Abstract
Defined by isolated del 5q and no excess of marrow blasts, the “5q– syndrome” is a specific type of myelodysplastic syndrome (MDS) with particular characteristics, including severe anemia, frequent thrombocytosis, typical dysmegakaryopoiesis and favorable outcome. Its pathogenesis remains uncertain, in particular the role of inactivation of gene(s) situated in 5q. It should be differentiated from other MDS with del 5q having an excess of marrow blasts and/or additional cytogenetic abnormalities, which carry a poor prognosis.
Until the advent of lenalidomide, repeated RBC transfusions were generally the only treatment of the 5q– syndrome, which was resistant to other therapeutic approaches. Lenalidomide can lead to RBC transfusion independence in at least two thirds of cases of the 5q– syndrome, two thirds of those responses persisting after 2 years of treatment. Importantly, not only reversal of anemia but also frequent complete pathological and cytogenetic responses are obtained. Grade 3 or 4 neutropenia and thrombocytopenia, especially during the first 6 to 8 weeks of treatment, are the major side effect of lenalidomide, justifying close monitoring of blood counts and regular patient visits.
Preliminary results suggest that lenalidomide is also very active in MDS with del 5q other than the 5q–syndrome. Although its mechanism of action remains uncertain, lenalidomide appears to target specifically the del 5q clone. By doing this, lenalidomide may have an effect on disease course and survival, which is currently being assessed in clinical trials.
Described by Van den Berghe et al,1 the “5q– syndrome” is a paradigm for myelodysplastic syndrome (MDS) due to its particular hematological characteristics, its still unknown pathogenesis and, until recently, by its truly “refractory” anemia, requiring in most cases frequent RBC transfusions. Its short-term and possibly long-term evolution has been, however, recently modified by the advent of lenalidomide.
I. MDS with del 5q and the “5q– Syndrome”
1. Hematological characteristics
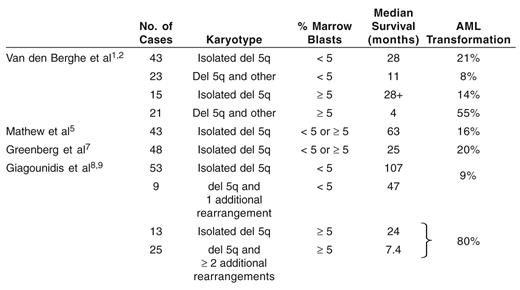
Van den Berghe et al reported, in 1974, a type of refractory anemia characterized by female predominance (unlike other MDS), pronounced macrocytosis, frequent erythroblastopenia (unusual for MDS), absent or mild leukopenia, normal or elevated platelet counts, abnormal large monolobulated megakaryocytes with excentered nucleus, usually normal (< 5%) bone marrow blast percentage, isolated deletion of the long arm of chromosome 5, rare progression to acute myeloid leukemia (AML) (about 10%) and prolonged survival (Table 1 ).1,2 Anemia, however, tended to be profound, requiring multiple RBC transfusions and resulting in frequent, often fatal, hemochromatosis. The authors also reported that isolated del 5q could be found in higher grade MDS (RAEB, RAEB-T) or AML. In RAEB, but not in RAEB-T and AML, other features of the 5q– syndrome (in particular thrombocytosis and monolobulated megakaryocytes) were often present. Finally, Van den Berghe et al found that MDS with del 5q and additional cytogenetic abnormalities generally did not have “typical” features of the 5q– syndrome, especially if there was more than one additional cytogenetic change.
The WHO classification more recently redefined the 5q– syndrome as a specific type of MDS, also with isolated del 5q but restricting the entity to cases without excess of marrow blasts (i.e., < 5% blasts).3 When those two restrictions (no chromosomal abnormality in addition to del 5q and no excess of marrow blasts) are applied, MDS with del 5q indeed generally have most of the features described in Table 1 , and as seen below, prolonged survival.
2. Cytogenetic and genetic characteristics
Large patient series have shown that, in MDS with del 5q, the chromosomal deletion was interstitial, of variable size, but with a predominance for large 5q13–33 deletions.1,2,4–9 Both proximal and distal breakpoints were variable, and the only region deleted in almost all reported cases was band 5q31–32 (“common deleted band”).
Cytogenetic and FISH analysis in marrow progenitors have shown, in MDS with del 5q, that the deletion was generally present in pluripotent hematopoietic stem cells (CD34+, CD38–), and the persistence of normal progenitors in the bone marrow of MDS patients with del 5q.10 On the other hand mature lymphocytes, in MDS patients with del 5q–, do not seem to be involved in the 5q clone, and del 5q in those patients is generally present in only 35 to 50% of erythroblasts.11 Those findings may have different explanations, including inability of dysplastic stem cells to differentiate in several pathways, or the possibility that del 5q may be a secondary genetic event.
The presence of a recurring deletion of band 5q31–32 suggested the presence of tumor suppressor gene(s) in that chromosomal region, whose inactivation (through deletion of one allele, the other being inactivated by different possible mechanisms) played a major pathogenetic role in development of the 5q– syndrome. Several groups, especially the Oxford group and the Chicago group, have identified by molecular analysis of the common deleted band 2 “common deleted regions,” containing in particular numerous genes coding for hematopoietic growth factors.12–15 However, in spite of at least two decades of considerable work, the gene(s) responsible for the development of the 5q– syndrome have not been identified. Several candidate genes have been suspected at some point by research groups, because they were inactivated in most if not all del 5q MDS patients. However, those findings were not confirmed later by other groups. This has eventually led to suggest that different genes on 5q could be involved (a theory possibly difficult to reconcile with the very specific hematological features of the 5q– syndrome, unless the different genes acted in the same or in a similar pathways), or that inactivation of only one allele of gene(s) located on 5q could be sufficient to pathogenesis (haploinsufficiency). Gene expression profiles of CD34+ cells in MDS with del 5q appear to be distinct from those of other MDS, with (not unexpectedly) downregulation of genes assigned to 5q, but also upregulation of the histone HIST1 gene cluster located in 6p21 and of genes related to the actin skeleton.16
In MDS with del 5q, it is also unclear if there are cytogenetic and/or genetic differences between the deletion observed in the 5q– syndrome and the deletion observed in other MDS with del 5q (i.e., with additional cytogenetic abnormalities and/or excess of marrow blasts), in particular if the common deleted region and the gene(s) involved in 5q31–32 are different. Presence of an excess of blasts or of additional chromosomal abnormalities, in MDS with del 5q, may indeed also result from the occurrence of secondary genetic events. The dramatic activity of lenalidomide on MDS with del 5q, irrespective of whether it is the 5q–syndrome or whether there is an excess of marrow blasts and/or additional cytogenetic abnormalities, possibly argues for a unique pathogenetic mechanism. Finally, N ras and p53 mutations are very rarely seen in MDS with del 5q.17 JAK2 mutations are also rare in our experience in MDS with del 5q (6% of the cases) and are associated with increased marrow cellularity, and a trend for high platelet and WBC count.18
3. Outcome and prognostic factors
All studies have confirmed the favorable outcome of MDS with isolated del 5q and no excess of marrow blasts, i.e., of the 5q– syndrome according to WHO classification (Table 2 ).1,2,4–9 The Dusseldorf group, however, showed that median survival of patients with the 5q– syndrome (107 months), was significantly less than that of a sex- and age-matched control population, in both men and women.8
By contrast, median survival of MDS with isolated del 5q and marrow blasts > 5 % is shorter (only 24 months in the Dusseldorf group) and their risk of progression to AML higher. Likewise, the risk of progression to AML dramatically increases, and median survival is greatly shortened when chromosomal abnormalities in addition to del 5q are seen, especially if there is more than one.8
II. Treatment of the 5q– Syndrome and Other MDS with del 5q before the Advent of Lenalidomide
It remained largely dominated by RBC transfusions, other treatment modalities being rarely applicable or having limited efficacy. Published results of those approaches (where different types of MDS with del 5q based on % marrow blasts and presence or not of additional cytogenetic findings were not always individualized) are summarized in Table 3.
1) RBC transfusions
Before the advent of lenalidomide, treatment of the 5q–syndrome indeed mainly consisted of repeated RBC transfusions. Transfusion requirement, in the 5q– syndrome, tends to increase with time, and is generally of at least 2 RBC concentrates/month after a few years of evolution.1,2,4–9 RBC transfusion treatment, although it transiently improves hemoglobin level, carries in MDS in general several disadvantages: (i) It is time consuming for the patient and makes him (her) dependent on regular hospital stays and medical staff; (ii) It represents a burden to health systems. For example, in France, the number of RBC concentrates transfused in MDS represents 3% of all RBC transfused in the country, and MDS account for 24% of hospital stays for transfusions (Groupe Français des Myélodysplasies [GFM], unpublished data); (iii) It has potential complications: iron overload (which, for its prevention, requires chelators), volume overload, frequently symptomatic in elderly patients, immunization and, even if they have become very rare, bacterial and viral infections; (iv) It is expensive: the mean monthly cost of RBC transfusions was evaluated at about 800 euros in transfused MDS patients by blood banks of the French region of Franche Comté (GFM, unpublished data). This cost includes the cost of transfusion itself (RBC concentrates, immunological and viral blood tests, staff salaries), iron chelators, cost of transportation to and from the hospital, etc.; (v) Finally, and perhaps more importantly, RBC transfusions only transiently improve Hb levels, and regularly transfused patients spend most of their time with Hb levels below 10 g/dL, a situation significantly associated with fatigue and other symptoms of anemia, and decreased quality of life.19 An improvement can be obtained by trying to transfuse patients at higher thresholds (i.e., 9 to 10 g/dL, instead of the “usual” 8 g/dL threshold), avoiding major symptoms of anemia. This is, however, not a general practice, especially for logistical reasons.
2) Recombinant erythropoietin, thalidomide and retinoids
Because of the drawbacks of RBC transfusion treatment, clinicians tend to use more and more frequently, in low-risk MDS, agents capable of improving RBC production, especially erythropoietin (EPO) and its derivatives. Response to EPO (alpha or beta) or darbepoetin alpha, with or without granulocyte colony-stimulating factor (G-CSF), however, appears to be lower in the 5q– syndrome than in other low risk MDS. By reviewing published series of MDS treated with EPO or darbepoetin alpha (+/− G-CSF) we found 11 responses in 41 treated patients.20–22 The proportion of major and minor responses and their duration were generally not known. In a retrospective analysis of 419 MDS patients treated with EPO or darbepoetin alpha (G-CSF) in France, 48 had del 5q (presented at ASH 2006). Of 17 cases that fulfilled criteria of the 5q– syndrome, 9 (52%) responded, all the responses being major according to IWG response criteria.23 However, the median duration of response was 12 months, significantly shorter than that of the other low-risk MDS in our series (24 months) (GFM, presented at ASH 2006). In addition, the response rate was 13 in 31 in other MDS with del 5q (with additional cytogenetic anomalies and/or > 5% blasts).
We treated with thalidomide, in two successive trials, 20 patients with the 5q– syndrome; 3 major and 3 minor erythroid responses were achieved (24 and GFM, presented at ASH 2006). All-trans retinoic acid (ATRA), which can improve cytopenias in some low-risk MDS, appears to have limited efficacy in the 5q– syndrome, with 12 generally minor responses in 60 patients treated by the Dusseldorf group.25,26
3) Chemotherapy and hypomethylating agents
Four responses were reported in 9 low-risk MDS with del 5q treated with low-dose Ara C. However, this drug, even at such low doses, is associated with relatively major myelosuppression.27 In the rare relatively young patients (capable of receiving intensive chemotherapy) having MDS with del 5q and high-risk characteristics (with or without progression to AML) poor response to high-dose chemotherapy was seen.28
Too few cases of MDS with del 5q treated with the hypomethylating agents 5-azacytidine and decitabine have been reported to determine their response to those drugs. Interestingly, however, complete hematological responses accompanied by cytogenetic responses with a progressive decrease of the clone over time have been reported with decitabine by Lubbert in 1/1 and 3/4 high-risk MDS with isolated del 5q and del 5q and additional chromosomal abnormalities, respectively.29 In the MD Anderson experience, 2 of 16 MDS with del 5q showed hematological response to decitabine.30
4) Allogeneic bone marrow transplantation
In relatively younger patients with an HLA-identical donor, allogeneic stem cell transplantation probably remains the only curative approach. In a large report, 30 patients with isolated del 5q had a relapse-free survival of 68% at one year, while this rate fell at 22% when additional karyotypic abnormalities were present.31
5) Conclusion
Before the advent of lenalidomide, treatments available were rarely effective, or for only short periods of time, in MDS with del 5q, and almost all those patients eventually required repeated RBC transfusions, often during years.
III. Treatment of the 5q– Syndrome and Other MDS with del 5q with Lenalidomide
1. Overall results
Lenalidomide is an immunomodulatory drug (IMiD) with an array of biological properties, including the suppression of pro-inflammatory cytokine production by monocytes, enhancement of T- and NK-cell activation and inhibition of angiogenesis. It has greater potency than thalidomide, especially for antiangiogenic and anti-TNF properties. Unlike thalidomide, it is nonteratogenic in animal models and does not lead to peripheral neuropathy in humans.32
In a landmark paper, List et al showed that lenalidomide gave an erythroid response (major or minor) in about 50% of RBC transfusion–dependent low-risk MDS, but that response was particularly marked (83% transfusion independence obtained) in patients with del 5q.33 Moreover, histological and cytogenetic responses including complete ones were reported, principally in del 5q patients. Myelosuppression, with neutropenia and thrombocytopenia, was the major side effect, requiring dose reduction from 25 mg/d to 10 mg/d or even less in most patients. Myelosuppression was particularly marked in patients with del 5q.
Results of a subsequent multicenter large phase II trial of lenalidomide in MDS with del 5q (MDS 003 study) have been reported at previous ASH and ASCO meetings, with a median follow-up of treatment now reaching 67 weeks, and with 42% of the patients having received at least 2 years of treatment.34 This study included 148 red blood cell transfusion–dependent patients. Median age was 71; 66% of the patients were females, 20% had ≥ 5% marrow blasts, 74% had isolated del 5q, 17% had 1 and 8% had ≥ 2 additional cytogenetic changes, respectively. Patients received lenalidomide 10 mg/d continuously or 10 mg/d 3 weeks on, 1 week off (the 2 doses were not randomized). Overall, 67% of the patients achieved transfusion independence (major erythroid response) and 9% had minor erythroid response (with ≥ 50% reduction of transfusion requirement). Median time to transfusion independence was 4.6 weeks. Most patients responded rapidly; however, a small percentage of patients became transfusion independent after as much as 11 months.
At last update, 63% of the major responders remained transfusion free, while 37% had relapsed (i.e., required RBC transfusions again). Therefore, median duration of major erythroid response was not reached. Median increase in Hb level was 5.4 g/dL (range 1.1 to 11.4). Interestingly, in our preliminary experience with lenalidomide in 20 RBC transfusion-dependent low and int 1 MDS with del 5q, 3 patients achieved Hb level ≥ 15.5 g/dL and required phlebotomies (Fenaux et al, unpublished). Lenalidomide did not only induce erythroid response. Indeed, complete cytogenetic response (disappearance of del 5q) was seen in 45% of patients and minor cytogenetic response (> 50% reduction in del 5q mitoses) in 28% of patients. Complete histological response was also seen in 36% of the patients.
Myelosuppression was a constant finding, with grade 3 or 4 neutropenia and thrombocytopenia in 55% and 44% of the cases, respectively. Neutropenia and thrombocytopenia, however, tended to improve after the first 6 to 8 weeks of treatment, in parallel with erythroid response. Three deaths, attributed to infection, however, occurred during the period of grade 4 neutropenia. Of note is that grade 3 or 4 thrombocytopenia occurred even in patients with thrombocytosis at inclusion. For example, 1 of our patients with 1,200,000/mm3 platelets at onset of lenalidomide developed grade 3 thrombocytopenia (Fenaux et al, unpublished). Other side effects reported in > 10% patients included diarrhea, muscle cramps, pruritus, rash, dizziness, but they rarely reached grade 3 or 4. Dose reduction was required at some point in 80% of the patients, mainly due to neutropenia and/or thrombocytopenia. Furthermore, in the 51 patients who remained transfusion free at the date of analysis, 21 (41%) received 5 mg/day and 16 (31%) received 5 mg every other day, indicating that response can be maintained at low dose of lenalidomide in many of the patients who require dose reduction for tolerability reasons. No significant difference in results between 10 mg/d and 10 mg/d, 3 weeks on, 1 week off were seen.
Finally, with the current price of lenalidomide in France, the monthly cost of this treatment would range from 900 euros (5 mg/every second day dosing) to 2600 euros (10 mg/day, 3 weeks every month dosing). By comparison, as seen above, the mean monthly cost of transfusions for MDS in France has been evaluated at 800 euros. However, this does not take into account the fact that MDS with del 5q are generally more transfused than the average MDS and the different effect on quality of life (especially fatigue) of having stable Hb levels above 12 g/dL versus being regularly transfused. In addition, having stable normal hemoglobin level may allow to pursue normal activities, including work.
2. Results in the 5q– syndrome and in other MDS with del 5q
Although transfusion independence was more frequently achieved in patients with IPSS low (71%) and int 1 (63%), 3 of the 8 patients with IPSS int 2 or high also achieved major erythroid response. In addition, cytogenetic response was similar in patients with the 5q– syndrome (about 50% complete and 30% partial responses) and in patients with excess of marrow blasts, and/or additional cytogenetic abnormalities. Finally, 12 of 16 patients with increased % of marrow blasts at treatment onset reached a bone marrow blast % of less than 5%. Still, 82% of patients with the 5q–syndrome who responded remained transfusion free, as compared to 56% of the responders with increased % marrow blasts and/or additional cytogenetic abnormalities.
3. Unanswered questions about lenalidomide treatment of MDS with del 5q
Lenalidomide therefore appears to be a major breakthrough in the treatment of the 5q– syndrome, and possibly also in MDS with del 5q and increased % marrow blasts and/or additional cytogenetic abnormalities. Because this drug has only recently become available, however, many questions remain regarding its use:
What is the optimal dose of lenalidomide? Contrary to myeloma, where 25 mg is a common daily dose, only lower doses are generally tolerated in MDS with del 5q, i.e., 10 mg/day, or 10 mg/day 3 weeks on, 1 week off or even less (5 mg/day or every second day). Such lower doses may be as effective as higher doses in many patients. A randomized European trial (MDS 004) is currently comparing a daily dose of 10 mg (3 weeks on/1 week off) and 5 mg (continuous schedule) in terms of efficacy and toxicity in low and int 1 risk MDS with del 5q.
How can lenalidomide-induced cytopenias be managed? Cytopenias are a common finding in myeloma treated with lenalidomide, but grade 3 or 4 (to extent transient) cytopenias after the initiation of treatment appear to be particularly pronounced in MDS with del 5q. In our experience and that of other groups, neutropenia can be corrected by the addition of G-CSF, which we recommend to start as soon as neutrophils decrease below 1000/mm3. Thrombocytopenia is more difficult to treat, except by platelet transfusions and/or transient treatment discontinuation or dose reduction. Current recommendations are to discontinue treatment if platelets are lower than 25,000/mm3, and restart at lower dose when platelets increase above that threshold. As thrombocytopenia and neutropenia are to some extent transient with lenalidomide in patients with the 5q–syndrome, it may be possible, in some cases, to increase the lenalidomide dose after their correction and improve erythroid responses. In our experience G-CSF, when required for neutropenia < 1000/mm3, can generally be stopped after 2 to 3 months of treatment.
How long should treatment be administered in responders? This question cannot be answered at the moment. First, it is not well known after what latency anemia recurs in responders who have discontinued treatment. Second, because lenalidomide appears to somewhat specifically target the 5q– clone, one may have to continue treatment for as long as possible in order to maintain the MDS clone in a quiescent state for prolonged periods. For the same reason, in patients who have excessive erythroid response, it is uncertain whether the lenalidomide dose should be reduced (with the risk of being less effective on clonal disease) or whether it should be maintained, and phlebotomies performed. When relatively high Hb levels are obtained, it is also recommended, in order to reduce potential thromboembolic risk, to administer low-dose aspirin, although the benefit of this treatment has not been thoroughly evaluated.35
Has lenalidomide an impact on disease course in MDS with del 5q? This is a major issue, for which no response is as yet possible. Other drugs active in low-risk MDS, like EPO, appear to have very limited effect if any, on disease course and survival. A large study with long-term follow-up indeed showed that EPO neither accelerated nor slowed progression to AML and did not modify survival.36
In the case of MDS with del 5q treated with lenalidomide, the cytogenetic responses (including complete ones) and the normalization of the blast percentage observed in a high proportion of patients with increased percent of marrow blasts suggest a specific (or at least preferential) activity of lenalidomide on the 5q– clone. Interestingly, in MDS 003 study, some complete cytogenetic responses were seen in patients with del 5q and additional cytogenetic findings, suggesting that clones with del 5q and additional (putatively secondary) abnormalities may also be specifically targeted by lenalidomide.34
Because del 5q appears to be an early genetic event in the pathogenesis of MDS with del 5q, occurring as seen above in pluripotent hematopoietic stem cells,10 it may be hypothesized that targeting the 5q– clone and reducing its size may slow disease progression and, possibly, prolong survival. Demonstrating this effect in the 5q– syndrome may require years of follow-up, due to the slow evolution and prolonged survival of this category of patients. In other MDS with del 5q, where the progression rate to AML is much higher and survival much shorter, it may however prove easier to demonstrate.
IV. Mechanism of Action of Lenalidomide in MDS with del 5q
Lenalidomide may have different mechanisms of action in MDS with del 5q. Buesche et al found that response to lenalidomide was correlated to inhibition of angiogenesis, but that this effect was not associated with VEGF and KDR (VEGF receptor) downregulation.38 In fact, the complete hematological and cytogenetic responses observed in a substantial proportion of MDS patients with del 5q suggest that lenalidomide may specifically target the del 5q clone. The transient period of profound neutropenia and thrombocytopenia seen after onset of lenalidomide also suggests disruption of a predominantly clonal hematopoiesis, resulting from the del 5q clone, subsequently replaced by expansion of normal residual clones. The disappearance of thrombocytosis with normalization of platelet counts, in responders, is in agreement with this hypothesis. Another argument comes from in vitro studies, showing that lenalidomide can selectively abrogate the growth of myeloid cell lines with del 5q.37,39
If confirmed, it would remain to determine how lenalidomide can selectively target cells with del 5q. One hypothesis could be interference of lenalidomide with one or several protein(s) encoded by gene(s) located in 5q and with a major role on cell survival. At reduced level resulting from haploinsufficiency, function of those proteins could be inhibited by lenalidomide, eventually leading to cell death. In a chromosome 5 deleted cell line very sensitive to lenalidomide (Namalwa CSN.10 cell line), Gandhi et al showed that the drug induced G0/G1 cell cycle arrest, inhibited Akt and Gab1 phosphorylation, and inhibited the ability of Gab1 to associate with a receptor tyrosine kinase.39
Such specific mechanisms of action, if confirmed, would place lenalidomide in the small group of targeted treatments currently available in the treatment of hematological malignancies.
V. Conclusion
Lenalidomide has a dramatic effect on anemia of MDS with del 5q, especially in patients with the 5q– syndrome, and has become the first-line treatment in those patients, approved by the FDA in December 2005 (indication: IPSS low and int 1 MDS with del 5q). However, lenalidomide can also normalize platelet counts and induce hematological and cytogenetic response, often complete, in those patients. Although its mechanism of action may be multiple, lenalidomide appears to specifically target the del 5q clone. This suggests that this drug may have also an effect on disease course. In particular, lenalidomide appears to be active in int 2 or high-risk MDS with del 5q (with increased percentage of blasts and/or cytogenetic abnormalities in addition to del 5q) and could particularly have an impact on disease course and survival in this subset of patients with poor prognosis. Larger studies will have to confirm this hypothesis, however. The effect of lenalidomide is also currently tested in low-risk MDS without del 5q. Studies combining lenalidomide and other drugs (especially EPO) in low-risk MDS with anemia (with or without del 5q) are also in progress.
Finally, lenalidomide remains an analogue of thalidomide. Although it has no teratogenetic effect in animal models, females should formally avoid pregnancy while on lenalidomide.
| Clinical and Biological Features |
| Female preponderance |
| Severe anemia |
| Pronounced macrocytosis |
| Normal or moderately decreased leucocytes |
| Normal or moderately increased platelets |
| Rare AML transformation (10%) |
| Prolonged survival |
| Bone Marrow Features |
| Characteristic dysmegakaryopoiesis (large monolobulated megakaryocytes with excentred nucleus) |
| No or moderate blast excess (restricted to marrow blasts < 5% in the WHO classification3) |
| Isolated 5q deletion |
| Clinical and Biological Features |
| Female preponderance |
| Severe anemia |
| Pronounced macrocytosis |
| Normal or moderately decreased leucocytes |
| Normal or moderately increased platelets |
| Rare AML transformation (10%) |
| Prolonged survival |
| Bone Marrow Features |
| Characteristic dysmegakaryopoiesis (large monolobulated megakaryocytes with excentred nucleus) |
| No or moderate blast excess (restricted to marrow blasts < 5% in the WHO classification3) |
| Isolated 5q deletion |
Progression to acute myeloid leukemia (AML) and survival in main published series of MDS with del 5q.
Treatment (other than with lenalidomide) of anemia in myelodysplastic syndrome (MDS) with del 5q (no. responders/no. treated patients).
| . | Type of MDS with del 5q . | Published Series . | GFM Experience . |
|---|---|---|---|
| Abbreviations: GFM, Groupe Francophone des Myélodysplasies; Epo, erythropoietin; G-CSF, granulocyte colony-stimulating factor; ATRA, all trans-retinoic acid | |||
| Epo or darbepoetin | 5q– syndrome | 4/8 (2 major, 2 minor) | 9/17 (9 major) |
| +/− G-CSF20–22 | Other MDS with del 5q | 7/37 | 13/31 (6 major, 7 minor) |
| Thalidomide24 | Low-risk MDS with del 5q | Not determined | 6/20 (3 major, 3 minor) |
| ATRA25,26 | Low-risk MDS with del 5q | 12/60 (mainly minor responses) | |
| Low-dose cytarabine27 | Low-risk MDS with del 5q | 4/9 (3 major, 1 minor) | |
| Decitabine | |||
| Lubbert et al29 | Isolated del 5q and > 5% blasts | 1/1 (1 complete hematological and major cytogenetic response) | |
| Del 5q and other cytogenetic abnormalities, and > 5% blasts | 3/4 (3 complete hematological and major cytogenetic responses) | ||
| Kantarjian et al30 | High-risk MDS with del 5q | 2/16 | |
| Allogeneic bone marrow transplantation31 | Isolated del 5q Del 5q and others | 13/20 relapse-free at 1 year 8/37 relapse-free at 1 year | |
| . | Type of MDS with del 5q . | Published Series . | GFM Experience . |
|---|---|---|---|
| Abbreviations: GFM, Groupe Francophone des Myélodysplasies; Epo, erythropoietin; G-CSF, granulocyte colony-stimulating factor; ATRA, all trans-retinoic acid | |||
| Epo or darbepoetin | 5q– syndrome | 4/8 (2 major, 2 minor) | 9/17 (9 major) |
| +/− G-CSF20–22 | Other MDS with del 5q | 7/37 | 13/31 (6 major, 7 minor) |
| Thalidomide24 | Low-risk MDS with del 5q | Not determined | 6/20 (3 major, 3 minor) |
| ATRA25,26 | Low-risk MDS with del 5q | 12/60 (mainly minor responses) | |
| Low-dose cytarabine27 | Low-risk MDS with del 5q | 4/9 (3 major, 1 minor) | |
| Decitabine | |||
| Lubbert et al29 | Isolated del 5q and > 5% blasts | 1/1 (1 complete hematological and major cytogenetic response) | |
| Del 5q and other cytogenetic abnormalities, and > 5% blasts | 3/4 (3 complete hematological and major cytogenetic responses) | ||
| Kantarjian et al30 | High-risk MDS with del 5q | 2/16 | |
| Allogeneic bone marrow transplantation31 | Isolated del 5q Del 5q and others | 13/20 relapse-free at 1 year 8/37 relapse-free at 1 year | |
