing clinical trial. Therapeutic plt transfusions to control or prevent bleeding with trauma or surgical procedures require higher transfusion triggers of 100,000/μL for neurosurgical procedures and between 50,000/μL and 100,000/μL for other invasive procedures or trauma.
Leukoreduction has been documented to reduce plt alloimmunization rates, cytomegalovirus (CMV) transmission by transfusion, and febrile transfusion reactions. Whether it reduces immunomodulatory effects of transfusion (i.e., decreases infection rates and cancer recurrence) is still controversial, as is universal leukoreduction.
Poor responses to plt transfusions are often multifactorial. For alloimmune plt refractoriness, HLA matching, cross-matching, and identification of the specificity of the patient’s antibodies with avoidance of mismatched donor antigens are all equally effective in identifying compatible plts for transfusion. Other causes of poor plt responses are splenomegaly, ABO mismatching, females with 2 or more pregnancies and males, use of heparin or amphotericin, bleeding, fever, graft-vs-host disease (GVHD), and vaso-occlusive disease (VOD).
Platelet Products Available for Transfusion
Platelets (plts) are obtained by two different methods: plt concentrates from whole blood or apheresis plts.
Plt concentrates from whole blood
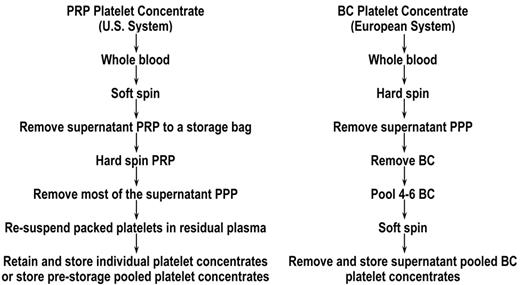
Plt concentrates can be prepared from whole blood by using the two different methods outlined in Figure 1 . These are referred to as the plt-rich plasma (PRP) method, which is used exclusively in the U.S.,1 and the buffy coat (BC) method2 that is predominantly used in Europe (Canada is converting to this method). Comparative studies have shown no difference in the quality of these plt concentrates when they are stored for up to 7 days.3,4 However, there is emerging evidence that, when plts are stored for extended time periods, the method of plt collection and the storage media influence post-transfusion plt viability.5 –7 The hard-spinning of the plts against a red cell layer in the BC method versus against the bottom of the bag in the PRP method, requiring resuspension of the plts, may induce a collection injury that could potentially compromise the long-term storage of PRP plts compared with BC plts. However, there is currently no direct evidence to substantiate this hypothesis.
Apheresis plts
The major advantage of apheresis plts is that enough plts can be collected from a single donor to constitute a transfusion dose. In contrast, to obtain an equivalent number of plts requires pooling 4 to 6 whole blood–derived plt concentrates.
The reduction in donor exposures by using apheresis plts has the potential advantages of reducing transfusion-transmitted infections and the incidence of plt allo-immunization. However, the current tests for detecting viral transmission by transfusion have reduced the infectious risk/donor exposure to very low levels.8 The bacterial risk associated with plt transfusions is high because plts are stored at 22°C rather than at the 4°C storage required by red cells. Some studies have suggested a reduction in bacterial transmission by transfusion with the use of single-donor plts.9 However, both the American College of Pathologists and the American Association of Blood Banks (AABB) have mandated testing of all plt products for bacteria,10 which should reduce this potential advantage of single donor plts versus pooled random-donor plts when prestorage pooling of random-donor PRP plt concentrates becomes widely available. With prestorage pooling of plt concentrates, the whole pool can be tested once. Currently, the requirement for bacterial testing has increased the use of apheresis plts because the costs for culture-based testing of an apheresis unit versus testing each plt concentrate when plts are pooled after storage has provided a significant cost-benefit to apheresis plts.
Concerning prevention of plt alloimmunization, there is no benefit to using leukoreduced single-donor plts compared with using leukoreduced pooled random-donor plts.11 There is a substantial increase in costs for single-donor compared with pooled random donor plts. As the quality of apheresis plts is similar to pooled random-donor plt concentrates,3,4 these two products can be used interchangeably based on availability and cost considerations.12
Leukoreduction
There are clear indications for providing leukoreduced plt products: (1) reduction of plt alloimmunization rates;11 (2) prevention of cytomegalovirus (CMV) transmission by transfusion;13 and (3) reduction in febrile transfusion reactions.14 In addition, there are studies that suggest that white cells that contaminate plt and red-cell transfusions may contribute to possible immunomodulatory effects of transfusion, such as an increased incidence of postoperative infections and metastasis formation in cancer patients. However, a great deal of controversy still surrounds whether transfusions have immunomodulatory effects.15
Another controversial issue is universal leuko-reduction.16 In spite of the increased costs associated with leukoreduction and a loss of up to 25% of the plts, many countries, organizations, and individual blood centers and hospitals have instituted universal leukoreduction of the blood supply rather than limit leukoreduction to the established indications.
γ-irradiation
γ-irradiation of plts is indicated to prevent transfusion-related graft-versus-host disease (GVHD), which is uniformly fatal. Proven situations where γ-irradiation should be performed are for patients receiving allogeneic stem cell transplants, for patients receiving blood products from related donors, and for patients who are severely immunocompromised, usually because of their disease or its treatment (e.g., patients with Hodgkin disease or other lymphomas).17
Volume reduction
Whenever plts are concentrated by centrifugation, there is likely to be some damage to the plts, and resuspension is often incomplete, involving the loss of plts; therefore, this extra processing should only be done if really necessary for patient care.18
Indications for Plt Transfusions
Prophylactic plt transfusions
Three aspects of prophylactic plt transfusions can be controlled by the physician: (1) whether to provide prophylactic plt transfusions to patients with chronic thrombocytopenia; (2) what plt count should initiate a plt transfusion (i.e., what is the appropriate plt transfusion trigger); and (3) what dose of plts should be used.19
Are prophylactic plt transfusions necessary?
The first issue that has not yet been resolved and that awaits the results of ongoing transfusion trials is whether prophylactic plt transfusions are indicated in patients with chronic thrombocytopenia to prevent bleeding, or whether an equally effective strategy would be to transfuse plts only with the onset of active bleeding. The latter strategy has been documented to be safe in a select group of patients: those undergoing autologous peripheral blood stem cell transplantations.20 In this study, the need for plt transfusions was reduced by as much as 50% when transfusions were given only for active bleeding.
Identification of a safe and effective plt transfusion trigger.
Two early studies performed in patients with chronic thrombocytopenia not receiving plt transfusions both suggested that significant spontaneous bleeding through an intact vascular system does not occur until the plt count is 5000 plts/μL or less.21,22 This fits with estimates of the daily loss of 7100 plts/μL/day that are needed to maintain the integrity of the vessel wall.23
Previously, a plt count of ≤ 20,000/μL was considered to be an indication for a prophylactic plt transfusion. However, four randomized prospective transfusion trials comparing prophylactic plt transfusion triggers of 10,000 plts/μL versus 20,000 plts/μL showed no differences in hemorrhagic risks (reviewed in Slichter19). Because fewer plt transfusions were used in the lower trigger arm, a cost-savings of 22% to 33% was achieved compared with patients in the higher trigger arm.
Standard-setting groups in both the U.S.24 and England25 have recommended a 10,000 plts/μl prophylactic plt transfusion trigger for all patients who are chronically thrombocytopenic due to chemotherapy, bone marrow transplantation, or for marrow conditions resulting in thrombocytopenia such as aplasia or myelodysplasia.
Plt Dose.
As opposed to the consensus that has been reached on an appropriate prophylactic plt transfusion trigger, well-designed prospective studies to evaluate the effects of plt dose on hemostasis and rates of plt utilization are not available. The effects of plt dose on transfusion outcomes has recently been reviewed.26 As the major cost of a plt transfusion is the plts themselves, low-dose, frequent transfusions can easily be given to hospitalized patients, and this should be the most cost-effective strategy as long as hemostasis is maintained. The issue of hemostasis is critical as low-dose plt transfusions will likely result in patients spending more time at lower plt counts. However, for outpatients, a better approach may be to give high-dose plt transfusions that will reduce their transfusion frequency and, thereby, the number of required clinic visits. An ongoing, large, prospective, randomized clinical trial sponsored by the National Heart, Lung, and Blood Institute of the National Institutes of Health is comparing three plt doses: a medium dose of 2.2 × 1011 plts/m2; a low dose of 1.1 × 1011 plts/m2 (half the medium dose); and a high dose of 4.4 × 1011 plts/m2 (twice the medium dose) in hospitalized patients with thrombocytopenia.27 This trial should provide definitive data on the most cost-effective dosing strategy for maintaining hemostasis while reducing plt utilization rates.
Thrombopoietin (TPO) adsorption by plt transfusions.
TPO has been recognized as the major hematopoietic growth factor that stimulates plt production, and it is constitutively made in the liver at a constant rate. TPO binds to its ligand (Mpl) on the surface of plts and megakaryocytes, and, in the absence of megakaryocytes or plts, TPO levels rise. It has been documented that TPO is adsorbed onto the surface of transfused plts.28 Therefore, reductions in the number of transfused plts by decreasing the plt transfusion trigger and the plt dose should further reduce the number of plt transfusions required, as elevated TPO levels will stimulate plt production as soon as megakaryocytes appear in the marrow. With endogenous TPO-stimulated megakaryocyte plt production, the duration of patients’ thrombocytopenia will be reduced, further decreasing the need for transfused plts.
Therapeutic plt transfusions
Chronically thrombocytopenic patients.
Plt transfusions are considered “therapeutic” if they are given to control active bleeding whether due to thrombocytopenia and/or plt dysfunction. Therapeutic plt transfusions in patients with chronic thrombocytopenia are usually indicated when bleeding is ≥ WHO grade 2. Generally accepted WHO bleeding grades are grade 0, none; grade 1, petechiae, ecchymosis, occult blood in body secretions, and mild vaginal spotting; grade 2, evidence of gross hemorrhage not requiring red cell transfusions over routine transfusion needs (e.g., epistaxis, hematuria, hematemesis); grade 3, hemorrhage requiring transfusion of 1 or more units of red cells/day; and grade 4, life-threatening hemorrhage, defined as massive bleeding causing hemodynamic compromise or bleeding into a vital organ (e.g., intracranial, pericardial, or pulmonary hemorrhage).19 WHO bleeding grades 1 and 2 are usually considered directly attributable to the degree of a patient’s thrombocytopenia, while more severe bleeding—WHO grades 3 and 4—is more often associated with contributing factors such as medications, an underlying disease state (e.g., uremia) that may interfere with plt function, anticoagulants, co-existent plasma clotting factor deficiencies, or disruption of the vascular system (e.g., necrotic tumors). Therefore, because of these other factors that may contribute to bleeding, it is not surprising that plt transfusions may not prevent or control all bleeding in thrombocytopenic patients. In fact, in spite of prophylactic plt transfusions given at transfusion triggers of 10,000 to 20,000 plts/μL, several clinical trials have demonstrated that bleeding still occurs. The risk of bleeding varies substantially among studies; depending on the study, WHO grade 1 bleeding was observed in 46% of patients, WHO grade 2 in 12% or 58% of patients, and WHO grades 3 and 4 in 5%, 11%, 20%, or 36% of patients (reviewed in Heddle et al29). In the SPRINT Trial, bleeding grades were assessed before and after 186 therapeutic plt transfusions given for active bleeding.30 WHO bleeding grades decreased following only 21% of the transfusions; they were unchanged after 69%, and they actually increased after 10%.
Invasive procedures.
If the vascular system is not intact, as may occur with a surgical procedure or following trauma, the consensus of medical opinion is that a plt count of at least 50,000/μL should be maintained.31 Unfortunately, there are no definitive studies to substantiate this plt transfusion trigger. Because of the potential for significant adverse outcomes associated with intracerebral bleeding, patients with intracerebral bleeding and during and following neurosurgical procedures should have plt counts maintained at > 100,000/μL. With plt counts between 50,000 and 100,000/μL, the decision to transfuse plts is based on the extent of surgery/trauma, ability to control bleeding with local measures, rates of bleeding, risk of bleeding, the presence of plt dysfunction, and other coagulation abnormalities.
Factors Affecting Poor Responses to Plt Transfusions
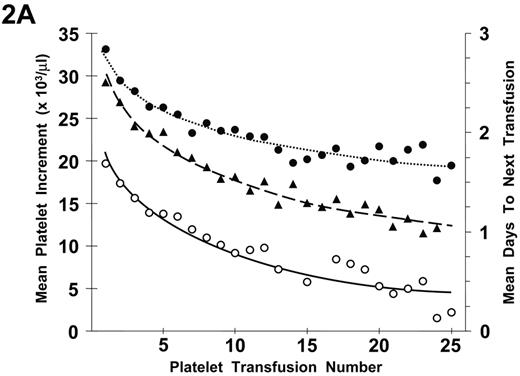
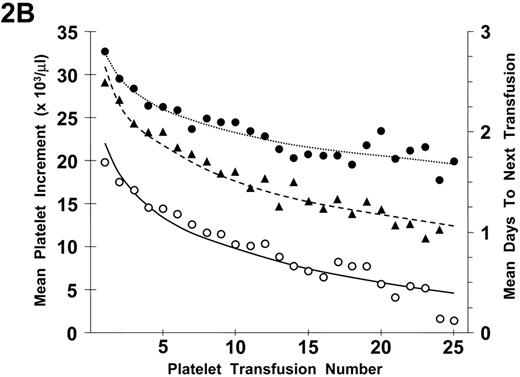
Refractoriness to plt transfusions can be separated into immune- and nonimmune-mediated mechanisms. Because isolated poor responses to an individual plt transfusion are not uncommon, a determination of plt refractoriness requires two serial plt transfusions with poor responses. In addition, in evaluating transfusion responses, it has been demonstrated that there is a progressive decrease in both plt increments and days to next transfusion with increasing numbers of plt transfusions (Figure 2A ). These changes were observed even in the absence of plt alloimmunization (Figure 2B ). There appears to be a logarithmic decrease in plt responses with the largest changes between transfusion events occurring with the earliest transfusions. The reason for these effects is not apparent but may be related to endothelial damage as a result of the induction therapy given to the patients with acute myeloid leukemia (AML) who were involved in the study.32 Whether such changes will also occur in other chronically transfused patients with thrombocytopenia with or without cancer chemotherapy is unknown.
Alloimmunization
Prevention of plt alloimmunization
ABO compatibility.
A and B red cell antigens are expressed on plts, and ABO-incompatible plts have reduced post-transfusion plt recoveries but normal survivals.33 ABO-compatible means the donor has no A or B antigens incompatible with the recipient’s A or B antibodies. A study in 1990 demonstrated the importance of ABO compatibility on rates of plt refractoriness and alloimmunization (Table 1 ).34 Recipients of ABO-incompatible plts become plt refractory at a higher rate than the ABO-compatible recipients (69% versus 8%, respectively; P = .001) because of the development of anti-HLA and plt-specific alloantibodies. The authors postulated that transfusion of ABO-incompatible plts not only increased their anti-A and/or anti-B antibody titers, depending on the mismatched A or B antigens they received, but also stimulated the recipients’ immune systems to make other alloantibodies. Therefore, providing ABO-compatible plts is important both to achieve the best post-transfusion plt increments but also to reduce the incidence of alloimmune plt refractoriness.
Leukoreduction.
Several prospective randomized plt transfusion trials have clearly documented the effectiveness of transfusing leukoreduced plts and red cells compared with standard blood products (control) in preventing the development of HLA antibodies (reviewed in Vamvakas35) However, several unanswered questions remain unanswered with regard to preventing plt alloimmunization. The actual effectiveness of the current methods of leukoreduction in preventing plt alloimmunization may very well be dependent on the immunocompetence of the patients who receive transfusions. As all of these trials were done in patients with AML undergoing induction; the role of leukocyte reduction in other patient populations remains to be determined. As the rates of antibody development in the TRAP trial11 were substantially different than the rates of alloimmune plt refractoriness, one might question the cost-effectiveness of modifying plts to prevent alloimmunization. HLA alloantibodies developed in 45% of the patients in the control arm and in 17% to 21% of the patients in the treated arms, and this compares with rates of alloimmune plt refractoriness of only 13% and 3% to 5%, respectively. In addition, it has been documented that HLA antibodies may not persist in up to 42% of patients, even with continued transfusions.36
Finally, there is some evidence from animal studies that prevention of plt alloimmunization may not just be related to a quantitative reduction in the number of transfused white cells. Rather, it may be important to document both what types of white cells are removed and which types remain.37 It is known that the types of white cells that are removed differ among filters and among apheresis machines that produce in-process leukoreduction.38
Management of alloimmunized patients
There are basically three strategies for managing allo-immunized plt refractory patients: (1) select HLA-compatible donors from an HLA-typed registry of apheresis donors; (2) identify HLA-antibody specificities and select antigen-compatible apheresis donors; and (3) perform plt cross-match tests to select compatible plts.36 All of these strategies are equally effective in selecting compatible donors. Unfortunately, even with donors selected by any of these techniques, 20% to 30% of the selected donor transfusions may give poor responses. These poor responses are likely related to: (1) nonimmune causes of plt refractoriness that may also be present in alloimmunized patients (see below); (2) drugs or autoantibodies; or (3) failure to detect relevant antibodies because of insensitivity of the assay systems. As patients may lose their antibodies over time (1 week to several months) despite continued plt transfusions, periodic assessments of antibody status may allow some patients to be returned to random donor plt transfusions with continued good responses to these plts for extended periods of time.36
Nonimmune Platelet Refractoriness
When plt refractoriness in the TRAP trial was defined as two sequential post-transfusion plt increments of 11,000 plts/mL or fewer at 1 hour after transfusion, 27% of the 533 patients receiving induction therapy for AML developed plt refractoriness. Analyses of the results of 6379 transfusions given to these TRAP trial patients were used to determine the clinically important patient- and product-related factors that affected transfusion outcomes (Table 2 ).32 Only two factors improved plt responses: splenectomy and giving ABO-compatible plts. Conversely, factors that reduced plt responses progressing downward from the most adverse were the following: patients who developed lymphocytotoxic antibodies; females with 2 or more pregnancies and males; splenomegaly; receiving heparin; bleeding; fever; and disseminated intravascular coagulation (DIC). Because of the known relationship between plt count and plt survival,23 those factors that reduced plt increments usually also reduced plt survivals, and these adverse factors also often caused plt refractoriness. In patients undergoing hematopoietic stem cell transplantation, factors specific to these patients that adversely affected plt transfusion outcomes were vaso-occlusive disease (VOD), GVHD, high bilirubin levels, total body irradiation (TBI), and high serum tacrolimus or cyclosporin levels.39
Management Strategies for Persistently Refractory Patients
Whether the cause of the refractoriness is immune or nonimmune, there are patients who remain plt refractory in spite of our best efforts to find compatible donors for alloimmunized patients or eliminate adverse clinical conditions that are associated with refractoriness. For patients who are having major bleeding that is considered life-threatening, several approaches may provide some benefit, but there is only anecdotal data supporting their use: (1) giving small-dose, frequent plt transfusions (e.g., 3–4 plt concentrates every 4–8 hours). These transfusions may be helpful in maintaining vascular integrity even though there is no increase in the patient’s post-transfusion plt count; (2) intravenous IgG may transiently increase post-transfusion plt increments (reviewed in Delafro-Weiss and Mintz36); (3) fibrinolytic inhibitors may help stabilize any clots that are being formed;36 and (4) recombinant factor VIIa may control bleeding in some patients.40
Refractoriness and alloimmunization rates after transfusing ABO-matched versus mismatched platelets.
| . | . | . | . | . | . | New antibodies . | ||
|---|---|---|---|---|---|---|---|---|
| Platelet transfusions . | Enrolled . | Female patients . | Possible prior sensitization* . | Platelet transfusions Median (range) . | Platelet refractoriness†P= .001 . | Anti-A/B‡ . | Anti-HLA . | Platelet-specific . |
| *Possible prior sensitization after pregnancy/transfusion. | ||||||||
| †1-hour post-transfusion corrected count increment less than 4500. | ||||||||
| ‡ ≥ three doubling dilution increase over their baseline. | ||||||||
| From Carr R et al. Br J Haematol. 1990;75:408–413.34 | ||||||||
| ABO Matched | 13 | 10 (77%) | 9 (69%) | 7 (5–19) | 1 (8%) | 0 | 1 (8%) | 1 (8%) |
| ABO Mismatched | 13 | 2 (15%) | 4 (31%) | 9 (4–30) | 9 (69%) | 7 (54%) | 5 (38%) | 4 (31%) |
| . | . | . | . | . | . | New antibodies . | ||
|---|---|---|---|---|---|---|---|---|
| Platelet transfusions . | Enrolled . | Female patients . | Possible prior sensitization* . | Platelet transfusions Median (range) . | Platelet refractoriness†P= .001 . | Anti-A/B‡ . | Anti-HLA . | Platelet-specific . |
| *Possible prior sensitization after pregnancy/transfusion. | ||||||||
| †1-hour post-transfusion corrected count increment less than 4500. | ||||||||
| ‡ ≥ three doubling dilution increase over their baseline. | ||||||||
| From Carr R et al. Br J Haematol. 1990;75:408–413.34 | ||||||||
| ABO Matched | 13 | 10 (77%) | 9 (69%) | 7 (5–19) | 1 (8%) | 0 | 1 (8%) | 1 (8%) |
| ABO Mismatched | 13 | 2 (15%) | 4 (31%) | 9 (4–30) | 9 (69%) | 7 (54%) | 5 (38%) | 4 (31%) |
Clinically important factors affecting platelet transfusion outcomes.
| . | 1-hour platelet increment (platelets/μL) . | 18- to 24-hour platelet increment (platelets/μL) . | Refractoriness (hazard ratio) . | Days-to-next transfusion . |
|---|---|---|---|---|
| *A clinically important change for 1-hour and 24-hour post-transfusion increments and days to next transfusion was considered to be a ≥20% difference (either an increased or a decreased response) from the overall responses observed in the trial. | ||||
| †For the hazard ratio, an increase of ≥2.0 was considered clinically important. | ||||
| ‡Value meets the criteria for a clinically important change. If a result is given but not noted with ‡, it is significantly different statistically but does not meet the clinically important criterion. If no value is listed (—), there was neither a clinically important nor statistically significant difference for the outcome measure. | ||||
| §The platelet increment was estimated to be 9300 platelets/μL less at 1 hour after transfusion for all study arms except UV-B. UV-B platelets were reduced by only 750 platelets/μL. The platelet increment was estimated to be 4000 platelets/μL less at 18 to 24 hours after transfusion for all arms. | ||||
| From Slichter SJ, et al. Blood. 2005;105:4106–4114.32 | ||||
| Factor | ||||
| Overall response | 24,900 | 12,000 | 1.75 | |
| Clinically important change | ≥ 5000* | ≥ 2400* | ≥ 2.0† | ≥ 0.35* |
| Improved Platelet Responses | ||||
| Splenectomy | +24,800‡ | +12,400‡ | — | — |
| ABO compatible | +4,600 | +6300‡ | — | — |
| Decreased Platelet Responses | ||||
| Lymphocytotoxic antibody-positive | −9300‡§ | −4000‡ | 3.48‡ | −0.36‡ |
| Females with ≥ 2 pregnancies, and males | −8900‡ | −5700‡ | 2.78‡ | −0.40‡ |
| Palpable spleen | −3500 | −4400‡ | — | −0.23 |
| Heparin | — | −3800‡ | 2.43‡ | −0.37‡ |
| Bleeding | −1700 | −3100‡ | 2.00‡ | −0.33 |
| Fever | −1600 | −2000 | 2.12‡ | −0.25 |
| Amphotericin | −2700 | −2500‡ | — | −0.28 |
| DIC | — | — | — | −0.40‡ |
| . | 1-hour platelet increment (platelets/μL) . | 18- to 24-hour platelet increment (platelets/μL) . | Refractoriness (hazard ratio) . | Days-to-next transfusion . |
|---|---|---|---|---|
| *A clinically important change for 1-hour and 24-hour post-transfusion increments and days to next transfusion was considered to be a ≥20% difference (either an increased or a decreased response) from the overall responses observed in the trial. | ||||
| †For the hazard ratio, an increase of ≥2.0 was considered clinically important. | ||||
| ‡Value meets the criteria for a clinically important change. If a result is given but not noted with ‡, it is significantly different statistically but does not meet the clinically important criterion. If no value is listed (—), there was neither a clinically important nor statistically significant difference for the outcome measure. | ||||
| §The platelet increment was estimated to be 9300 platelets/μL less at 1 hour after transfusion for all study arms except UV-B. UV-B platelets were reduced by only 750 platelets/μL. The platelet increment was estimated to be 4000 platelets/μL less at 18 to 24 hours after transfusion for all arms. | ||||
| From Slichter SJ, et al. Blood. 2005;105:4106–4114.32 | ||||
| Factor | ||||
| Overall response | 24,900 | 12,000 | 1.75 | |
| Clinically important change | ≥ 5000* | ≥ 2400* | ≥ 2.0† | ≥ 0.35* |
| Improved Platelet Responses | ||||
| Splenectomy | +24,800‡ | +12,400‡ | — | — |
| ABO compatible | +4,600 | +6300‡ | — | — |
| Decreased Platelet Responses | ||||
| Lymphocytotoxic antibody-positive | −9300‡§ | −4000‡ | 3.48‡ | −0.36‡ |
| Females with ≥ 2 pregnancies, and males | −8900‡ | −5700‡ | 2.78‡ | −0.40‡ |
| Palpable spleen | −3500 | −4400‡ | — | −0.23 |
| Heparin | — | −3800‡ | 2.43‡ | −0.37‡ |
| Bleeding | −1700 | −3100‡ | 2.00‡ | −0.33 |
| Fever | −1600 | −2000 | 2.12‡ | −0.25 |
| Amphotericin | −2700 | −2500‡ | — | −0.28 |
| DIC | — | — | — | −0.40‡ |
Preparation of platelet (plt) concentrates from whole blood. Two methods of preparing plt concentrates from whole blood have been developed. The main differences are related to the centrifugation steps that are used when proceeding from whole blood to a plt concentrate. Specific details of the methods are described in Slichter and Harker1 for plt-rich plasma (PRP) method plt concentrates and Pietersz et al2 for buffy coat (BC) method plt concentrates.
Preparation of platelet (plt) concentrates from whole blood. Two methods of preparing plt concentrates from whole blood have been developed. The main differences are related to the centrifugation steps that are used when proceeding from whole blood to a plt concentrate. Specific details of the methods are described in Slichter and Harker1 for plt-rich plasma (PRP) method plt concentrates and Pietersz et al2 for buffy coat (BC) method plt concentrates.
Relationship between number of platelet (plt) transfusions and plt increments at 1 hour and 18 to 24 hours after transfusion and days-to-next transfusion.
A) The mean 1-hour post-transfusion plt increments are plotted for the first 25 transfusions given to all study patients. These data represent 6334 transfusions given to 533 patients (•). Similar data for the 18- to 24-hour post-transfusion plt increments are shown for 5555 transfusions given to 531 patients (○). Data for days-to-next transfusion for 5955 transfusions given to 530 patients (▴).
B) When the same analyses are plotted for only lymphocytotoxic antibody-negative patients, the results are similar. One-hour increments for 5484 transfusions given to 477 patients (•), 18- to 24-hour increments for 4833 transfusions given to 475 patients (○), and days to next transfusion for 5144 transfusions given to 474 patients (▴). Dotted lines are best fit of the data for 1-hour post-transfusion increments; dashed lines, for 24-hour post-transfusion increments; and solid lines for days to next transfusion.
This research was originally published in
Relationship between number of platelet (plt) transfusions and plt increments at 1 hour and 18 to 24 hours after transfusion and days-to-next transfusion.
A) The mean 1-hour post-transfusion plt increments are plotted for the first 25 transfusions given to all study patients. These data represent 6334 transfusions given to 533 patients (•). Similar data for the 18- to 24-hour post-transfusion plt increments are shown for 5555 transfusions given to 531 patients (○). Data for days-to-next transfusion for 5955 transfusions given to 530 patients (▴).
B) When the same analyses are plotted for only lymphocytotoxic antibody-negative patients, the results are similar. One-hour increments for 5484 transfusions given to 477 patients (•), 18- to 24-hour increments for 4833 transfusions given to 475 patients (○), and days to next transfusion for 5144 transfusions given to 474 patients (▴). Dotted lines are best fit of the data for 1-hour post-transfusion increments; dashed lines, for 24-hour post-transfusion increments; and solid lines for days to next transfusion.
This research was originally published in
Director, Platelet Transfusion Research, Puget Sound Blood Center; and Professor of Medicine, University of Washington School of Medicine, Seattle, WA
Acknowledgments
The author gratefully acknowledges the excellent administrative support of Ginny Knight.