Abstract
As the overall health of patients with sickle cell anemia (SS) improves and diagnostic techniques become more sensitive, physicians are seeing patients with an increasingly wide range of subtle and not-so-subtle brain injury. The major breakthrough in the field of sickle-related brain injury has been the unprecedented success of transcranial Doppler ultrasonography (TCD) to identify asymptomatic patients at high risk of stroke, coupled with chronic transfusion therapy to prevent it. The evidence for TCD screening and preventive treatment is strong and compelling, but there are still important unanswered questions regarding the implications of “silent infarcts” found in the magnetic resonance images (MRIs) of asymptomatic individuals, and the growing awareness of the burden of neuropsychiatric dysfunction in otherwise apparently healthy individuals.
Acute Stroke
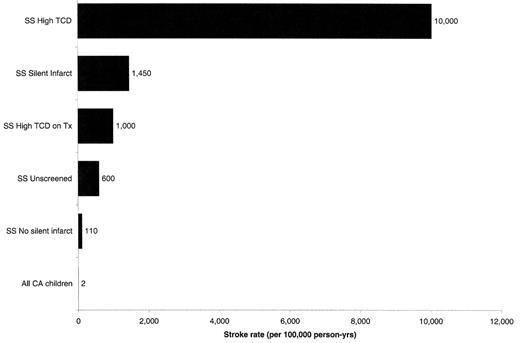
As shown in Figure 1 , there is a wide spectrum of brain injury, and a wide spectrum of impact of that injury on overall morbidity and mortality in patients with sickle cell anemia (SS). The most catastrophic is the classical acute stroke. Typically presenting with hemiplegia with or without aphasia, seizures, and altered consciousness, acute stroke occurs at a rate of ~600/100,000 patient-years (Figure 2), resulting in a prevalence approaching 10% by the age of 50.1 These patients may have ongoing neurologic abnormalities and the poorest outcomes in neuropsychological testing of all patients with SS.2,3
Treatment
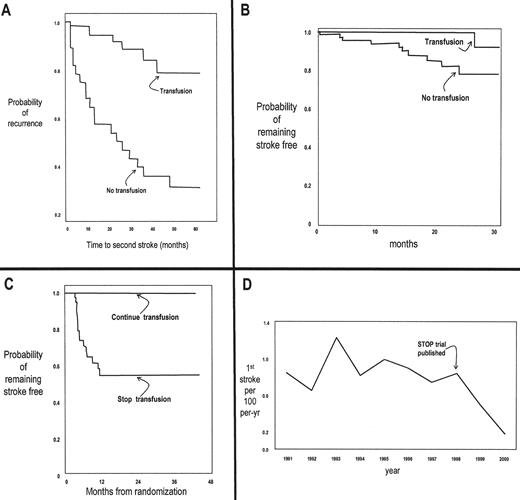
It is no surprise that acute stroke became an early target for clinical intervention and investigation. Investigators in the 1970s made some key observations: 1) that the underlying pathology is occlusion of large vessels in the circle of Willis; 2) that acute mortality and morbidity could be minimized with the aggressive use of exchange transfusion at presentation (see Dr. Swerdlow’s paper in this volume for more on exchange); 3) that patients with one stroke are at very high risk of recurrence; and 4) that chronic maintenance transfusion could prevent recurrence (Figure 3 , panel A) and normalize previously stenotic cerebral vessels.4,5
Prevention: TCD and transfusion
The success that resulted from the acceptance of emergency exchange and chronic maintenance transfusions for stroke led to a new goal—primary stroke prevention. The critical factor for what has turned out to be one of the most important innovations in sickle cell care is the use of transcranial Doppler ultrasonography (TCD) to identify a group at high risk of stroke.
TCD is a reproducible, noninvasive technique to find narrowed internal carotid or middle cerebral arteries in asymptomatic children by detecting a signature high-flow pattern.6 Children with elevated velocities (≥ 200 cm/sec) have an astonishingly high rate of stroke: ~10,000/100,000 patient-years (Figure 2 ).7,8 When asymptomatic children with abnormally high flow velocities demonstrated by using screening TCD are preemptively treated with maintenance transfusion, 90% of strokes are prevented (Figure 2 , Figure 3 panel B).8 During the transfusion period, most of the TCD studies revert to or toward normal; unfortunately, the risk of stroke reappears once the transfusion regimen is stopped (Figure 3 , panel C).
Based on the data available to date, the following is considered to be rational practice:
Screen all children with SS ages 2–16 using TCD. Repeat annually for children with normal studies, and every 6 months for those in the “conditional” range.
Once confirmed with a repeat study, children with abnormal TCD should be placed on a chronic maintenance transfusion program using blood matched for ABO, C, D, E, and Kell antigens, and timed and dosed to keep the [HbS] ≤ 30%.
The impact of this approach has been amazing. It has been known for decades that SS is one of the major risk factors for stroke in children. As shown in Figure 3 panel D, the implementation of TCD screening and transfusion therapy has been so successful in California that the overall rate of stroke in SS children has declined since the publication of the SS data.9
Prevention: TCD and alternatives to transfusion?
The beneficial and adverse effects of chronic maintenance transfusion to prevent stroke are shown in Table 1 . The patients benefit not only because their stroke rates are dramatically decreased, but also because their growth is improved, and their morbidity is decreased in terms of the hemolysis and acute vaso-occlusive events.10,–12 The most serious and predictable adverse effect or transfusion is iron overload.13 At this point it is difficult to predict how the new chelation agents and protocols will ultimately influence the impact of this adverse effect.14 The most serious unpredictable adverse effect would be the emergence of a new HIV-like pathogen in the blood supply. The hassle factor of transfusion was a moderate problem for patients who were already on transfusion, as only 70% elected to remain transfused, in contrast to those who were never on transfusions, where only 45% elected to go on transfusion when offered.13
Nonetheless, chronic indefinite transfusion remains a major undertaking with serious known and unknown risks. Two other important alternatives are being studied, and hold considerable promise: hydroxyurea (HU), and bone marrow transplant (BMT). Chronic long-term HU has been widely used and has well-documented benefits and adverse effects (see Table 2 ). Several groups have studied (or are continuing to study) HU for primary and secondary stroke prevention, and the results are encouraging.15,–18 Less is known about the impact of BMT on stroke, but as regimens improve, the potential increases.19 An interesting decision analysis comparing BMT with chronic transfusion for stroke failed to identify either as better than the other. 20
Prevention: other risk factors as alternatives to TCD?
As the skull matures with age, the TCD window to the cerebral vessels closes down, making it inappropriate as a screening tool for some older children and most adults. As more research is done in older patients, especially in those who were screened using TCD as children, the importance of other known risk factors such as the presence of “silent infarcts” (see section below), elevated blood pressure,21 absence of alpha thalassemia,22 presence of moyamoya,23 or genetic profile24 will become useful guides to therapy.
Hemorrhagic Stroke
As expected,25 in the age group most carefully studied prospectively with TCD, fewer than 20% of strokes were hemorrhagic.26 This relatively small group included an assortment of intraparenchymal, subarachnoid, epidural, and mixed lesions. Although the numbers are small, it appears that TCD is not as useful a predictor of hemorrhage as infarct.
“Silent infarct”
As soon as magnetic resonance imaging (MRI) became readily available, it was immediately clear that brain abnormalities in SS patients are not restricted to those with clinically evident acute stroke and abnormal neurological exams. As more neurologically normal patients began to get MRIs because of trauma, headache workups, seizures, etc., unexpected abnormal bright spots started turning up—particularly in the deep white matter and, in contrast to the MRIs of patients with stroke, sparing the cortex.27 Clinical investigators started to look at these “unexplained bright objects,” as they were originally called, in a systematic way and found that they occurred in ~20% of SS children—more or less dependent on the sensitivity of the MRI being used.27 –30 The term “silent infarct” came into use to describe these MRI findings, although the classic clinical/pathological study to support a precise anatomic/pathophysiological diagnosis underlying these observed lesions is (fortunately) lacking.
What we do know about these lesions is that they are not really “silent”—even though patients with them have normal neurological exams, they tend to perform poorly on neuropsychological tests, albeit not as poorly as patients who have had a clinical stroke.2,3,31,–34 Some children with normal MRIs also have abnormal neuropsychological exams, a group that may require more sensitive imaging techniques, such as positron emission tomographic imaging, to identify a brain abnormality.35
We also know that the group of patients with silent infarcts are at high risk of developing stroke30 (Figure 2 ); that the group of neurologically normal patients with elevated TCD is enriched for those with silent infarcts; that the stroke risk for those with both is even higher than for either group alone; and that transfusion for those with both elevated TCD and silent infarcts not only provides protection from stroke, but also new silent lesions.36
Some questions on the pathogenesis, treatment, and prevention of stroke remain open:
Can clues on the pathogenesis of the vascular lesions associated with stroke in SS and their overlap with stroke lesions in AA open up new approaches?
Can genetic signatures increase the sensitivity of TCD screening?
Can data from MRIs be used to improve stroke prediction?
Can temporal changes in TCD be used to improve stroke prediction?
Is a stroke model appropriate for thinking about treatment of neuropsychologic abnormalities in the absence of stroke?
What about hemorrhagic stroke?
What about stroke in adults?
| Favorable Effects . | Adverse Effects . |
|---|---|
|
|
| Favorable Effects . | Adverse Effects . |
|---|---|
|
|
| Favorable Effects . | Adverse Effects . |
|---|---|
|
|
| Favorable Effects . | Adverse Effects . |
|---|---|
|
|
Estimates of morbidity/mortality, and impact on neurological exam and neuropsychological test performance in patients with sickle cell anemia (SS).
The different SS clinical subgoups are represented by circles that approximate their relative numbers in a typical SS population in care at US institutions.
Estimates of morbidity/mortality, and impact on neurological exam and neuropsychological test performance in patients with sickle cell anemia (SS).
The different SS clinical subgoups are represented by circles that approximate their relative numbers in a typical SS population in care at US institutions.
Approximate stroke rates (incidence per 100,000 person-years) in different sickle cell anemia (SS) clinical populations.
Approximate stroke rates (incidence per 100,000 person-years) in different sickle cell anemia (SS) clinical populations.
Growth in knowledge about stroke in sickle cell anemia (SS).
Panel A: Effect of chronic transfusion on recurrence after an initial episode of stroke.39 Panel B: Effect of chronic transfusion on stroke rate in an asymptomatic population without history of stroke shown to have elevated TCD.8
Panel C: Effect of discontinuing chronic transfusion on stroke rate in asymptomatic patients with elevated TCD who had been maintained on transfusion as shown in Panel B.40
Panel D: Incident rate of stroke in SS children living in California (CA), before and after publication of the paper illustrated in Panel B.9 (all figures were modified from the original papers)
Growth in knowledge about stroke in sickle cell anemia (SS).
Panel A: Effect of chronic transfusion on recurrence after an initial episode of stroke.39 Panel B: Effect of chronic transfusion on stroke rate in an asymptomatic population without history of stroke shown to have elevated TCD.8
Panel C: Effect of discontinuing chronic transfusion on stroke rate in asymptomatic patients with elevated TCD who had been maintained on transfusion as shown in Panel B.40
Panel D: Incident rate of stroke in SS children living in California (CA), before and after publication of the paper illustrated in Panel B.9 (all figures were modified from the original papers)