Abstract
JAK2V617F, a somatic gain-of-function mutation involving the JAK2 tyrosine kinase gene, occurs in nearly all patients with polycythemia vera (PV) but also in a variable proportion of patients with other myeloid disorders; mutational frequency is estimated at approximately 50% in both essential thrombocythemia (ET) and myelofibrosis (MF), up to 20% in certain subcategories of atypical myeloproliferative disorder (atypical MPD), less than 3% in de novo myelodysplastic syndrome (MDS) or acute myeloid leukemia, and 0% in chronic myeloid leukemia (CML). Accordingly, there is now molecular justification for grouping PV, ET, and MF together in a distinct MPD category (i.e., classic, BCR-ABL− MPD) that is separate from chronic myeloid leukemia (CML), MDS, and atypical MPD. To date, JAK2V617F has not been described in patients with reactive myeloproliferation, lymphoid disorders, or solid tumor. Therefore, the presence of JAK2V617F strongly suggests an underlying MPD and it is therefore reasonable to consider JAK2V617F-based laboratory tests for the evaluation of polycythemia, primary thrombocytosis, unexplained leukocytosis, bone marrow fibrosis, or abdominal vein thrombosis. Current information on disease-specific prognostic relevance of JAK2V617F is inconclusive and confounded by inter-study differences in the performance of mutation screening assays. Regardless, the discovery of JAK2V617F has reinforced the pathogenetic contribution of JAK-STAT signaling in MPD and identifies JAK2 as a valid drug target.
In 1960, chronic myeloid leukemia (CML) became the first cancer to be associated with a specific cytogenetic marker (the Philadelphia chromosome), which subsequently was shown to harbor a reciprocal chromosomal translocation, t(9;22)(q34;q11).1 This seminal observation ultimately led to the identification of the disease-causing mutation, establishment of highly accurate diagnostic tests, development of molecularly targeted therapy, and the possibility of molecular monitoring of treatment response and minimal residual disease (MRD). The recent discovery of JAK2V617F2 –5 has raised the prospect of a similar scenario in polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis (MF).
The Mutation
JAK2V617F represents a G to T somatic mutation of JAK2, at nucleotide 1849, in exon 14, resulting in the substitution of valine to phenylalanine at codon 617.5 The association of JAK2V617F with myeloproliferative disorders (MPDs), including PV, ET, and MF, was first reported in 2005.2,–5 Subsequently, the mutation has been demonstrated in other myeloid disorders including atypical MPDs and MDS6,7 as well as acute leukemia cell lines including the human erythroleukemia (HEL) cell line.3 Therefore, at present, JAK2V617F cannot be regarded as equivalent to BCR-ABL in terms of either pathogenetic relevance or utility as a diagnostic marker.
In cell lines, JAK2V617F promotes cytokine-independent3,–5 and erythropoietin (Epo)-3,5,8 or interleukin-3 (IL-3)4-hypersensitive growth accompanied by constitutive activation of JAK2,3,5,8,9 STAT5,4,5,8,9 and PI3K,5 ERK,5,8 and Akt8 pathways. Similarly, HEL cells, which carry a homozygous JAK2V617F mutation, display constitutive activation of JAK2, STAT5, and ERK and their growth is inhibited by a small molecule JAK2 inhibitor.3
In mice, JAK2V617F induces a PV-like disease with erythrocytosis, low serum Epo level, splenomegaly, extramedullary hematopoiesis, granulocytosis, megakaryocytic hyperplasia, and delayed-onset bone marrow fibrosis and anemia.5,9,10 Interestingly, thrombocytosis is conspicuously absent in JAK2V617F-induced “PV” in mice whereas it is a prominent feature of MPLW515L-induced murine “MF.”11 Consistent with this observation, a significantly lower platelet count has been shown in JAK2V617F-positive, as opposed to mutation-negative, patients with ET.12,13 Primary blood and spleen cells from mice with JAK2V617F-induced “PV” display constitutive STAT5 activation9 and endogenous and Epo-hypersensitive erythroid colony formation.9,10
In man, it is becoming increasingly evident that JAK2V617F is not an essential pathogenetic component for MPD other than PV14 and might not be the initial clonogenic event even in PV.15 Regardless, the mutation has been traced to a primitive stem cell that is capable of erythroid-weighted trilineage myeloid differentiation16 and potentially involving natural killer17 but not T and B lymphocytes.18 Furthermore, homozygosity for JAK2V617F, reflecting loss of heterozygosity as a result of mitotic recombination,2,–4 is relatively specific to PV.19 Therefore, JAK2V617F probably contributes to a PV-weighted MPD phenotype.
Screening Tests
Several laboratory methods of JAK2V617F mutation detection are currently available, and one must interpret test results in the context of assay sensitivity. For example, in one of the first reports on JAK2V617F, mutational frequency in PV increased from 73% to 97% by using an allele-specific PCR assay (~3% sensitivity) as compared to direct sequencing (~20% sensitivity).2 On the other hand, false positive results could arise from the utilization of ultra sensitive assay systems (~0.01% sensitivity) that could detect very low levels of JAK2V617F even in healthy individuals.20 In general, quantitative PCR methods are preferred because of their potential value in measuring mutant allele burden and monitoring treatment response.21
Conventional DNA sequencing
A PCR amplified DNA sequence of interest (in this instance the mutated DNA region of JAK2) is processed through an automated sequencer that is based on the Sanger DNA sequencing principle. The method is semiquantitative, can distinguish a homozygous from a heterozygous pattern of mutation in the absence of mixed clonality, and the reported sensitivity is above 5%.22
Pyrosequencing (sequencing-by-synthesis)
The technique of pyrosequencing is based on the detection of light signal (bioluminescence) from a luciferase reaction using pyrophosphates that are released by the incorporation of nucleotides by polymerase during in vitro DNA synthesis as source of ATP. Specific nucleotides are added stepwise to the reaction in order to determine the sequence of the DNA strand of interest that is used as a template for DNA synthesis. The method is suitable for quantitative measurements and can distinguish homozygous from heterozygous mutation pattern, and assay sensitivity is estimated at 5%.7
Melting curve assay
A fluorescence-tagged primer that is complementary to either wild-type (WT) or mutated sequence is incorporated in a standard PCR reaction and differences in the dissociation temperature of the primer from either the WT or mutant sequence is used to differentiate between the two. In other words, mismatched binding dissociates at a lower temperature. The method is semiquantitative with a 1% to 10% reported assay sensitivity.23
Allele-specific PCR
There are several different methodologies that utilize allele-specific (AS) primers, in the context of both qualitative and quantitative platforms. One such assay is very sensitive (0.01%–0.1%) and involves the use of a mutation-specific forward primer that results in the amplification of only the mutated sequence that is detected by capillary electrophoresis.23 Another AS-PCR method, referred to as amplification refractory mutation system (ARMS; reported sensitivity of 1%–2%),7 uses two primer pairs to specifically amplify both WT and mutant DNA sequence in a single reaction. In general, AS-PCR methods are suitable for quantitative measurements on a real-time PCR platform, using either genomic DNA or cDNA.14,24
BsaXI restriction analysis
The JAK2V617F mutation interferes with a BsaXI restriction enzyme recognition site and therefore results in restriction digests with altered size following site-specific restriction analysis.2 The method can discriminate homozygous from heterozygous mutation patterns.
Impact on Disease Classification
Myeloid disorders are classified into acute myeloid leukemia (AML) and chronic myeloid disorders (CMDs) based on the presence or absence, respectively, of AML-defining features that include the presence of ≥ 20% blasts either in the bone marrow or peripheral blood.25 The MPDs constitute a subcategory of CMD and include CML, ET, PV, and MF.26 These four clinicopathologic entities were described between 1845 and 1934 and it was the substantial overlap in their clinical and laboratory manifestations that inspired William Dameshek to group them together as “MPD” in 1951.26 Between 1967 and 1981, Fialkow and colleagues used the X-linked glucose-6-phosphate dehydrogenase locus as a cell marker to establish classic MPD as clonal stem cell disorders.27
The recent revelation that JAK2V617F clusters with the classic, BCR-ABL− MPD validated their consideration as a distinct MPD category separate from CML, MDS and atypical MPD (Table 1 ). At the same time, certain CMDs, including CML, are now molecularly well characterized, paving the way for a semi-molecular classification system (Table 1 ).28 However, at the present time, it is premature to attempt sub-classification of ET and MF based on the presence or absence of JAK2V617F, because neither prognostic nor therapeutic implications have been attached to the specific mutation, although mutation-positive patients have been shown to display PV-characteristic bone marrow features and low serum Epo.12 Furthermore, mutation status designation is dependent on assay sensitivity and the issue is further confounded by the occurrence of marked interpatient variation in mutation burden.24
Impact on Diagnostic Approaches
In the pre-JAK2V617F era, diagnosis in the classic, BCR-ABL− MPD, was based on consensus criteria that relied primarily on either measured variables (e.g., red cell mass, hematocrit, platelet count, serum Epo level) or subjective technologies (e.g., bone marrow histology); the two internationally recognized diagnostic criteria in this regard were fostered by the Polycythemia Vera Study Group (PVSG) and the World Health organization (WHO).25,29 Proponents of each approach were concerned primarily about suboptimal sensitivity associated with the alternative approach. Others have effectively argued that the use of arbitrary threshold levels for either platelet count or red cell mass compromises the detection of early-phase disease that is otherwise biologically relevant.30 The issue is further confounded by the definitions of what constitutes “PV,” “ET,” or “MF” and therefore uncertainty regarding test specificity.
Recent observations from the Mayo Clinic and Harvard have raised the possibility that all patients with PV might carry JAK2V617F.14,31,32 This contention is supported by some2,24 but not other33 studies. In an attempt to reconcile the discrepancies in mutational frequency among different reports, 17 cases from three major academic institutions, which were previously reported as being JAK2V617F-negative PV were reexamined, and it was demonstrated that the diagnosis of PV was inaccurate in 4 patients, questionable in 1, and the mutation was detected by more sensitive techniques in 5 patients.34 Among the remaining 7 patients, 4 were either receiving or had received interferon alpha therapy that resulted in excellent hematological remission that might have affected mutation detection.35 Mutation screening was performed at the time of leukemic transformation in two patients whose antecedent diagnosis of PV was not well documented. In the last patient, there was not enough material available for re-testing. Similarly, mutational frequency increased from 74%3 to 99%14 in the Harvard experience when more sensitive assays were utilized. Therefore, it is likely that diagnostic imprecision and inadequate assay sensitivity are the major causes of JAK2V617F-negativity in reported cases of PV. Additional causes of false-negative results include utilization of suboptimal tissue for mutation screening and treatment effect on mutant allele burden.21,35
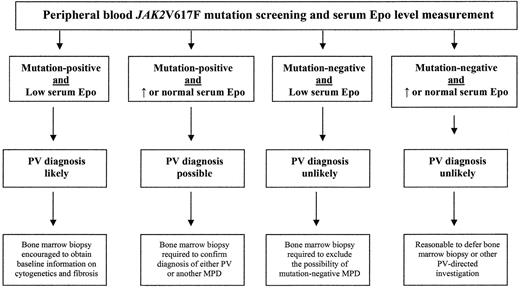
These data support the recommendation that peripheral blood mutation screening for JAK2V617F be incorporated into the initial evaluation of a patient with suspected PV (Figure 1 ). One cannot exclude the possibility of legitimate instances of JAK2V617F-negative PV at the present time, however, and therefore it is appropriate to obtain concomitant measurement of serum Epo level to minimize the chances of both false-negative and false-positive results. Accordingly, if clinical suspicion for PV is low, a negative peripheral blood test for JAK2V617F accompanied by normal or elevated serum Epo argues against the specific diagnosis and should allow deferral of further investigation with either bone marrow biopsy or red cell mass measurement. However, additional evaluation by a bone marrow biopsy is indicated in the presence of either a high clinical suspicion for PV or discrepancy between the two test results (Figure 1 ). For comparative purposes, the current WHO criteria for PV diagnosis are presented in Table 2 .
Unlike the case with PV, the utility of mutation screening for JAK2V617F for the diagnosis of ET, MF, or other MPD is limited by suboptimal negative predictive value and lack of diagnostic specificity. Regardless, it is reasonable to consider the test for the evaluation of otherwise unexplained thrombocytosis in order to streamline further investigation. Accordingly, a positive test strongly suggests an underlying MPD and makes the possibility of occult CML unlikely. However, a bone marrow biopsy is still required to differentiate ET from cellular-phase MF as well as other causes of clonal thrombocytosis including MDS and atypical MPD. On the other hand, the presence of homozygous JAK2V617F favors the diagnosis of PV over that of ET.19,24
Other current indications for JAK2V617F mutation screening include unusual thrombotic complications including abdominal36 or cerebral vein thrombosis, arterial events at young age, and other MPD-characteristic clinical manifestations including erythromelalgia and aquagenic pruritus. In contrast, currently available information does not warrant testing of family members.17 Finally, one must confirm JAK2V617F-suggested diagnosis by alternative means if the clinical presentation is not felt to be consistent with the specific diagnosis. Similarly, although the use of adequately sensitive mutation screening assays facilitates diagnosis, it must be recognized that the possibility of false-positive results increases with the use of highly sensitive techniques.20
Impact on Prognosis and Management
There are currently a limited number of reports with more than 100 study patients that have looked into the prognostic relevance of JAK2V617F in MPDs. In terms of survival, two large studies from the Mayo Clinic, one in ET37 (n = 150; median follow-up, 11.4 years) and the other in MF38 (n = 117; median follow up, ~37 months), did not identify JAK2V617F mutational status as an independent prognostic factor.37,38 In contrast, another multicenter study from Europe reported inferior survival, possibly linked to increased risk of leukemic transformation, in patients with mutation-positive MF (n =152; median follow-up, 38 months).39 However, the suggested connection between the presence of the mutation and either blastic or fibrotic transformation was not confirmed by other studies in both MF40 and ET.12,37 On the other hand, the evidence that links JAK2V617F in ET to propensity for conversion to polycythemia is stronger.12,37
With regard to the risk of thrombosis, the largest relevant study in ET (n = 806) suggested that JAK2V617F was associated with venous but not arterial events.12 Similarly, an increased prevalence of thrombotic episodes has been reported in JAK2V617F-positive patients with MF.38 However, the overall risk of thrombosis was not affected by the presence of the mutation in two other relatively large studies (n = 15037 and n = 13013) of ET and another study of MF.39 Regardless, it is conceivable that the significantly higher age distribution,12,37,38 hematocrit,12,13,37 and leukocyte12,37,39 levels in mutation-positive patients contributed to the apparent association between JAK2V617F and thrombosis reported in some studies. One also wonders about possible confounding from the inadvertent inclusion of PV patients in the mutation-positive ET category.
In PV, because of the small number of JAK2V617F-negative cases described, valid comparison between mutation positive and negative cases is not possible. On the other hand, one study has suggested a higher level of hemoglobin and an increased risk of fibrotic transformation in the presence of high mutant allele burden.31 However, the validity of the latter association is confounded by the demonstrated increase in clonal load over time that is seen in some patients with PV.41
Taken together, it is obvious that current information regarding the prognostic relevance of JAK2V617F is largely inconclusive and does not warrant a change in current treatment strategies that are summarized in Table 3 .
Conclusion
The year 2005 might become as important to BCR-ABL-negative MPD as was the year 1960 to CML.1 Like BCR-ABL, JAK2V617F is a constitutively activated cytoplasmic tyrosine kinase that results in hyperactivity of the corresponding signal transduction pathway as well as induction of the relevant disease phenotype in mice. However, whereas BCR-ABL, in the context of chronic myeloid disorders, is found exclusively in CML, JAK2V617F has been demonstrated in a spectrum of myeloid disorders and the association with a specific disease category is less than perfect (with the possible exception of PV). Similarly, whether or not JAK2-targeted small molecule drug therapy will be as successful as imatinib therapy in CML remains to be seen. Nevertheless, the discovery of JAK2V617F has already had a major impact on the diagnostic approach to MPDs as well as research strategies in terms of both molecular pathogenesis and drug development.
Semi-molecular classification of myeloproliferative disorders (MPD).
| . | Main Categories . | Subcategories . |
|---|---|---|
| I. Classic MPD | 1. BCR-ABL-positive 2. BCR-ABL-negative | Chronic myeloid leukemia (CML)
|
| II. Atypical MPD | 1. Chronic myelomonocytic leukemia | |
| 2. Juvenile myelomonocytic leukemia (frequent PTP11, NF1, and RAS mutations) | ||
| 3. Chronic neutrophilic leukemia (~20% JAK2V617F+) | ||
| 4. Chronic eosinophilic leukemia/eosinophilic MPD |
| |
| 5. Hypereosinophilia syndrome | ||
| 6. Chronic basophilic leukemia | ||
| 7. Systemic mastocytosis |
| |
| 8. Unclassified MPD (~20% JAK2V617F+) |
|
| . | Main Categories . | Subcategories . |
|---|---|---|
| I. Classic MPD | 1. BCR-ABL-positive 2. BCR-ABL-negative | Chronic myeloid leukemia (CML)
|
| II. Atypical MPD | 1. Chronic myelomonocytic leukemia | |
| 2. Juvenile myelomonocytic leukemia (frequent PTP11, NF1, and RAS mutations) | ||
| 3. Chronic neutrophilic leukemia (~20% JAK2V617F+) | ||
| 4. Chronic eosinophilic leukemia/eosinophilic MPD |
| |
| 5. Hypereosinophilia syndrome | ||
| 6. Chronic basophilic leukemia | ||
| 7. Systemic mastocytosis |
| |
| 8. Unclassified MPD (~20% JAK2V617F+) |
|
World Health Organization (WHO) criteria for polycythemia vera (PV). Diagnosis of PV requires the presence of the first two A criteria together with either any one other A criterion or two B criteria.
| A Criteria |
|
| B Criteria |
|
| A Criteria |
|
| B Criteria |
|
Current treatment in myeloproliferative disorders.
| . | . | . | Myelofibrosis . | |
|---|---|---|---|---|
| Risk Categories . | Essential Thrombocythemia . | Polycythemia Vera . | Age < 50 years . | Age ≥ 50 years . |
| *Clinically significant acquired von Willebrand disease should be excluded before the use of aspirin in patients with platelet count over 1000 X 109/L. | ||||
| Abbreviations: RIC, reduced-intensity conditioning | ||||
| Low | Low-dose aspirin | Low-dose aspirin + Phlebotomy | Observation or Experimental drug therapy | Observation or Experimental drug therapy |
| Intermediate | Low-dose aspirin* | Low-dose aspirin* + Phlebotomy | Experimental drug therapy or RIC transplant | Experimental drug therapy or Conventional therapy |
| High | Low-dose aspirin + Hydroxyurea | Low-dose aspirin + Phlebotomy + Hydroxyurea | Experimental drug therapy or Full transplant | Experimental drug therapy or RIC transplant |
| . | . | . | Myelofibrosis . | |
|---|---|---|---|---|
| Risk Categories . | Essential Thrombocythemia . | Polycythemia Vera . | Age < 50 years . | Age ≥ 50 years . |
| *Clinically significant acquired von Willebrand disease should be excluded before the use of aspirin in patients with platelet count over 1000 X 109/L. | ||||
| Abbreviations: RIC, reduced-intensity conditioning | ||||
| Low | Low-dose aspirin | Low-dose aspirin + Phlebotomy | Observation or Experimental drug therapy | Observation or Experimental drug therapy |
| Intermediate | Low-dose aspirin* | Low-dose aspirin* + Phlebotomy | Experimental drug therapy or RIC transplant | Experimental drug therapy or Conventional therapy |
| High | Low-dose aspirin + Hydroxyurea | Low-dose aspirin + Phlebotomy + Hydroxyurea | Experimental drug therapy or Full transplant | Experimental drug therapy or RIC transplant |
Diagnostic algorithm for suspected polycythemia vera (PV) in routine clinical practice.
Abbreviations: Epo, erythropoietin; MPD, myeloproliferative disorder.
Diagnostic algorithm for suspected polycythemia vera (PV) in routine clinical practice.
Abbreviations: Epo, erythropoietin; MPD, myeloproliferative disorder.