Abstract
During the last decade much progress has been made toward better understanding of the underlying reasons causing thromboembolism in children. A considerable number of acquired and hereditary thrombotic risk factors have been identified which may also have an impact on therapeutic decisions and prognosis concerning outcome and the risk of a second event. However, indications for therapeutic interventions, such as thrombolysis and prophylactic anticoagulation with respect to the different clinical conditions and their combination with other risk factors, are not yet well defined. The following article describes the causes, clinical presentation and management of thrombosis in neonates, infants and older children, focusing on the clinically most relevant conditions.
Thromboembolism (TE) is still regarded as a rare event in childhood and therefore knowledge of diagnostics, therapy and prophylaxis is limited among general pediatricians. During the past years, however, it is increasingly recognized as having significant impact on mortality, chronic morbidity and the normal development of children, which has led to an enhanced sensitivity toward considering such events in respective patients. Besides the greater awareness, an objective increase in childhood thrombosis is due to the medical progress in the treatment of critically ill patients. This seemingly contradictory observation is easily explained by the increasing use of central catheters and innovative interventional procedures in the treatment of premature infants, neonates and older children who are critically ill, suffering from complex cardiac defects, and from malignant disease, respectively. Therapeutic and prophylactic measures have subsequently become increasingly important, but in addition to the complexity of the clinical background and the heterogeneity in the pattern of acquired and inherited risk factors for TE among patients, the physiological significant differences of the coagulation system between newborns, young children and adolescents and differences in drug metabolism do not allow general recommendations for therapeutic interventions like thrombolysis and prophylactic anticoagulation for the different clinical conditions. This situation is further complicated by a lack of availability of pediatric formulations and pediatric data for new drugs.
The increasing knowledge of exogenous and endogenous thrombophilic risk factors has initiated a number of studies to assess the impact of such factors with respect to their contribution to the thrombophilic state, both individually but also in concert with other factors. In addition to their impact on a first thrombotic event, much of the interest is now focused on their importance for thrombotic relapses. Only such studies will give us an answer to questions concerning the indications for treatment, prophylaxis and its optimal duration. All management recommendations are reflecting the authors’ experiences and opinions and are not based on evidence gained by controlled trials as such trials are either completely lacking or still ongoing.
Epidemiology
The annual incidence of TE in childhood in general is considerably lower than in adults, with a reported frequency of 0.07 to 0.14 per 10.000 children or 5.3 per 10,000 referrals of children to the hospital. The results of a prospective German study suggested an incidence of 5.2 per 100,000 neonates, and a prospective Dutch study resulted in an estimate of 1.4 per 100,000 children and adolescents (referenced in 1). More than 80% of TE in childhood were on a background of a severe preceding illness or other comparable predisposing factors.2 Arterial TE in children is less common than venous thrombosis2 with the exception of stroke. The estimated yearly incidence of stroke in childhood is between 3–8 per 100,000.3,4 The highest incidence of 25–35 per 100,000 live births has been reported for neonates (reviewed in 5). In addition to its impact on the development of children, stroke also quantitatively plays the most important role.
The reasons for the lower incidences of TE in children compared to adults are not completely understood; an intact vascular endothelium, the lower capacity of thrombin generation6 and elevated levels of α-2-macroglobulin, an inhibitor of thrombin, are possible age-dependent modifying factors in children. There are two age-related peaks in the frequency of thromboembolic disorders in children and adolescents: the first peak corresponds to the perinatal/neonatal period, with the highest relative incidence, and the second is observed post puberty in adolescents, with a higher frequency in females.2,7
The relatively higher incidence in neonates as compared to older children may be due to higher hematocrit, and the greater lability of the hemostatic system in neonates due to the generally decreased levels of both coagulation factors and their inhibitors in this age group, except factor VIII (FVIII) and von Willebrand factor (VWF) which are normal or even elevated.8 In adolescents the incidence equals that of young adults, probably due to the hormonal status, the use of contraceptives or pregnancy in young women, obesity and smoking.7
Clearly, these epidemiological data have to be considered when assessing the individual absolute thrombotic risk of children with thrombophilia.
Diagnosis
Clinical presentation
Pain, swelling and discoloration of extremities are acute symptoms of deep vein thrombosis (DVT). Vena cava inferior thrombosis manifests with prominent cutaneous veins and possibly liver or renal dysfunction depending on the site and extension of the thrombus. Superior vena cava thrombosis leads to cyanosis and swelling of the head and upper thorax with prominent collateral veins and may finally result in acute cardiac failure. Portal vein thrombosis, in most cases due to central catheters, and renal vein thrombosis with hematuria as a frequent sign may result in functional impairment or even failure of liver and renal function, respectively. Acute chest pain and dyspnea could suggest pulmonary embolism. Acute headache, visual impairment, cerebral convulsions and signs of venous congestion may indicate sinus venous thrombosis. Signs and symptoms of central venous catheter (CVC)-associated DVT are loss of CVC patency, the need for local thrombolytic therapy or CVC replacement, CVC-related sepsis, or prominent collateral circulation over chest, neck and head.
Childhood arterial ischemic stroke (AIS) manifests in neonates preferentially with seizures and abnormalities of muscle tone, whereas in elder children hemiparesis is the most frequent neurologic sign.9 Acquired or inherited severe deficiencies of protein S and protein C are disorders involving both the microcirculation and arterial vessels and may manifest with characteristic symptoms such as deep skin necrosis (purpura fulminans), blindness due to retinal vessel occlusion and arterial embolism followed by necrosis of distal extremities or whole limbs. Thrombotic thrombocytopenic purpura (TTP), a severe microangiopathic disorder is characterized by nonimmunologic hemolytic anemia and thrombocytopenia, neurologic symptoms, and renal, pulmonary and cardial involvement.
Laboratory parameters
Every thrombotic event initiates a particular response to re-establish the balance of the hemostatic system, e.g., by fibrinolysis. Subsequently markers of fibrinolysis such as D-dimers can be detected in the circulation. The specificity of these markers is low; however, the negative predictive value of the D-dimer test to correctly exclude DVT is as high as 89% in adult patients with likely DVT compared to 99% in patients who were categorized as unlikely to have DVT.10 In a study on the outcome of TE in children, elevated D-dimer and/or FVIII:C were found in only 67% of the patients; however, elevation of these markers at diagnosis and during follow-up are significantly correlated with persistence or recurrence of TE and/or a post-thrombotic syndrome.11
Imaging
Color Doppler ultrasound, conventional and MRI angiography, lineograms and echocardiography are the diagnostic means of imaging the occlusion of vessels. Pulmonary embolism of proximal pulmonary arteries can be visualized by echocardiography and by CT scan; however, the specificity and sensitivity are low in detecting more distal clots. In such cases ventilation and perfusion scintigraphies are the recommended techniques for children.12 Transcranial Doppler ultrasound is used to assess the risk of stroke in patients with sickle cell disease. All techniques can be regarded as equally specific, sensitive and precise; their application, however, differs with respect to the region of interest, age and therapeutic options. Table 1 lists the different techniques with respect to their application.
Prothrombotic Risk Factors
Assessment of prothrombotic risk factors is by no means suitable for diagnosing TE. It may possibly help to explain unusual manifestations of TE; however, the predictive power concerning outcome, thereby providing a basis for therapeutic and prophylactic decisions is still a matter of ongoing studies and debate. Interpretation of laboratory data is strongly age dependent since normal ranges may differ considerably between newborns, young children and adolescents.
Hereditary prothrombotic factors
The most important factors involved in the genetic predisposition to thrombophilia are the factors of the coagulation cascade and in particular their natural inhibitors. It is not clear if genetic defects of fibrinolysis also contribute to the hypercoagulable state. Certain metabolic defects also cause thrombophilia.
1. Coagulation factors
Fibrinogen (FI)
In addition to being the final substrate for thrombin, FI is also an acute-phase protein that may lead to acquired thrombophilia and may also contribute to the risk of arterial TE.13 Genetic defects causing dysfibrinogenemia associated with thrombophilia are rare.
Prothrombin (FII)
Heterozygosity for the 20210A allele of the common FII polymorphism 20210G/A in the untranslated 3′ region of the Prothrombin (FII) gene14 is found at a prevalence of 2.7% in the normal Caucasian population (n = 11.932, cumulative data from several studies). This mutant correlates with slightly elevated FII levels, suggesting a quantitative contribution to thrombophilia, and is found at a frequency of 7.1% in unselected patients with thombosis (n = 2884, cumulative data from several studies). The derived relative risk for thrombosis is 2.6. FII 20210A also seems to play a role in childhood stroke. Published data, however, do not give a clear picture.15,16 At least, FII 201210A does not seem to be involved in re-infarction.17
Factor V (FV)
The FV mutation Arg506 to Gln506 (R506Q or FV Leiden) causes relative resistance against cleavage by the activated protein C (PC) complex.18,19 It has been identified as the most common significant genetic risk factor for thrombosis to date. The prevalence in the normal Caucasian population is on the average 5%, with prevalences in particular populations of up to 15%.20 The relative thrombotic risk for heterozygotes is 6- to 8-fold, whereas homozygotes carry an 80-fold relative risk.21 In children with venous thrombosis, FV Leiden was identified in up to 30%.22 In contrast to adults it may also play a role in childhood stroke.15
Factor VIII (FVIII)
Elevated FVIII seems to contribute to the risk of TE in children. Furthermore, persistence of elevated FVIII after TE may also predict an unfavorable prognosis (11, see Laboratory parameters).
Von Willebrand factor
Due to its key position in platelet adhesion and aggregation under conditions of high shear forces, VWF plays a most important hemostatic role in arterial vessels and in the microcirculation.23 This suggests a significant contribution of VWF to arterial TE and to microangiopathies such as thrombotic thrombocytopenic purpura (TTP). An elevated level of VWF is an independent risk factor for myocardial infarction and stroke in adults.24 It has not yet been shown whether elevated VWF also plays a role in arterial thrombosis of childhood. In the neonate, supra large VWF multimers, which are the most active in primary hemostasis, are more abundant than later in life and correlate with a very effective platelet dependent function of VWF in newborns.25 It can be speculated if these large multimers contribute to the higher rate of stroke in the perinatal period, but respective data have not been reported yet. However, it is now clear that supra large VWF multimers are responsible for the life-threatening condition of TTP (reviewed in 26).
2. Inhibitors of hemostasis
The hemostatic process is tightly regulated by specific inhibitors that act on coagulation factors and on the factors of primary hemostasis. Functionally most important are tissue factor pathway inhibitor, the PC system, antithrombin (AT) and the VWF cleaving protease ADAMTS13. Clinically, to date only the latter three are important. Involvement of the coagulation inhibitors AT, PC and Protein S (PS) is rare with a prevalence in unselected patients with thrombosis of 0.019 for AT, 0.037 for PC, and 0.023 for PS deficiency.26,28 Recently, severe deficiency of ADAMTS13 has been identified as the causative factor of the rare TTP in most TTP patients (reviewed in 26).
Protein C system
The PC system comprises PC, PS and FV as co-factors. PC is activated to APC by thrombin, which changes its substrate specificity from FI to PC by being bound to thrombomodulin at the endothelial cell surface. APC cleaves and inactivates aFV and aFVIII at specific proteolytic sites, thereby regulating the formation of thrombin. Severe PC deficiency as well as severe PS deficiency correlates with purpura fulminans, a life-threatening thromboembolic disorder of the microcirculation and larger vessels. Heterozygous deficiency of either inhibitor correlates with venous TE. PC also binds plasminogen activator inhibitor 1 (PAI1) which then facilitates fibrinolysis. This dual function of PC suggests a central role in the regulation of thrombus formation.
Antithrombin
When bound to heparan sulfate on endothelial cells, AT inhibits thrombin but also aFXI, aFIX and aFX. Its action on thrombin is enhanced 1000-fold by heparin through an allosteric conformational change. In contrast, low-molecular-weight heparin makes AT more aFX specific. These effects are the basis for prophylactic or therapeutic anticoagulation by heparin. Even mild hereditary deficiency of AT function may correlate with thrombophilia with a penetrance higher than in PC and PS deficiency.
ADAMTS13
ADAMTS13 regulates the size of VWF multimers and thereby its functional activity in primary hemostasis. Its deficiency has clearly been assessed as playing the causative role in TTP.26 An acquired form, caused by autoantibodies against ADAMTS13, and an inherited form called Upshaw Schulman syndrome (USS) due to mutations in the gene, exist. Lack of the protease correlates with persistence of supra large VWF multimers (Figure 1 ) and, on an adequate trigger (infection, stress, hypoxia), these large multimers will induce platelet adhesion and aggregation in the microcirculation with subsequent microangiopathy, finally resulting in organ failure and death in 80% of cases when untreated. Thrombosis of larger venous and arterial vessels has also been observed. In childhood, TTP is rare and seems more often inherited.29 Oligo-symptomatic courses have been observed, however, their long-term prognosis is not clear. In addition to the obvious causative role of severe ADAMTS13 deficiency in TTP, the impact of milder ADAMTS13 deficiency as thrombophilic factor has not been assessed yet, but is subject of ongoing studies. ADAMTS13 has been identified as a potent antithrombotic in an animal model,30 which may be of future therapeutic interest.
3. Metabolic conditions
MTHFR polymorphism 677C/T
The rare condition of classical homocystinuria is most often caused by a deficiency of either cystathionine-β-synthetase or 5-methyltetrahydrofolate-homocysteine-methyltransferase and correlates with frequent TE due to severe homocysteinemia causing endothelial cell damage. The activity of 5-methyl tetrahydrofolate-homocysteine-methyltransferase in turn depends on the availability of 5-methyl-tetrahydrofolate, regulated by 5, 10-methyl tetrahydrofolate-reductase (MTHFR). A common thermolabile MTHFR-variant (MTHFR, 677C>T) correlates with a slightly elevated level of homocysteine. Although repeatedly claimed in many studies, this variant does not seem to be an independent risk factor for TE.
Lipoprotein (a)
Lipoprotein (a) is considered a significant venous and arterial risk factor for TE in children.15,31 However, other reports could not confirm these findings.32 Levels of Lp(a), though genetically determined, vary considerably among different populations. Lp(a) has structural homology to plasminogen, suggesting a possible competitive mechanism of Lp(a) in fibrinolysis. However, the lack of correlation between severe plasminogen deficiency and TE speaks against this hypothesis.
Acquired prothrombotic risk factors
1. Central venous catheters
CVCs have become critically important as medical and supportive management of various diseases and have greatly improved quality of life. They bear two serious complications: thrombotic occlusion and CVC-associated DVT as well as systemic infections. CVCs seem to be the most important risk factor for DVT. The range of reported CVC-related DVT ranges from 1% to nearly 70%, reflecting the problem of different definitions, diagnostic methods and alertness.33,34 However, the estimated contribution of CVCs to all thromboembolic events in newborns is as high as 90% and over 50% in older children.1 There are only a few controlled studies on the prevalence of CVC-related DVT and infection rate as well as the efficacy of antithrombotic measures to prevent catheter occlusion and infection.
2. Childhood cancer
TE is a well known complication in adult patients with cancer. With the exception of acute lymphoblastic leukemia (ALL), the knowledge about TE in childhood cancer is still limited. ALL has the highest rate of TE in childhood that is not necessarily related to the use of a CVC. In contrast, brain tumors have a rather low incidence of thrombosis with or without CVC.35 An overall estimation looks at a risk of up to 16%.36
TE in cancer is the result of complex interactions of a variety of factors such as the malignancy itself, chemotherapy and its side effects including infections or dehydration, CVCs, the unbalanced hemostatic system with predominant hypercoagulability as well as possible hereditary thrombophilia. The impact of the different types of childhood malignancy on the hemostatic system is still not well understood. Most reports are regarding ALL and show the highest risk for TE under ALL/non-Hodgkin lymphoma (NHL) treatment is during induction and re-induction therapy that contains l-asparaginase, the most common site being the upper deep venous system and the cerebral veins.
3. Thrombosis and antiphospholipid syndrome (APS)
APS is an antibody-mediated thrombophilic state characterized by specific clinical manifestations of venous, arterial or small vessel TE at any site as well as the presence of antiphospholipid antibodies (APA) in the blood. In addition to DVT, acute ischemic stroke or transient ischemic attack are characteristic. APS is often associated with a number of autoimmune disorders.37 APS in women causes adverse pregnancy outcome including unexplained still birth or prematurity because of severe placental insufficiency (multiple infarction) or severe (pre)eclampsia. APS is classified as primary and secondary; the clinical picture, however, is the same. Patients with no underlying disease are diagnosed as primary APS. Secondary APS refers to patients with underlying autoimmune (mainly rheumatologic) disorders as well as viral and bacterial infections or cancer.
All proposed pathophysiological mechanisms share the binding of the APA to anionic protein-phospholipid-complexes, leading to activation of endothelial cells, platelets and prothrombin, interference with natural inhibitory pathways and fibrinolysis, and disruption of the binding of annexin V to phospholipids coating the vascular system.38,39 There are clinical/laboratory diagnostic and therapeutic criteria for adults37 that do not apply equally for children. There have been recent reports on gene expression profiles to identify subtle distinctions in order to define the clinical relevance of different APA.40,41 Apart from DVT as the most frequent clinical symptom in children along with the presence of LAC and high risk of recurrence without adequate long-term anticoagulation, there is a subgroup of children presenting with perinatal stroke and no risk of recurrence independent of secondary antithrombotic prophylaxis.42 This underlines the discordance to adults and the need for diagnostic and therapeutic guidelines to be defined for pediatric patients.
APA along with decreased activity of various coagulation factors, mainly F XII, are found in about 50% of otherwise healthy children with multiple viral infections, screened for prolonged aPTT preceding tonsillectomy or adenotomy.42,44 APA in this context are in association to the repeated infections and do not appear to be clinically relevant, carry no risk for bleeding or TE, and hence do not influence perioperative management. They usually disappear after tonsillectomy and/or with decreasing frequency of infectious episodes. In contrast, life-threatening TE including purpura fulminans may occur with varicella, which have been shown to have a increased prevalence of APA and associated PS deficiency.43 Bleeding is rare and responds to corticosteroids.
4. Heparin-induced thrombocytopenia type 2 (HIT)
The overall incidence of HIT type 2 is estimated around 1% of patients hospitalized in pediatric intensive care units.45,46 Most often it is observed in neonates and infants after cardiac surgery and in adolescents treated with unfractionated heparin (UFH) for venous thrombosis. HIT-associated TE is mainly venous but arterial events may occur.
5. Other acquired prothrombotic conditions
Perinatal asphyxia, systemic infections/sepsis/DIC, congenital heart disease (CHD) and hypovolemia are the main risk factors in neonates, the latter particularly prone to arterial events in association with CHD and/or arterial catheters frequently used in an intensive care setting.47 There are additional factors in older children: trauma, major surgery, immobilization, estrogen containing contraceptives in adolescent girls, corticosteroid therapy, nephrotic syndrome, hemolytic uremic syndrome, inflammatory bowel disease, and rheumatic and other chronic disorders. To date, it remains an individual decision if and which antithrombotic prophylaxis should be offered considering additional and individual risk factors.
Therapy and Prophylaxis
Irrespective of an underlying disease, every thromboembolic manifestation should be treated, aiming at the complete recanalization of the occluded vessel and stopping the thrombotic process. In the vast majority of cases thrombosis will resolve under heparin given for 5–14 days. Other therapy options with a higher risk such as thrombolytic therapy or surgical embolectomy should be limited for patients with extensive thrombosis and/or threatened organ function. As LMWH show considerable advantages over UFH for therapeutic as well as prophylactic purposes, the following recommendations are in favor of LMWH. Yet evidence shows no difference in the antithrombotic efficacy. For detailed recommendations refer to Table 2 and reference 53.
Commonly used anticoagulants
Unfractionated heparin
The following disadvantages should be considered: the need for venous access for therapy and monitoring, age-dependent unpredictable pharmacokinetics; normal AT levels required; monitoring by aPTT prone to pre-analytic errors; risk for bleeding; risk for HIT. Intravenous UFH should only be given in the initial phase of antithrombotic therapy and then switched to LMWH.
Low-molecular-weight heparin
Advantages are easy subcutaneous administration once daily without need of venous access, predictable pharmacokinetics, minimal monitoring, minimized bleeding complications, reduced risk of HIT. Infants < 5 kg required about 50% higher doses than older children to reach equivalent anti-FXa levels.54 As a general guideline we recommend LMWH with therapeutic anti-Xa levels for 4–6 weeks, followed by prophylactic dosage up to ≤ 6 months. For the treatment duration of different sites, types and age groups refer to references 53,55.
Thrombolytic agents
The agent of choice is rt-PA. Streptokinase should not be used because of its allergic reactions. The use of urokinase at least in the USA is restricted for safety concerns. rt-PA may be indicated if thrombosis is extensive or organ/life threatening. The established contraindications in adults apply for children as well but should be considered relative.53 Therapeutic recommendations are listed in Table 3 .
Vitamin K antagonists
Warfarin and phenprocoumon are usually administered for oral anticoagulation and inhibit g-carboxylation of vitamin K–dependent proteins. Considerable variation due to nutrition, co-medication, intercurrent illness and difficult monitoring requires close supervision and dose adjustment. We administer vitamin K antagonists in cases of prophylaxis exceeding 6 months (Table 4 ).
Infusion of deficient inhibitors of hemostasis
In cases of thrombosis with hereditary or acquired deficiencies of coagulation inhibitors, replacement therapy may be an option. Concentrates of AT and PC are commercially available and are life saving in conditions of purpura fulminans due to inhibitor deficiency. PC concentrate also proved to be effective in heterozygous or acquired PC deficiency (Figure 2 ). Fresh frozen plasma is the only but effective option of treating patients with purpura fulminans or hereditary TTP due to PS or ADAMTS13 deficiency, respectively.
New anticoagulants
The limitations of the traditional anticoagulants are particularly obvious in pediatrics; hence, the promotion of the new drugs already approved in adults urgent. Yet there is but individual experience in children with the following substances: the pentasaccharides fondaparinux and idraparinux, and the direct thrombin inhibitors hirudin, bivalirudin, argatroban; ximelagatran has been withdrawn from the market because of hepatic toxicity.56,57
Special conditions
Prophylaxis of CVC occlusion
UFH
Prophylactic UFH seems to significantly decrease CVC-related DVT as well as bacterial colonization of the catheter.58 Heparin-bonded catheters do not reduce clot formation and bacterial colonization beyond 24 hours after CVC insertion.
Thrombolytic agents (urokinase, rt-PA)
Thrombolytic therapy is widely and safely used for the management of occluded catheters. There are only a few studies using thrombolytic agents prophylactically in order to reduce catheter infections and occlusions. Some studies show a substantial benefit of thrombolytic agents over UFH or no prophylaxis58 whereas others get contradictory results.60,61
LMWH
Oral anticoagulation with vitamin K-antagonists
There are no data for children on using low-dose oral anticoagulation to prevent CVC-associated DVT and to maintain catheter patency. Considering the heterogeneous pediatric population requiring a CVC with respect to age, thrombogenic risk profile, underlying disease, intensity and duration of treatment, the use of vitamin K–antagonists must remain a decision on a strictly individual base.
Management of Thrombosis in Children with Cancer
The main challenge is to keep the balance of benefit and risk of an antithrombotic treatment, as most children are being treated with chemotherapy with intermittent thrombocytopenia and an unbalanced hemostatic system, both of which lead to potential bleeding complications. It is therefore strongly recommended not to use antithrombotic agents with potentially serious side effects such as thrombolytic agents, UFH or vitamin K antagonists.
Prophylaxis of TE in children with cancer
Since a high percentage of TE seems to be directly CVC-related, it is of primary importance to maintain its patency. Though there is a lack of clear evidence based indications the following situations for primary prophylaxis may be individually considered: 1) children with hereditary thrombophilia under intensive chemotherapy, 2) adolescents in the presence of additional risk factors such as major surgery or immobilization, 3) patients with prior TE in their history and 4) children with tumors compressing large vessels. Because ALL carries the highest risk for TE an efficient prophylaxis would be of major importance. To date there are no controlled trials that allow the extrapolation of prophylactic strategies. The German BFM-Study Group is conducting the first randomized interventional trial comparing three different antithrombotic strategies during ALL-induction therapy (Thrombotect). This ongoing trial is expected to provide the basis for risk adapted prophylaxis guidelines.
Antithrombotic therapy for APS
Long-term prognosis depends on the risk of recurrent TE, which seems to be the highest within 6 months of discontinuation of anticoagulation.64 Duration and intensity of therapy are still controversial, at least for subgroups. After the first DVT, secondary prophylaxis for 12 months is indicated. Lifelong anticoagulation is to be considered after a very serious first event and recurrent TE with persistence of APA. After arterial TE the optimal secondary prophylaxis remains controversial.64,65 In children consideration should be given to performing and/or extending first/second line antithrombotic treatment on an individual basis, depending on the presence of underlying disorders.
Treatment-related Indications for Thrombophilia Screening
It makes a difference if children are diagnosed and treated as study patients or if they are individually seen. In the latter case, laboratory work-up of thrombosis in childhood should pertain to the following basic questions: i) is there a specific therapy and ii) what are the consequences of a particular finding concerning future management and counseling of the patient and the family?48 Keeping this in mind, the necessary investigations are only a few (see Table 5 ) which is at odds with the current recommendations published by the Subcommittee on Perinatal/Pediatric Hemostasis of the Scientific and Standardization Committee (SSC) of the International Society on Thrombosis and Hemostasis (ISTH).49 However, since there is no consensus on management guidelines yet, laboratory testing may also vary between different institutions. It is well accepted that the coagulation inhibitors AT, PC and PS should be part of the diagnostic program. Though rare, their deficiencies can be compensated for by commercially available concentrates (AT, PC) and by fresh frozen plasma (PS). In cases of TE accompanied by hemolytic anemia and thrombocytopenia, Upshaw Schulman syndrome should be suspected and ADAMTS13 activity should be determined, since fresh-frozen plasma (FFP) is a life-saving replacement therapy in this condition and plasma exchange is the method of choice in the acquired form. Fasting homocysteine may be determined, since its elevation can be treated by folic acid substitution. However, two recent studies on lowering homocysteine by folate administration in patients with vascular disease did not show a reduction of re-infarction or stroke in adults.50,52 HIT type 2 should be ruled out in patients with thrombosis who show a drop of the platelet count under heparin administration. APA should be determined, since the respective patients require a longer lasting prophylaxis against a relapse. There is no specific treatment for patients with Factor V Leiden or PT G20210A. Although these established hereditary risk factors are the most common, therapeutic and prophylactic measures are not necessarily different for children with or without these risk factors. Indeed, many studies on adults and a few on children have shown that these factors have only minor or even no impact on re-TE in unselected patients with or without these risk factors.17,52 As some studies have suggested, combined thrombophilic factors may enhance the risk of thrombosis. However, the risk of a second event in unselected patients does not seem to be high enough to justify more intense and prolonged anticoagulation, compared to patients without these risk factors. Deviations from this “minimalistic” diagnostic approach may be indicated with respect to the individual case and to the particular institutional management guidelines. Many other factors are part of diagnostic programs, although their contribution to the thrombotic risk seems to be very low or even absent.
Concluding Remarks
Thromboembolism in a child is a serious condition that, in addition to the complications also seen in adults, may adversely effect the child’s further development. Better predictors of prognosis in relation to risk factors, therapy and prophylaxis are therefore urgently needed. However, in spite of many efforts over the last decade to address the problem of TE and the thrombophilic state in children and the almost “logarithmic” increase in novel risk factors, there has not been much progress toward evidence-based risk-factor adapted guidelines for treatment and prophylaxis of TE. Decisions have to be made on an individual basis. The large number of established and less established, known and also as yet unknown risk factors for TE does not allow to individually predict a patient’s outcome or prognosis for an additional event. Recent data on the prognostic value of FVIII and D-dimers seem promising.11 Future respective studies may help to assess the optimal duration of anticoagulation in particular cases better than a “risk profile” on the basis of many different prothrombotic factors. However, independent from these considerations one should always consider the overall low absolute risk of TE in a child compared to adults. Concerning costs and possible over-interpretation of laboratory tests, investigation of risk factors in the individual patient should be limited according to their usefulness in the particular setting.
Imaging methods for thromboembolism in neonates and children.
| Method . | Indication . | Limitations . |
|---|---|---|
| Abbreviations: CVC, central venous catheter, DVT, deep vein thrombosis, SVT, Sinus venous thrombosis | ||
| * in young infants through the patent fontanella. | ||
| Lineograms | CVC related thrombosis | Only clots at the tip of the CVC and the distal adjacent vessel wall |
| Color Doppler ultrasound | DVT, SVT* | Exception: subclavian vein, use venography |
| Bilateral venography | DVT, SVT | Exception: jugular vein, use color Doppler ultrasound conventional or MRI |
| Echocardiography | CVC-related thrombosis, intracardial thrombus, pulmonary embolism | Distal clots in PE |
| Scintigraphy | Pulmonary embolism | — |
| Method . | Indication . | Limitations . |
|---|---|---|
| Abbreviations: CVC, central venous catheter, DVT, deep vein thrombosis, SVT, Sinus venous thrombosis | ||
| * in young infants through the patent fontanella. | ||
| Lineograms | CVC related thrombosis | Only clots at the tip of the CVC and the distal adjacent vessel wall |
| Color Doppler ultrasound | DVT, SVT* | Exception: subclavian vein, use venography |
| Bilateral venography | DVT, SVT | Exception: jugular vein, use color Doppler ultrasound conventional or MRI |
| Echocardiography | CVC-related thrombosis, intracardial thrombus, pulmonary embolism | Distal clots in PE |
| Scintigraphy | Pulmonary embolism | — |
Recommended dosing of UFH and LMWH in neonates and children.
| UFH i.v. . | Neonates < 5kg . | Children > 5kg . | Target aPTT at 4h . |
|---|---|---|---|
| * 1 mg Enoxaparin = 110 anti-FXa units | |||
| For UFH: aPTT 4 hours after loading dose and 4 hours after each dosage adjustment, at least once daily; keep AT level within normal range; daily blood count (platelets!). For LMWH: anti-FX activity 4 hours after injection | |||
| loading dose | 1 × 75 U/kg/10 min | 1 × 75 U/kg/10 min | |
| maintenance | 25–30 U/kg/h | 20 U/kg/h | 60–85 sec. |
| LMWH s.c. | Neonates < 5kg | Children > 5kg | Target anti-FXa at 4 h |
| initial treatment dose | |||
| Enoxaparin* | 1 × 2.0 mg/kg/d | 1 × 1.5 mg/kg/d | 0.4–0.8 U/mL |
| Dalteparin | 1 × 200 U/kg/d | 1 × 150 U/kg/d | 0.4–0.8 U/mL |
| Reviparin | 2 × 150 U/kg/d | 2 × 100 U/kg/d | 0.5–1.0 U/mL |
| initial prophylactic dose | |||
| Enoxaparin* | 1 × 1.5 mg/kg/d | 1 × 1.0 mg/kg/d | < 0.4 U/mL |
| Dalteparin | 1 × 100 U/kg/d | 1 × 50 U/kg/d | < 0.4 U/mL |
| Reviparin | 2 × 50 U/kg/d | 2 × 30 U/kg/d | < 0.5 U/mL |
| UFH i.v. . | Neonates < 5kg . | Children > 5kg . | Target aPTT at 4h . |
|---|---|---|---|
| * 1 mg Enoxaparin = 110 anti-FXa units | |||
| For UFH: aPTT 4 hours after loading dose and 4 hours after each dosage adjustment, at least once daily; keep AT level within normal range; daily blood count (platelets!). For LMWH: anti-FX activity 4 hours after injection | |||
| loading dose | 1 × 75 U/kg/10 min | 1 × 75 U/kg/10 min | |
| maintenance | 25–30 U/kg/h | 20 U/kg/h | 60–85 sec. |
| LMWH s.c. | Neonates < 5kg | Children > 5kg | Target anti-FXa at 4 h |
| initial treatment dose | |||
| Enoxaparin* | 1 × 2.0 mg/kg/d | 1 × 1.5 mg/kg/d | 0.4–0.8 U/mL |
| Dalteparin | 1 × 200 U/kg/d | 1 × 150 U/kg/d | 0.4–0.8 U/mL |
| Reviparin | 2 × 150 U/kg/d | 2 × 100 U/kg/d | 0.5–1.0 U/mL |
| initial prophylactic dose | |||
| Enoxaparin* | 1 × 1.5 mg/kg/d | 1 × 1.0 mg/kg/d | < 0.4 U/mL |
| Dalteparin | 1 × 100 U/kg/d | 1 × 50 U/kg/d | < 0.4 U/mL |
| Reviparin | 2 × 50 U/kg/d | 2 × 30 U/kg/d | < 0.5 U/mL |
Recommendations for systemic thrombolysis in neonates and children.
| Contraindications . | |
|---|---|
| Strong | within 10 days after hemorrhage or major surgery |
| within 7 days after severe asphyxia | |
| within 3 days after invasive procedure | |
| Soft | within 48 hours after cerebral convulsion |
| prematurity < 32 weeks of gestation | |
| sepsis | |
| active minor hemorrhage | |
| refractory thrombcytopenia and hypofibrinogenemia | |
| Contraindications . | |
|---|---|
| Strong | within 10 days after hemorrhage or major surgery |
| within 7 days after severe asphyxia | |
| within 3 days after invasive procedure | |
| Soft | within 48 hours after cerebral convulsion |
| prematurity < 32 weeks of gestation | |
| sepsis | |
| active minor hemorrhage | |
| refractory thrombcytopenia and hypofibrinogenemia | |
| Therapy . | Loading Dose . | Maintenance . | Monitoring . |
|---|---|---|---|
| Indications: extensive and/or life/organ-threatening thrombosis. Contraindications: on an individual basis to be considered relative, not absolute; keep fibrinogen > 0.5 g/L and platelets > 50 g/L; increasing D-dimers indicate effective fibrinolysis; dose reduction or cessation of rt-PA if major bleeding occurs; minor bleeding (oozing from catheter puncture site or wound) treat with local pressure; optimal duration of rt-PA therapy uncertain, mostly up to 7 days, shorter/longer courses | |||
| rt-PA | 0.1–0.2 mg/kg/10 min. | 0.8–2.4 mg/kg/24 h | FI, platelets, D-dimers |
| UFH | none | 5–10 U/kg/h | Apt |
| Therapy . | Loading Dose . | Maintenance . | Monitoring . |
|---|---|---|---|
| Indications: extensive and/or life/organ-threatening thrombosis. Contraindications: on an individual basis to be considered relative, not absolute; keep fibrinogen > 0.5 g/L and platelets > 50 g/L; increasing D-dimers indicate effective fibrinolysis; dose reduction or cessation of rt-PA if major bleeding occurs; minor bleeding (oozing from catheter puncture site or wound) treat with local pressure; optimal duration of rt-PA therapy uncertain, mostly up to 7 days, shorter/longer courses | |||
| rt-PA | 0.1–0.2 mg/kg/10 min. | 0.8–2.4 mg/kg/24 h | FI, platelets, D-dimers |
| UFH | none | 5–10 U/kg/h | Apt |
Recommended dosing of oral anticoagulants (OAC) in neonates and children.
| OAC . | Day 1 . | Day 2 . | From Day 3 . | Target INR . |
|---|---|---|---|---|
| Phenprocoumon | 6 mg/m2 | 3 mg/m2 | 1–2 mg/m2 | 2.0–3.0 |
| Warfarin | 0.2 mg/kg | 0.2 mg/kg | 0.1–0.3 mg/kg | 2.0–3.0 |
| OAC . | Day 1 . | Day 2 . | From Day 3 . | Target INR . |
|---|---|---|---|---|
| Phenprocoumon | 6 mg/m2 | 3 mg/m2 | 1–2 mg/m2 | 2.0–3.0 |
| Warfarin | 0.2 mg/kg | 0.2 mg/kg | 0.1–0.3 mg/kg | 2.0–3.0 |
| Reversal of oral anticoagulant therapy . | |
|---|---|
| Coumarin therapy always to begin with concomitant heparin therapy (UFH or LMWH); to stop heparin, INR within therapeutic range for 2 days, concomitant medication at least 5 days; attention to multiple drug interactions | |
| no bleeding, slow reversal | vitamin K 0.5–2.0 (−5.0) mg orally (s.c., i.v.) |
| no bleeding, rapid reversal | vitamin K 0.5–2.0 (−5.0) mg s.c. or i.v. |
| significant bleeding, not life threatening | vitamin K 0.5–2.0 (−5.0) mg s.c. or i.v. + FFP 20 mL/kg |
| significant bleeding, life threatening | vitamin K 5 mg i.v. over 20 min. (risk of anaphylactic shock) + prothrombin concentrate (Prothomplex) 50 U/kg i.v. |
| Reversal of oral anticoagulant therapy . | |
|---|---|
| Coumarin therapy always to begin with concomitant heparin therapy (UFH or LMWH); to stop heparin, INR within therapeutic range for 2 days, concomitant medication at least 5 days; attention to multiple drug interactions | |
| no bleeding, slow reversal | vitamin K 0.5–2.0 (−5.0) mg orally (s.c., i.v.) |
| no bleeding, rapid reversal | vitamin K 0.5–2.0 (−5.0) mg s.c. or i.v. |
| significant bleeding, not life threatening | vitamin K 0.5–2.0 (−5.0) mg s.c. or i.v. + FFP 20 mL/kg |
| significant bleeding, life threatening | vitamin K 5 mg i.v. over 20 min. (risk of anaphylactic shock) + prothrombin concentrate (Prothomplex) 50 U/kg i.v. |
List of relevant, established and potential thrombophilic factors.
| 1 . | 2 . | 3 . |
|---|---|---|
| Column 1: factors of therapeutic and/or prognostic relevance; column 2: established risk factors with possible therapeutic and prognostic relevance for the individual patient; column 3: potential thrombophilic factors. Their therapeutic and prognostic relevance for the individual patient is doubtful. Laboratory tests for HIT type 2 and ADAMTS13 are only indicated when additional data suggest their involvement (see text). | ||
| Antithrombin | APC resistance (FV Leiden) | PAI-1 polymorphism |
| Protein C | Prothrombin G20210A | Plasminogen |
| Protein S | Lipoprotein (a) | Heparin-cofactor II |
| Antiphospholipid-Ab | Dysfibrinogenemia | FIX |
| Homocysteine | FVIII | FXI |
| HIT Type 2 | D-Dimer | FXIII |
| ADAMTS13 | VWF | |
| 1 . | 2 . | 3 . |
|---|---|---|
| Column 1: factors of therapeutic and/or prognostic relevance; column 2: established risk factors with possible therapeutic and prognostic relevance for the individual patient; column 3: potential thrombophilic factors. Their therapeutic and prognostic relevance for the individual patient is doubtful. Laboratory tests for HIT type 2 and ADAMTS13 are only indicated when additional data suggest their involvement (see text). | ||
| Antithrombin | APC resistance (FV Leiden) | PAI-1 polymorphism |
| Protein C | Prothrombin G20210A | Plasminogen |
| Protein S | Lipoprotein (a) | Heparin-cofactor II |
| Antiphospholipid-Ab | Dysfibrinogenemia | FIX |
| Homocysteine | FVIII | FXI |
| HIT Type 2 | D-Dimer | FXIII |
| ADAMTS13 | VWF | |
Supra large VWF multimers in 3 siblings (thrombotic thrombocytopenic purpura [TTP]) with hereditary ADAMTS13 deficiency compared to normal plasma (NP). Activation of ADAMTS13 in normal plasma (NP +a) results in complete proteolytic cleavage of VWF multimers, whereas VWF in the TTP patient (TTP +a) remains unchanged due to the lack of ADAMTS13.
Supra large VWF multimers in 3 siblings (thrombotic thrombocytopenic purpura [TTP]) with hereditary ADAMTS13 deficiency compared to normal plasma (NP). Activation of ADAMTS13 in normal plasma (NP +a) results in complete proteolytic cleavage of VWF multimers, whereas VWF in the TTP patient (TTP +a) remains unchanged due to the lack of ADAMTS13.
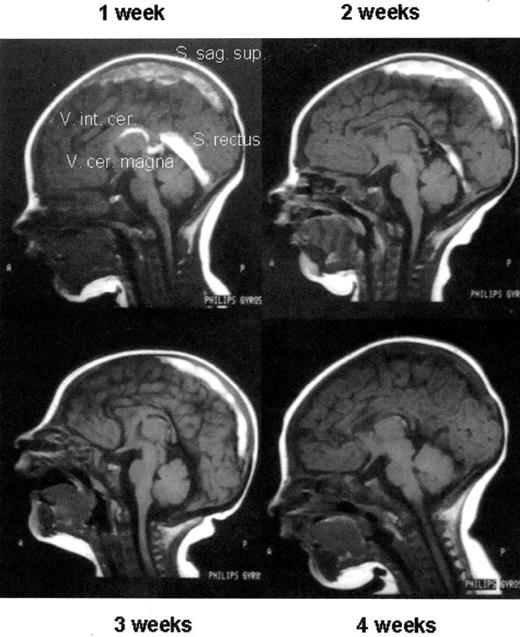
Thrombolytic therapy of an extensive sinus venous thrombosis in a newborn with heterozygous protein C deficiency by protein C concentrate after 1 week of ineffective UFH therapy (1 week) and, after initiating protein C replacement, at week 2, 3, and 4 of therapy, respectively. Note the almost complete re-canalization.
Abbreviations: UFH, unfrationated heparin; FAI, FM
Thrombolytic therapy of an extensive sinus venous thrombosis in a newborn with heterozygous protein C deficiency by protein C concentrate after 1 week of ineffective UFH therapy (1 week) and, after initiating protein C replacement, at week 2, 3, and 4 of therapy, respectively. Note the almost complete re-canalization.
Abbreviations: UFH, unfrationated heparin; FAI, FM
RS: University Medical Center Hamburg-Eppendorf, Dept. of Pediatric Hematology and Oncology, Hamburg, Germany
JG: Children’s Hospital of Eastern Switzerland, St. Gallen, Switzerland

![Figure 1. Supra large VWF multimers in 3 siblings (thrombotic thrombocytopenic purpura [TTP]) with hereditary ADAMTS13 deficiency compared to normal plasma (NP). Activation of ADAMTS13 in normal plasma (NP +a) results in complete proteolytic cleavage of VWF multimers, whereas VWF in the TTP patient (TTP +a) remains unchanged due to the lack of ADAMTS13.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2006/1/10.1182_asheducation-2006.1.86/5/m_schneppenheim_fig1.jpeg?Expires=1766005726&Signature=OwvldnWbLk-VLPQd4wFm0NIhQ~-fpmV0YpS7EWzjEsd9~0u30EcUi2m1DtjwsTFmxDNkw8Y7hflp3hLJuGkeIhmKPMwY3VUGBIE7tCbseH5ZqydbNkkSp4GiaP9hPDklxr~QrqGkuT1ReICINtX2zinZjVHO2DUxHDMFmggdtu-Lo-CRj3DoYzAZTeSipoP9tPXnz8AkODV~WcO~7l-QbTr3i-IKtlyFUTDkrAPxffLfF98eM75F9KnYYqT8ttZ2JpoX8T2s~PIAwergK22pjnxUHm-EJ7ThUYDpFtbTwiUKj19IbKtX0JleXglmyipR3bqTC8xUqZWY1BJkiQ-ULA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)