Abstract
Autologous stem-cell transplantation is widely accepted as effective therapy for patients with relapsed aggressive B-cell non-Hodgkin lymphomas. Although 40–60% of younger patients with diffuse large cell lymphoma can expect to be cured, substantial numbers will experience a relapse. In addition, certain histologic subtypes are associated with particularly poor prognoses with combination chemotherapy alone (e.g., mantle cell lymphoma). Relatively few of these patients will experience long-term responses. Although other NHL subtypes are associated with more favorable prognoses in terms of overall survival, they are rarely cured (e.g., follicular lymphoma, chronic lymphocytic leukemia). Allogeneic transplantation has been increasingly utilized in patients with lymphoid malignancies but is associated with high toxicity. Recently, reduced-intensity conditioning regimens have shown encouraging results, attributed to graft-versus-lymphoma effects. This article discusses changes in the way autologous and allogeneic transplants may be carried out in the future to treat patients with lymphoid malignancies.
Recurrent non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL) have a poor prognoses with conventional chemotherapy.1,2 Intensive research with novel chemotherapy regimens has been conducted, but patients with recurrent disease typically have only a transient response to salvage therapies and ultimately die of their disease. Autologous and allogeneic blood and marrow transplantations have decreased morbidity and mortality rates as a result of improvements in stem cell transplantation (SCT) procedures and supportive care. As a result, there has been an increase in the use of SCT in lymphoid malignancies.
Improvements in SCT for NHL and CLL
Toxicity related to intense preparative regimens, graft-versus-host-disease (GVHD), and infections remain the main cause of failure after high-dose chemoradiotherapy and allogeneic SCT. The treatment-related mortality (TRM) rate of this approach has been substantial, with a 30–40% risk of death within 100 days of the transplantation. The use of recently introduced reduced-intensity and truly nonmyeloablative conditioning regimens has shifted some or all of the burden of tumor-cell kill from the conditioning regimens to the graft-versus-tumor effects. These regimens are less toxic than conventional regimens and allow for the treatment of older patients and patients with comorbid conditions.
The inclusion of targeted therapies that are focused on the recipient’s abnormal cells and hematopoietic system while sparing all other organs; better donor selection, with improvements in histocompatibility typing procedures; new prophylactic antifungal agents; and early detection of cytomegalovirus reactivation by sensitive methods have all contributed to the decreased TRM risk of allogeneic SCT.
Unlike allogeneic SCT, autologous SCT has been associated with a TRM of < 5% but has also been associated with a higher risk of relapse related to contamination of the graft and persistence of minimal residual disease due to the lack of graft-versus-lymphoma or -leukemia (GVL). Recurrences after autologous SCT are due to the treatment resistance of the lymphoma but may also result from infusion of occult lymphoma cells contained in the cell infusate. The observation that detection of minimal residual disease at the time of transplantation and during follow-up can be used to predict relapse3 has stimulated the development of other treatment strategies. New strategies designed to reduce the incidence of relapse after autologous SCT must take into consideration patients’ limited hematopoietic reserve during the early posttransplantation period. One commonly practiced approach to improving the results of autologous SCT without increasing toxicity is the incorporation of anti-B-cell monoclonal antibodies before, during, and after transplantation. The other approach is allogeneic SCT.
Role of the anti-CD20 antibody
SCT is no longer considered a single shot of high-dose chemotherapy. It is now an integral part of comprehensive programs, including intensive debulking, stem cell mobilization and harvesting, and stem cell autografting, with or without consolidation chemotherapy.
Rituximab is an anti-CD20 antibody chimeric molecule that significantly increases response rates when used in combination with standard or salvage chemotherapy to treat patients with B-cell lymphoid malignancies. It can be used as part of the cytoreductive salvage chemotherapy to attain a disease-free status before autologous SCT and as part of the mobilization protocol to provide an effective in vivo purging method prior to transplantation. Moreover, it can be used as posttransplantation immunotherapy in an attempt to eliminate residual disease and prevent relapse (Table 1 ).
In a recent study, concurrent administration of high-dose rituximab before and after autologous SCT for relapsed DLCL was associated with a significant improvement in both OS and DFS rates. Rituximab was administered during stem-cell mobilization (1 day before chemotherapy at 375 mg/m2 and 7 days after chemotherapy at 1000 mg/m2).4 A dose of 1000 mg/m2 was administered again on days 1 and 8 after transplantation. The OS and DFS rates were 80% and 67% at 2 years, significantly higher than the 53% (P = 0.002) and 43% (P = 0.004) observed in a historical control group. The efficacy of this strategy was also evaluated in FL.5 Twenty-one patients were included in the study. Most were experiencing their first chemosensitive relapse after their disease had failed to respond to conventional chemotherapy. With a median follow-up time of 34 months, the estimated OS and DFS rates at 3 years were both 84%, a significant improvement compared with a historical control group. The causes of failure were secondary leukemia (2 cases) and viral encephalitis; no patient experienced a relapse.
The use of rituximab in conjunction with nonmyeloablative regimens seems to be a promising approach to allogeneic SCT as well. Studies have shown that, by recruiting host immune effector mechanisms, rituximab may enhance an effective GVL effect after allogeneic SCT. Rituximab has been used with donor lymphocyte infusions (DLIs) after allogeneic SCT,6 inducing durable responses in patients experiencing relapse. Some studies have reported a lower incidence of GVHD in conjunction with the use of rituximab. The anti-CD20 radioimmunoconjugates Y-90 ibritumomab tiuxetan and I-131 tositumomab have been associated with high response rates, durable remissions, and limited toxicity, apart from myelosuppression, making them ideal candidates for use in transplantation.7,8 Radioimmunoconjugates have been used with various dosing strategies to replace total body irradiation in some studies and to augment standard high-dose chemotherapy regimens in others. Thus far, the results have been promising, with combinations of radioimmunoconjugates and chemotherapy producing long-lasting responses in high-risk patients with no more toxicity than that caused by standard conditioning regimens. Whether this increase in the radiation dose to the targeted lymphoma translates into more durable remissions and an improvement in OS requires further investigation.
Autologous versus Nonmyeloablative Allogeneic SCT: Disease-Specific Outcome
The use of autologous versus allogeneic SCT depends on several factors, including disease status, age, performance status, comorbidities, and donor availability. In addition, because NHL is a heterogeneous group of tumors with varied biologic behavior, the histologic tumor type is an important factor.
Diffuse Large Cell Lymphoma (DLCL)
Autologous SCT
The PARMA prospective randomized multicenter trial1 provided the strongest evidence supporting the use of autologous SCT over salvage chemotherapy in patients with chemoresponsive relapsed disease. A significant improvement in OS and progression-free survival (PFS) rates occurred with the addition of anti-CD20 antibody, making autologous SCT the treatment of choice for these patients. However, new treatment strategies are needed to improve the current outcome for patients in the poor-prognosis group and for those who experience a relapse after autologous SCT.
Allogeneic SCT
Overall, the use of allogeneic SCT for DLCL is still limited. Most patients with DLCL who are referred for stem cell allografting have generally poor prognostic features, refractory disease, or disease that has failed a prior autologous SCT, or are precluded from stem cell autografting because of difficulties with autologous stem cell collection. Comparative studies have shown a lower relapse rate and a longer DFS after allogeneic SCT than after autologous SCT.9 The high TRM rate often offsets any potential survival benefits. However, the response to DLI and withdrawal of immunosuppression in some patients lends credence to the existence of a GVL effect in DLCL.
One of the most compelling lines of evidence of a graft-versus-lymphoma effect is the success of allogeneic SCT after autologous SCT has failed to produce a durable response or the use of allogeneic SCT as consolidative therapy after autologous SCT.10,11 We studied 20 patients with recurrent NHL (10 with DLCL) after autologous SCT, who had undergone nonmyeloablative transplantation.10 After fludarabine-based conditioning, all patients experienced engraftment, and 19 (95%) experienced complete remission. The remaining patient had progressive disease at day +115, was treated with DLI, experienced GVHD, and achieved a complete remission at day +220. The estimated 3-year progression-free survival rate was 95%, although all patients included in the study had either chemosensitive disease or low-bulk disease at the time of relapse.
Branson11 reported a different outcome in 38 patients who had been treated with alemtuzumab, fludarabine, and melphalan and allogeneic SCT after their disease had failed to experience a durable remission to a prior autologous SCT. The OS and PFS rates at 14 months were 53 and 50%, respectively. However, a significant number of post-procedure relapses continued to occur, and there was no plateau on the PFS. Others have reported a 57% TRM rate after a BEAM (BCNU [carmustine], etoposide, cytarabine, melphalan)-alemtuzumab regimen in patients with NHL who had previously undergone stem cell autografting.12 The TRM rate was only 8% in those not previously treated with autografting. Thus, conflicting data exist regarding the efficacy of nonmyeloablative transplantation after autologous SCT, with more encouraging results seen in patients with chemosensitive disease and those treated with non-alemtuzumab-containing truly nonmyeloablative regimens.
Follicular Lymphoma
Autologous SCT
In the pre-rituximab era, there was little evidence that autologous SCT conferred any survival advantage compared with conventional treatment for follicular lymphoma (FL). Furthermore, the observed 5- to 15-fold increased incidence of secondary myelodysplasia13 was of major concern particularly for patients with indolent lymphoma, whose major benefit from autologous SCT was the prolongation of remission, not a cure. However, the outcome improved with the incorporation of monoclonal antibody within the treatment strategy, as described above,5 making the use of autologous SCT in FL a subject of increasing interest.
Choice of autologous versus allogeneic SCT
Conventional allogeneic SCT in FL has been found to be particularly effective in patients who experience a relapse and those with inadequate bone marrow reserve or massive bone marrow involvement.14 Nonmyeloablative conditioning regimens may offer similar results in chemosensitive patients, with less toxicity (Table 2 ).5,15,16 The risk of relapse after non-T-cell depleted nonmyeloablative transplants for FL has been 15% or less at 3 years.5,15 A recent report from the CIBMTR17 revealed that since 2000, nonmyeloablative transplantations have been more common than myeloablative transplantations for FL. Despite these improvements, the risk of other toxicities and chronic GVHD cannot be underestimated in patients with this indolent disease, complicating the choice of autologous or allogeneic SCT.
The approach used at M. D. Anderson Cancer Center for relapsed chemosensitive FL is to treat patients with nonmyeoablative allogeneic SCT if they have a matched sibling donor, especially if the patients are experiencing their second or later relapse, have undergone more than two lines of therapy, or have undergone a prior autologous SCT.5 Otherwise, patients with chemosensitive disease receive high-dose chemotherapy and autologous SCT with rituximab, before and after transplantation, as described for aggressive lymphoma.
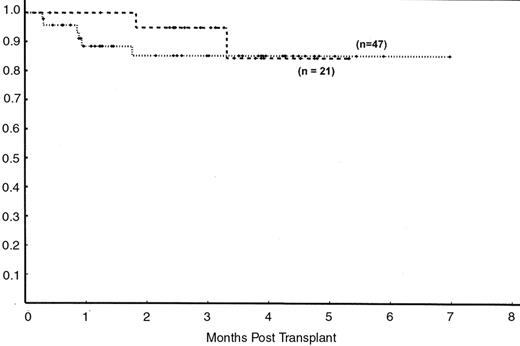
Between March 1999 and April 2005, 68 consecutive patients were treated at M. D. Anderson Cancer Center. Forty-seven underwent nonmyeloablative allogeneic SCT, and the other 21 underwent autologous SCT with rituximab. The median age of the patients (53 years), time from diagnosis to transplantation (3 years), and percentage of patients who were in partial remission (57%) at study entry were similar in both groups. There were no statistically significant differences in sex distribution, histologic subtypes of FL grades, history of bone marrow involvement, beta-2 microglobulin (β2-M), International Prognostic Index scores and LDH levels between the two. However, more patients in the allogeneic SCT group experienced > 1 relapse (38% vs 19%), or underwent > 3 lines of therapy (28% vs none). Eight patients (17%) in the allogeneic SCT group had disease that had experienced a relapse after a prior autologous SCT. With a median follow-up time of 34 months, the OS rates at 3 years were 88% and 84% for the allogeneic and autologous groups, respectively (P = 0.8). DFS rates and the risk of progression were comparable between the two groups (85% vs 84% and 3% vs 5%, respectively) (Figure 1 ). The DFS rate of the 8 patients who had undergone allogeneic SCT after failing a prior autologous SCT was 87% at 4 years. The causes of death varied between the autologous SCT (2 secondary leukemia and 1 viral encephalitis) and the allogeneic SCT (2 acute GVHD , 2 chronic GVHD , 1 unknown) groups. These data suggest that nonmyeloablative SCT has a safety spectrum that is comparable to that of autologous SCT and that the addition of rituximab appears to improve the outcome significantly after autologous SCT in patients with chemosensitive disease experiencing their first relapse.
Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) is now recognized as a distinct clinicopathologic subtype of B-cell NHL and continues to pose a significant challenge to oncologists. There is currently no standard therapy for newly diagnosed or relapsed disease. Unlike in DLCL, the addition of rituximab to the CHOP regimen does not improve the outcome.18 Romaguera et al19 recently reported a complete remission rate of 87% with the addition of rituximab to the hyper-CVAD regimen. The median follow-up time was 40 months, and the 3-year failure-free survival and OS rates were 67% and 81%, respectively. Age, β2-M, and gastrointestinal involvement were adverse prognostic factors. Although there was no plateau on the curves, the results were encouraging compared with historic controls.
Only randomized, prospective studies can determine whether survival is truly extended by the addition of rituximab to hyper-CVAD schedules. Furthermore, it should be noted that the toxicities associated with this therapy are clinically significant: 5% of patients died of toxicities, and 4 other patients had myelodysplastic syndrome or acute leukemia after treatment. Moreover, the continuing occurrence of relapses suggests that minimal residual disease persists and needs to be managed with additional therapy. The use of radioimmunoconjugates after hyper-CVAD with rituximab was discontinued prematurely at M. D. Anderson Cancer Center because of unacceptable early toxicity.
Autologous SCT
Strategies for consolidation with autologous SCT have been addressed in numerous studies. We have reported our experience with autologous SCT after 4 courses of the hyper-CVAD regimen.20 At a median follow-up of 49 months, the OS rate at 5 years was estimated to be 94% in the patients with a β2-M level of ≤ 3 mg/L compared with 34% (P = 0.005) in patients with levels of 3 mg/L at the time of transplantation. Only 1 trial has randomly assigned patients who had responded to CHOP-like chemotherapy to autologous SCT or interferon maintenance.21 The 3-year PFS rates were 54% and 25% for the autologous SCT and interferon arms, respectively (P = 0.01). The fact that patients who experienced a relapse on interferon could cross over to the transplantation arm complicated the OS analysis. It should be noted, however, that the outcome curves did not show a plateau, and there could be many reasons for that.
As mentioned earlier, the transplantation procedure is a complex strategy involving the pretransplantation cytoreductive therapy, stem cell collection with purging, and a conditioning regimen and maintenance therapy after transplantation. In a retrospective analysis, Vose et al22 reported an improved outcome in patients with MCL who received an autologous SCT consolidation after hyper-CVAD with or without rituximab rather than CHOP-like chemotherapy with or without rituximab. With a median follow-up of 38 months, the OS rates at 3 years were 97% and 68% for the hyper-CVAD and CHOP-like groups, respectively (P = 0.01), whereas the PFS rates were 78% and 55%, respectively (P = 0.05).
Current approaches to improve the preparative regimen for autologous SCT include adding rituximab, radioimmunoconjugates, or proteosome inhibitors. Gianni et al23 used rituximab as part of the preparative regimen for autologous stem cell collection. They treated 28 patients, and 24 remained in complete remission with a median follow-up time of 35 months. Mangel et al24 treated 20 patients with newly diagnosed MCL with CHOP followed by stem cell collection with rituximab. Patients underwent standard autologous SCT with chemotherapy conditioning, followed by rituximab maintenance. The PFS rate at 3 years was 89% for autologous SCT compared with 29% for matched historical controls; the OS rates at 3 years were 88% and 65%, respectively. Other studies incorporating either 131I-tositumomab, or 90Yttrium-ibritumomab tiuxetan, or bortezomib with chemotherapy and autologous SCT for patients experiencing first remission are in progress.
Allogeneic SCT
Allogeneic SCT has been reserved to treat patients with relapsed or recurrent disease.25,26 To date, this has been the only treatment capable of inducing long-term remissions in these patients. The demonstration of the GVL effect through observed responses to DLI is encouraging, particularly in conjunction with nonmyeloablative T-cell-depleted conditioning regimens.25
In a study at M. D. Anderson, 18 patients were treated with nonmyeloablative transplantation. Their median age was 56.5 years. Patients were heavily pretreated with a median of 3 prior chemotherapy regimens. Autologous SCT had failed to produce a response in 28% of patients. Most patients received a conditioning regimen containing fludarabine, cyclophosphamide, and rituximab. The PFS rate at 3 years was 82%.
Maris et al26 reported on 33 patients with relapsed and refractory MCL after nonmyeloablative conditioning with fludarabine and 2 Gy of total body irradiation. The median patient age was 53.5 years, and the median number of treatment regimens was 4. Forty-two percent of patients failed to experience a durable response to autologous SCT. The authors reported OS and DFS rates of 65% and 60%, respectively. Given that MCL is incurable with conventional chemotherapy and that no plateau exists in the survival curve after autologous SCT in patients with relapsed MCL, these preliminary results are encouraging.
Autologous versus Allogeneic SCT
In summary, in the absence of randomized trials, autologous SCT is recommended in patients with newly diagnosed MCL. Patients with relapsed or recurrent disease and some newly diagnosed patients with high-risk disease features, such as those with blastoid cytologic findings or elevated β2-M levels, should be considered for nonmyeloablative allogeneic SCT after cytoreductive therapy.
Chronic Lymphocytic Leukemia
Although B-cell CLL is a disease with an often indolent and prolonged clinical course, more than 90% of young patients die as a result of causes directly related to CLL.27 Important prognostic factors have been recently identified in patients with CLL, making it “1 diagnosis for different diseases.” The recent introduction of new drugs and antibodies has increased the rate of remissions.2 However, the quality of these remissions is unknown, because computed tomographic scans are not routinely used to assess response. In addition, the effect of these strategies on CLL cells with abnormal chromosomes, unmutated immunoglobulin variable heavy-chain gene status, or 70-kD zeta-associated protein (ZAP-70) expression is unknown. In a recent report from the German CLL group28 on patient outcome after fludarabine versus fludarabine plus cyclophosphamide, a 17p deletion, which predicts poor prognosis and resistance to treatment in CLL, was observed in 5 of 8 patients who did not experience a response to the combination drugs. Six of 7 nonresponders had unmutated immunoglobulin heavy-chain variable-region genes. Therefore, while those strategies may prolong survival in some patients with CLL, none of them has been curative, and better treatments are needed, especially for patients with aggressive subtypes.
Autologous SCT
Autologous SCT has been tested as a strategy to improve survival in patients with advanced CLL; however results have shown a constant rate of relapse (50% to 60% at 3 years), without suggestion of a cure.29 A 6-year PFS rate of 30% was also recently reported from the Dana Farber Cancer Center,30 where investigators studied patients early in the course of their disease, with a low tumor burden after in vitro purging with a cocktail of anti-B cell antibodies. Most patients had an unmutated immunoglobulin variable heavy-chain gene, by retrospective analysis. The 9% risk of myelodysplasia, in the absence of a plateau on the curves raises significant concerns about the use of autologous SCT in CLL.
Allogeneic SCT
Early experience with high-dose chemoradiotherapy and allogeneic SCT in patients with heavily pretreated disease demonstrated high non-relapse mortality rates (10% to 40%), but the suggestion of plateau on survival curves. Recent data available on 38 patients who had undergone transplantations from unrelated donors from the National Marrow Donor Program revealed a 5-year failure-free survival rate of 32%.31
Because CLL is generally a disease of older, often debilitated patients, only a minority have been considered for high-dose myeloablative therapies. A nonmyeloablative approach based on a combination of fludarabine and cyclophosphamide with rituximab has been reported.6 The study schema was designed to maximize the chance of GVL activity in those patients with persistent or progressive disease by early tapering and discontinuation of tacrolimus followed by rituximab and DLI. The initial results continue to be promising with more patients and a longer follow-up. Currently, 39 patients have been treated. The median age was 57 years (range, 34–70 years). The median time from diagnosis to transplantation was 4.5 years. All patients had recurrent advanced CLL and had been previously treated with fludarabine-rituximab-based regimens. Each patient had received 2 to 8 (median, 3) chemotherapy regimens. At the time of transplantation, 9 patients (23%) had evidence of Richter’s transformation. Thirty-four patients (87%) had active disease at the time of study entry, 16 (41%) had an elevated serum lactate dehydrogenase level, and 19 (49%) had an elevated beta-2 microglobulin of > 3 mg/L. Seven of 26 patients (27%) tested by either positron emission tomography or Gallium-67 scanning before transplantation had scanning avidity. Thirty-two patients underwent an allogeneic SCT from a human leukocyte antigen-identical donor, 3 from a matched unrelated donor, 1 from a phenotypically identical family member, 2 from a 1-antigen-mismatched sibling donor, and 1 from a 1-antigen-mismatched unrelated donor. Fourteen patients required immunomodulation with rituximab and DLI for persistent or progressive disease after transplantation. One patient died early. Of the 38 evaluable patients, 27 (71%) experienced a complete response by CT scans, bone marrow biopsies, and flow cytometry. Among the patients who achieved complete response, serial bone marrow specimens were available for 21 of 61 to assess the presence of clonal B-cells by polymerase chain reaction (PCR) technique for clonal CDRIII rearrangements. On the last analysis, PCR were results were negative after therapy for all these patients. The estimated OS and current PFS rates at 4 years were 48% and 44%, respectively. The incidence of acute grade II–IV GVHD was 45%, and the incidence of chronic extensive GVHD was 58%.
Sorror et al32 recently reported the outcome of 64 patients with advanced CLL who were treated with nonmyeloablative conditioning consisting of 2 Gy of total body irradiation with (n = 53) or without (n = 11) fludarabine from related (n = 44) or unrelated (n = 20) donors. The median age was 56 years, and the median interval between diagnosis and transplantation was 4.4 years. There was a median of 4 prior treatment regimens, and chemotherapy resistance to pretransplantation salvage treatment was present in 53% of patients. The incidence of acute II–IV GVHD was 61%. The 2-year estimated OS and DFS rates were 60% and 52%, respectively.
Allogeneic SCT and prognostic markers in CLL
To date, few data have addressed whether the potential GVL effect of allogeneic SCT can overcome the prognostic influence of the newer markers of adverse biologic disease characteristics in CLL. In a study by Moreno et al,33 the significance of an unmutated immunoglobulin variable heavy-chain gene in CLL patients undergoing myeloablative allogeneic SCT was addressed. The risk of relapse at 5 years in patients who underwent autologous SCT (n = 20) or allogeneic SCT (n= 14) were 66% and 17%, respectively (P = 0.01), suggesting that allogeneic SCT may overcome the unfavorable effect of unmutated CLL.
The effect of nonmyeloablative SCT is still unknown. To address this question, we retrospectively examined the ZAP-70 status of 39 patients with CLL who had undergone received nonmyeloablative SCT, as reported above.34 Using immunohistochemical techniques on bone marrow biopsies, we determined that 25 patients were ZAP-70 positive, 13 were ZAP-70 negative, and 1 was of indeterminate status. Patients who were ZAP-70 positive had a median age of 54 years. All had received prior fludarabine with or without cyclophosphamide, 44% had disease that had been exposed to prior therapy with alemtuzumab, 84% had been previously exposed to rituximab, and 31% had been treated with hyper-CVAD for rapidly growing bulky lymphadenopathy. With a median follow-up time of 41 months (range, 4–80 months), their OS and current PFS rates at 4 years were 56% and 53%, respectively. By multivariate analysis, chemorefractory disease at transplantation (P = 0.01) and mixed T-cell chimerism at day 90 (P = 0.02) but not ZAP-70 status were correlated with the risk of progression after transplantation. These results are promising an important role for immunotherapy in the management of this disease.
Issues Related to the Choice of Nonmyeloablative Transplants: Regimens, Disease Status, GVHD Prophylaxis and the Use of DLI
Numerous series have reported promising response rates to nonmyeloablative transplantations in patients with lymphoid malignancies. The current medical literature is limited, however, by the heterogeneity of nonmyeloablative transplantation regimens, the patients who receive them, and the current lack of randomized trials. Despite those limitations, some patterns of outcomes after nonmyeloablative and reduced-intensity approaches have become apparent.
Several conditioning regimens with reduced doses of chemotherapy have been developed. There has been wide variation in regimen components and intensity, GVHD prophylaxis, and the application of DLI. In addition, there is a marked heterogeneity of patients’ specific disease characteristics. The truly nonmyeloablative regimens have mainly been highly immunosuppressive. Examples include the cyclophosphamide and fludarabine regimen, low-dose TBI with or without fludarabine, and the total lymphoid radiation with thymoglobulin. Others have used more intense regimens, such as fludarabine and melphalan or the BEAM combination, to provide cytoreduction and allow donor cell engraftment. To date, there is no evidence to support the use of the more toxic reduced-intensity regimens in patients with chemosensitive disease. On the other hand, reduced-intensity regimens have been associated with significant mortality in patients who have undergone a prior autologous SCT, and should be avoided under these circumstances. Although some exceptions do exist, it should be noted that most regimens have appeared to be incapable of controlling chemorefractory disease. Chemorefractoriness should be currently viewed as the only indication for the use of high-dose myeloablative treatment and should be limited to young patients with good performance status.
The risk of GVHD after nonmyeloablative transplantations varies. However, the risk after reduced-intensity regimens appears to be comparable to be that of traditional myeloablative regimens, whereas conditioning with truly nonmyeloablative regimens such fludarabine and low-dose cyclophosphamide is more associated with mixed chimera and a comparatively low incidence of GVHD.5,15 Studies have shown that grade 2 to 4 acute GVHD had no significant impact on the risk of relapse or progression but was associated with increased risk of nonrelapse mortality. Conversely, patients with extensive chronic GVHD had been noted in some reports to be associated with a decreased risk of progression or relapse.35 An additional feature in some studies has been in vivo T-cell depletion for both host immunosuppression and GVHD prophylaxis. Although the risk of GVHD was low, T-cell depletion has generally been associated with a higher risk of relapse. Morris et al16 reported a relapse rate of 44% at 3 years in 41 patients with low grade lymphoma (follicular, n = 29; CLL, n = 9; and lymphoplasmacytoid NHL, n = 3) after conditioning with fludarabine, melphalan, and alemtuzumab (Table 2 ). All but 1 had chemosensitive disease, suggesting that T-cell depletion may have a negative effect on outcome, and one should be cautious about using this strategy.
The precise criteria for DLI administration are not always clear. Selected patients receive DLI for persistent disease or relapse, but DLI is commonly provided for mixed chimerism after nonmyeloablative SCT, even in the absence of measurable disease. This represents a high risk for GVHD and a major cause of mortality and morbidity after allogeneic SCT. We recently demonstrated that this practice may not be routinely needed in patients with FL who continue to have stable mixed chimera after non-T cell-depleted nonmyeloablative SCT, because we found it had no effect on the relapse rate and final responses, including the molecular ones.36 Whether this same strategy can be applied to other histologic types remains to be determined.
Summary
The current data collectively suggest that addition of anti-CD20 monoclonal antibodies significantly improves the outcome of autologous SCT in patients with NHL who have chemosensitive disease (Table 3 ). It is recommended for patients with DLCL or FL undergoing their first relapse and MCL during first remission, but not in patients with CLL. A significant GVL effect can be achieved clinically through the use of nonmyeloablative transplantation in patients with chemosensitive advanced disease. Yet the applicability of nonmyeloablative SCT appears dependent on disease histologic type, individual patient, and disease characteristics. The current data suggest an increased risk of relapse with the use of alemtuzumab. Randomized studies may allow the comparison of various nonmyeloablative transplantation strategies. High-dose conditioning regimens should be considered for patients with chemorefractory disease who are young and have a good performance status. The field offers significant opportunity for clinical research, and we strongly encourage participation in clinical trials whenever possible.
Rituximab in stem cell transplantation (SCT).
| Rituximab . | Purpose . |
|---|---|
| Abbreviations: DLI, donor lymphocyte infusion; EBV, Epstein-Barr virus; GVHD, graft-versus-host disease; GVL, graft-versus-lymphoma; MRD, minimal residual disease | |
| Cytoreduction for relapsed disease | Attain a state of minimal disease before SCT |
| In autologous SCT | |
| During stem cell harvest | In vivo purge |
| Maintenance after SCT | Treat MRD |
| In allogeneic SCT | |
| With conditioning regimen | Enhance GVL and tumor kill, decrease risk of GVHD |
| With DLI | Treat MRD, enhance GVL |
| Post-transplantation complications | |
| Chronic GVHD | Treat manifestations |
| EBV-associated lymphoma | Treat and prevent |
| Rituximab . | Purpose . |
|---|---|
| Abbreviations: DLI, donor lymphocyte infusion; EBV, Epstein-Barr virus; GVHD, graft-versus-host disease; GVL, graft-versus-lymphoma; MRD, minimal residual disease | |
| Cytoreduction for relapsed disease | Attain a state of minimal disease before SCT |
| In autologous SCT | |
| During stem cell harvest | In vivo purge |
| Maintenance after SCT | Treat MRD |
| In allogeneic SCT | |
| With conditioning regimen | Enhance GVL and tumor kill, decrease risk of GVHD |
| With DLI | Treat MRD, enhance GVL |
| Post-transplantation complications | |
| Chronic GVHD | Treat manifestations |
| EBV-associated lymphoma | Treat and prevent |
Outcome after nonmyeloablative stem cell transplantation (SCT) for follicular lymphoma (FL).
| Author . | No. of Patients . | Regimen . | Sibling/Unrelated . | Median Follow-up (months) . | Acute II–IV GVHD (%) . | Chronic GVHD (%) . | Relapse (%) . | PFSc (%) . |
|---|---|---|---|---|---|---|---|---|
| a 38 with FL, 5 small lymphocytic lymphoma, and 2 with marginal zone lymphoma | ||||||||
| b 29 with FL, 9 with CLL, and 3 with lymphomplasmacytoid NHL | ||||||||
| c Including responses post donor lymphocyte infusion after transplantation. | ||||||||
| Abbreviations: FCR, fludarabine, cyclophosphamide, rituximab; F/TBI, fludarabine, low-dose total body irradiation; FM, fludarabine, melphalan; NA, information not available. | ||||||||
| Khouri5 | 47 | FCR | 45/2 | 34 | 11 | 51 | 2 | 85 |
| Maris15 | 45a | F/TBI | 22/23 | 24 | 60 | 51 | 15 | 51 |
| Morris16 | 41b | FM/alemtuzumab | NA | 16 | 15 | 6.8 | 44 | 65 |
| Author . | No. of Patients . | Regimen . | Sibling/Unrelated . | Median Follow-up (months) . | Acute II–IV GVHD (%) . | Chronic GVHD (%) . | Relapse (%) . | PFSc (%) . |
|---|---|---|---|---|---|---|---|---|
| a 38 with FL, 5 small lymphocytic lymphoma, and 2 with marginal zone lymphoma | ||||||||
| b 29 with FL, 9 with CLL, and 3 with lymphomplasmacytoid NHL | ||||||||
| c Including responses post donor lymphocyte infusion after transplantation. | ||||||||
| Abbreviations: FCR, fludarabine, cyclophosphamide, rituximab; F/TBI, fludarabine, low-dose total body irradiation; FM, fludarabine, melphalan; NA, information not available. | ||||||||
| Khouri5 | 47 | FCR | 45/2 | 34 | 11 | 51 | 2 | 85 |
| Maris15 | 45a | F/TBI | 22/23 | 24 | 60 | 51 | 15 | 51 |
| Morris16 | 41b | FM/alemtuzumab | NA | 16 | 15 | 6.8 | 44 | 65 |
Autologous versus allogeneic stem cell transplantation (SCT) for lymphoid diseases.
| . | . | Allogeneic SCT . | |
|---|---|---|---|
| Disease . | Autologous SCT . | Nonmyeloablative . | High-dose Chemotherapy . |
| Abbreviations: DLCL, diffuse large-cell leukemia; FL, follicular lymphoma; MCL, mantle cell lymphoma; CLL, chronic lymphocytic leukemia | |||
| DLCL | Treatment of choice for first chemosensitive relapse |
|
|
| FL | First chemosensitive relapse |
|
|
| MCL | First remission |
|
|
| CLL | No defined role |
|
|
| . | . | Allogeneic SCT . | |
|---|---|---|---|
| Disease . | Autologous SCT . | Nonmyeloablative . | High-dose Chemotherapy . |
| Abbreviations: DLCL, diffuse large-cell leukemia; FL, follicular lymphoma; MCL, mantle cell lymphoma; CLL, chronic lymphocytic leukemia | |||
| DLCL | Treatment of choice for first chemosensitive relapse |
|
|
| FL | First chemosensitive relapse |
|
|
| MCL | First remission |
|
|
| CLL | No defined role |
|
|
Disease-free survival (DFS) after nonmyeloablative (• • •) versus autologous ( - - - - ) stem cell transplantation (SCT) for follicular lymphoma using rituximab-containing regimens.
Disease-free survival (DFS) after nonmyeloablative (• • •) versus autologous ( - - - - ) stem cell transplantation (SCT) for follicular lymphoma using rituximab-containing regimens.
Department of Blood and Marrow Transplantation, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030, USA