Abstract
Hematopoietic stem cell transplantation (HSCT) has proven successful for the treatment of a host of genetic and malignant diseases of the blood, but immune barriers to allogeneic tissue transplantation have hindered wider application. Likewise, gene therapy now appears effective in the treatment of various forms of immune deficiency, and yet insertional mutagenesis from viral gene transfer has raised safety concerns. One strategy for addressing the limitations of both gene therapy and allogeneic transplantation entails the creation of pluripotent stem cells from a patient’s own somatic cells, thereby enabling precise in situ gene repair via homologous recombination in cultured cells, followed by autologous tissue transplantation. In murine model systems, the methods of somatic cell nuclear transfer, parthenogenesis, and direct somatic cell reprogramming with defined genetic factors have been used to generate pluripotent stem cells, and initial efforts at therapeutic gene repair and tissue transplantation suggest that the technology is feasible. Generating patient-specific autologous pluripotent stem cells provides an opportunity to combine gene therapy with autologous cell therapy to treat a host of human conditions. However, a number of technical hurdles must be overcome before therapies based on pluripotent human stem cells will appear in the clinic.
Cellular transplantation therapy has enormous potential for treating a variety of degenerative, genetic, and malignant conditions. Hematopoietic stem cell transplantation (HSCT) is the most widely used form of cellular therapy, but many patients do not benefit from HSCT because they lack a suitable HLA-matched donor or because the morbidity of the HSCT procedure precludes its use in all but the most acutely fatal conditions. HSCT is freely exploited in acute leukemia, but despite evidence of success for treating a number of genetic bone marrow disorders,1 patients with “benign” conditions such as sickle cell anemia and thalassemia are typically given transplants only in the most severe cases. Allogeneic HSCT carries a major complication: acute and chronic graft-versus-host disease (GVHD) that causes significant morbidity and mortality. In contrast, autologous HSCT, in which a patient’s own stem cells are harvested prior to high-dose chemotherapy, is considerably less taxing because there are fewer of the highly morbid immunologic consequences of delayed engraftment and no GVHD. Autologous HSCs are an important target for gene therapy, and yet success has been illusory because of the challenge of maintaining HSCs in culture, the intrinsic difficulty of expressing genes in HSCs, and the risk of insertional mutagenesis with viral vectors.2 If gene therapy methods were more successful and safe, then gene correction coupled to autologous HSCT would be the treatment of choice for genetic bone marrow disorders.
Nearly two decades of research in mice has established that embryonic stem cells (ESCs) derived from the blastocyst can give rise to all differentiated cells in the adult organism, a property called developmental pluripotency. Under certain conditions of tissue culture in vitro, ESCs will aggregate and spontaneously differentiate to form embryoid bodies (EBs). These teratoma-like structures consist of an array of tissues from which specific cells can be selected for further study or transplantation. In the last decade and a half, in vitro differentiation of ESCs has been exploited to study genetic pathways leading to formation of a number of tissues, including cardiac and skeletal muscle, endothelium, and blood. Not only have these studies proven invaluable for illuminating mechanisms in developmental biology, they provide a framework for engineering directed tissue formation. With the availability of human ESCs,3 basic knowledge of developmental pathways might enable replacement cell therapies for patients with a host of degenerative diseases. Harnessing ESCs as a source of HSCs would accelerate basic investigation into genetic and epigenetic influences on hematopoietic cell function, facilitate genetic modification of stem cell populations precisely through homologous recombination, and enable combined gene and cell therapy for hematopoietic disorders.
This paper will outline the major challenges and opportunities for creating patient-specific pluripotent stem cells and using them for hematopoietic therapy. First, I will review the three dominant methods that appear feasible for generating pluripotent stem cells: (1) somatic cell nuclear transfer (SCNT); (2) parthenogenesis; and (3) direct reprogramming with defined genetic factors. Then, I will review strategies for directing the differentiation of HSCs from pluripotent stem cells, and success to date in preclinical murine models of hematopoietic transplantation.
Methods for Creating Autologous Pluripotent Stem Cells
Somatic cells exist in a variety of highly specialized, differentiated states, with some 200-odd distinct cell types recognizable in the adult human organism. A small fraction of these cells represent stem cells that can self-renew and differentiate to maintain and repair tissue. Such somatic or organ-specific stem cells (sometimes called “adult” stem cells) are important objects for research and therapy, as exemplified by the HSCs. In contrast to somatic stem cells, which are restricted in their differentiation potential to the tissues in which they reside, the stem cells of the early embryo (ESCs) are pluripotent and can form all tissues in the developing organism. For investigations of human infertility and miscarriage, early human development, and genetic disorders of aneuploidy, the cells of the early human embryo represent an invaluable resource. However, most human ESCs are derived from embryos left over from in vitro fertilization procedures, and as such represent generic cell lines unrelated to patients with specific diseases of interest (except in the rare cases where ESCs are derived from embryos identified by pre-implantation genetic diagnosis). To generate ESCs or similarly pluripotent cells from specific patients, several methods have been proposed and reduced to practice in murine systems.
SCNT has had a long history in developmental biology and only recently has gained interest among scientists interested in modeling disease. Classical studies by Briggs and King and Gurdon in the 1950s and 1960s used nuclear transfer to explore the competence of cellular DNA to direct development. By transferring the nucleus of differentiated somatic cells from embryonic and ultimately adult frogs, these early pioneers of SCNT proved that somatic tissues retained the genetic material sufficient for orchestrating the development of the complete organism. They showed that tissue differentiation proceeds not by a progressive loss of DNA through rearrangement or deletion, but rather due to selective expression and repression of segments of an intact genome. When extracted from its natural niche in the adult tissue and placed into the cytoplasm of an egg (whose own nucleus is first removed), the somatic nucleus is reset or “reprogrammed” back to its embryonic state. This process entails the silencing of genes responsible for the differentiated state of the somatic cells, and the re-expression of genes normally involved in early embryonic development. How the genome becomes remodeled during SCNT remains a poorly understood and complex process, but clearly entails extensive modifications of DNA and chromatin by enzyme complexes normally resident in the egg. The result of SCNT is a reconstructed pseudo-zygote, with the somatic nucleus encapsulated by the enucleate egg. The egg is then activated by chemical means, mimicking fertilization by sperm, and mitotic cleavage ensues. Remarkably, the dividing cells resemble embryonic blastomeres and, with subsequent cell culture, form a blastocyst—an embryo that carries the nuclear DNA of the donor cell and, as such, represents a genetic clone. In over more than a dozen mammalian species, uterine transfer of these cloned embryos has resulted in the birth of cloned animals, the most famous of which, Dolly the sheep, brought to prominence the concept that SCNT could be used for reproductive cloning.4
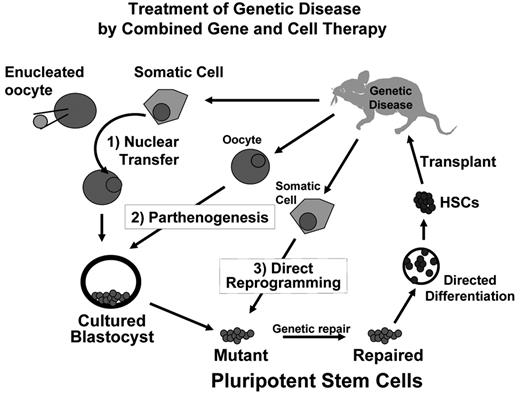
Whereas reproductive applications of SCNT have important uses in animal husbandry and agriculture, there are currently no legitimate efforts to pursue SCNT for human reproductive purposes, in large part due to the serious health deficiencies of cloned animals that result from faulty reprogramming and widespread ethical concerns.5 However, embryos created by SCNT can be used to generate pluripotent ESCs,6 thereby providing a means of generating genetically matched patient-specific stem cells that could serve to model disease and create rejection-proof “autologous” tissues for cell replacement therapies. Such a theoretical approach to the treatment of disease in a mouse model is illustrated in Figure 1 and has been reduced to practice using SCNT in the mouse.7 Although cloned organisms have health defects due to faulty reprogramming, studies of ESCs derived by SCNT (so-called ntESCs) suggest that they are as normal as ESCs derived from naturally fertilized embryos.8 The process of establishing ESCs in culture entails a winnowing of incompletely reprogrammed clones, and a selection for those clones that have sustained effective reprogramming and pluripotency. In studies of mouse embryo chimeras made with ntESCs, tissues develop normally, even when the entire mouse pup is derived from the input ntESCs.5,7 Although ntESCs can be readily generated from the mouse, it has proven difficult to generate ntESCs in primates, and early reports at success in humans turned out to be fraudulent. Importantly, a recent presentation by the Oregon group of Mitalipov and colleagues at the annual meeting of the International Society for Stem Cell Research held in Cairns, Australia, in June of 2007 claimed success in generating two ntESC lines from rhesus macaques. This report, if validated, suggests that SCNT might indeed be feasible in primates. There is no reason to suspect SCNT cannot be successful with human cells.
Despite success in numerous mammalian species, SCNT remains a cumbersome and highly inefficient process, resulting in success in at most a low percentage of attempts. Given this inefficiency, the chief limitation to human SCNT remains access to a supply of oocytes. This has driven the quest for alternative approaches to SCNT that might be more efficient at using the rare oocytes available for research, or that might avoid the use of oocytes altogether.
The first such alternative is parthenogenesis, which entails the development of an embryo directly from an unfertilized oocyte. This is made possible by artificially activating the egg. Even without fertilization by sperm, the activated oocyte will divide and form a diploid embryo, either through retention of one of the polar bodies or duplication of the haploid genome sometime during early embryo development. Although such parthenogenetic embryos do not sustain full organismal development due to the requirements for male-determined patterns of gene expression (“imprinting”), they can serve as a source of parthenogenetic ESCs (pESCs).9,10 The efficiency of derivation of pESCs from the mouse is significantly higher than derivation of ntESCs (e.g., 65%).11 Primate pESCs have been derived from cynomolgous monkeys,12 and the first human ESC line purportedly made by SCNT has actually proven to be the first human pESC line.13 Recently, an independent group has confirmed the isolation of six new human pESCs at quite robust efficiency (6 lines out of 44 activated oocytes; 14%).14
Parthenogenesis generates embryos that are substantially but not completely genetically identical to the oocyte donor. During the process of oocyte maturation, genetic recombination occurs between homologous pairs of chromosomes. The most efficient methods for parthenogenetic embryo development use drugs that block cytokinesis, which maintains diploidy by inhibiting the extrusion of the second polar body. By this method, parthenogenetic embryos retain the duplicated sister chromatids within the same cell. This results in substantial genetic homozygosity at regions adjacent to the centromeres and, as a consequence of crossover events, genetic heterozygosity at the distal genetic loci. Indeed, because of genetic recombination and retention of the recombinant chromosomes within the parthenogenetic embryos, ESCs derived from these embryos retain substantial proportions of both copies of the parental chromosomes and thus maintain complete genetic identity to the oocyte donor at most genetic loci (for a pictorial account of the chromosomal dynamics of parthenogenesis, see Kim et al11). Genetic screening of mouse pESCs can identify clones with more than 90% identity to the oocyte donor.11 Genetic screening can also be used to identify the pESC lines that retain complete identity to the oocyte donor at the major histocompatibility complex (MHC) loci that control tissue graft rejection, or alternatively to identify those lines that harbor duplicated copies of either parental MHC haplotype. Cells carrying duplicated MHC haplotypes represent a histocompatible tissue match for siblings, parents, or offspring of the oocyte donor, and tissue engraftment from pESCs has been successfully modeled in the mouse.11 An advantage of pESCs is that they maintain not only substantial nuclear identity but also complete mitochondrial identity with the oocyte donor and her siblings and offspring due to mitochondrial inheritance through the egg. Because tissues with duplicated MHC genotypes present only 3 antigens to be matched against the 6 routinely surveyed in tissue transplantation (e.g., class I A and B and class II DR antigens), it might be feasible to create banks of pESCs to provide “off-the-shelf” cell products for tissue transplantation. Computational models based on pools of transplant donors and recipients suggest that a relatively small number of highly selected master cell lines that are homozygous within the MHC regions (perhaps as few as 10) could provide a beneficial match for a majority of the population.15,16
Despite the theoretical feasibility of generating patient-specific ESCs from females by parthenogenesis for use in research and potential therapy, concerns about their safety and differentiation efficiency remain. Mouse parthenogenetic embryos are unable to complete full development due to the absence of paternally expressed imprinted genes, and tissues derived from pESCs appear to have growth defects.17 However, stable and functional hematopoietic engraftment has been reported from parthenogenetic cells in mice18 and in a rare human parthenogenetic chimera.19 If careful genetic and functional analyses of tissues derived from human pESCs show them to be safe and effective, then pESCs might represent a favorable source for tissue replacement therapies.
Given the hardships involved in donating oocytes, alternatives that avoid the need for oocytes altogether are ultimately preferable, but depend on being able to recapitulate the reprogramming process in somatic cells. A remarkable leap forward was reported and confirmed only recently as a result of a surprisingly bold and ingenious experiment. The Japanese group of Shinya Yamanaka, exploring a set of candidate genes known to play important roles in maintaining the pluripotency of ESCs, discovered that retroviral transduction of only four genes (Myc, KLF4, Oct4, and Sox2) was sufficient to induce a state of pluripotency in mouse fibroblasts.20 Within a year, the Yamanaka group and two others had independently confirmed and extended this original startling report by showing that these cells, dubbed induced pluripotent stem (iPS) cells, were the functional equivalents of embryo-derived ESCs capable of contributing to the formation of all tissues in the developing mouse.21–23 The four “Yamanaka factors” appear to initiate the reprogramming process, which evolves over several weeks in culture, and are required only transiently. Ultimately, in all cases the retroviruses were silenced, and the pluripotent state of the reprogrammed cells depended upon the activity of endogenous genes.
Will iPS cells provide a means of generating patient-specific tissues for regenerative medicine? While the promise is great, significant hurdles remain. Two of the factors, Myc and KLF4, have been closely linked with oncogenesis. Indeed, 20% of the mice generated from iPS cells by the Yamanaka group developed tumors that had reactivated expression of the retroviral Myc gene,22 highlighting safety concerns from the stable genetic modification of the cells. An important next step will be to evaluate whether methods for transient expression of the Yamanaka factors might be sufficient to induce iPS cell formation, and whether chemicals might replace one or more of the gene functions. And it remains to be shown whether these or other factors will efficiently reprogram human cells.
Taken together, the three methods outlined above have all proven technically feasible in murine systems as a means of generating pluripotent stem cells that might be useful in research and therapy, and might serve as a platform for combined gene and cell therapy (Figure 1 ). For applications in blood diseases, a second major hurdle remains the efficient production of HSCs that will faithfully engraft and sustain blood production. Progress to date in the murine system is outlined below.
Blood Development from Pluripotent Stem Cells
For more than two decades, differentiation of ESCs into embryoid bodies has been observed to result in blood cell production, yet achieving hematopoietic reconstitution of irradiated mice from ESCs has proven more challenging. The difficulty has been ascribed to the fact that EB differentiation recapitulates the early yolk sac stages of blood formation, and that the developmentally naïve blood island progenitors do not engraft in the adult stem cell niche. Classic studies once held that the primitive HSCs that formed in the yolk sac were destined to mature into definitive lymphoid-myeloid HSCs after migrations through the fetal liver and bone marrow. However, more recent evidence suggests that definitive HSCs—those that show lymphoid potential and can engraft in irradiated adults—arise primarily within the aorta-gonad-mesonephros (AGM) region of the embryo proper.24 While these data are compelling, they do not resolve the ultimate fate of the yolk sac progenitors and do not exclude the possibility that, if matured under favorable experimental conditions, yolk sac progenitors might manifest definitive HSC potential.
Indeed, significant evidence exists from several experimental contexts that yolk sac–type blood progenitors can contribute long-term to adult hematopoiesis. When marked yolk sac cells from one embryo are injected transplacentally into other embryos, yolk sac progeny contribute to blood formation in the adult.25,26 When directly injected into the liver of myeloablated newborn mice, highly purified CD34+/c-Kit+ yolk sac stem cells reconstitute blood production throughout adulthood,27 suggesting that the neonatal liver retains the fetal liver microenvironment that supports developmental maturation of yolk sac stem cells. When taken from precirculation embryos (to exclude contamination from circulating AGM-type precursors), yolk sac cells can engraft in irradiated mice after culture on a supportive stromal cell line taken from the paraaortic region of the embryo.28 Here, the yolk sac cells appear to be “educated” by the stromal cells to adopt an adult profile. Likewise, we have genetically modified precirculation yolk sac cells with the homeobox gene hoxb4, and observed long-term lymphoid-myeloid repopulation in irradiated primary and secondary mice.29 A recent paper reported an elegant method for genetically marking early yolk sac cells and following their derivatives in adult mice. This classic lineage tracing experiment showed that precirculation yolk sac cells could indeed contribute to definitive lymphoid-myeloid hematopoiesis in the adult.30 These data suggest that the full hematopoietic potential of yolk sac progenitors is latent within the embryonic microenvironment and can be exposed through cell culture or genetic modification.
A major challenge for the use of pluripotent cells in hematopoietic therapy is coaxing the development of adult-type definitive HSCs from the primitive stem cells. Previous studies that differentiated pluripotent ESCs into EBs suggested a predominance of primitive or early embryonic type hematopoiesis, with little evidence for formation of definitive HSCs. It is not clear how precisely EB hematopoiesis correlates with blood formation in the embryo, or whether EBs recapitulate the yolk sac or AGM microenvironment (or both). Several independent groups have reported success in engrafting mice with the differentiated progeny of ESCs,18,31–35 although a robust and efficient method is not yet widely practiced. Our group has shown that we can achieve definitive, long-term lymphoid-myeloid engraftment of irradiated primary and secondary mice from differentiated hematopoietic progeny of embryonic stem cells, albeit requiring stable genetic modification of the pluripotent cells.29,36,37 Thus, the significant challenge remains to identify key signals within the in vitro system that might facilitate the formation of engraftable HSCs without having to resort to genetic modification.
Our investigations began by asking whether a HSC could be identified clonally in differentiating cultures of ESCs. We dissociated EBs and infected with a retrovirus carrying the BCR/ABL oncogene, noting that BCR/ABL transforms bone marrow HSCs without hindering their lymphoid-myeloid differentiation. Indeed, BCR/ABL enabled us to culture and single-cell–clone lines of blast-like cells that formed primitive erythroid cells in vitro, and reconstituted definitive lymphoid-myeloid-erythroid hematopoiesis in irradiated mice.38,39 This confirmed the previous demonstration by Keller and colleagues of a common progenitor for primitive and definitive hematopoiesis in ESC cultures40 while extending the differentiation potential of these cells to include the lymphoid lineage. BCR/ABL-engrafted mice ultimately succumbed to leukemia. We thus tested whether ectopic expression of STAT5, a target of BCR/ABL signaling, might enable normal blood engraftment. STAT5 stimulated hematopoietic proliferation from ESCs and enabled lymphoid-myeloid engraftment in irradiated mice, though only transiently.37 We then tested hoxb4, prompted by data that hoxb4 was expressed in bone marrow HSCs but not in yolk sac, and studies from Humphries, Sauvageau and colleagues that showed enhanced reconstitution potential of hoxb4 -transduced HSCs.41 We expressed hoxb4 in ESCs, cultured hematopoietic blasts on supportive bone marrow stromal cells, and transplanted irradiated syngeneic mice. We observed long-term lymphoid-myeloid hematopoiesis in primary and secondary recipients, thereby suggesting that hoxb4 could enhance definitive HSC production from ESCs.29
These early mice showed incomplete lymphoid engraftment and overall low degrees of hematopoietic chimerism. Some evidence suggests that overexpression of hoxb4 hinders lymphoid differentiation,36 but alternatively, we speculate that the in vitro system for development of HSCs from ESCs lacks critical signals for the appropriate developmental maturation of the cells. Therefore, a major goal has been to interrogate the elements upstream of the hox genes and their roles in specifying hematopoiesis during embryonic development. Our understanding of how hox genes promote hematopoietic specification has been greatly advanced by insights into the role of a family of caudal-related homeobox-containing transcription factors that specify posterior tissue fates and mediate anterior-posterior patterning through modulation of hox gene expression. Cdx4 was previously shown by the Zon group to be necessary for blood formation in the zebrafish, and that ectopic expression of specific hox gene targets could rescue blood formation in Cdx4-deficient embryos.42 In collaboration with the Zon group, we showed that ectopic Cdx4 expression promotes hematopoietic colony formation from ESCs. These findings demonstrate that a genetic pathway involving cdx and hox genes plays an essential role in blood formation, and provide a central mechanism for driving hematopoietic specification from ESCs. Using a murine ESC line with tetracycline-inducible Cdx4, we have demonstrated that Cdx4 promotes commitment to hematopoietic mesoderm, stimulates hematopoietic progenitor formation from ESCs, and promotes lymphoid potential of ESC-derived HSCs. Using ESCs engineered to ectopically express both Cdx4 and HoxB4, we have demonstrated radioprotection and stable engraftment of hematopoietic lineages in irradiated mice. Moreover, we have applied proviral integration analysis in fractionated myeloid and lymphoid lineages of primary and secondary mice to document the clonal derivation of self-renewing, multi-potential HSCs from ESCs.36 In recent unpublished studies, we have found that transplantation of large numbers of in vitro–derived hematopoietic progenitors (5–10 × 106 cells/mouse) are required to achieve robust and consistent high-level hematopoietic chimerism. Nevertheless, the extent of lymphoid engraftment remains highly variable, suggesting that more work will be required to promote formation of a cell population from pluripotent cell sources that will faithfully mimic bone marrow in transplantation.
In the mouse, the ability to engraft blood from ESCs has allowed for initial demonstrations of combined gene and cell therapy in a therapeutic model. In collaboration with Rudolf Jaenisch’s laboratory, we have used ESCs generated by SCNT to treat a murine model of genetic immune deficiency due to deficiency of a gene required for immunoglobulin and T-cell receptor gene rearrangement.7 This model of therapeutic cloning represents a first proof-of-principle and demonstrates the theoretical feasibility of performing gene therapy in the context of cell therapy. Such an approach would be a general platform for a large number of genetic disorders of the bone marrow, and might be performed with pluripotent cells generated by any of the three methods described above (Figure 1 ). Achieving success in a human patient must await methods for generating patient-specific human pluripotent cells and for forming human HSCs that faithfully engraft. An accompanying paper by M. Bhatia in this volume reviews the significant but incomplete progress to date in achieving directed differentiation of HSCs from human ESCs.
Combining gene therapy with cell therapy for the treatment of genetic bone marrow disorders. Three strategies for generating autologous pluripotent stem cells are shown and described in the text: (1) Somatic cell nuclear transfer (SCNT); (2) parthenogenesis; and (3) direct somatic cell reprogramming with defined genetic factors. Each of these methods produces a pluripotent stem cell that can be cultured in vitro, which facilitates safe and effective correction of single gene defects. Repaired cells can then be differentiated into desired tissues for transplantation. Differentiation into hematopoietic stem cells (HSCs) enables autologous transplantation and genetically normal hematopoiesis.
Combining gene therapy with cell therapy for the treatment of genetic bone marrow disorders. Three strategies for generating autologous pluripotent stem cells are shown and described in the text: (1) Somatic cell nuclear transfer (SCNT); (2) parthenogenesis; and (3) direct somatic cell reprogramming with defined genetic factors. Each of these methods produces a pluripotent stem cell that can be cultured in vitro, which facilitates safe and effective correction of single gene defects. Repaired cells can then be differentiated into desired tissues for transplantation. Differentiation into hematopoietic stem cells (HSCs) enables autologous transplantation and genetically normal hematopoiesis.
Division of Hematology/Oncology, Children’s Hospital Boston; Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School; Harvard Stem Cell Institute, Boston, MA