Abstract
While imatinib is highly effective therapy, with improving prospects over time for sustained remission and potential to severely limit or eliminate disease progression and transformation, a minority of patients either fail or respond suboptimally to imatinib; as well, disease eradication may not be possible with imatinib. Distinct patterns of resistance have evolved with the use of imatinib, and Abl kinase mutations, which alter imatinib binding or favor kinase conformations inaccessible to imatinib, are a common finding associated with clinical resistance. Dasatinib and nilotinib, alternate Abl kinase inhibitors, restore hematologic and cytogenetic remission in the majority of patients with primary failure or acquired resistance in chronic phase disease; in advanced disease and Philadelphia chromosome (Ph)+ ALL, responses are more limited and relapse is common. Future studies with these agents will focus on further optimizing imatinib response, reduction of minimal residual disease, and prevention of resistance. Still newer inhibitors active against T315I mutant BCR-ABL may overcome primary and secondary resistance to dasatinib and nilotinib.
Imatinib mesylate has revolutionized the way we treat chronic myelogenous leukemia (CML); its high level of activity, low toxicity, and ongoing durability have set a dramatic example by which future therapies in CML and cancer therapy overall will be judged. Five-year data from the pivotal trial of imatinib, the IRIS trial,1 suggests an impending plateau in progression-free survival, due to reduction and near elimination of the incidence of imatinib failure over time. The natural history of CML, particularly for patients in the chronic phase, has been dramatically altered and “chronic” has taken on a new meaning. This being said, imatinib does have its limitations; as much as it has taught us about the possibility of success based on rational drug design and centrality of a disease target, it also promptly stimulated research into how and why resistance to kinase inhibitors occurs, allowing for rapid development and validation of alternative therapies to circumvent known mechanisms. While the number of imatinib failures in CML is small, decreasing over time, and may diminish more with newer therapies, the quest to define, unravel, and address imatinib resistance continues; this chapter will review key elements of this effort.
Imatinib Resistance, Past and Present
The scope of the problem of imatinib resistance in CML is best understood when framed by an understanding of the natural history of CML prior to, and currently in, the “imatinib era,” as well as evolving mechanisms of resistance and milestones of response with current therapy for CML. Prior to imatinib, the main options for therapy were allogeneic stem cell transplant, interferon (IFN)-based therapy, and simple cytoreductive therapy with hydroxyurea. If feasible, transplant was advised to occur without delay beyond 1–2 years given reduction in success rates thereafter. With these options, resistant disease was characterized by persistence or relapse of Philadelphia chromosome (Ph)-positive hematopoiesis despite therapeutic maneuvers such as interferon therapy or allografting. In addition, advancing disease was heralded by in many cases acquisition of secondary chromosome abnormalities in the Ph(+) clone, or clonal evolution. Response to IFN-based therapy sufficient to protect against progressive disease and prolong survival occurred in only a minority of patients, forming the urgency to transplant early, ahead of impending progression. In IFN-responsive patients, observation of Ph(−) clonal cytogenetic abnormalities was exceedingly rare, with fewer than 10 cases in the medical literature. It is important to note that while further cytogenetic abnormalities in Ph(+) cells (Ph-positive clonal evolution) herald disease progression and are a marker of accelerated phase disease, Ph(−) clonal cytogenetic abnormalities occur in the setting of CML response, may become apparent only after significant reduction of the Ph(+) clone, and the etiology and significance of this phenomenon remains unclear.2
The introduction and widespread application of imatinib for the treatment of CML has brought about a new era in the disease, with unprecedented response rates and resistance patterns evolving in parallel to therapy. Whereas delivery of IFN at doses targeted to optimize response was often difficult or impossible due to toxicity, imatinib offers therapy with modest early and minimal late toxicity. Imatinib, as of now, is seemingly deliverable over an indefinite period of time, and places the overwhelming majority of patients into minimal residual disease status with expectation of stability and, even after 5 years of therapy, ongoing improvement in depth of response. Discontinuation of imatinib due to toxicity occurs in less than 5% of patients in all phases of disease, making “imatinib intolerance” a rare event. Rates of cumulative best response in chronic-phase patients from the IRIS trial after 5 years are 98% for complete hematologic response, 92% for major cytogenetic response (MCR) and 87% for complete cytogenetic response (CCR).1 With similar follow-up for patients initially studied in “late chronic phase” disease with intolerance or failure during prior IFN, MCR was achieved in 66% and CCR in 55%,3 reflecting the remarkable ability of imatinib to salvage such patients but also the negative effect of longer disease duration and improvement gained with front-line imatinib, now standard.
Gaps in this highly effective therapy, i.e., failures of imatinib or inadequate response, where risk of relapse or progression are deemed unacceptably high by current standards, can be divided into the broad categories of primary and secondary resistance. Primary resistance to imatinib, defined as an inability to achieve landmark response, is comprised of the 2% of patients who fail to achieve hematologic response and 8–13% who fail to achieve major or complete cytogenetic response using early chronic phase CML treated with imatinib at diagnosis as a benchmark.1 Strictly defining patients with secondary resistance—those who achieve but subsequently lose relevant response—is most straightforward for overt relapse such as loss of cytogenetic or hematologic response and progression from chronic to advanced-stage disease. Again per the benchmark IRIS trial of chronic phase patients with primary imatinib therapy,1 the rate of all progression events, including cytogenetic and hematologic relapse within chronic phase and transformation to advanced phase, is 18% after a median of 5 years. However, such events appear to be most evident in the first 3 years of treatment in the IRIS trial, where progression to advanced phases of disease averaged 2% per year and progression within chronic phase 5% per year; year 4 then showed diminished rates and in year 5 both risks are less than 1%. For chronic-phase patients with intolerance or failure during prior IFN, early, significant response to imatinib predicts for maximal protection from progression3; after 5 years of follow-up, 69% of patients overall have remained free of progression; however, those achieving CCR or MCR by 3 months are 94% and 87% free from progression to AP/BC, respectively, versus only 55% for the remaining patients. The relapse rate in this cohort appeared to be fixed at approximately 7% per year. Lastly, for patients with advanced disease treated with imatinib as salvage therapy, rates of resistance and relapsing disease is dramatically higher, occurring in 75% or more of AP patients and 95% of myeloid BC patients.4
Ph(−) clonal cytogenetic abnormalities, not representing “resistance” per se, nonetheless have been observed to a greater degree with imatinib therapy, likely due to increased response and survival of “late” chronic phase and advanced-phase patients, where prior therapy may have impacted residual normal hematopoiesis. Alternatively, underlying abnormalities or susceptibility to damage in the progenitor cells in which the Ph(+) translocation arises have also been thought to explain this phenomenon. With primary imatinib therapy, most effective at suppressing Ph(+) hematopoiesis, one might expect to unmask such abnormalities to a greater degree if present; to date, however, reports of this finding in primary imatinib-treated patients are less common.2,5 While associated with isolated reported cases of secondary hematopoietic disorders including MDS and Ph(−) acute leukemias,6 this phenomenon is usually benign in course and simply requires careful observation in most cases.
What Is the Basis for Resistance in the Current Era?
Persistence or re-emergence of Ph+ hematopoiesis continues to firmly define clinical resistance; when it occurs despite imatinib, or moreover ABL kinase inhibitors as a class, a large proportion of cases (generally in the rage of ~50%) have a consistent feature observed: acquisition of a point mutation in the Abl kinase domain. Other mechanisms are suspected in patients with wild-type ABL or those with Abl mutations predicted to respond to alternate kinase inhibitors who fail to respond. BCR-ABL amplification at the genomic or transcript level7,8 has been implicated in imatinib failure, overexpression of other tyrosine kinases such as the Scr-related LYN kinase has been observed in the case of BCR-ABL independent resistance,9 and variability in the amount and function of the drug influx protein OCT-1 has been linked to relative insensitivity to kinase inhibition by imatinib.10 It is certainly worthwhile to add to this list the notion of CML “stem cell resistance,” based in the ability of CML progenitors to exchange between a cycling and resting or “quiescent” (G0) state, the latter associated with minimal or no BCR-ABL expression and resulting lack of effect of Abl kinase inhibitors.11
Regarding tyrosine kinase mutation, early investigation into advanced phase CML cases of relapse first revealed critical single amino acid substitutions (mutations)7 within BCR-ABL and reactivation of the kinase. Numerous reports followed, and the spectrum of Abl kinase domain mutations observed in the setting of imatinib spans the entire kinase domain with over 40 mutations identified.12 Abl kinase mutations generally cluster into four main categories and are associated with particular numbered amino acid residues13: ATP binding loop (p-loop), particularly Y253 and E255 mutants; T315 mutants; M351 mutants; and activation loop (a-loop), particularly H396 mutants. Modeling of imatinib and other kinase inhibitors with the crystal structure of the catalytic region of the Abl kinase suggests that mutations may interrupt critical drug contact points or induce or favor a conformation of the Abl kinase in which drug binding is reduced or precluded. Now termed the “gatekeeper” position, mutations at threonine 315 confer resistance both to imatinib and “second generation” Abl kinase inhibitors nilotinib and dasatinib and represent a new challenge already being engaged by newer inhibitors.
While it is accepted that expansion of a Ph(+) CML clone bearing an Abl kinase domain mutation may be associated with resistance to imatinib7,14,–16 and also may herald progression to advanced-phase disease,17,18 the fact that mutations may be identified prior to imatinib exposure and do not strictly correlate with clinical resistance suggests a role for additional mechanisms to trigger outgrowth of mutants, or that genesis of mutant clones reflects greater genetic instability.19 Cytogentic clonal evolution has been linked to mutation detection prior to imatinib and resistance to second generation Abl kinase inhibitors where identified Abl mutations would predict response. These observations support a continued role, past and present, for clonal evolution in resistance and progressive disease.
Controversy remains over the point in time when such mutations are acquired; the same mutant clone has been observed at relapse and in archived samples prior to relapse, speaking to the theory of outgrowth with selection pressure; however, this is not a universal finding and different mutant clones have been observed pre-therapy and at relapse. Beyond the “signature” of a kinase domain mutation (the particular amino acid substitution and kinase region being predictive), the “fitness” of mutant clones—their ability to sustain proliferation with a relative advantage over Ph(−) clones or wild type BCR-ABL—is most relevant to risk; screening for mutations prior to imatinib and for those with stable minimal residual disease are thus scenarios that may be misleading.19,20
One of the most relevant issues for the bulk of current patients and where efforts are intense is the ability to recognize and clarify secondary resistance occurring at the molecular (qPCR) level, i.e., a significant change in minimal residual disease, and to correlate this with risk of kinase domain mutation. This area is “muddied” by variable precision in qPCR assays, with fluctuations in patient results common, and the fact that the threshold of “complete molecular response”—where BCR-ABL transcripts are no longer detectable—is as much a reflection of assay sensitivity as level of patient response. Absence of detectable BCR-ABL transcripts in laboratories with a high degree of sensitivity was previously felt to not be a consistent finding even in the best responding patients21; however, with time and continued improvement in depth of molecular response, a cohort of patients consistently without detectable transcripts is emerging (Timothy P. Hughes, IMVS, Adelaide, Australia, personal communication, 9/27/06). As a result, there is concern that while kinase inhibitor-based therapy options improve and longitudinal responses deepen for CML, the gap between depth of response and ability to confidently quantify minimal residual disease may unfortunately widen. For those patients with change in minimal residual disease status, BCR-ABL transcript level increases as small as a single observed22 or confirmed23 twofold (2 ×) rise may represent proliferating disease and have been associated with increase prevalence of kinase domain mutations and impending clinical resistance. However, a less subtle change such as a fivefold (5 ×) increase or a one-log increase (10 ×) confirmed in a second sample may be more readily identifiable, predictive with the majority of current PCR labs’ techniques, and should warrant investigation for molecular causes and closer follow-up. The depth of BCR-ABL reduction from which change is detected is relevant, and loss of threshold levels of response, such as loss of a major molecular response (e.g., increase to a level representing less than a 3 log reduction in BCR-ABL transcripts) may define patients in need of change in therapy, particularly if linked to a molecular cause such as kinase mutation.
Milestones in Therapy and Defining Failure/Suboptimal Response
NCCN guidelines24 and a recent European LeukemiaNet consensus paper25 cite achievement of CHR by the 3-month mark of therapy as a minimum initial response and lack of hematologic response by 3 months as failure. With current therapies and monitoring strategies, complete hematologic response is quickly established in > 95% of patients in chronic phase. Ongoing peripheral blood count monitoring remains useful to screen for and manage hematologic toxicity, the most common adverse event during therapy, most notably early in the course; relapsing disease should be and can be identified in almost all cases prior to hematologic relapse. Cytogenetic response was with IFN-based therapy and remains now crucial to altering the natural history of CML; risk of any progressive disease and moreover risk of transformation to advanced disease increases in proportion to residual cytogenetic positivity after imatinib. IRIS trial data examining the predictive value of cytogenetic testing early in treatment (3, 6, 9, and 12 months)26 established minimums for cytogenetic response, which are agreed upon.25 Failure to achieve any reduction in Ph(+) cells by cytogenetic testing after 6 months of imatinib and failure to achieve MCR after 12 months of imatinib therapy predicts for less than 20% chance of subsequently achieving CCR. In contrast, much earlier (3 mo) cytogenetic response had been required to optimized outcome in late chronic phase (post-IFN) patients.3 Response beyond these minimums, specifically CCR achieved by 12 months, certainly offers further risk reduction. At key time points, target responses have been identified which incorporate cytogenetic and molecular response and can triage patients into categories of failure, suboptimal and optimal response, based on variance in risk of relapse or progression; a summary of generally accepted response targets for imatinib therapy is listed in Table 1.
Early reduction in BCR-ABL transcript levels by qPCR, other novel early predictors and optimizing monitoring in CML are the subject of another section of this education program. Overall, very early prediction (< 12 mo of therapy) is desired to optimize outcomes but challenging due to variable kinetics of response, increased significance of ultimate level of response rather than speed, and concern over “overcalling” inadequate response. Premature or erroneous labeling of patients as inadequate responders or in “molecular relapse” would have multiple consequences, including the potential to skew clinical trial results and increase the cost and morbidity of therapy of CML. Despite these uncertainties, one threshold level of BCR-ABL transcript reduction, agreed upon to be a 3-log or greater reduction below standard baseline (a major molecular response) occurring in the first 12–24 months of imatinib therapy in the setting of CCR, confers maximal protection from progression to advanced disease (projected transformation-free survival 100%) and the lowest rate of any disease progression with 5 years’ follow-up.1 Major molecular response has emerged as a new target response to achieve and is gaining acceptance as a surrogate for long-term benefit in clinical trials. It may be that despite persistence of minimal residual disease, the concern over relapse stemming from Ph(+) quiescent progenitors, and the fact that relapse is likely upon cessation of imatinib, critical levels of reduction in disease burden have indeed begun to translate to prolonged remission and protection for the majority of patients.
New Therapy to Address Imatinib-Resistant Disease
The addition of dasatinib and impending arrival of nilotinib to the armamentarium for the treatment of CML is like lightning striking twice or three times in the same place; no other cancer has seen rapid development of two highly active second-line therapies following such highly effective, novel front-line therapy. The ingredients for such success stem from the centrality of BCR-ABL in imatinib sensitive and resistant CML, the latter evidenced by selection or genesis of clones in the case of resistant disease with restored BCR-ABL kinase activity. Dasatinib (Sprycel; formerly BMS354825) and nilotinib (formerly AMN107) were brought to clinical trials nearly simultaneously to address the gaps left by imatinib for Ph+ leukemias and as well for potential use in other conditions with relevant inhibitable kinases. Phase I trials in CML for both agents were recently reported27,28 simultaneously in paired articles, and remarkably the results with both agents appear similarly active and reminiscent of early results with imatinib treatment for IFN-resistant CP and advanced phase CML. Both compounds are noted in vitro to be more potent inhibitors of Abl; nilotinib was developed from imatinib, modified to bind the Abl kinase with higher affinity and with less stringent bonding requirements,29 whereas dasatinib was developed as an inhibitor of the Src kinase but found to inhibit BCR-ABL avidly and in the active, as well as the inactive conformation30 required by imatinib for binding. Both compounds inhibit Abl as well as all known mutant Abl kinases in vitro except for one bearing threonine-to-isoleucine substitution at position 315 of Abl (T315I).31
Phase I studies for both agents27,28 included patients with resistant chronic phase disease (n = 40 for dasatinib, n = 17 for nilotinib), with slightly different entry criteria (mainly allowance for imatinib intolerant patients [20% of the total] in the dasatinib study and patients with cytogenetic resistance only [i.e., still in CHR] in the nilotinib trial). The rate of complete hematologic response was identical for both at 92%, as was CCR at 35%, with an additional 10% of patients on dasatinib achieving partial cytogenetic response, bringing the totals for MCR to 45% for dasatinib and 35% for nilotinib. No dose-limiting toxicity was observed for dasatinib, with a range of 15–240 mg per day administered; for nilotinib, dosing at 600 mg BID was limiting, with associated liver (predominantly grade 3 indirect bilirubin and transaminase) and pancreatic enzyme elevations (including grade 2 pancreatitis), as well as one grade 3 subdural hematoma. Extensive monitoring for electrocardiographic changes from nilotinib revealed a 5–15 msec increase in the corrected QT (QTcF). Pleural effusions deemed therapy related were observed in 15 of 84 dasatinib treated patients overall in phase I (13% grade 3–4 in the myeloid blast crisis cohort) and were treated with diuretics and/or drainage. Other higher-grade toxicity from dasatinib included edema, headache, and elevated transaminase levels. Myelosuppression was observed beyond the level seen with imatinib for both agents, and was more pronounced with dasatinib; however, comparison may be difficult due to the fact that patients with imatinib failure and intolerance may be at greater risk due to longer disease duration or other factors. Activity was seen for advanced phases of CML and Ph+ ALL with both agents in phase I. Of note, 70% of patients studied on dasatinib and 41% studied on nilotinib had Abl kinase mutations prior to therapy; in both studies, presence of T315I mutant clones prior to therapy precluded any response and at relapse, detection of T315I was a common finding; patients with other mutations responded to both agents, and patients without mutations responded as well.
Phase II studies for dasatinib32,–35 in all phases of CML and Ph+ ALL have been reported and supported rapid approval of the compound (named Sprycel) on 6/29/06 for both indications; the recommended dose is 70 mg BID. In the “Start-C” trial of dasatinib in CP CML,32 60% of patients required dose reductions over time for toxicity and the median dose was closer to 100 mg per day; ongoing trials continue to explore dosing options for dasatinib, including varying total dose and QD versus BID dosing. Phase II data has been presented for nilotinib, expanding experience with the 400 mg BID dosing,36 –38 and both sets of data are summarized in Table 2 . Results for both agents in chronic phase remain impressive, with the majority of patients achieving sustained hematologic response and approximately one-half MCR and one-third CCR. Advanced-phase results show more limited salvage capability for both agents, particularly for the Ph+ acute leukemias, with early relapse common; in accelerated-phase disease with both agents a subset of responders remains fairly durable, albeit with limited follow-up.
High-dose imatinib early in disease continues to be studied in comparison to standard dose, with randomized trials ongoing and data forthcoming; further update of previously published single center experience39 now shows similar ultimate depth of response for both 400 and 800 mg dosing, yet increased rapidity of response and also potentially lower risk of progression for higher dose imatinib. A randomized trial of dasatinib (70 mg BID) versus imatinib 800 mg for patients with hematologic or cytogenetic resistance to lower dose imatinib (400–600 mg)40 reported early improvement in CCR for dasatinib over high dose imatinib (21% vs. 8% at 3 months) and prolonged time to treatment failure, prompting greater interest in planned studies comparing dasatinib or nilotinib to dose escalation of imatinib at earlier recognition of resistant or suboptimally responding disease.
In addition to exploration earlier in the course of CML, the ubiquitous issue of “stem cell resistance” remains a challenge for new Abl kinase inhibitors; with a goal of more definitive disease reduction or potential elimination, dasatinib has been studied, and while able to “reach” deeper into the earlier progenitor pool, the most primitive CML cells remain resistant to both imatinib and dasatinib.41
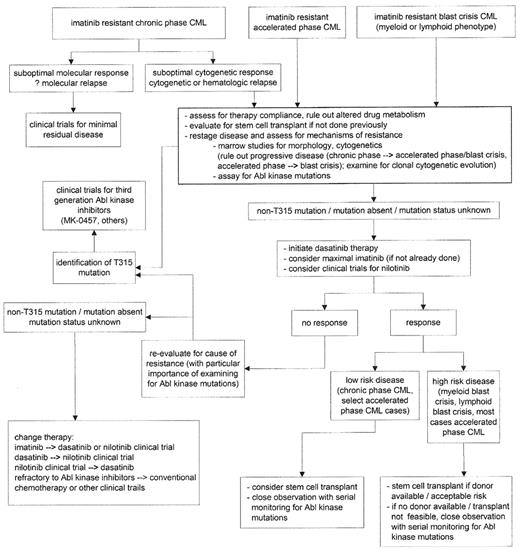
With the ability to utilize Abl kinase and kinase mutant structure-function analysis, the aurora kinase inhibitor MK-0457 (formerly VX-680)42 and others are emerging with the expectation of ability to overcome the T315I mutant kinase; MK-0457 is active in patient cell samples in vitro bearing the T315I mutation and clinical trial reports are imminent from these compounds, filling the most current gap in targeted therapy for CML. The use of combinations of agents to circumvent resistance has strong rationale from in vitro studies, including combinations of imatinib with both second-generation inhibitors43 and combinations of nilotinib and dasatinib,44 and clinical trials are planned in order to explore development of a potential “cocktail” of kinase inhibitors to obviate development of resistance. The role and timing of stem cell transplant in the course of CML is the topic of another section of this volume; however, allogeneic SCT remains an option offering long-term remission/”cure” for CML. Although utilized differently in the current era of Abl kinase inhibitors for CML, the potency of the graft-versus-leukemia effect cannot be overlooked as a proper consolidation option after disease salvage with second-generation inhibitors, or as an alternative to unacceptably high relapse/progression risk most often associated with disease unstable or unresponsive during nontransplant therapy. A potential algorithm for navigating treatment options for patients with imatinib-resistant disease is presented in Figure 1 .
The Future
Of course, anticipation of the potential to use more aggressive and comprehensive targeted therapy up front in CML is great; currently the overwhelming majority of patients treated with imatinib have excellent disease control, low risk of progression, but not disease eradication. The forces at play in clinically resistant disease, especially Abl kinase mutations, however, do not seem to pose risk to such patients currently in response; goals of incorporating therapy beyond imatinib for chronic phase CML might include optimization of molecular response, given that timely reduction of BCR-ABL transcripts by 3 logs or greater currently offers maximal protection from relapse, or better prospects for the ability to cease therapy at some point. Data from emerging trials will be crucial to help understand the risk/benefit balance of such options against the current 5+ years of success with imatinib. Without a doubt, despite the concern for all and reality for some of resistance, the chinks in the CML therapy armor continue to be filled.
Suggested thresholds defining failure, suboptimal response, and optimal response.
| . | 3 Months . | 6 Months . | 12 Months . | 18 Months . |
|---|---|---|---|---|
| Failure | No hematologic response | > 95% Ph+ | > 35% Ph+ | > 0% Ph+ |
| Suboptimal Response | No complete hematologic response | 35–95% Ph+ | 1–35% Ph+ | 0% Ph+, < 3 log ⇓ in BCR-ABL transcripts |
| Optimal response | 1–2 log ⇓ in BCR-ABL transcripts | < 35% Ph+ | 0% Ph+, ≥ 3 log ⇓ in BCR-ABL transcripts | 0% Ph+, ≥ 3 log ⇓ in BCR-ABL transcripts |
| . | 3 Months . | 6 Months . | 12 Months . | 18 Months . |
|---|---|---|---|---|
| Failure | No hematologic response | > 95% Ph+ | > 35% Ph+ | > 0% Ph+ |
| Suboptimal Response | No complete hematologic response | 35–95% Ph+ | 1–35% Ph+ | 0% Ph+, < 3 log ⇓ in BCR-ABL transcripts |
| Optimal response | 1–2 log ⇓ in BCR-ABL transcripts | < 35% Ph+ | 0% Ph+, ≥ 3 log ⇓ in BCR-ABL transcripts | 0% Ph+, ≥ 3 log ⇓ in BCR-ABL transcripts |
Phase II results for nilotinib and dasatinib across all phases of chronic myeloid leukemia (CML).
| Drug . | Disease State . | N . | Hematologic Response (%) . | CHR (%) . | Any Cytogenetic Response (%) . | Minor CyR (%) . | MCR (%) . | CCR (%) . | Median F/U (mos) . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|---|
| *Nilotinib response in Ph+ ALL was recorded as “complete response” (= hematologic recovery + < 5% marrow blasts); | ||||||||||
| Abbreviations: ALL, acute lymphoblastic leukemia; AP, accelerated phase; CCR, complete cytogenetic response; CP, chronic phase; LBC, lymphoid blast crisis; MBC, myeloid blast crisis; MCR, major cytogenetic response; NR, not reported. | ||||||||||
| Nilotinib (AMN107) | ||||||||||
| Imatinib refractory / intolerant CP CML | 81 | NR | 69 | 68 | 11 | 46 | 32 | 6 | 36 | |
| Imatinib refractory / intolerant AP CML | 25 | 40 | 16 | 56 | 8 | 28 | 16 | 5.5 | 37 | |
| Imatinib refractory / intolerant MBC CML | 13 | 8 | NR | NR | NR | 16 | 8 | 2.5 | 38 | |
| Imatinib refractory / intolerant LBC CML | 6 | NR | 17 | NR | NR | 50 | 33 | 2.5 | 38 | |
| Imatinib refractory / intolerant Ph+ ALL | 15 | 27* | NR | NR | NR | NR | NR | 2.3 | 38 | |
| Sprycel (Dasatinib) | ||||||||||
| Imatinib refractory / intolerant CP CML | 387 | NR | 90 | NR | NR | 51 | 40 | 8 | 32 | |
| Imatinib refractory / intolerant AP CML | 174 | 59 | 34 | 39 | 5 | 34 | 25 | 7 | 33 | |
| Imatinib refractory / intolerant MBC CML | 109 | 49 | 25 | 44 | 13 | 31 | 25 | 3.5 | 34 | |
| Imatinib refractory / intolerant LBC CML | 48 | 39 | 29 | NR | NR | 44 | 38 | 2.3 | 35 | |
| Imatinib refractory / intolerant Ph+ ALL | 46 | 48 | 33 | NR | NR | 46 | 44 | 2.7 | 35 | |
| Drug . | Disease State . | N . | Hematologic Response (%) . | CHR (%) . | Any Cytogenetic Response (%) . | Minor CyR (%) . | MCR (%) . | CCR (%) . | Median F/U (mos) . | Ref. . |
|---|---|---|---|---|---|---|---|---|---|---|
| *Nilotinib response in Ph+ ALL was recorded as “complete response” (= hematologic recovery + < 5% marrow blasts); | ||||||||||
| Abbreviations: ALL, acute lymphoblastic leukemia; AP, accelerated phase; CCR, complete cytogenetic response; CP, chronic phase; LBC, lymphoid blast crisis; MBC, myeloid blast crisis; MCR, major cytogenetic response; NR, not reported. | ||||||||||
| Nilotinib (AMN107) | ||||||||||
| Imatinib refractory / intolerant CP CML | 81 | NR | 69 | 68 | 11 | 46 | 32 | 6 | 36 | |
| Imatinib refractory / intolerant AP CML | 25 | 40 | 16 | 56 | 8 | 28 | 16 | 5.5 | 37 | |
| Imatinib refractory / intolerant MBC CML | 13 | 8 | NR | NR | NR | 16 | 8 | 2.5 | 38 | |
| Imatinib refractory / intolerant LBC CML | 6 | NR | 17 | NR | NR | 50 | 33 | 2.5 | 38 | |
| Imatinib refractory / intolerant Ph+ ALL | 15 | 27* | NR | NR | NR | NR | NR | 2.3 | 38 | |
| Sprycel (Dasatinib) | ||||||||||
| Imatinib refractory / intolerant CP CML | 387 | NR | 90 | NR | NR | 51 | 40 | 8 | 32 | |
| Imatinib refractory / intolerant AP CML | 174 | 59 | 34 | 39 | 5 | 34 | 25 | 7 | 33 | |
| Imatinib refractory / intolerant MBC CML | 109 | 49 | 25 | 44 | 13 | 31 | 25 | 3.5 | 34 | |
| Imatinib refractory / intolerant LBC CML | 48 | 39 | 29 | NR | NR | 44 | 38 | 2.3 | 35 | |
| Imatinib refractory / intolerant Ph+ ALL | 46 | 48 | 33 | NR | NR | 46 | 44 | 2.7 | 35 | |
Potential algorithm for treatment options, imatinib-resistant chronic myelogenous leukemia (CML).
Potential algorithm for treatment options, imatinib-resistant chronic myelogenous leukemia (CML).