Abstract
Immune (or idiopathic) thrombocytopenic purpura (ITP) is commonly encountered by the practicing hematologist. Clinical management decisions have traditionally been guided by individual training and past experience. Input from the literature has been more from observational reports of case series than from scientific results of hypothesis-driven research. Practice guidelines and several surveys of clinical hematology practice have highlighted important questions in the field, and in the past 5 to 10 years both clinical and laboratory investigations have produced valuable new information. Thrombopoietin levels are normal or only slightly increased in ITP, and stimulation of thrombopoiesis appears to be a promising new therapeutic approach in clinical trials. Chronic, refractory ITP in children or adults remains a challenge for the hematologist. It is this group that has the greatest risk of serious bleeding, particularly among the elderly. The anti-B–cell monoclonal antibody, anti-CD20, has shown benefit in phase I/II clinical trials in patients who had failed a number of previous therapeutic modalities. The standard for clinical research into therapy for ITP has become evidence-based medicine, and more prospective, randomized clinical trials are being completed by multi-institutional study groups.
Ten years ago the American Society of Hematology published immune (or idiopathic) thrombocytopenic purpura (ITP) practice guidelines based upon a systematic and comprehensive review of the literature.1 The authors acknowledged that hematologists regularly faced clinical management decisions with too little published scientific information about the natural history of ITP. Many of the treatments employed in ITP had been discovered empirically, and therapeutic choices were often guided by published results in case series of selected patients without a control group. Today, a decade later, we benefit from work by many investigators addressing the natural history of ITP and the pathogenic mechanisms contributing to autoimmune platelet destruction. Practice guidelines and a number of expert and consensus opinions in the literature have better defined the field.2–6 The recent era has also brought reports of new prospective clinical trials including multicenter, randomized studies that promise better guidance for the clinical hematologist in the 21st century.
Recent clinical investigations of ITP assess outcome measures in addition to platelet count increments including quality of life,7 severity of bleeding,8 and complications of therapy. In addition, there are published reports of systematic analyses that help interpret previous literature. Hypothesis-driven studies of ITP patients and experimental animal models have also improved our understanding of the basic cellular and molecular immune mechanisms that contribute to ITP and have thus revealed new therapeutic opportunities.
The present contribution will cover some of the recent basic research that has advanced our understanding of the pathogenesis of ITP and will highlight some of the clinical trials that offer insights into the diagnosis, natural history, and management of ITP in adults and children. Promising new therapeutic modalities for chronic refractory ITP will be discussed. Emphasis is placed upon explicitly designed trials. Several important clinical topics could not be included: secondary ITP, immune thrombocytopenia in pregnancy and infancy, the role of viral infection in ITP, and drug-related thrombocytopenia. Space limitations and editorial requirements also direct that the focus is on work published in the past 5 years, making it impossible to cite many valuable contributions made to this field.
Natural History of ITP and Clinical Outcome Measures
Several prospective studies and cooperative registries have provided further information on the natural history of ITP. The natural history of ITP in childhood has been studied collaboratively for a number of years. The first Intercontinental Childhood ITP Study Group (ICIS-I) enrolled more than 2000 children.9 Initial study questions focused upon demographic data, recent infections, platelet counts, initial management, and status 6 months after diagnosis, the time at which a normal platelet count without therapy defined a diagnosis of acute ITP. The presenting features of 2031 children from 38 countries enrolled by 209 participating physicians from 136 institutions from 1997 to 2000 included a peak age of 1–5 years, presenting platelet count < 20,000/μL, and slightly more boys (54.8%) than girls (45.2%). A seasonal fluctuation for a diagnosis of ITP was noted, with a peak in the spring and early summer and a nadir in the winter. The ICIS investigators found a higher than predicted rate of persistent thrombocytopenia at 6 months (31%), and an earlier UK study had reported that 22% of 427 children with ITP remained thrombocytopenic at 6 months.10 Longer follow-up of the ICIS-I cohort published in 2006 suggests that a 6 month definition of chronic ITP may be too restrictive, since 25.6% of their 308 chronic ITP patients achieved a normal platelet count between 6 and 12 months after diagnosis.11 Adult subjects are included in the current Pediatric and Adult Intercontinental Registry on Chronic ITP (PARC-ITP), and the UK ITP registries also enroll patients of all ages.
The findings of a number of studies of the natural history and complications of ITP in adults have been published recently. Portielje et al12 followed 152 consecutive adults with ITP managed at a single European center. In their series, 9% of patients were refractory to standard management in that they failed to maintain a platelet count > 30,000/μL; this group had a 4.2% mortality rate over a 2-year period, four times that of the general population. Several authors have reported a trend toward older age of ITP patients at diagnosis. A Danish group reported on a cohort of ITP patients > 15 years old from 1973 to 1995.13 The annual incidence of adult ITP in that population was 3.2 cases per 100,000 per year (1.94 per 100,000 for people < 60 years of age and 4.62 per 100,000 for the > 60 year age group). The median age of patients in the Danish study was 56.4 years, but there was a greater age at presentation in the second half of the study. In 2003, Neylon et al14 published findings from a prospective study of 245 adult ITP patients in a Northern England health care region. They found a median age at diagnosis of 56 years and noted an increased incidence in the > 60 year age group. Cohen et al15 performed a systematic analysis of 17 previously published case series that included 1917 adults with ITP; calculated age-adjusted mortality rates and loss of quality-adjusted life years (QALYs) indicated that a 30-year-old woman with persistent, severe thrombocytopenia (< 30,000/μL) would lose 20.4 years of life expectancy and 14.9 QALYs. The projected rate of fatal bleeding varied considerably with age—0.004, 0.012, and 0.130 events per patient-year for patients who are < 40, 40–60, and > 60 years of age, respectively. Elderly patients seem to be at greater risk for fatal hemorrhage, although further studies are needed to determine the true incidence of serious bleeding. This group is also more likely to have co-morbidities that might increase the risk of ITP therapies. Many randomized clinical trials in the present era are wisely including important outcome measures other than platelet count such as quality of life, assessment of bleeding, and cost analysis for the management approaches being compared.
Not all clinical series of ITP patients have reported race or ethnicity among the demographic data for the population, but it has recently been noted that African Americans seem to be underrepresented among patients diagnosed with ITP.16 If confirmed, this observation may suggest unknown genetic factors that are protective against platelet autoimmunity.
Thrombopoiesis in ITP
For more than 50 years, the underlying problem in ITP has been recognized as immune platelet destruction, and it was assumed that thrombopoiesis was maximized in response to the increased platelet clearance. The platelet count in any state is determined by a balance between platelet destruction and the rate of platelet production. It has now been shown that the rate of thrombopoiesis in ITP is inadequate to offset the increased rate of platelet destruction. In fact, platelet production may actually be decreased in ITP. Circulating and tissue-bound thrombopoietin (TPO) or megakaryocyte-derived growth factor (MGDF) levels in ITP are normal or only slightly increased in ITP in contrast to the elevated levels found with thrombocytopenia due to bone marrow failure. Using a liquid culture system, Chang et al17 studied TPO-driven megakaryocyte growth in vitro in the presence of anti-GP antibody–positive plasma from children with ITP and found that antiplatelet antibodies—particularly anti-GPIb—interfered with megakaryocyte proliferation. Experiments using CD34+ human hematopoietic stem cells in culture and plasma from adults with chronic ITP also inhibited megakaryocyte production.18
By analogy with autoimmune neutropenia, growth factor stimulation of megakaryopoiesis might be expected to increase the platelet count in patients with ITP. Indeed, clinical trials of investigational thrombopoietic agents report promising responses in adults with chronic ITP refractory to other treatments. Agents under investigation include PEG-recombinant human MDGF and the thrombomimetic agent AMG 351.19–20
Platelet Phagocytosis
It is well accepted that autoantibody-mediated phagocytosis of platelets is involved in the pathogenesis of ITP. Human macrophages express several Fc receptors that bind IgG specifically (Table 1 ).21 FcγRI, the high affinity receptor, does not appear to play a role in ITP. Engagement of FcγRIIA on the surface of human macrophages by anti-GPIIb/IIIa–coated platelets triggers intracellular signaling through the tyrosine kinase, Syk, that leads to engulfment of the opsonized platelets. FcγRIIB shares considerable homology with FcγRIIA, but lacks the intracellular immunoreceptor tyrosine activation motif (ITAM) domain; instead it promotes inhibitory signaling upon binding of the Fc portion of an immune complex.
Antiplatelet Antibodies in ITP
The most commonly identified antigenic targets of the platelet autoantibodies in ITP are GPIIb/IIIa and GPIb/IX, and a number of ITP patients have circulating or platelet-bound antibodies directed to multiple platelet antigens.22 Historically, assays for antiplatelet antibodies have not proven to be pathognomonic tests for a diagnosis of ITP, and their routine use has not been recommended by practice guidelines. In particular, routine measurement of platelet-associated IgG is rarely indicated due to the low specificity of an abnormal result and the variability of commercials tests of antiplatelet antibodies. However, some glycoprotein-specific antigen capture assays have demonstrated superior sensitivity and specificity and may be useful to support a diagnosis of ITP in cases with atypical presentation or clinical course.23 The presence of antiplatelet glycoprotein antibodies has also been shown to correlate with a need for more intensive therapy and also with increased frequency of hospitalization for bleeding.24
Antibody-mediated Platelet Destruction in ITP
Patients with ITP due to anti-GPIb antibodies have been reported by several groups to have special clinical manifestations. Early case reports that patients with anti-GPIb antibodies may be more refractory to therapy (particularly IVIg) suggested the possibility of direct cytotoxicity or complement fixation as a mechanism of platelet destruction rather than antibody-dependent, FcR-mediated phagocytosis by macrophages. In support of this hypothesis, recent studies by Webster et al25 using a murine model for ITP demonstrated that IVIg was unable to ameliorate the thrombocytopenia caused by most rat anti-mouse GPIb antibodies, although IVIg could reliably protect against anti-GPIIb/ IIIa–mediated thrombocytopenia. The therapeutic mechanism by which IVIg interrupts antibody-mediated platelet destruction has been reported to be related to increased expression of the inhibitory macrophage receptor, FcγRIIB, in animal models.26–27 However, the mechanism of therapeutic effect of IVIg in the clinical setting of human ITP is probably more complex and may include contributions from anti-idiotypic antibodies, FcR blockade, and alteration of autoantibody production and clearance.
Emergence of Antiplatelet Antibodies
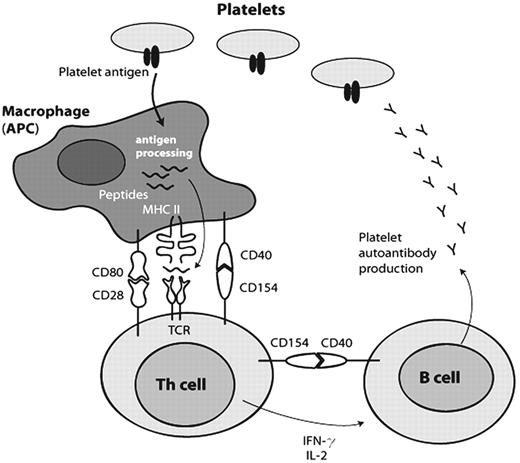
Emergence of antiplatelet autoantibodies requires a failure of the immunological tolerance for self antigens. The precipitating etiology for this loss of tolerance in ITP remains unclear. However, the pathogenic mechanism involves interaction between platelets, antigen-presenting cells (APCs), T cells, and B cells. As illustrated in Figure 1 , platelet proteins are cleaved to peptides by an APC and expressed on the APC cell surface via MHC class II molecules. The T-cell receptor (TCR) of the Th cell can then bind the peptide-MHC complex and signal activation that upregulates CD154 (CD40 ligand) to interact with CD40 on the APC and cause additional costimulatory interactions to occur. The activated Th cell produces cytokines that promote B-cell differentiation and antibody production.
Ongoing interaction between T cells and B cells through CD40-CD40L (CD154) is necessary to maintain active platelet autoimmunity. It has been recently reported that platelets themselves express CD154 and may thus play a more active role in the autoimmune process than simply bearing antigens and accepting a fate of destruction.28
A number of investigators have studied the pattern of cytokine production in ITP. While there are conflicting results, most reports favor the interpretation that adult chronic ITP represents a predominantly Th1 type immune response characterized by production of interleukin-2 (IL-2) and interferon-γ (IFNγ). Conversion to a more Th2-like response after therapy has been associated with remission.
Attempts to Interrupt Platelet Autoimmunity
The CD40-CD154 axis is an attractive therapeutic target for patients with refractory chronic ITP, and monoclonal antibodies against CD154 have undergone clinical trials in ITP. Although encouraging increases in platelet counts were seen, unacceptable adverse events (thromboembolism) resulted in premature termination of the trial.29 The immunosuppressive effect of interrupting CD40-CD154 interactions would not be expected to be specific for platelets, but further dissection of the cellular and molecular events contributing to ITP should reveal new immunosuppressive avenues that could be specific for platelet autoimmunity. Not all cases of ITP respond to the usual first- or second-line therapies. It is likely that heterogeneous immune mechanisms contribute to some cases of ITP. Recently Olsson et al have documented evidence for direct T cell–mediated cytotoxicity against platelets in chronic ITP.30
Clinical Management of ITP in the 21st Century
Acute ITP
Currently there is no single optimal management for the child who is newly diagnosed with ITP. Most hematologists in the United States choose to treat a child with a platelet count <10,000/μL or with mucous membrane bleeding, although observation and education are appropriate for the child with mild thrombocytopenia and no clinical bleeding. Initial treatment options for childhood ITP include IVIg, anti-Rh immunoglobulin (“anti-D”), steroids (oral or IV), or combination therapy, and randomized trials do not support a single therapy for all clinical situations. In a 2001 survey pediatric hematologists in the US were queried about their usual management of a child with newly diagnosed ITP and severe thrombocytopenia. When the responses were compared to those from a similar survey performed in 1997 more hematologists reported “observation only” as their preferred management (from 16% to 29%.)31 (See Table 2 , which includes contrasting treatment preferences of UK/European pediatric hematologists.) If treatment was recommended for the hypothetical patients in the US surveys, there was a striking preference for anti-D in 2001 (from 10% to 33%), reflecting the rapid acceptance of anti-D in the late 1990s. There have been recent case reports of severe, even fatal, hemolysis32 and renal failure33 as a complication of anti-D. However, at present the actual frequency of these rare side effects is not known, and the medication has been well tolerated by the great majority of ITP patients. It is recommended that patients treated with anti-D be monitored for unexpectedly severe hemolysis and that Rh(D)-negative red blood cells be chosen if erythrocyte transfusion is necessary.
Intermittent treatment with IVIg or anti-D can be used to minimize the risk of bleeding due to severe thrombocytopenia while awaiting a spontaneous remission. This approach is common for children, many of whom will remit over time, and one single-arm prospective trial suggested that 40% of adults who failed an initial course of steroids could avoid splenectomy by intermittent treatment with anti-D.34 A multi-institutional, randomized trial was conducted to compare “standard management” (oral steroids followed by early splenectomy) to aggressive anti-D for adults with ITP.35 In this trial, treatment with anti-D was able to delay, but not avoid, splenectomy. However, the intensive anti-D regimen (given at intervals of 4 ± 1 days) was found to be too onerous for many patients and their clinicians, resulting in numerous protocol violations.
Splenectomy for ITP
The spleen is a major site for antiplatelet antibody production as well as destruction of opsonized platelets. Surgical splenectomy, the first curative treatment for ITP, usually results in a remission and is generally considered second-line therapy for adults with ITP after the use of steroids. Splenectomy is typically postponed for at least 6 to 12 months in children, management that is well supported by data from studies of the natural history of ITP. Results of 47 published series of splenectomy in 2599 adults with ITP reveal a response rate of 66% with a median follow-up of 29 months as reported recently in the analysis of an exhaustive systematic review by George et al.36
Chronic refractory ITP
Patients who fail to maintain a platelet count above 30,000/μL after 6 to 12 months of standard therapy are often defined as refractory to therapy; these cases present particularly challenging management decisions for the hematologist. Numerous single-agent and combination immunosuppressive treatments have been reviewed elsewhere. Design of prospective, randomized clinical trials has been particularly difficult, since this group is a small subset of ITP patients (9% in one study)12 and there is considerable clinical variability within the same diagnostic group. Collaboration between institutions toward completion of randomized controlled trials has been facilitated by creation of the NIH Transfusion Medicine and Hemostasis (TMH) network, which is expected to tackle important questions in this area.
Anti-CD20 antibody off-label use in ITP
Case reports and clinical trials of the humanized anti-B–cell monoclonal antibody, anti-CD20 (Rituximab), in patients with chronic refractory ITP have had encouraging results. Given the key role for the humoral immune system in most cases of ITP, it was reasonable to hypothesize that anti-CD20 might offer a benefit. Indeed, more than 40 reports of off-label use of anti-CD20 in ITP have been published. In three large cohorts of adults who had failed multiple therapeutic modalities, patients were treated with the regimen of anti-CD20 used to treat B-cell lymphoma—375 mg/m2 weekly for 4 weeks.37–39 Approximately 50% of patients had a partial or complete response, and about 33% had durable remissions. Case reports have also documented remissions in children with refractory ITP, and a large (36 patient), multicenter, prospective phase I/II trial of anti-CD20 for children with ITP has been completed.40 A sustained platelet count above 50,000/μL was achieved by 31% of the subjects, and the anti-CD20 therapy was well tolerated. This pediatric study population had particularly severe ITP, which may explain why the number of responders was less than reported in the three large adult cohorts. The mechanism by which anti-CD20 leads to a remission of ITP may be through the elimination of B-lymphocytes necessary for autoantibody production. However, some patients experience early responses after only 1 week of the treatment course, raising the possibility that B cells opsonized by anti-CD20 may contribute to Fc receptor blockade.
Summary and Future Directions
The current century has brought a number of informative prospective clinical trials in ITP. Clinical studies as well as basic cellular and molecular investigations have also advanced our understanding of the pathogenesis of ITP. The NHLBI Transfusion Medicine and Hemostasis network has been established to rigorously assess therapeutic regimens. Large registries for children and adults with ITP continue to address important questions about natural history and patient management. In the future, new therapeutic strategies to specifically target the pathogenic mechanisms contributing to ITP are expected to emerge from active investigations—both in the laboratory and in the clinic.
Fc receptors for human IgG on macrophages.
| Human . | Affinity . | Subclass Specificity . |
|---|---|---|
| FcγRI | High | IgG1,3,4 |
| FcγRIIA | Low | IgG1,2,3 |
| FcγRIIB | Low | IgG1,2,3 |
| FcγRIII | Low | IgG1,3 |
| Human . | Affinity . | Subclass Specificity . |
|---|---|---|
| FcγRI | High | IgG1,3,4 |
| FcγRIIA | Low | IgG1,2,3 |
| FcγRIIB | Low | IgG1,2,3 |
| FcγRIII | Low | IgG1,3 |
Initial management of acute childhood immune thrombocytopenic purpura (ITP).*
| . | Observation Only . | Steroids . | IVIg . | Anti-D . | Other or Combination . |
|---|---|---|---|---|---|
| * See references 5, 9–11, 31 | |||||
| Registry Reports | |||||
| UK Survey 1997 n = 425 | 168 (40%) | 104 (24%) | 96 (23%) | 57 (13%) | |
| ICIS-I Registry 1997–2000 n = 303 | 117 (39%) | 90 (30%) | 82 (27%) | 3 (1%) | 11 (3%) |
| UK Survey 2000 n = 304 | 192 (63%) | NA | NA | NA | NA |
| Opinion Surveys | |||||
| 1997 ASPHO (hypothetical 5 year old with plt ct 3000) | 16% | 19% | 45% | 10% | 10% |
| 2001 ASPHO (hypothetical 5 year old with plt ct 7000) | 29% | 15% | 24% | 33% | |
| . | Observation Only . | Steroids . | IVIg . | Anti-D . | Other or Combination . |
|---|---|---|---|---|---|
| * See references 5, 9–11, 31 | |||||
| Registry Reports | |||||
| UK Survey 1997 n = 425 | 168 (40%) | 104 (24%) | 96 (23%) | 57 (13%) | |
| ICIS-I Registry 1997–2000 n = 303 | 117 (39%) | 90 (30%) | 82 (27%) | 3 (1%) | 11 (3%) |
| UK Survey 2000 n = 304 | 192 (63%) | NA | NA | NA | NA |
| Opinion Surveys | |||||
| 1997 ASPHO (hypothetical 5 year old with plt ct 3000) | 16% | 19% | 45% | 10% | 10% |
| 2001 ASPHO (hypothetical 5 year old with plt ct 7000) | 29% | 15% | 24% | 33% | |
Emergence of antiplatelet autoantibodies.
Platelet proteins are cleaved to peptides by an antigen-presenting cell (APC) and expressed on the APC cell surface via MHC class II molecules. The T-cell receptor (TCR) of the Th cell can then bind the peptide-MHC complex and signal activation that upregulates CD154 (CD40 ligand) to interact with CD40 on the APC and cause additional costimulatory interactions to occur. The activated Th cell produces cytokines (interleukin-2 and interferon-γ) that promote B-cell differentiation and antibody production.
Emergence of antiplatelet autoantibodies.
Platelet proteins are cleaved to peptides by an antigen-presenting cell (APC) and expressed on the APC cell surface via MHC class II molecules. The T-cell receptor (TCR) of the Th cell can then bind the peptide-MHC complex and signal activation that upregulates CD154 (CD40 ligand) to interact with CD40 on the APC and cause additional costimulatory interactions to occur. The activated Th cell produces cytokines (interleukin-2 and interferon-γ) that promote B-cell differentiation and antibody production.
Departments of Pediatrics and Internal Medicine, Yale University School of Medicine