Abstract
The mature T/natural killer (NK) lymphoma/leukemias represent 5–15% of all non-Hodgkin lymphoma. These diseases have a geographic variation, with more nodal disease in North America and Europe, including peripheral T cell lymphomas, unspecified, anaplastic large cell lymphoma, and angioimmunoblastic T cell lymphoma; and more extranodal disease in Asia due to Epstein-Barr virus–related nasal NK/T lymphoma and human T-cell leukemia virus (HTLV)-1–associated adult T cell leukemia/lymphoma. The prognosis in most peripheral T/NK neoplasms is poor, with 5-year survival less than 30%. Progress has been slow due to the rarity of the diseases, geographic variation, relative chemoresistance, and lack of randomized trials. There is no consensus about optimal therapy in T/NK neoplasms, and recommendations are based on anecdotal reports, small series, and phase II trials. In this review, topics include the question of CHOP as standard therapy, prognostic factors, disease-adapted therapy, novel approaches, monoclonal antibody therapy, and stem cell transplantation.
Therapeutic advances in peripheral T/natural killer (NK) neoplasms have been slow due not only to difficulty in diagnosis but also to early disagreements about the importance of immunophenotyping. The Working Formulation (WF) devised in the 1980s failed to recognize the B and T cell origins and grouped together disorders of different biologies. Present day classification identifies disease entities based upon the cell of origin by immunophenotyping along with clinical, morphologic, and genotypic data when available. Despite improvements in diagnosis, survival has remained less than 30% for most types of peripheral T/NK neoplasms (Table 1 ).
Clinical factors of T/NK neoplasms impeding progress in therapy include the rarity of the diseases, geographic variation, relative chemoresistance, absence of a common marker for monoclonal antibody therapy, and lack of randomized trials. In the Western hemisphere, nodal diseases tend to predominate, with peripheral T cell lymphoma, unspecified (PTCL/U), anaplastic large cell lymphoma (ALCL), and angioimmunoblastic T cell lymphoma (AITL) as the more common subtypes.1,2 In Asia, extranodal disease, particularly of the nasal type, which is associated with Epstein-Barr virus, is more common; and adult T cell leukemia/lymphoma (ATLL) is present where HTLV-1 is endemic. ALCL associated with anaplastic lymphoma kinase (ALK) expression is considered chemosensitive, while other systemic PTCL are relatively chemoresistant and have an inferior prognosis compared to diffuse large B cell lymphoma.3,4
There is no consensus about the optimal therapy for T/ NK neoplasms, although recent reviews provide suggestions that are based on results of anecdotal reports, small series, or phase II trials.5,6 Another vexing problem is clinicopathologic dissociation where the pathology may appear aggressive but the clinical course is indolent. This disparity is common in T/NK lymphomas that have cutaneous only involvement, such as primary cutaneous ALCL, primary cutaneous small or medium-sized PTCL,7 and subcutaneous panniculitis-like PTCL (SCPTCL), although the latter often has a fulminant course when associated with hemophagocytosis.8
Questions to address regarding therapy for T/NK neoplasms are: 1) Should CHOP chemotherapy be the standard for therapy? 2) Are there clinical or tumor factors that can predict prognosis and influence type or intensity of therapy? 3) What are the features of specific disease entities that warrant disease-adapted strategies? 4) Are there novel approaches and agents that can improve outcome? 5) Can patients be selected for either dose-intensive regimens or early stem cell transplantation (SCT)?
CHOP as Standard?
The intergroup trial in the United States comparing CHOP to three newer regimens established CHOP as the standard therapy for WF intermediate-grade lymphoma, which presumably included subsets of PTCL. The complete remission rate of T/NK lymphomas to CHOP is highly variable, but the 5-year survival is less than 30% except in ALK-positive ALCL and some types with low bulk, limited-stage disease. Adding etoposide to CHOP or shortening the cycle to 2 weeks is uncertain to have a significant improvement in PTCL.
More intensive regimens such as those utilized in Europe (LNH programs) and MD Anderson (HyperCVAD) have not shown a clear benefit in PTCL,4,9 although a randomized trial in poor-prognosis elderly patients with different types of aggressive lymphoma did favor the more intensive ACVBP over CHOP despite greater treatment-related mortality.10 Risk stratification that utilizes more intensive regimens as adverse features increase in number has been successful in improving survival for pediatric ALCL. There is general agreement that results with CHOP are so poor in adults with most PTCL that new approaches are warranted.
Prognostic Factors
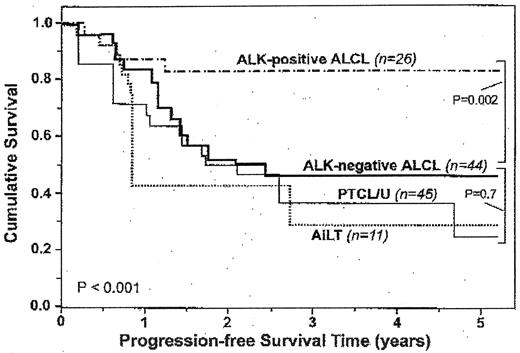
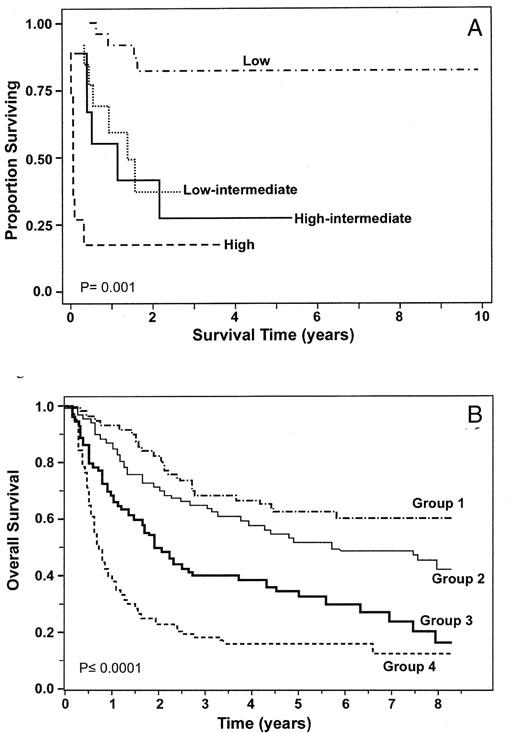
Although pathology has not consistently predicted prognosis due to small numbers in each disease, there are clear trends, with the best prognosis in ALK-positive ALCL, an intermediate-prognosis in PTCL/U, ALK-negative ALCL, and AITL; and the worst in the systemic extranodal subtypes, including nasal type, hepatosplenic and intestinal (Figure 1 ).1,2,11 The International Prognostic Index (IPI) has been validated for PTCL in multiple series (Figure 2a ) and a new model adding bone marrow involvement to some of the IPI factors (age, PS, LDH) has been proposed.12 The 5-year survival varies from 62% (0 factors) to 18% (3–4 factors) (Figure 2b ). Bulky disease (≥ 7cm), B symptoms, and an elevated β2 microglobulin level have also been associated with a worse prognosis.9 Elevated levels of serological markers, nm23-H1 (involved in regulation of metastasis), interleukin-2 receptor, and soluble CD44 may be additive to the IPI to better identify poor prognosis patients.13
Tumor-specific features under evaluation to assess prognosis include expression of cytotoxic molecules, Ki-67, p53, chemokine receptors, and gene profiles. Cytotoxic molecules (CM), TIA-1 and granzyme B, in PTCL/U, are associated with B symptoms, a higher IPI, an inferior CR rate (30% vs 63%), and a worse overall survival (P = 0.004) when compared to a CM-negative group.14 The proliferation-associated antigen, Ki-67, was additive to IPI factors (age, PS, LDH) in a series of PTCL/U (Figure 3 ).15 Immunohistochemistry detection of p53 overexpression was detected in a minority (29%) of nodal PTCL cases; was associated with Ki-67, Bcl-2 and p-glycoprotein; and was a better predictor of poor survival than IPI.16
Preliminary data indicate chemokine receptor expression that distinguishes subsets of T helper cells, Th1 and Th2, and correlates with histology and prognosis.17 CXC chemokine receptor 3 (CXCR3) and CC chemokine receptor 5 (CCR5) are selective for Th1 while CCR4, CCR3 and CCR8 are associated with the Th2phenotype. CXCR3 expression was observed in AILT while CCR4 was observed with poor prognosis histologies, including ALCL and ATLL. Moreover, CCR4 expression in PTCL/U was an independent poor prognostic factor (P < 0.001).18
Gene expression profiles can distinguish subsets of DLBCL and are likely to be able to subdivide PTCL. Genes involved in the NF-κB pathway are activated in PTCL and not in T-lymphoblastic lymphoma.19 Preliminary data show that gene profiles can discriminate AILT and ALCL.20 AILT is represented by overexpression of B cell markers including immunoglobulin genes. The ALCL signature involves genes active in the early immune response, extracellular matrix and cell proliferation. More importantly, three molecular profiles were identified in PTCL/U: 1) over-expression of genes with an aggressive course, including CYCLIN D2; 2) genes involved in T-cell activation and apoptosis, NF-κB1 and BCL-2; and 3) overexpression of genes in the interferon γ/JAK/STAT pathway.20
Disease-AdaptedTherapy
ALCL can be subdivided into three primary diseases (systemic ALK-positive, systemic ALK-negative, primary cutaneous ALCL) and may occur secondary to lymphomatoid papulosis, mycosis fungoides and Hodgkin lymphoma. ALK-positive ALCL occurs at a young age (median age < 30 years) and has a better prognosis than systemic ALK-negative ALCL (median age, 50–60 years). Five-year overall survival following anthracycline therapy is 60–93% for ALK-positive compared to 11–46% for ALK-negative ALCL.1,3 A worse prognosis can be seen in ALK-positive patients with B symptoms, a high IPI, small cell variant histology, and CD56 or survivin (a member of the inhibitor of apoptosis family) expression.21,22 More intensive therapy could be justified in selected ALK-positive patients with these adverse features.
AITL is difficult to diagnose and treat because there may be the presence of both B and T clones, as well as Epstein-Barr virus; it has a variable clinical course with autoimmune features.5,6 AITL usually occurs in the elderly (median age, 57–68 yrs) as a systemic nodal disease with B symptoms, a pruritic rash, arthritis, eosinophilia and immunologic abnormalities (Coombs-positive hemolytic anemia, cold agglutinins, cryoglobulinemia, polyclonal hypergammaglobulinemia, antinuclear antibodies, and rheumatoid factors).
AITL rarely spontaneously regresses and it can respond to single agents, including steroids, cyclosporine, methotrexate, interferon, nucleoside analogs, and denileukin difitox. Combination chemotherapy is warranted once a diagnosis is made with a CR rate of 50–70% following anthracycline-based therapy, but patients have frequent and early relapses or deaths due to infections, with 5-year survival at 10–30%.5 Cyclosporine has been effective in patients who had relapsed after either steroids or chemotherapy.23
Extranodal NK/T lymphomas have unique presentations depending upon the site of origin and usually have a poor prognosis. Facial swelling, nasal obstruction, nasal discharge and epistaxis are common symptoms in nasal NK/T lymphomas. Prognosis is variable and depends upon whether the disease is small and confined to the nasopharynx as opposed to disseminated disease. There is a role for radiation usually combined with chemotherapy and CNS prophylaxis in these patients. In a recent series, patients with disease confined to the nasal cavity, nasopharynx, larynx, pharynx or oral cavity had 54% 5-year survival compared to 20% (P = 0.0068) with more advanced disease.24
Enteropathy-associated intestinal T-cell lymphoma is often associated with celiac disease. Patients present with abdominal pain and weight loss, and frequently have small bowel obstruction or perforation. Diagnosis is made at laparotomy where jejunal involvement is found with multiple circumferential ulcers. Aggressive nutritional support is required. Prognosis is poor, with median survival of 7.5 months and failure-free survival less than 20% at 1 year.25
Hepatosplenic T-cell lymphoma (HSTCL) is rare and usually arises from the γ/δ T cells of the sinusoidal area, or red pulp, of the spleen. HSTCL occurs in young males (median age, 29–35 years) who present with B symptoms, hepatosplenomegaly, minimal lymphadenopathy, lymphocytosis, anemia, and severe thrombocytopenia. Most patients are refractory or have brief responses to anthracycline therapy and have less than 15% long-term survival. There are anecdotal reports of responses to pentostatin and prolonged survival with allogeneic (allo) SCT.6,26
Novel Therapy in T/NK Leukemia
Leukemia presentations of T/NK cell neoplasms are aggressive although they can rarely have an indolent phase. They include T-prolymphocytic leukemia (T-PLL), ATLL and aggressive NK leukemia. Because all are relatively chemoresistant and have median survivals less than 1 year, they can serve as models for innovative therapy. Nucleoside analogs have been active in T-PLL with pentostatin having a response rate up to 45% (9% CR) in patients who had received prior chemotherapy27; however, alemtuzumab (Campath-1H, anti-CD52 antibody) is the most active single agent in T-PLL, with a 76% response rate (60% CR) in previously treated patients.28 Preliminary data indicate prolonged CR and survival in patients who have received alemtuzumab followed by either an autologous (auto) or alloSCT.28 Ongoing trials for T-PLL patients utilize combination chemotherapy, including a nucleoside analog, followed by alemtuzumab and then SCT.
There has been minimal improvement in chemotherapy regimens in ATLL despite it being recognized nearly 30 years ago.29 The molecular pathogenesis revolves around Tax, a potent HTLV-1 transcription activator. The Tax protein induces proliferation and inhibits apoptosis of HTLV-1 cells through induction of IκB-alpha degradation, which activates the NF-κB pathway; however, as the disease advances, Tax expression diminishes and the ATL cells acquire the ability to proliferate independently. A multistep process is proposed with ongoing genetic instability, including mutation of p53, deletion of tumor suppressor genes p15 and p16, and DNA methylation.29
Because of its chemoresistance and its viral leukemo-genesis, ATLL has been an unique disease for investigating therapy. Interferon-α (IFN) plus zidovudine (AZT) has had a higher response rate (67–92%) than chemotherapy regimens in ATLL. One of the best survivals (17 months) was reported in a study in which patients were debulked with 2 cycles of CHOP followed by AZT/IFN plus etopside.30 NF-κB inhibition has been proposed as a therapeutic target in ATLL, and preliminary data has shown in vitro activity of the proteosome inhibitor, bortezomib, and the histone deacetylase inhibitor, depsipeptide. Conjugated and unconjugated monoclonal antibody therapy directed at the IL-2 receptor, CD25, has activity in ATLL and is being evaluated in conjunction with chemotherapy.
AlloSCT was first reported as a curative option in ATLL in 1996. Although the median survival with alloSCT (9.6 months in a review of 40 patients from 7 centers) does not appear superior to that achieved with other therapies, the 3-year estimated overall survival of 45.3% and relapse-free survival of 33.8% suggests SCT may offer the best chance for long-term survival.31
Despite the new therapies under investigation in ATLL, the ultimate goal is prevention of the disease. Avoiding breast-feeding in mothers infected with HTLV-1 can reduce infection in the newborn by 80%.29 Other proposed methods to prevent ATLL are antiretroviral therapy, monoclonal antibody therapy, and a Tax-targeted vaccine.
Novel Chemotherapy
Trials in cutaneous T cell lymphoma (CTCL) as well as the aggressive T/NK leukemias have introduced agents that have activity in PTCL. Most of the data have been derived by single-agent phase I/II trials in relapsed CTCL, but some of the trials have included a minority of patients with PTCL. Gemcitabine, pentostatin, and liposomal doxorubicin have had the highest responses in CTCL and are under investigation in PTCL.
Gemcitabine is a pyrimidine analog with stuctural similarities to cytarabine. The overall response rates in series with both relapsed CTCL and PTCL were 60–70%, with a CR rate of 11–20%.32 A response rate of 75% (22% CR) was obtained in patients earlier in the course of disease.33 Gemcitabine has been effectively combined with platinum derivatives and with liposomal doxorubin plus vinorelbine in relapsed non-Hodgkin (NHL) and Hodgkin lymphoma (HL). Clinical trials in PTCL are comparing CHOP plus gemcitabine to CHOP alone.
The purine analogs, pentostatin, fludarabine and clardribine, inhibit adenine deaminase by different mechanisms and all have had activity in CTCL. Dosing for pentostatin has been variable and response rates have been in the 36–71% (2–25% CR) range.34 The nucleoside analogs are being combined with other agents, and a recent trial in PTCL (excluding ALK positive ALCL and primary cutaneous ALCL), which combined alemtuzumab with fludarabine, cyclophosphamide and doxorubicin, reported a CR rate of 78% in untreated patients.35
T-cell lymphoma has been proposed as a model for histone deacetylase inhibitors.36 Depsipeptide induces hi-stone acetylation, p21 expression and apoptosis without significant cell cycle arrest. It also increases expression of the IL-2 receptor, which can serve as a target for denileukin difitox. A phase II trial of depsipeptide in PTCL had a response rate of 26% in relapsed patients.37
Immunotherapy
Monoclonal antibodies have been active as single agents in PTCL and are being investigated in combination with other therapy (see T-PLL). The response rate of alemtuzumab was 36% in heavily treated patients with PTCL; infectious complications were cytomegalovirus reactivation (43%), pulmonary aspergillosis (14%), and EBV-related hemophagocytosis (14%).38 Case reports and small trials have shown responses to the IL-2 diphtheria toxin fusion protein, denileukin difitox (anti-CD25), and to antibodies directed at CD2 or CD4.39,40 Both alemtuzumab and denileukin difitox are being combined with chemotherapy in phase II trials. Several different antibodies directed at CD30, which is present in all types of ALCL, most HL and in a subset of PTCL/U, are in phase II trials. In vitro data indicate that anti-CD30 antibodies activate NF-κB and sensitize the malignant cells to chemotherapy agents.41
Stem Cell Transplantation
The role of SCT in T/NK neoplasms is uncertain other than that it should be considered in patients who relapse and are chemosensitive as indicated by the PARMA trial. The usefulness of SCT should not be overestimated, because reports usually do not include an intent-to-transplant analysis and many patients are ineligible due to progressive disease. The main controversy is whether SCT should be used in first remission or best early response for patients who have adverse prognostic factors or pathology (Table 2 ). For first remission patients with age-adjusted IPI ≥ 2 who were randomized to transplantation in the LNH-87 and LNH-93 trials, survival was not improved in non-ALCL PTCL but was in B cell NHL.42 The failure to show an advantage for the transplantation arm could be due in part to the problem that only a small number of patients with PTCL achieved a remission.
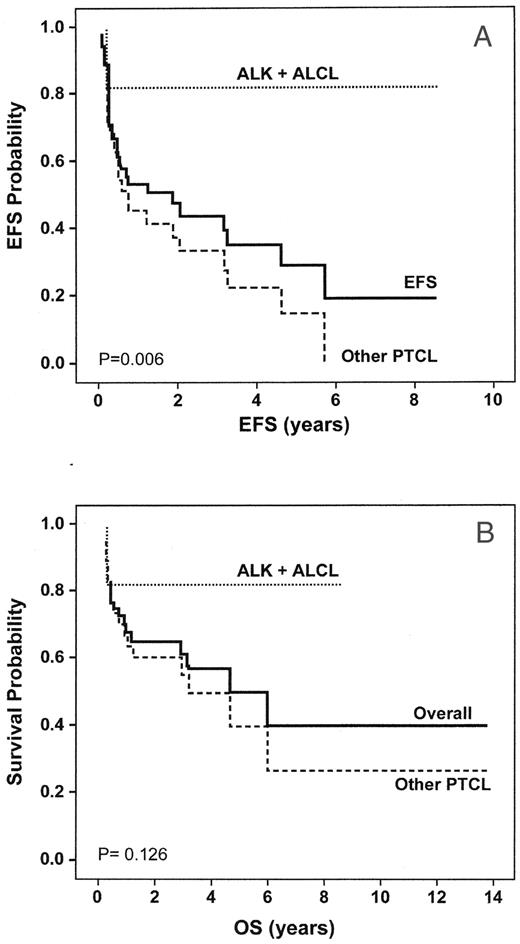
Most reports of SCT in PTCL are small, retrospective series from single institutions.43,44 Outcomes depend on patient prognostic factors (age, B symptoms, pretransplant IPI, disease status: first CR vs > CR1, chemosensitivity, type of SCT) and the proportion of different histologies. Series with a large number of ALK-positive ALCL will have better results (Figure 4 ). ALK-positive ALCL should not routinely be transplanted in first CR, because it can be cured with initial chemotherapy and can be salvaged by SCT after relapse. Alternatively, early SCT may be warranted in ALK-negative systemic ALCL because prognosis has been poor with autoSCT in relapsed patients.45 Small, prospective series have had good results with autoSCT in patients with AILT (5 of 8 prolonged CR) and EATL (6 of 10) in first remission.43 The SCT literature for most of the rare systemic extranodal histologies is limited to case reports; however, the prognosis is so poor with chemotherapy that early SCT should be considered.
The data for alloSCT is scant, but the principles for alloSCT are similar to its use in other hematologic neoplasms. There is a role in T/NK neoplasms with marrow involvement, particularly the leukemias, and there are case reports for systemic extranodal subtypes, including HSTCL. There is evidence of a graft-versus-leukemia effect and there are fewer relapses with alloSCT than autoSCT; but there is a higher treatment related mortality (TRM), 25–44%, with alloSCT using myeloablative regimens than the TRM of 4–10% with autoSCT.43 A trial with a reduced-intensity conditioning regimen reported a TRM mortality of 6% with a 3-year overall survival and progression-free survival of 81% and 64%, respectively.46 Results are better with reduced-intensity conditioning if there has been debulking prior to alloSCT.
Progress in the therapy of T/NK neoplasms remains a challenge due to their rarity, relative chemoresistance, and lack of a single type of effective monoclonal antibody. Clinicopathologic factors along with genomic profiles may further subdivide these neoplasms into prognostic groups. Novel approaches utilizing new agents and monoclonal antibodies are under evaluation. Randomized trials have not shown that early SCT is better than chemotherapy, but the prognosis is so poor in some patients that SCT may be warranted. Well-designed phase II trials and international cooperation are required to make advances in the therapy of T/NK neoplasms.
Clinical subdivision of noncutaneous, mature T/NK neoplasms, unique features, and expected 5-year survival.
| . | Unique Features . | Survival (%) . |
|---|---|---|
| *Survival pertains to the acute leukemia and lymphoma presentations of ATLL | ||
| Nodal | ||
| Anaplastic large cell, ALK-positive | t(2;5)(p23;q35) and variants; extranodal involvement (50–80%), skin (21–35%) | 60–90 |
| Anaplastic large cell, ALK-negative | Distinguish from primary cutaneous anaplastic large cell lymphoma (ALCL) | 10–45 |
| Angioimmunoblastic | Autoimmunity | 10–30 |
| Peripheral T-cell lymphoma, unspecified | Most common, survival dependent on IPI | 15–35 |
| Extranodal | ||
| Nasal | Epstein-Barr virus association, central nervous system risk | |
| Localized | 50–70 | |
| Disseminated (nasal type) | Sites: skin, gastrointestinal tract, testis, orbit | 5–10 |
| Enteropathy associated | Celiac disease; small bowel obstruction | 5–20 |
| Hepatosplenic, γδ | Isochromosome 7, trisomy 8; can occur in organ transplants | 5–15 |
| Subcutaneous panniculitis-like | Aggressive with hemophagocytosis; may be indolent | 10–30 |
| Leukemia | ||
| T-Prolymphocytic leukemia | Chromosome 14 abnormalities | 10–20 |
| Adult T-cell lymphoma/leukemia | HTLV-1 association, hypercalcemia. Four types: acute (55–65%) chronic, smoldering leukemia and lymphoma (20–25%) | 0–15* |
| Large granular lymphocytic leukemia | Rheumatoid arthritis, neutropenia | 50–75 |
| Aggressive NK leukemia | May represent leukemic phase of extranodal NK neoplasms (nasal type) | 0–10 |
| . | Unique Features . | Survival (%) . |
|---|---|---|
| *Survival pertains to the acute leukemia and lymphoma presentations of ATLL | ||
| Nodal | ||
| Anaplastic large cell, ALK-positive | t(2;5)(p23;q35) and variants; extranodal involvement (50–80%), skin (21–35%) | 60–90 |
| Anaplastic large cell, ALK-negative | Distinguish from primary cutaneous anaplastic large cell lymphoma (ALCL) | 10–45 |
| Angioimmunoblastic | Autoimmunity | 10–30 |
| Peripheral T-cell lymphoma, unspecified | Most common, survival dependent on IPI | 15–35 |
| Extranodal | ||
| Nasal | Epstein-Barr virus association, central nervous system risk | |
| Localized | 50–70 | |
| Disseminated (nasal type) | Sites: skin, gastrointestinal tract, testis, orbit | 5–10 |
| Enteropathy associated | Celiac disease; small bowel obstruction | 5–20 |
| Hepatosplenic, γδ | Isochromosome 7, trisomy 8; can occur in organ transplants | 5–15 |
| Subcutaneous panniculitis-like | Aggressive with hemophagocytosis; may be indolent | 10–30 |
| Leukemia | ||
| T-Prolymphocytic leukemia | Chromosome 14 abnormalities | 10–20 |
| Adult T-cell lymphoma/leukemia | HTLV-1 association, hypercalcemia. Four types: acute (55–65%) chronic, smoldering leukemia and lymphoma (20–25%) | 0–15* |
| Large granular lymphocytic leukemia | Rheumatoid arthritis, neutropenia | 50–75 |
| Aggressive NK leukemia | May represent leukemic phase of extranodal NK neoplasms (nasal type) | 0–10 |
Suggested role for stem cell transplantation (SCT) in noncutaneous, mature T/NK neoplasms.
| Disease . | Type and Timing of SCT . |
|---|---|
| *4 prognostic factors in PTCL: age, performance status, LDH and bone marrow involvement (alloSCT will need to be considered in the latter). Group 3 had 2 or more factors and group 4 had 3 or 4 factors.12 | |
| ** Because 5-year survival > 60% has been observed in PTCL/ U only with IPI ≤ 1, early SCT should be investigated in PTCL/U with IPI ≥ 2. | |
| Anaplastic large cell, ALK-positive | Auto – after relapse |
| Anaplastic large cell, ALK-negativ | Auto – if IPI ≥ 3 or ≥ group 3* |
| e | |
| Angioimmunoblastic | Auto – if IPI ≥ 3 or ≥ group 3 |
| PTCL, unspecified | Auto – if IPI ≥ 3** or ≥ group 3 |
| Nasal, localized | Auto – after relapse |
| disseminated | Auto or allo |
| Enteropathy associated | Auto |
| Subcutaneous panniculitis-like | Auto if hemophagocytosis |
| Hepatosplenic, γδ | Allo over auto |
| T prolymphocytic leukemia | Allo over auto |
| Adult T cell leukemia/lymphoma | Allo over auto |
| Aggressive NK leukemia | Allo |
| Disease . | Type and Timing of SCT . |
|---|---|
| *4 prognostic factors in PTCL: age, performance status, LDH and bone marrow involvement (alloSCT will need to be considered in the latter). Group 3 had 2 or more factors and group 4 had 3 or 4 factors.12 | |
| ** Because 5-year survival > 60% has been observed in PTCL/ U only with IPI ≤ 1, early SCT should be investigated in PTCL/U with IPI ≥ 2. | |
| Anaplastic large cell, ALK-positive | Auto – after relapse |
| Anaplastic large cell, ALK-negativ | Auto – if IPI ≥ 3 or ≥ group 3* |
| e | |
| Angioimmunoblastic | Auto – if IPI ≥ 3 or ≥ group 3 |
| PTCL, unspecified | Auto – if IPI ≥ 3** or ≥ group 3 |
| Nasal, localized | Auto – after relapse |
| disseminated | Auto or allo |
| Enteropathy associated | Auto |
| Subcutaneous panniculitis-like | Auto if hemophagocytosis |
| Hepatosplenic, γδ | Allo over auto |
| T prolymphocytic leukemia | Allo over auto |
| Adult T cell leukemia/lymphoma | Allo over auto |
| Aggressive NK leukemia | Allo |
Comparison of progression-free survival (PFS) according to lymphoma subtype: anaplastic large cell lymphoma (ALCL) versus peripheral T-cell lymphoma, unspecified (PTCL/U) versus angioimmunoblastic (AILT) and anaplastic lymphoma kinase (ALK) expression.
Adapted with permission from
Blackwell Publishing.
Comparison of progression-free survival (PFS) according to lymphoma subtype: anaplastic large cell lymphoma (ALCL) versus peripheral T-cell lymphoma, unspecified (PTCL/U) versus angioimmunoblastic (AILT) and anaplastic lymphoma kinase (ALK) expression.
Adapted with permission from
Blackwell Publishing.
Overall survival of patients with peripheral T-cell lymphoma (PTCL) according to the International Prognostic Index
(Adapted with permission from
(b) Overall survival according to a prognostic index for PTCL/U
(Adapted with permission from
Overall survival of patients with peripheral T-cell lymphoma (PTCL) according to the International Prognostic Index
(Adapted with permission from
(b) Overall survival according to a prognostic index for PTCL/U
(Adapted with permission from
(A) Overall survival of patients with nodal peripheral T-cell lymphoma, unspecified (PTCL/U). (B) Prognostic index includes age, performance status, LDH, and Ki67 (>80%): group I (0,1 risk factors), median survival 37 months; group II (2 factors), median survival 23 months; group III (3,4 factors), median survival 6 months
(
(A) Overall survival of patients with nodal peripheral T-cell lymphoma, unspecified (PTCL/U). (B) Prognostic index includes age, performance status, LDH, and Ki67 (>80%): group I (0,1 risk factors), median survival 37 months; group II (2 factors), median survival 23 months; group III (3,4 factors), median survival 6 months
(
Event-free survival (EFS) (a) and overall survival (b) of primarily relapsed patients with PTCL who underwent stem cell transplantation. ALK-positive ALCL had a statistically significant better EFS than other PTCL.
(Update from ref. 44, courtesy of Madan Jagasia, MBBS)
Event-free survival (EFS) (a) and overall survival (b) of primarily relapsed patients with PTCL who underwent stem cell transplantation. ALK-positive ALCL had a statistically significant better EFS than other PTCL.
(Update from ref. 44, courtesy of Madan Jagasia, MBBS)