Abstract
Improvements in anticancer treatments, the ability to modify myelosuppression profiles, greater duration and intensity of immunosuppression, and the variety of available antimicrobial therapies have influenced the spectrum of pathogens associated with invasive fungal infection complicating treatment of hematological malignancies and hematopoietic stem cell transplantation. The approaches to the management of these infections encompass strategies of prevention for all those at risk, pre-emptive therapy based upon surrogates of infection before the onset of clinical disease, empirical therapy for patients with clinical evidence of early disease, and directed or targeted therapy for infected patients with established disease. Chemoprophylaxis is effective if applied to the highest risk patients over the duration of the risk. Pre-emptive strategies, while promising, have yet to be validated and linked to reliably predictive nonmicrobiological diagnostic techniques. Empirical antifungal therapy, as it is currently applied, now seems questionable. Patients with probable or proven invasive fungal infection still have suboptimal outcomes despite the availability of promising anti-fungal agents. Strategies examining the concept of dose-intensity and combination regimens require careful study and cannot yet be regarded as an acceptable standard of practice.
Changing Epidemiology of Invasive Fungal Infection in Hematological Malignancies and Stem Cell Transplants
Interest in the field of medical mycology has expanded considerably as the numbers of immuno- and myelosuppressed patients susceptible to opportunistic invasive fungal infections (IFI) increase. While the majority (95–97%) of invasive yeast infections are due to five species, predominantly Candida albicans, followed by C. tropicalis, C. glabrata, C. parapsilosis, and C. krusei, emerging importance of infections due to non-albicans Candida spp. including C. glabrata, C. parapsilosis, and C. krusei in high-risk cancer patients is noteworthy.1 The remaining 3% to 5% of pathogens include other non-albicans Candida spp.. and non-Candida yeasts such as Trichosporon spp., Cryptococcus spp., Blastoschizomyces spp., and Malassezia spp..2,3 The risks for IFI among hematopoietic stem cell transplants (HSCT) increases as the disparity of the donor:recipient pair (autologous HSCT, 0.6%; matched related donor HSCT, 3.7%; mismatched related or unrelated donor HSCT, 5.9%, P < 0.0001).4 The changing character of the HSCT populations together with effective anti-Candida agents contribute to a changing spectrum of opportunistic mycoses. In a comparison of transplant patients with IFI to a more general population of patients at risk, the proportion of Candida infections decreased by approximately 45% (77% to 42%); however, that due to Aspergillus spp. has more than doubled (13% to 29%) and that due to other environmental moulds including Fusarium spp., Scedosporium spp., the zygomycetes, and the less common dematiacious fungi, Trichoderma spp., Paecilomyces spp., and Scopulariopsis spp., more than tripled (4% to 14%).4,5
First-order risk groups for IFI include those with underlying conditions such as cancer or myelosuppression, those undergoing abdominal surgery, prematurity, and advanced age. Additional second-order risk factors applicable to these groups include indwelling vascular catheters, broad-spectrum antibacterial therapy, renal insufficiency, fungal colonization, prolonged ICU admissions, and parenteral nutrition.1 Among HSCT recipients, additional risk factors include conditioning regimen (myeloablative versus non-myeloablative); source of stem cells; donor:recipient disparity; graft-versus-host disease; augmented combination immunosuppression with agents such as calcinurin inhibitors, mycophenolate, corticosteroids and infliximab; prolonged severe neutropenia; and environmental exposures.
Even though the event rates for these infections are low by comparison to bacterial diseases, the mortality rates are disproportionately high. Higher clinical indices of suspicion, improved diagnostic imaging techniques, and molecular diagnostic strategies have improved our ability to identify patients at risk for these diseases.3,6–8
Diagnostic criteria have been developed to provide consistency in the conduct of clinical trials of antifungal therapy9; however, clinicians have been discouraged from using these criteria in clinical circumstances.10 The availability of newer antifungal agents with differing mechanisms of action has encouraged their application to a spectrum of clinical circumstances. This review is intended to highlight some aspects of treatment of invasive fungal infection (IFI) important to the management of patients with hematological malignancies and stem cell transplant (SCT) and to expand upon several previous excellent reviews presented at the American Society of Hematology.11,12
The Continuum of Antifungal Therapy: Prophylactic, Pre-emptive, Empiric, or Directed
An individual patient may be eligible for one or more of these approaches at different times during the trajectory of the course of treatment for the underlying disease. Prophylactic therapy targets a population of patients at risk for IFI over a defined period of risk, but who do not yet have evidence of either infection or clinical disease. Pre-emptive or presumptive therapy targets a population of patients with evidence of IFI based upon a surrogate marker such as an antigen or genomic detection test, but without evidence of clinical disease. Empiric antifungal therapy, on the other hand, may be regarded as a variant of pre-emptive therapy where persistent neutropenic fever despite broad-spectrum antibacterial therapy is considered the surrogate marker of IFI. Directed or targeted therapy applies to patients with evidence of infection and clinical disease. It remains unclear whether the array of available imaging and molecular diagnostic techniques should be applied for screening purposes to identify high-risk patients eligible for pre-emptive therapy or for non-culture–based diagnosis in the presence of signs and symptoms suggestive of invasive fungal infection.
Antifungal Chemoprophylaxis
What are the current chemoprophylaxis practices?
Table 1 details the proportions of centers in the US, Japan, and Europe reporting the administration of antifungal prophylaxis with fluconazole or extended-spectrum mould-active azoles in high-risk patients between 2001 and 2005. Most HSCT centers in these regions report using antifungal chemoprophylaxis, fluconazole still being the most commonly prescribed. Of note, however, the higher proportion of European centers reporting the administration of mould-active azoles in 2005 reflects the growing concern for invasive mould infections.
Fluconazole and itraconazole have been administered over a range of oral doses (50–400 mg and up to 10 mg/kg/d, respectively); however, evidence suggests that among highest risk patients higher doses are more effective.13,14 The ability to switch from the oral to an intravenous formulation for the same antifungal product in the setting of severe oral mucositis is an advantage. Prophylactic voriconazole in allogeneic HSCT recipients (oral or IV 200 mg BID) is currently under investigation. Two large randomized, fluconazole- or itraconazole-based controlled clinical trials in acute leukemia patients15 and in HSCT recipients with graft-versus-host disease16 have demonstrated the efficacy of oral posaconazole (200 mg thrice daily) for reducing invasive mould infections. Prophylactic micafungin17 and caspofungin18 have been studied at daily IV doses of 50 mg.
When should antifungal prophylaxis begin and end?
Table 2 shows the distribution of reported start and end dates for antifungal prophylaxis reported in the literature.13,15,16,19–23 Prophylaxis should usually be initiated in parallel with the administration of cytotoxic therapy in order to ensure a protective effect at the time of maximal neutropenia and intestinal epithelial damage. Concerns over drug interactions have compelled some investigators to modify the application of triazoles-based prophylaxis until after the administration of cytotoxic therapy.15,16,21 The end date should be dictated by the termination of the specific risk. Mould-active prophylaxis may require administration into the late post-engraftment period in allogeneic HSCT for those patients with higher risk due to acute or chronic graft-versus-host disease requiring augmented immunosuppressive therapy.16,21
How effective are these strategies?
Several systematic reviews evaluating the published clinical trials of antifungal prophylaxis efficacy are available for review.24 Moreover, two recent large randomized controlled trials have been published in abstract form15,16 demonstrating treatment effects for a variety of clinically important outcomes, including use of empirical antifungal therapy for persistent neutropenic fever, mucosal colonization, superficial fungal infection, proven IFI, IFI-related mortality, and all-cause mortality (particularly in subsets of patients with prolonged severe neutropenia13).
Pre-emptive Antifungal Therapy
The interest in this strategy is based upon the observation that early detection is associated with better outcomes. A multicenter randomized German study compared preemptive and empirical antifungal therapy with liposomal amphotericin B among allogeneic HSCT recipients receiving fluconazole prophylaxis25 based on serial serum PCR fungal DNA detection studies. The pre-emptive group received more antifungal therapy than the empirical therapy group (109 of 196, 56%, vs 76 of 207, 37%, respectively, P < 0.001). Although the numbers of documented IFI were similar (5.6% versus 7.7%, P = 0.396, respectively), the 30-day mortality was reduced by 67% in the pre-emptive group (2.0% versus 6.3%, P = 0.034).
A second study in acute leukemia patients receiving fluconazole prophylaxis examined an algorithm-based preemptive approach based upon serial diagnostic testing and clinical monitoring.26 Only patients with ≥ 2 positive serum galactomannan assays or CT and or bronchoscopic evidence for mould infection received antifungal therapy. A total of 41 of 117 febrile neutropenic episodes (35%) had persistent neutropenic fever; however, only 9 patients (22% of the 41 persistent neutropenic fevers; 8% of the original febrile neutropenic episodes) satisfied the pre-defined criteria for antifungal therapy—a 78% relative risk reduction in antifungal therapy use.
Despite these promising observations, the appearance of a clinical or radiological marker such as a suggestive nodular pulmonary infiltrate on computerized thoracic tomography in a high-risk patient will compel the anxious physician to initiate antimould therapy independent of molecular markers. Pending validation in larger randomized studies, these important studies not only argue the feasibility of the pre-emptive approach but also challenge the empirical approach as a standard of practice.
Empirical Antifungal Therapy
During the early 1980s one quarter to one third of severely neutropenic cancer patients with persistent or recrudescent fever despite receiving broad-spectrum antibacterial therapy developed IFI. Empirical amphotericin B deoxycholate reduced the incidence of IFI and overall mortality by 50%–80% and 23%–45%, respectively. The early seminal reports have become the rationale for the current standard of practice despite the lack of statistical robustness in the original observations for defervescence and survival (P = 0.08 and P = 0.23, respectively).27
Fever is a poorly predictive surrogate upon which to base an intervention such as empirical antifungal therapy.28 Between 35% to 69% of leukemia patients and 56% to 82% of HSCT receive empirical antifungal therapy, yet proven IFI occurs in only 2% to 15%,13,15,16,29 suggesting that the current guidelines30 based upon persistent neutropenic fever are significantly flawed and may result in unjustifiable excess treatment-related toxicities and resource expenditures.28
Directed Antifungal Therapy
Invasive Candidiasis
Myeloid recovery is an independent predictor of outcome among patients with Candida spp. bloodstream infections. Very few studies of invasive candidiasis (IC), particularly bloodstream infections, include data on neutropenic patients.
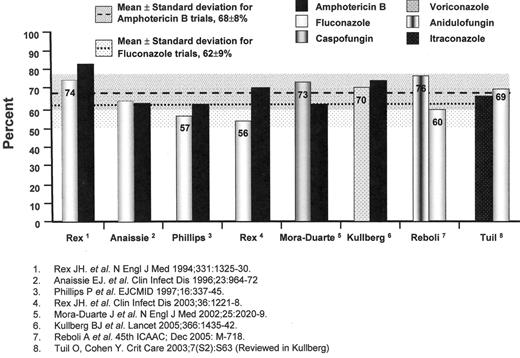
The results of individual trials have suggested that fluconazole at daily doses of 400 milligrams and amphotericin B deoxycholate at daily doses of 0.5 to 0.6 milligrams per kilogram are similarly effective in non-neutropenic patients; however, a pooled analysis of randomized-controlled trials comparing the efficacy of fluconazole and amphotericin B deoxycholate for IC suggests the possible superiority of amphotericin B (62% of 345 patients versus 70% of 343 patients, χ2 = 5.206, P = 0.023, OR 1.47, 95%CI 1.07 to 2.03) (Figure 1 ),31–34 despite the greater amphotericin B-related renal toxicity noted in previous pooled analyses (OR 3.20, 95%CI 1.61 to 5.01).35
Voriconazole, an extended-spectrum azole, offers a safe and effective alternative to amphotericin B-based regimens for the treatment of IC36( Figure 1 ). Itraconazole has been recommended for maintenance treatment and secondary prophylaxis of invasive aspergillosis, the primary treatment of dimorphic fungal infections, and for the empirical treatment of persistent neutropenic fever, but not for primary treatment of invasive aspergillosis (IA) or IC.37
The echinocandin antifungal agents offer yet another alternative to either the azole- or amphotericin B-based regimens.38,39 These cyclic hexapeptide agents inhibit the enzymatic biosynthesis of 1,3-beta glucans in the fungal cell wall of Candida spp., Aspergillus spp., Pneumocystis jiroveci, but not Cryptococcus neoformans or the zygomycetes. A study of caspofungin and amphotericin B deoxycholate in 224 patients with IC suggested a trend favouring caspofungin (73.4% of 109 vs 61.7% of 115, OR 1.71, 95%CI 0.97 to 3.01)40 (Figure 1 ); however, more caspofungin recipients had persistently positive blood cultures with C. parapsilosis (P = 0.02). Another echinocandin, anidulofungin, was found to be superior to fluconazole for IC (75.6% vs 60.2%; difference 15.4%, 95%CI, 3.85 to 26.99, P = 0.01)41(Figure 1 ). A third echinocandin agent, micafungin, is also under study. The safety, efficacy, and availability of these products have diminished the role of polyenes as initial therapy for IC.
Invasive aspergillosis
Primary therapy
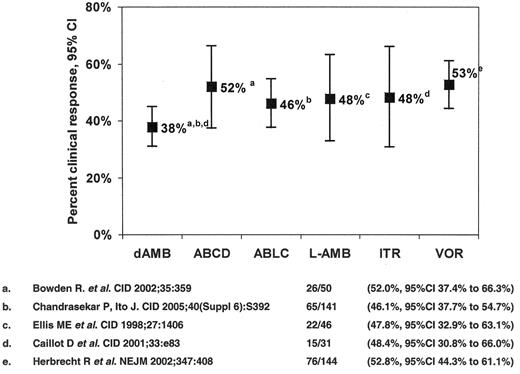
The only antifungal agents approved for the treatment of IA are conventional amphotericin B deoxycholate (CAB) and voriconazole, the response rates for the former being of the order of 1 in 3. The seminal study comparing voriconazole to CAB in 277 patients42 demonstrated higher response rates among voriconazole recipients (52.8% versus 31.6%), a 67% improvement (Figure 2 ). Patients with early lesions characterized by pulmonary nodules with halos had higher treatment response rates (52.4% versus 29.1%).43 Moreover, a survival advantage for voriconazole recipients was observed (70.8% compared to 57.9%, a 22% improvement (χ2 = 5.063, P = 0.024)). Despite this, response among allogeneic HSCT recipients remained suboptimal (32.4% for voriconazole versus 13.3% for CAB). A study of dose-intense liposomal amphotericin B (10 mg/kg/d for 14 days followed by 3 mg/kg/d vs 3 mg/kg/d ) as primary therapy for IA demonstrated similar response rates (46% vs 50%, respectively), but more nephrotoxicity (31% versus 14%), hypokalemia (30% versus 16%), and higher mortality in the dose-intense group (41% versus 28%, OR 0.55, 95%CI 0.33 to 0.99).44 Based upon this experience, the value of dose-intensity for IA appears limited.
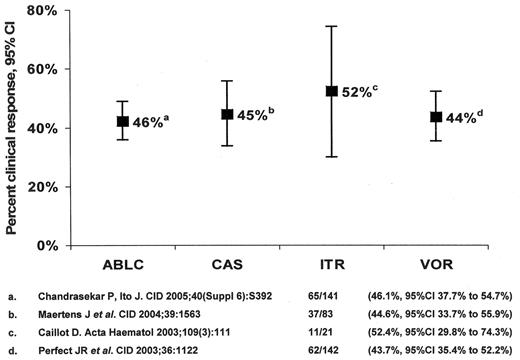
Salvage monotherapy
Combination therapy
Poor response rates for primary and salvage monotherapy therapy for IFI and the availability of agents with differing mechanisms of action have fueled recent reviews.49–52 Arguments for considering combination therapy include enhanced fungal killing (synergy), an enhanced spectrum of activity, prevention of development of resistance, and reduction of drug-related toxicities.50 Response rates from retrospective reports in heterogeneous patient populations have been inconsistent.45,53,54 Favorable responses were observed among HSCT patients failing polyene-based therapy for invasive aspergillosis with a combination of voriconazole and caspofungin compared to voriconazole monotherapy.55 Only one recent randomized-controlled trial in IC suggested that a combination of fluconazole and amphotericin B deoxycholate may have advantages over fluconazole monotherapy.34 A preliminary report on a French multicenter randomized study comparing combination therapy with liposomal amphotericin B (3 mg/kg/d) plus caspofungin (70 mg day 1 and 50 mg/d thereafter) versus high-dose liposomal amphotericin B monotherapy (10 mg/kg/d) for primary treatment of invasive aspergillosis was recently presented.56 A favorable overall response was observed in 67% (95%CI 38% to 88%) combination therapy recipients compared to 27% (95%CI 8% to 55%) high-dose therapy recipients (P = 0.028). The results of this small pilot, representing the first prospective study of combination therapy in IA, are encouraging but need confirmation.
Combination antifungal therapies are expensive and potentially toxic and, despite some recent encouraging reports, there are limited well-designed randomized-controlled trials to guide the practicing clinician faced with managing these problems.
Non-Aspergillus mould infections
These have been recently reviewed2,57,58 and include infections due to Fusarium spp., Scedosporium spp., the Zygomycetes, the dematiacious (dark-walled) fungi, Trichoderma spp., Paecilomyces spp., and Scopulariopsis spp. The extremely poor outcomes of infections due to the first three, the most commonly reported pathogens, have been largely a function of selection bias of most profoundly immunosuppressed patients and of reduced antifungal susceptibility profiles to conventional antifungal agents.58 Voriconazole has been effective for infections due to Fusarium spp. and Scedosporium apiospermum.48,59 While concerns have been raised with regard to increased use of voriconazole and risk for Zygomycete infections,59,60 a cause-and-effect relationship remains unproven. Moreover, the rising incidence of Zygomycoses antedated the introduction of this agent into clinical practice.61 Outcomes of posaconazole therapy, an extended-spectrum azole, appear to compare favorably with more standard lipid-based amphotericin B in the treatment of zygomycete infections.62
Azole-based antifungal prophylaxis in high-risk patients by region and type of agent (fluconazole versus mould-active extended-spectrum azoles).
| . | USA,* 2001 . | Japan,** 2001 . | Europe,† 2005 . | |||
|---|---|---|---|---|---|---|
| . | FLU . | ESA . | FLU . | ESA . | FLU . | ESA . |
| * Trifilio S et al. (A survey of 31 transplant centers in the United States encompassing over 3400 transplants performed in 2001. Almost all (93%) reported using some form of antifungal prophylaxis). Bone Marrow Transplant . 2004 ;33 :735 –9 | ||||||
| ** Imataki O et al. (A survey among 63 transplant centers encompassing 48% of all HSCT performed in Japan in 2001. All (100%) patients undergoing autologous, myeloablative and nonmyeloablative HSCT received antifungal chemoprophylaxis). Bone Marrow Transplant . 2004 ;33 :1173 –9 | ||||||
| † Round Table, European Conference on Infections in Leukemia, 14th Symposium on Infections in the Immunocompromised Host, Crans-Montana, Switzerland, July 2–5, 2006. | ||||||
| Abbreviations: Auto HSCT, autologous hematopoietic stem cell transplant; Allo HSCT, allogeneic hematopoietic stem cell transplant; FLU, fluconazole; ESA, extended-spectrum mould-active azoles (itraconazole, voriconazole, posaconazole). | ||||||
| Auto HSCT | 84% | 3% | 72% | 18% | 53% | 36% |
| Allo HSCT | 67% | 13% | 79% | 4% | 57% | 33% |
| Acute leukemia | — | — | — | — | 55% | 35% |
| . | USA,* 2001 . | Japan,** 2001 . | Europe,† 2005 . | |||
|---|---|---|---|---|---|---|
| . | FLU . | ESA . | FLU . | ESA . | FLU . | ESA . |
| * Trifilio S et al. (A survey of 31 transplant centers in the United States encompassing over 3400 transplants performed in 2001. Almost all (93%) reported using some form of antifungal prophylaxis). Bone Marrow Transplant . 2004 ;33 :735 –9 | ||||||
| ** Imataki O et al. (A survey among 63 transplant centers encompassing 48% of all HSCT performed in Japan in 2001. All (100%) patients undergoing autologous, myeloablative and nonmyeloablative HSCT received antifungal chemoprophylaxis). Bone Marrow Transplant . 2004 ;33 :1173 –9 | ||||||
| † Round Table, European Conference on Infections in Leukemia, 14th Symposium on Infections in the Immunocompromised Host, Crans-Montana, Switzerland, July 2–5, 2006. | ||||||
| Abbreviations: Auto HSCT, autologous hematopoietic stem cell transplant; Allo HSCT, allogeneic hematopoietic stem cell transplant; FLU, fluconazole; ESA, extended-spectrum mould-active azoles (itraconazole, voriconazole, posaconazole). | ||||||
| Auto HSCT | 84% | 3% | 72% | 18% | 53% | 36% |
| Allo HSCT | 67% | 13% | 79% | 4% | 57% | 33% |
| Acute leukemia | — | — | — | — | 55% | 35% |
Reported initiation and cessation times for antifungal prophylaxis by patient risk group.
| Patient Group . | Start Date . | End Date . |
|---|---|---|
| * Day of sustained absolute neutrophil count > 0.5 × 109/L | ||
| Abbreviations: Auto HSCT, autologous hematopoietic stem cell transplant; Allo HSCT, allogeneic hematopoietic stem cell transplant. | ||
| Acute leukemia | Day 1 of induction therapy | Myeloid reconstitution* |
| Auto HSCT | Day 1 of conditioning therapy | Myeloid engraftment* |
| Allo HSCT | Day 1 of conditioning therapy Transplant day 0 Transplant day 1 | Myeloid engraftment* Protocol specified day (+75, +100, +120, +180) Cessation of immunosuppressive therapy |
| Patient Group . | Start Date . | End Date . |
|---|---|---|
| * Day of sustained absolute neutrophil count > 0.5 × 109/L | ||
| Abbreviations: Auto HSCT, autologous hematopoietic stem cell transplant; Allo HSCT, allogeneic hematopoietic stem cell transplant. | ||
| Acute leukemia | Day 1 of induction therapy | Myeloid reconstitution* |
| Auto HSCT | Day 1 of conditioning therapy | Myeloid engraftment* |
| Allo HSCT | Day 1 of conditioning therapy Transplant day 0 Transplant day 1 | Myeloid engraftment* Protocol specified day (+75, +100, +120, +180) Cessation of immunosuppressive therapy |
Treatment trials of invasive candidiasis. Overall success at end-of-treatment. Also shown are the mean success rates (shaded areas represent the standard deviation of the mean) for trials with amphotericin B deoxycholate and fluconazole arms for comparison.
Treatment trials of invasive candidiasis. Overall success at end-of-treatment. Also shown are the mean success rates (shaded areas represent the standard deviation of the mean) for trials with amphotericin B deoxycholate and fluconazole arms for comparison.
Antifungal therapy of invasive aspergillosis in myelosuppressed and immunosuppressed cancer patients. Clinical response to primary therapy.
Abbreviations: dAMB, amphotericin B deoxycholate; ABCD, amphotericin B colloidal dispersion; ABLC, amphotericin B lipid complex; L-AMB, liposomal amphotericin B; ITR, itraconazole; VOR, voriconazole.
Antifungal therapy of invasive aspergillosis in myelosuppressed and immunosuppressed cancer patients. Clinical response to primary therapy.
Abbreviations: dAMB, amphotericin B deoxycholate; ABCD, amphotericin B colloidal dispersion; ABLC, amphotericin B lipid complex; L-AMB, liposomal amphotericin B; ITR, itraconazole; VOR, voriconazole.
Antifungal therapy of invasive aspergillosis in myelosuppressed and immunosuppressed cancer patients. Clinical response to salvage monotherapy.
Abbreviations: ABLC, amphotericin B lipid complex; CAS, caspofungin; ITR, itraconazole; VOR, voriconazole.
Antifungal therapy of invasive aspergillosis in myelosuppressed and immunosuppressed cancer patients. Clinical response to salvage monotherapy.
Abbreviations: ABLC, amphotericin B lipid complex; CAS, caspofungin; ITR, itraconazole; VOR, voriconazole.
Sections of Infectious Diseases and Haematology/Oncology, Professor and Head, Section of Haematology/Oncology, Department of Internal Medicine, The University of Manitoba; Head, Department of Medical Oncology and Haematology, Director, Infection Control Services, CancerCare Manitoba, Winnipeg, Manitoba, Canada