Abstract
The rate of venous thromboembolism (VTE) in patients with acute leukemia or lymphomas is comparable with that of other “high-risk” cancer types. Chemotherapy and anti-angiogenic drugs increase the thrombotic risk in patients with lymphomas, acute leukemias and multiple myeloma (MM). Patients with hematologic malignancies often present with a hypercoagulable state or chronic disseminated intravascular coagulation (DIC) in the absence of active thrombosis and/or bleeding. Malignant cell procoagulant properties, cytotoxic therapies, and concomitant infections are major determinants for clotting activation in hematologic malignancies. In acute leukemia, clinical manifestations range from localized venous or arterial thrombosis to a diffuse, life-threatening thrombohemorrhagic syndrome (THS). All-trans retinoic acid (ATRA) has greatly improved the management of acute promyelocytic leukemia (APL), but has not significantly changed the rate of early hemorrhagic deaths and may actually promote thrombosis. Randomized, controlled trials (RCTs) of different prophylactic regimens to prevent VTE or THS in hematologic malignancies are urgently needed, particularly in patients with lymphoma or MM during chemotherapy and in patients with APL. Anticoagulant therapy is a particular challenge in patients with hematologic malignancies, since these patients are at very high risk for hemorrhage. No guidelines are available for the prophylaxis or treatment of VTE; extrapolations can be made from existing guidelines for management of patients with other malignancies; prolonged periods of treatment-induced thrombocytopenia in patients with hematologic malignancies, however, require a more judicious application of standard anticoagulant approaches. Use of the newer anticoagulants will require careful assessment of hemorrhagic risk in this group of high-risk patients but may be justified under special circumstances.
Overview
Patients with cancer are at high risk for both thrombosis and hemorrhage.1 Venous thromboembolism (VTE) has been a more frequent complication in patients with solid tumors, whereas hemorrhage and uncompensated disseminated intravascular coagulation (DIC) have been more frequent complications in patients with hematologic malignancies, particularly acute leukemia.2 Recent large epidemiologic studies indicate, however, that the rate of VTE in patients with hematologic malignancies is at least as high (if not higher) as in patients with so-called “high-risk” types of solid tumors3–5 (e.g., an adjusted odds ratio of 28.0; 95% CI 4.0–199.7,3 and an incidence rate varying between 3.87% and 5.79%5).
As in patients with solid tumors, the risk for thrombosis is increased by both chemotherapy and surgical intervention. Common risk factors for VTE in patients without cancer, such as immobility, advanced age, history of previous thromboses, venous stasis and sepsis, further complicate the natural history of hematologic malignancies.5,6 In recent years, attention has been drawn to the increased risk of VTE in patients with multiple myeloma (MM) treated with the anti-angiogenesis drugs thalidomide or lenalidomide, particularly in combination with chemotherapy and steroids.7,8
While the use of central venous catheters (CVCs) contributes to thrombotic risk in patients with hematologic malignancy, two recently published studies demonstrated a much reduced rate of symptomatic CVC-associated VTE than was predicted by the older literature, i.e., 3.1% and 4.4%, respectively,9,10 recognizing that venographic rates may be much higher.
Nearly all patients with malignancy show evidence of subclinical activation of clotting, or chronic DIC, in the absence of active bleeding and/or thrombosis.11,12 However, patients with acute leukemia are unique in that they often present with a range of laboratory abnormalities consistent with DIC and a variety of clinical manifestations, ranging from localized venous or arterial thrombosis to diffuse life-threatening bleeding. The incidence of these complications varies according to the type of leukemia and the phase of treatment. Thrombosis is more common than previously appreciated in individuals with all types of adult acute leukemias,13 including patients with acute promyelocytic leukemia (APL), in whom hemorrhage is usually predominant.14 Thrombosis and bleeding can occur concomitantly as a part of the same thrombohemorrhagic syndrome (THS) of APL. Introduction of all-trans retinoic acid (ATRA) has reduced the risk for fatal hemorrhage in patients with APL1,2 and has produced a high rate of complete remission with rapid resolution of the coagulopathy.2
Major determinants of the pathogenesis of clotting activation in hematologic malignancies include: (1) tumor cell–derived procoagulant, fibrinolytic and proteolytic factors and inflammatory cytokines; (2) cytotoxic therapies; and (3) infectious complications.
In contrast to patients with solid tumors, for whom guidelines for the prevention and treatment of VTE have recently been published15–17 or are about to be published,18 no such guidelines are readily available to indicate optimal strategies for patients with hematologic malignancies. While low-molecular-weight heparin (LMWH) has improved the outcome for VTE treatment in patients with solid tumors,19 no similar experience from large, randomized controlled trials has been published for patients with hematologic malignancies. It is likely that the high risk of hemorrhage due to severe thrombocytopenia is the primary deterrent to investigators who might wish to study both VTE prophylaxis and treatment in leukemia.
The Hypercoagulable State, DIC and Thrombosis in Hematologic Malignancies
Patients with cancer commonly demonstrate laboratory evidence for subclinical activation of clotting (hypercoagulability) even in the absence of overt clinical thrombosis.2,11,12 Consideration of the pathophysiology of this hypercoagulability is critical to the design of appropriate measures for intervention—either to prevent or treat thrombohemorrhagic complications of the hematologic malignancies.
Up until quite recently, activation of clotting in patients with cancer was considered an epiphenomenon—an inadvertent and unintended consequence of the effects of cancer and its treatment on host defense mechanisms. Several recent molecular studies of experimental models of human cancer (including hepatoma, brain tumors and colon cancer) have demonstrated oncogene- and repressor gene–mediated activation of clotting as an integral feature of neoplastic transformation (activation of Met, loss of PTEN, induction of K-ras and loss of p53; Table 1 ).20–22 In APL cells the t15–17 translocation, which results in the fusion of the nuclear retinoic acid receptor (RARα) gene on chromosome 17 with part of the PML (promyelocytic leukemia) gene on chromosome 15, induces hyperexpression of tissue factor (TF) in the leukemic cell, again linking the primary oncogenic event with induction of hypercoagulability.2 Another gain-of-function mutation, the JAK2V617F mutation, present in 90% of patients with polycythemia vera and 50% of patients with essential thrombocythemia (ET), appears to result in increased procoagulant properties of platelets from JAK2-positive patients.23
The pathophysiology of thrombosis in patients with leukemia, lymphoma or MM is complex, but is simplified and illustrated in Figure 1 , corresponding to abnormalities of one or more of the three classical categories of host defense mechanisms as described by Virchow: blood flow (stasis); blood vessel wall function (injury); and dysfunction of the blood elements (both soluble and cellular). This process, in which tumor cell products interact with host cells (monocytes/macrophages, endothelial cells, platelets, fibroblasts, parenchymal cells, etc.) to produce the hypercoagulable state, is further complicated in patients with acute leukemia by the prolonged periods of therapy-induced cytopenias and the rapid induction by chemotherapy of malignant cell destruction (with elaboration of tumor products). Thus, it is not surprising that in spite of the epidemiologic evidence suggesting a relatively high frequency of VTE in some of these patients,3 there is little enthusiasm for routine anticoagulant prophylaxis (vide infra).
In MM, in addition to the role of the paraprotein in producing stasis through hyperviscosity, at least four other possible mechanisms leading to hypercoagulability have been suggested, including (1) interference with fibrin assembly, (2) production of a procoagulant autoantibody, (3) procoagulant effects of inflammatory cytokines, and (4) acquired activated protein C resistance (APC-R).24 Direct injury to the endothelium, either by tumor cells or by chemotherapy, may also predispose patients with MM to thrombosis by upregulation of adhesion molecules. The net result is that these adhesion molecules mediate increased interaction between tumor cells and vascular endothelial cells, platelets and leukocytes, and localize the secretion of thrombogenic and angiogenic peptides expressed by tumor cells and local inflammatory cells (Figure 1 ).
Blast cells isolated from patients with acute leukemia express procoagulant, fibrinolytic and proteolytic mediators, as well as inflammatory cytokines,1,2,25 including tissue factor and cancer procoagulant. All subtypes of acute myelogenous leukemia express some procoagulant activity. Cellular differentiation of APL induced by ATRA is associated with rapid loss of procoagulant activity expression by leukemic blasts in association with rapid, albeit partial correction of the coagulopathy in patients,1,2,25 further supporting the close link between the molecular events in neoplastic transformation and activation of clotting. Furthermore, this demonstration that the procoagulant activity loss parallels improvement of the hypercoagulable condition provided the first rational approach to treating the THS of APL and, by extrapolation, perhaps other malignancies.
Leukemic cells also express fibrinolytic and other proteolytic enzymes, as well as inflammatory cytokines, all of which have been implicated in the pathogenesis of the bleeding in some patients (Figure 1 ). Chemotherapy and use of biological agents (including steroids, growth factors such as erythropoietin [Epo] and angiogenesis inhibitors) increases the risk of thrombosis,1,2,7,8,12–14 and may require the concomittant use of anticoagulants, antiplatelet agents or both. Profound changes in the levels of markers of endothelium activation (e.g., von Willebrand factor [VWF], TM, and PAI-1) in patients receiving chemotherapy also suggests direct endothelial damage. Another important prothrombotic mechanism in these patients involves the reduction in levels of physiologic coagulation inhibitors (antithrombin, protein C and protein S), which may occur as a consequence of the hepatotoxicity of chemotherapy.
Hepatic veno-occlusive disease (VOD) occurs in approximately 50% of patients undergoing allogeneic hematopoietic stem cell (HSC) transplantation, but is also associated with autologous transplantations. Hepatic VOD, which represents an important cause of mortality (>30%), is characterized by activation of blood coagulation (most likely secondary to endothelial damage).26
Acquired Disorders of Coagulation in Hematologic Malignancies
A variety of acquired coagulation disorders have been reported in patients with hematologic malignancies, many of which are due to the unusual properties of paraproteins generated by the malignant clone. Among these are lupus anticoagulants and/or antiphospholipid antibodies, factor VIII inhibitors, VWF inhibitors and, less commonly, inhibitors to factor V, factor X, factor XI, factor XII or factor XIII. With the exception of the lupus anticoagulant/anti-phospholipid antibody syndrome (LAC/APAS), acquired von Willebrand syndrome (AVWS) and inhibitors to factor VIII (acquired hemophilia), the remainder of these disorders are exceptionally uncommon in patients with hematologic malignancies and are documented principally in isolated case reports.27
Patients with the LAC/APAS usually manifest thrombotic complications, although occasional patients exhibit high titer antibody specificity for prothrombin and develop hypoprothrombinemia and a profound bleeding diathesis. Nearly all of these inhibitors respond best ultimately to effective treatment of the underlying disorder with reduction or disappearance of the offending antibody or antibodies. Patients with hematologic malignancies (usually lymphoma or myeloma) and inhibitors to factor VIII usually require use of bypassing factor concentrates or activated recombinant factor VII (VIIa) to achieve hemostasis in the short term.27 In patients with hematologic malignancies and AVWS, multiple pathogenic mechanisms are described, including selective, pathologic absorption of VWF by tumor cells or aberrant vasculature, increased proteolysis of VWF and the presence of specific or nonspecific anti-VWF antibodies.28,29 Again, successful treatment of the underlying disease is usually required to achieve long-term hemostasis in these patients, who often have a profound bleeding disorder. Short-term hemostasis can be accomplished with use of DDAVP (desmopressin), a synthetic analog of antidiuretic hormone that releases VWF from storage sites, or VWF/factor VIII concentrates. Failure to achieve hemostatic levels of VWF in patients with high titer antibodies (e.g., in association with a monoclonal gammopathy) may be overcome in some with high-dose intravenous immune globulin (1 g/kg daily × 2 days).28 In a single-institution series of 22 patients with AVWS seen over 25 years, most of whom had a hematologic malignancy (69%), DDAVP was effective in fewer than 50% of the patients, while use of VWF-containing plasma-derived concentrates achieved hemostasis in all of the patients.29 Occasional patients may require rVIIa for cessation of bleeding, and immunosuppressive therapy (e.g., corticosteroids, cytotoxic drugs and/or rituximab) is almost always needed to control antibody production (and is variably effective). Plasmapharesis or extracorporeal immunoabsorption to remove antibody can be used to temporize until immunosuppression takes effect.29
Prophylaxis and Therapy of Thrombosis in Hematologic Malignancies
We have established that thrombotic complications are common in patients with both hematologic and nonhematologic cancers.3–6 In earlier studies, recently validated,6 thrombosis was listed as the second leading cause of death in hospitalized patients with cancer, a sobering statistic, particularly since included in this mortality analysis were patients who were responding well to primary cancer therapy. Ultimately, we should expect that a better understanding of the molecular events leading to the development of hematologic malignancies will provide appropriate targets for the production of bifunctional drugs—agents that will attack the malignant process as well as reverse the coagulopathy. The closest example of targeted bifunctional therapy today is, of course, ATRA, which we have discussed in some detail for the treatment of APL. Until more specific targeted therapies are available for the treatment of all hematologic malignancies, however, we must be satisfied with developing a reasonable temporizing approach—anticoagulant drugs and supportive therapy to prevent bleeding. No ad hoc studies or guidelines are available to help us with best practices for either prophylaxis or treatment of VTE in hematologic malignancies. While the use of LMWH has improved VTE management in patients with solid tumors,19 no similar experience has been accumulated in patients with acute leukemia. Since patients with acute leukemia have a high risk of hemorrhage related to thrombocytopenia from their primary disease and/or secondary to chemotherapy, the administration of anticoagulant therapy for VTE poses serious challenges. Thus, in this patient population the prevention of VTE takes on added significance.
Prophylaxis
As noted, thromboprophylaxis in patients with acute leukemia, lymphoma or MM has not been well studied, with the exception, perhaps, of CVC-related VTE.9,10 In the original randomized trial of very low dose prophlaxis (1 mg warfarin) versus placebo for prevention of CVC-related VTE, approximately 80% of the patients had a hematologic malignancy.10 While the “mini-dose” warfarin regimen was not effective in preventing CVC-related VTE, it did prove safe with regard to bleeding complications. In the study conducted by the Italian CATHEM group, 14.2% of patients who entered the trial were receiving thromboprophylaxis (principally LMWH, but with some use of unfractionated heparin, aspirin, or warfarin).9 In the subgroup of patients with hematologic malignancy, no increase in hemorrhagic complications was reported.
More information is available on thromboprophylaxis in patients with MM due to the high thrombotic risk reported with the use of thalidomide or lenalidomide. Considering the advantages to patients provided by these new drugs, the search for effective strategies for thomboprophylaxis has become a high priority. To date, no randomized, controlled trials have been reported to support the use of a particular regimen, although data are available from uncontrolled studies, as noted in Table 2 . Enoxaparin (40 mg/day) has been reported to be effective in reducing the risk for VTE in newly diagnosed patients with MM enrolled in a trial of combination therapy of thalidomide with doxorubicin during the first 3 months of treatment; again, it must be emphasized that this was not a randomized, controlled trial (RCT). Fixed low-dose warfarin (1 mg/day) did not appear to be effective compared with historic data in this study.30 Similar results with another preparation of LMWH (nadroparin) were reported in a study of 209 newly diagnosed patients with MM who received prophylaxis during treatment with thalidomide, dexamethasone and doxorubicin. The incidence of VTE was 10% without evidence for increased bleeding risk.31 Clinicians should be cautioned, however, regarding the use of standard doses of LMWH in patients with MM and renal failure—in these patients frequent measurement of peak anti- Xa levels is required to avoid overdosing. Fixed low-dose warfarin (1.25 mg/day) was studied for the prevention of VTE in newly diagnosed patients with MM treated with thalidomide and dexamethasone in the absence of a control arm; the results reported (13% VTE rate) appeared better than those in patients not treated with warfarin (again, not randomized).32 Finally, the efficacy of aspirin prophylaxis has been suggested in patients given thalidomide in combination with chemotherapy33 (Table 2 ). Aspirin prophylaxis has been used with promising results also in patients treated with the thalidomide derivative, lenalidomide.34,35 Recently, Palumbo and colleagues provided a retrospective analysis of different thromboprophylaxis regimens.36 Although all of these reports are encouraging, in the absence of a better understanding of the primary mechanisms for the high thrombosis rate observed with these drugs, it is critical to determine the best approach to VTE prophylaxis from prospective RCTs. Until such trial results are available, no definitive recommendations can be made.
Therapy of VTE
No published results of prospective RCT studies have specifically addressed the issue of VTE treatment in patients with acute leukemia. As previously noted for patients with solid tumors, a therapeutic strategy based on LMWH administered for 6 months after a VTE episode (1 month at full dose and 5 months at approximately 75% of full dose) has proved safe and superior to warfarin in preventing VTE recurrence;19 this regimen has been recommended by several expert panels.15–18 A similar approach was tested in a small group of patients with hematologic malignancies and VTE.37,38 The use of LMWH in these patients is an attractive choice due to the safety profile, lack of requirement for laboratory monitoring, and reduced risk (compared with warfarin) for drug and food interactions. In view of the high risk for bleeding in patients with hematologic malignancies, however, greater effort should be made to standardize dose-reduction regimens and provide guidelines for temporary suspension of LMWH administration according to the degree of thrombocytopenia. In the absence of data, we would recommend the initial use of standard doses of LMWH preparations, but with frequent monitoring of peak anti-Xa levels, as in other high-risk groups for whom good pharmacokinetic parameters are not yet available (e.g., renal failure, obesity, pregnancy, children). Tight maintenance of peak levels between 0.5 and 1.0 IU/mL may improve the risk-benefit ratio for patients with hematologic malignancies and VTE. When the platelet count is reduced below 50,000/μL, we would recommend using only 50% of the therapeutic dose of LMWH; below 20,000/μL, we would temporarily discontinue the use of LMWH.
Use of newer antithrombotic agents (e.g., the factor Xa inhibitors fondaparinux or idraparinux, or the direct thrombin inhibitors hirudin, bivalrudin or argatroban.) has not been reported in this group of patients. Particular caution would be needed for the use of any of these agents in the absence of a published experience. However, anecdotal reports suggest that bleeding complications associated with their use can be treated effectively in some patients with hematologic malignancies by using recombinant factor VIIa concentrate. Again, no data from RCTs are available to guide therapy.
Inferior vena cava (IVC) filters are being deployed with greater frequency, in part due to the ease with which they can be placed and retrieved by invasive radiologists. Retrievable or temporary filters play a valuable role in the management of high-risk patients with absolute contraindications to anticoagulant therapy. However, in spite of the proven efficacy of filters in preventing symptomatic pulmonary embolism (PE), the risk for DVT is significantly increased, even when patients receive concurrent anticoagulant therapy.39 Therefore, most hematologists recommend that the use of permanent IVC filters be reserved for those patients in whom a permanent risk factor for bleeding is demonstrated. Temporary filters, if used, should be removed as soon as it is possible to safely begin the patient on anticoagulant therapy.
Treatment of the Thrombohemorrhagic Syndrome in Acute Leukemia
A recent retrospective analysis of 771 consecutive patients with acute myeloid leukemia (including APL) admitted to a single institution in the past 30 years revealed a risk for hemorrhagic death of approximately 7% (55 of 771 patients).40 Analyzing that same population for the risk of thrombosis revealed a rate of 5.1% (39 of 759 patients),40 supporting further the results of the large, population-based studies noted earlier. The role of heparin therapy in the treatment of this complex coagulopathy complicating acute leukemia, especially APL, remains uncertain. Older studies, which used unfractionated heparin (UH), were small, retrospective, and uncontrolled. The benefit of UH therapy has never been proven in a prospective randomized trial. In a retrospective analysis of 268 patients with acute leukemia, no benefit was demonstrated of UH for the prevention of early hemorrhagic deaths, and no increase was observed in complete remission rate or overall survival.41 Neither LMWH nor any of the newer anticoagulants have been used to treat the THS of APL. A RCT using either LMWH or fondaparinux in an effort to reduce the remaining death rate in APL due to THS2,3 would seem appropriate. It would also seem reasonable to test the hypothesis that the anti-adhesive properties of LMWHs, which have been observed to reduce the interaction of tumor cells with the endothelium in vitro, might prevent some of the manifestations of the retinoic acid syndrome in patients with APL with high white blood cell counts.2
Therapeutic regimens including antifibrinolytic agents, such as epsilon-aminocaproic acid (EACA; Amicar), tranexamic acid, or protease inhibitors such as aprotinin, have been considered, based on a few small studies,1,2 but no data from large-scale RCTs have been published. In the large PETHEMA group study, tranexamic acid (100 mg/ kg/day) administered by continuous intravenous infusion until the platelet count was greater than 50,000/μL, “failed to demonstrate any impact on hemorrhage-associated mortality.”42 None of these agents has been tested in a RCT, where control subjects were not exposed to antifibrinolytic therapy.
Finally, prophylactic platelet transfusion therapy is universally applied in the supportive care of patients with acute leukemia. This practice has resulted in a decrease in bleeding complications and translated into prolonged survival by permitting intensification of therapy.1 Current recommendations for patients with APL suggest that platelets should be transfused to maintain the platelet count above 20 × 109/L in those not actively bleeding and above 50 × 109/L with active bleeding.2 However, the use of ATRA for remission induction has changed the natural history of APL and has helped to resolve THS in most patients. While some of the mechanisms by which ATRA regulates the aberrant hemostatic system in patients with APL have been elucidated,2,25 the rate of early hemorrhagic deaths in APL (3% to 10%) has not changed significantly.
Additional efforts to develop therapies that rapidly correct the coagulopathy in patients with hematologic malignancies are required. New approaches using both anticoagulant and anti-inflammatory drugs should be considered. As the molecular basis for activation of clotting in hematologic malignancies becomes better elucidated, we anticipate the development of drugs that will target both the malignant process and the resultant THS.
Molecular genetics of thrombohemorrhagic syndromes associated with human tumors.
| Oncogene/tumor suppressor gene . | Signaling pathway . | Tumor . | Vascular outcome . | Gene products regulated . |
|---|---|---|---|---|
| Abbreviations: DIC, disseminated intravascular coagulation; PAI-1, plasminogen activator inhibitor type 1; COX-2, cyclooxygenase type 2; TF, tissue factor; VEGF, vascular endothelial growth factor; TSP, thrombospondin. | ||||
| Modified from Rickles43 with permission of the publisher. | ||||
| MET | Tyrosine kinase receptor | Hepatoma | Thrombosis; DIC | PAI-1, COX-2 |
| PTEN | MEK/ERK | Glioblastoma | Thrombosis; necrosis | TF |
| K-ras; p53 | MEK/MAPK/PI3K | Colon cancer | Tumor angiogenesis | TF, VEGF, TSP |
| Oncogene/tumor suppressor gene . | Signaling pathway . | Tumor . | Vascular outcome . | Gene products regulated . |
|---|---|---|---|---|
| Abbreviations: DIC, disseminated intravascular coagulation; PAI-1, plasminogen activator inhibitor type 1; COX-2, cyclooxygenase type 2; TF, tissue factor; VEGF, vascular endothelial growth factor; TSP, thrombospondin. | ||||
| Modified from Rickles43 with permission of the publisher. | ||||
| MET | Tyrosine kinase receptor | Hepatoma | Thrombosis; DIC | PAI-1, COX-2 |
| PTEN | MEK/ERK | Glioblastoma | Thrombosis; necrosis | TF |
| K-ras; p53 | MEK/MAPK/PI3K | Colon cancer | Tumor angiogenesis | TF, VEGF, TSP |
Thrombosis rates in patients with multiple myeloma: prospective phase 2 trials of thromboprophylaxis.
| Reference, disease status . | No. patients . | Therapy . | Thrombosis, n (%) . |
|---|---|---|---|
| Abbreviations: CT, chemotherapy; DX, dexamethasone; T, thalidomide; ASA, aspirin. | |||
| Modified from Falanga and Marchetti44 with permission from the publishers. | |||
| Zangari M et al, 200430 | 68 | CT + DX + T + Enoxaparin (40 mg/d) | 10 (14.7) |
| Newly diagnosed | 35 | CT + DX + T + Warfarin (1 mg/d) | 11 (31.4) |
| Minnema MC et al, 200331 | 209 | CT + DX + T + Nadroparin | 21(10) |
| Newly diagnosed | |||
| Cavo M et al, 200432 | 19 | DX + T | 5 (26) |
| Newly diagnosed, age < 65 years | 52 | DX + T + Warfarin (1.2 mg) | 7 (13) |
| Baz R et al, 200533 | 19 | CT + Dx + T | 11 (58) |
| Newly diagnosed and CT refractory | 84 | CT + Dx + T + ASA | 15 (17.8) |
| Reference, disease status . | No. patients . | Therapy . | Thrombosis, n (%) . |
|---|---|---|---|
| Abbreviations: CT, chemotherapy; DX, dexamethasone; T, thalidomide; ASA, aspirin. | |||
| Modified from Falanga and Marchetti44 with permission from the publishers. | |||
| Zangari M et al, 200430 | 68 | CT + DX + T + Enoxaparin (40 mg/d) | 10 (14.7) |
| Newly diagnosed | 35 | CT + DX + T + Warfarin (1 mg/d) | 11 (31.4) |
| Minnema MC et al, 200331 | 209 | CT + DX + T + Nadroparin | 21(10) |
| Newly diagnosed | |||
| Cavo M et al, 200432 | 19 | DX + T | 5 (26) |
| Newly diagnosed, age < 65 years | 52 | DX + T + Warfarin (1.2 mg) | 7 (13) |
| Baz R et al, 200533 | 19 | CT + Dx + T | 11 (58) |
| Newly diagnosed and CT refractory | 84 | CT + Dx + T + ASA | 15 (17.8) |
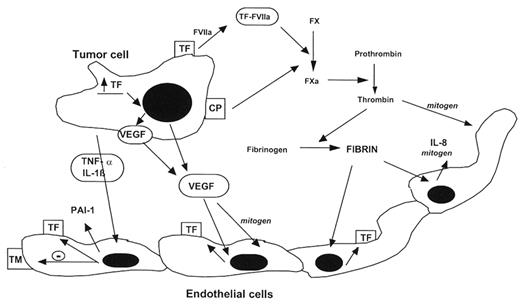
Host-tumor cell interaction and the hypercoagulable state of cancer. Tissue factor (TF) and cancer procoagulant (CP) are synthesized and expressed on the surface of tumor cells. The effects of these tumor cell procoagulants (made by both solid tumor and leukemic cells) are enhanced by the production of proangiogenic cytokines such as interleukin-8 (IL-8) and vascular endothelial growth factor (VEGF), both by the tumor cells and local endothelial cells. Release of proinflammatory cytokines, such as tumor necrosis factor (TNF-α) and interleukin-1 (IL-1b), by the tumor cells and host inflammatory cells further stimulates the hypercoagulable state, as follows. These cytokines are indirect procoagulants by virtue of their ability to convert the anticoagulant endothelium to a procoagulant endothelium by (1) down-regulation of thrombomodulin (TM) expression and (2) increased endothelial cell synthesis of TF and plasminogen activator type 1 (PAI-1). Generation of fibrin at the endothelium enhances thrombogenesis by inducing additional TF and IL-8 production by the injured endothelial cells. (Figure modified and reproduced from Falanga and Rickles,2 with permission of the publishers).
Host-tumor cell interaction and the hypercoagulable state of cancer. Tissue factor (TF) and cancer procoagulant (CP) are synthesized and expressed on the surface of tumor cells. The effects of these tumor cell procoagulants (made by both solid tumor and leukemic cells) are enhanced by the production of proangiogenic cytokines such as interleukin-8 (IL-8) and vascular endothelial growth factor (VEGF), both by the tumor cells and local endothelial cells. Release of proinflammatory cytokines, such as tumor necrosis factor (TNF-α) and interleukin-1 (IL-1b), by the tumor cells and host inflammatory cells further stimulates the hypercoagulable state, as follows. These cytokines are indirect procoagulants by virtue of their ability to convert the anticoagulant endothelium to a procoagulant endothelium by (1) down-regulation of thrombomodulin (TM) expression and (2) increased endothelial cell synthesis of TF and plasminogen activator type 1 (PAI-1). Generation of fibrin at the endothelium enhances thrombogenesis by inducing additional TF and IL-8 production by the injured endothelial cells. (Figure modified and reproduced from Falanga and Rickles,2 with permission of the publishers).
Reference
Author notes
Hematology Division, Ospedali Riuniti di Bergamo, Italy
Professor of Medicine, Pediatrics and Pharmacology and Physiology, The George Washington University, Washington, DC; Center for Science and Technology, Noblis