Abstract
Recombinant factor VIIa (rFVIIa) was developed in the early 1990s to provide “bypassing” hemostatic therapy for hemophilia A and B patients with inhibitors. More recently, it has been licensed for use in patients with inherited deficiency of factor VII. Since it was licensed for use in hemophilia with inhibitors in the US, Europe, and other countries for these specific indications, it has been used selectively but in a wide array of clinical settings for uncontrolled hemorrhage in individuals without an inherited bleeding disorder. Many of these uses have been described in the medical literature as case reports or small, uncontrolled series. Several randomized clinical trials (RCT) for these “off-label” medical uses have been published in recent months and will serve as the focus of this review. In particular, a review of an RCT for spontaneous intracranial hemorrhage that has demonstrated clinical efficacy in reducing both mortality and volume of central nervous system hemorrhage will be offered. A brief discussion of hypothesized physiologic mechanisms of supraphysiologic doses of rFVIIa will introduce the clinical discussion of these broad off-label uses. Since rFVIIa is a very expensive therapy, possible strategies for optimizing its use in the these settings will be presented.
Recombinant factor VIIa (rFVIIa) has been licensed by the U.S.A. and European regulatory agencies (FDA and EMEA, respectively) for nearly a decade to treat patients with congenital or acquired hemophilia A or B and high responding inhibitors to factor VIII (FVIII) and factor IX (FIX). Recently (2004–2005), rFVIIa has been similarly licensed for replacement therapy in factor VII (FVII) deficiency.1 In addition, both research investigators and clinicians treating patients with severe hemorrhaging have employed rFVIIa as a potential “universal hemostatic agent” in diverse scenarios for which there is no licensed indication.2 These have included treating patients with presumed deficiencies or antibody-neutralization of other clotting factors besides FVII, FVIII or FIX (e.g., patients with congenital or acquired FXI deficiency3); patients with qualitative or quantitative platelet defects (e.g., alloimmune thrombocytopenia after chronic platelet transfusion or Glanzmann’s throm-basthenia4) or vascular injury states (e.g., spontaneous ICH or blunt trauma5–11) in which either no identifiable coagulopathy is present or in which all efforts to correct associated cellular and/or biochemical defects have failed to stop hemorrhaging. These “off-label” uses of rFVIIa will be the focus of this discussion.
A review of the medical and scientific literature up to March 2006 yields a total of 948 publications that list rFVII in the title or as a key word. The original clinical description of the use of the molecule as therapy of a hemophilic inhibitor patient was by Dr. Ulla Hedner in 1983.12 Of all manuscripts about rFVIIa published since, over half (483) describe off-label uses of rFVII. This is more than all pre-clinical studies, mechanisms of action studies and clinical uses in hemophilic inhibitors and FVII deficiency combined. Such magnitude is indicative of both the perceived need for a universal hemostatic by physicians across clinical specialties and a paucity of definitive randomized prospective clinical trials (RCT) that prove efficacy of rFVIIa for treating bleeding in patients with these myriad underlying diseases. Some limited number of RCTs (including placebo-controlled studies) have been performed. These investigations will be the primary focus of this review. In addition, strategies for positively influencing the appropriate use of rFVIIa among inexperienced clinicians of all specialties will be discussed in the context of reducing morbidity risks and maximizing the potential benefits of this costly resource. Initially, however, a brief discussion of the current knowledge of the role rFVIIa plays in initiating the procoagulant phase of hemostasis will be presented.
rFVIIa: Mechanisms of Action
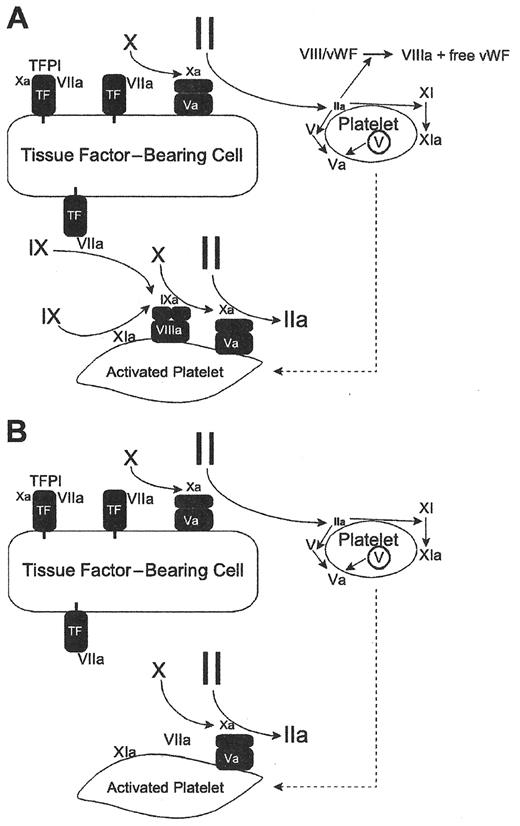
In vitro investigations by Roberts, Hoffman and Monroe have helped to elucidate the mechanisms of action in purified systems that mimic normal physiology or procoagulant deficiency states such as hemophilia A or B.13,14Figure 1a from the Blood paper of Roberts and Monroe shows the role FVIIa plays in initiating thrombin generation in patients who have a normal hemostatic function. In this context rFVIIa activates FXa in a tissue factor-dependent fashion.14 Physiologically, FVIIa represents approximately 1% of all circulating FVII. It is available to bind wherever tissue factor is expressed on activated inflammatory cells. (tissue factor [TF] upregulation is an NFκB-dependent process that results in expression and conversion to surface monomeric TF in response to cytokine stimulation of the inflammatory cell15–17.)
By contrast, superphysiologic levels of rFVIIa can overcome the drastically reduced rate of thrombin generation in patients with hemophilia A or B (Figure 1B ) independent of tissue factor.14 In the latter scenario very high plasma concentrations of infused rFVIIa can remarkably augment the slow rate of thrombin generation in a tissue-factor independent fashion on the surface of activated platelets, with amplified activation of FX. This may explain why enhanced dosing of rFVIIa (≥ 270 μg/kg) beyond recommended doses (90–120 μg/kg) for hemophilic patients with inhibitors has, in selected circumstances, resulted in enhanced hemostasis.18 (Nonetheless, it is notable that, in controlled studies of rFVIIa therapy of hemarthroses in hemophilia patients with inhibitors, this increased clinical response with very high doses (270 μg/kg) has not, in single dose administration, proven superior to sequential dosing of 90 μg/kg/ dose every 3 hours for 3 doses19.)
Most patients who are administered rFVIIa in off-label circumstances (with exception of those with acquired or inherited deficiencies/inactivations of other clotting factors besides FVII, FVIII or FIX) have inherent capacity to generate physiologic amounts of thrombin by Xa conversion of prothrombin in a tissue-dependent fashion. Naturally when acquired causes for procoagulant deficiencies coexist (e.g., liver disease), this may not be true.20–23 However, in the latter case where there is depletion of most procoagulant proteases along with FVIII and/or fibrinogen, it is unlikely that administration of rFVIIa alone will enhance hemostasis. Of course, replacement of these other factors, especially fibrinogen, is requisite for improved clotting even when high concentrations of FVIIa are administered.24,25 Accordingly, without replacement of these deficient procoagulant factors, hemorrhage persists despite rFVIIa administration. This will be emphasized during the subsequent discussion of how to maximize the potential benefits of rFVIIa for use outside of labeled indications.
By contrast, there are clinical circumstances (e.g., spontaneous intracranial hemorrhage [ICH]) where no underlying coagulopathy exists; yet bleeding occurs. In these circumstances, infused rFVIIa in a supraphysiologic dose may work in both a TF-dependent and a TF-independent manner, creating an extraordinarily large thrombin burst.14 If all the activated platelets are at the site of the ICH, the clinical result may be quite beneficial. However, if there are activated platelets elsewhere (e.g., a rupturing atheromatous plaque), the thrombin excess may potentially induce morbid thrombogenicity (e.g., myocardial infarction [MI]). Since TF upregulation on inflammatory cells in response to injury is, along with platelet activation, one way that the hemostatic response is localized, in circumstances like spontaneous ICH (with normal underlying thrombin generation capacity) dosing may need to be primarily targeted to enhance TF-dependent thrombin generation. Conversely, augmenting TF-independent thrombin generation by dramatically increasing rFVII dosing may increase the risk for thrombotic complications.5 This possibility will be discussed within the context of the recently reported ICH trial in the following section.
Experience with Off-Label Use of rFVIIa
To date, prospective randomized trials proving efficacy of off-label use of rFVIIa in securing hemostasis have been few. Initially such studies reported clinical endpoints such as reduction of acute blood loss since they were underpowered to address mortality or severe long-term morbidity endpoints.26 The first published randomized placebo controlled study indicating a reduction in bleeding with rFVIIa was in a cohort of patients undergoing prostatectomy.27 The hypothesis tested (for which the study was adequately powered) was whether a single dose of either 20 μg/kg or 40 μg/kg rFVIIa would reduce transfusion requirements compared to the placebo arm. Recombinant FVIIa infusion significantly reduced the volume of PRBC transfusions required to replace post-operative hemorrhaging, from a mean of 2500 mL to approximately 1000 mL and 800 mL for 20 μg/kg and 40 μg/kg rFVIIa, respectively (P = 0.001).
A similar design with a non-morbidity/mortality endpoint was employed in the first randomized prospective, placebo-controlled trauma trial.28 Among patients with blunt trauma, the percentage requiring ≥ 20 units of packed red blood cells (PRBC) was approximately 10% (n = 52) in the rFVIIa arm compared to approximately 20% (n = 59) in the placebo arm (P = 0.019). However, in a comparably sized and powered cohort no significant reduction in PRBC requirements was demonstrated for patients experiencing penetrating trauma.
A recent large phase II rFVIIa study in intracerebral hemorrhage was the first randomized, multidosed, placebo-controlled trial to approach a significant reduction in mortality (n = 399).29 The two higher of the three randomized doses of rFVIIa (80 μg/kg and 160 μg/kg but not 40 μg/kg) reduced 90-day mortality from 29% to 18% (P = 0.02), a 38% reduction. In addition, the highest dose arm significantly had a lower hematoma volume at 24 hours compared to the placebo arm. Notably, however, the incidence of morbid thromboembolic events was increased more then threefold (7% vs 2%) in the rFVIIa arms compared to the placebo-treated group. Whether this is some way related to enhanced TF-independent thrombin generation is conjecture. However, as discussed previously, patients with spontaneous ICH presumably have intact and normal TF-dependent rFVIIa capacity to generate thrombin with normal activation of tenase (rFVIIa-TF-Xa) and normal levels of factor V, prothrombin and fibrinogen. It is possible that in the ICH circumstances that the better clinical hemostasis (and perhaps the greater hypercoagulability as well) may involve enhancement of both TF-dependent and TF-independent pathways; however, more pre-clinical experimentation is required to determine whether there is relevance in vivo to this ex vivo mechanistic explanation.
Another RCT of rFVIIa in a non-hemophilia/non-FVII deficient cohort was reported recently by Pihusch et al.30 In this trial 100 patients who had undergone a hematopoetic stem cell transplantation (HSCT) with moderate or severe bleedings were randomized to receive either 7 doses of rFVIIa at 40, 80 or 160 μg/kg per dose) or placebo every 6 hours. The primary efficacy endpoint was the change in a pre-established bleeding score between first administration and hour 38. No significant reduction in bleeding was demonstrated. A post-hoc analysis indicated that the 80 μg/kg dose group but not the 160 μg/kg did show a reduction in the score but this did not translate into fewer transfusions. A total of 6 thromboembolic events were reported: all were the rFVIIa treatment arms. Events were too few to demonstrate a dosage effect. Compared to the ICH RCT, participants in this HSCT trial likely were more heterogeneous. This may have obscured a clinical effect or rFVIIa. However, it may also have obscured a dose-effect for thrombo-embolic risk as well. This is particularly relevant since patients post-HSCT may be at particular higher risk for thrombotic complications.31
A non-placebo controlled RCT has also been reported for prophylaxis against bleeding following percutaneous liver biopsy for advanced liver disease. Hemostasis was described as adequate during and following the procedure.32 Similarly a European placebo-controlled RCT study of 245 cirrhotic patients with upper GI bleeding demonstrated less bleeding from varices among patients with the most severe liver disease.28,33
Lodge et al have reported results from a double-blind placebo-controlled multicenter study of rFVIIa in 204 patients undergoing partial hepatectomy. Less perioperative blood loss was observed with rFVIIa, but the study was underpowered to show a morbidity/mortality endpoint.34
Risks for Thromboembolic Complications
The risks of a potential interaction between rFVIIa and either the acquired coagulation diverse diseases or between rFVIIa and other potentially procoagulant therapies for these disease states has not been adequately evaluated with regard to thromboembolic potential in preclinical or clinical studies. As the Mayer ICH study implies, these concerns are not trivial.29 Over the last decade, rFVIIa infusions in congenital/acquired hemophilia and FVIIa deficiency have produced quite rare TE events.35 Through 2003 there had been 18 specific thromboembolic events in patients with hemophilia reported after more than 400,000 doses.36 Several of these have occurred when rFVIIa has been combined therapeutically or in close time approximation with other procoagulant replacement products (e.g., activated prothrombin complex concentrates). By contrast, other than the Mayer study and selected case reports, the risk for TE associated with rFVII off-label use is not well characterized at the present. A recent study by O’Connell et al examined the number of TEs reported to the US FDA over a 5-year period (1999–2004).37 These included TE occurrence of subjects on a rFVIIa clinical trial as well as spontaneous post-licensure serious adverse event (SAE) reports from patients in the clinical setting. The arterial spectrum of TE events comprised 54% of the reports and included non-ICH strokes (21%), MIs (21%), and other arterial TEs (18.6%). The venous TEs (40.4%) included deep venous thromboses (23%), pulmonary embolism (17.5%), and device associated thrombi (5.5%). Most notably, the rate of spontaneous TEs reported has increased nearly 7-fold (from 5 to 35 per year) over a 5-year period during which the aggregate use of rFVIIa in hemophilia with inhibitors and Factor VII deficiency likely did not increase substantially. By contrast, reports from US hospital pharmaceutical purchasing consortia indicate an exponential increase in rFVIIa use for surgical hemostasis and other off-label use (University Hospital Consortium—Blood Products Survey). Hence, it is reasonable to conclude that the increase in TEs reported by O’Connell is related to off-label uses of rFVIIa. Importantly the reported rFVIIa-associated TEs have been associated with a similar striking increase in reported deaths over the period.37 These realities and associated cost considerations have led a number of healthcare institutions to examine the risk benefit of off-label use of rFVIIa.38 Examples of strategies to address this are discussed in the following section.
Monitoring/Controlling Access to Use of rFVIIa for Off-Label Use
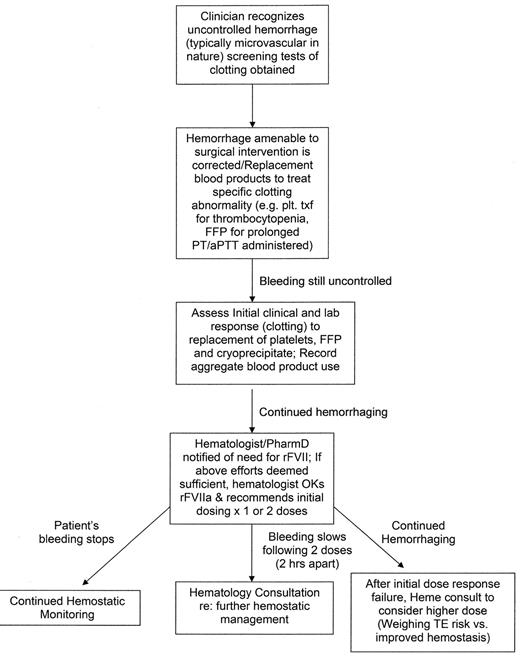
As the preceding review of off-label uses of rFVIIa implies, choosing an effective dose for the myriad of diseases and diverse therapeutic scenarios is a challenge (Figure 2 ). Many of the previous descriptions of use for such indications have been case reports and have chosen dosing predicated on the hemophilia experience.26,39 Examples include use of rFVII in FXI deficiency with or without an associated anti-FXI antibody.40 However, in vitro data suggest that one half of the hemophilia A/B inhibitor dosing may be sufficient.41 The implied higher risk for thrombotic complications in the off-label setting should enjoin clinicians treating these patients to establish realistic risk:benefit assessments. The latter should engender cautious dosing strategies commensurate with the perceived balance between excessive bleeding and risk for thromboembolic events. Maintaining such a delicate balance for diverse clinical bleeding circumstances requires expertise in hemostasis as well as a broad experience in rFVIIa use. This argument serves as the basis for developing strategies in the hospital setting to influence off-label use of rFVIIa.
As discussed, with the exception of the randomized clinical trials of rFVIIa in ICH, prostate surgery and the other limited settings, there is little evidence from randomized controlled trials (or even well-controlled trials) for rFVII efficacy.26 Further, at a cost of ≥ $1.00 per μg of rFVIIa (with dosing ranging from 1.2 mg to 9.6 mg in some adult off-label uses) indiscriminate use can impact significantly on health resource allocation in a hospital or care setting.38 One study calculated that the cost of each dose averaged $6805.38 This becomes potentially magnified when repeated dosing occurs in the context of marginal therapeutic effect. It is for all these reasons that hematologists, doctors of pharmacy, blood banking specialists, and pharmacy and therapeutics committees have recently joined forces in some institutions to limit off-label access to rFVIIa to situations deemed appropriate by designated expert “gate-keepers.”38
In order to provide a valid “gate-keeper” function for multiple clinical services that may identify individuals with life-threatening bleeding who may potentially benefit from off-label rFVIIa, certain criteria should be met: 1) a knowledge of rFVIIa pharmacology, physiology in hemostasis and its development for use in acquired and congenital hemophilia; 2) a knowledge of alternative therapies (e.g., prothrombin complex concentrate use in warfarin reversal); 3) broad expertise to provide hemostasis consultation to the requesting clinical service (e.g., surgery, medicine); and 4) specialized knowledge of confounders in the bleeding patient (e.g., liver disease versus consumptive coagulopathy, TF-dependent versus TF-independent rFVIIa activity), coagulation diagnostic capabilities and expertise in interfacing with the dispenser of the product (pharmacy or blood bank). Such expertise will help to ensure that each off-label use of rFVII follows a considered, cautious evaluation of the patient and his/her clinical situation in the context of therapeutic index of rFVIIa.
Several overriding principles should apply to implementation of an institutional “gatekeeper.” Specifically, off-label use of rFVIIa is as an adjunct therapy that is administered only after standard hemostatic maneuvers and medications have been employed unsuccessfully (with the exception of situations such as ICH where RCTs have demonstrated benefit for such patients). Further, the requesting physician should be a senior staff/faculty care-provider of the bleeding patient. Longitudinal laboratory assessment of the bleeding should be ongoing with the almost universally available screening tests of coagulation (i.e., PT, aPTT, platelet count, fibrinogen and as measure of fibrin cross-linking such as D-dimer/fibrin degradation product). For trauma patients warming strategies should have been implemented. For all hemorrhaging patients efforts to restore depleted procoagulant proteins and platelets should be ongoing in a strategy to improve both the clinical bleeding and the abnormal laboratory parameters. Dosing of fresh frozen plasma to replace procoagulants and anticoagulants can be maximized in the setting of severely critical patients by limiting maintenance replacement fluids and replacing them mL for mL with fresh frozen plasma.42 The sum of these therapies should be provided to the hemato-logic “gatekeeper” at the time rFVIIa is requested. Certainly cryoprecipitate (or fibrinogen concentrates if available) should be given to correct hypofibrinogenemia levels < 100 mg/dL since thrombin bursts initiated by rFVIIa therapy require fibrinogen substrate in order to form a clot. Additionally, every effort should be made to achieve a platelet count > 50,000/μL since rFVIIa activates FX best on the surface of activated platelets and the latter serve to localize the thrombin burst—perhaps decreasing thromboembolic risk.
Once a decision is made to administer rFVIIa, specific coagulation monitoring should continue. In particular, since rFVIIa does generate thrombin serial monitoring for DIC (using the above global tests) is prudent. In addition, both clinical response (attenuation of hemorrhage) and scrutiny for drug-related toxicity should continue—at least after every 2 doses. Because most off-label uses will be somewhat empirical, a commitment to greater than 2 doses (given 2–3 hours serially) should not be made until these careful assessments have been made. Dosage recommendations for off-label uses for which specific data on safety and efficacy are wanting may need to be adjusted based on the results of the screening laboratory tests (both pre- and post-initial rFVIIa as well as the observed clinical response.
At our institution, Memorial Hermann Hospital (the primary teaching hospital for the University of Texas Medical School at Houston), we instituted such a “gatekeeper” function for off-label uses of rFVIIa approximately 5 years ago.43 The number of off-label requests has increased from 16 in 2002 to 88 in 2006. Since we are also one of only two level 1 trauma centers in the metropolitan Houston area with large stroke and cardiovascular surgery centers, we have had 54, 59 and 42 requests for rFVIIa to treat bleeding during and after trauma, neurosurgery, and heart surgery, respectively, since 2002. A total of 203 off-label uses have been approved during this timeframe and 114 (56%) of patients receiving the drug survived their disease or injury. Approximately 60% of all requests have been for either trauma (CNS injury and systemic injury) or perioperative bleeding associated with cardiovascular surgery. A case control comparison between similar bleeding patients in whom rFVIIa was or was not utilized is planned, and data on thromboembolic events is being tallied.
In summary, the complex interplay between inflammation and coagulation in very ill, hemorrhaging patients makes it very difficult to predict the toxicity of rFVIIa when it is used in disease and injury states which exhibit “ramped up” inflammation. Therefore, a clear estimate of inflammatory and procoagulopathic upregulation in individual bleeding patients along with precision timing and dosing of rFVIIa in such situations will likely enhance clinical outcome. To date, the methodology for achieving such precision in the use of this drug is wanting, making an accurate determination of risk:benefit for use of rFVIIa in such settings presently difficult, if not impossible. Only continued randomized clinical trials of rFVIIa across the spectrum of nonhemophilic/non-FVII deficient disease states will provide the essential data to optimize the contribution of rFVIIa as a marginal versus more complete universal hemostatic agent.
Models of coagulation and hemophilia.
Reprinted with permission from Roberts HR, et al. Blood. 2004;104:3858–3864.
Models of coagulation and hemophilia.
Reprinted with permission from Roberts HR, et al. Blood. 2004;104:3858–3864.