Abstract
FLT3 is a receptor tyrosine kinase with important roles in hematopoietic stem/progenitor cell survival and proliferation. It is mutated in about 1/3 of acute myeloid leukemia (AML) patients, either by internal tandem duplications (ITD) of the juxtamembrane domain or by point mutations usually involving the kinase domain (KD). Both types of mutation constitutively activate FLT3. Many studies have shown that AML patients with FLT3/ITD mutations have poor cure rates due to relapse. This has led to the development of a number of small molecule tyrosine kinase inhibitors (TKI) with activity against FLT3. Many of these are still in preclinical development, but several have entered clinical phase I and II trials as monotherapy in patients with relapsed AML. Patients with FLT3 mutations in these trials have shown clinical responses, most often a clearing of peripheral blasts, but rarely major reductions of bone marrow blasts. Several studies have shown that FLT3 was successfully inhibited in most patients. However, complete remissions have rarely been achieved in these trials. The difference in responses of chronic myeloid leukemia (CML) patients to BCR-ABL inhibitors compared to FLT3 mutant AML patients to FLT3 inhibitors may be reflective of treating a single gene disease in CML versus multiply altered gene disease in AML. This has led to clinical testing of FLT3 TKI in combination with conventional chemotherapy, with trial designs based on preclinical testing showing synergistic effects between these agents in inducing cytotoxic responses. Several combination trials are ongoing or planned in both relapsed and newly diagnosed FLT3-mutant AML patients.
FLT3 in Hematopoiesis
FLT3 is a receptor tyrosine kinase that belongs to the same family as FMS, KIT and the two genes encoding PDGFRα and β. The murine gene was first cloned by two groups and named “FMS-like tyrosine kinase 3 “ (FLT3) by one group who cloned it from a testis cDNA library and “fetal liver kinase 2” (FLK-2) by another group who cloned it from a fetal liver cDNA library. The human FLT3 homolog was cloned shortly thereafter from a human CD34+ cDNA library and initially named STK-1 for “stem cell tyrosine kinase 1.” Over time the generally accepted name for the receptor has come to be FLT3. FLT3 is normally expressed by hematopoietic stem/progenitor cells (HSPCs) and expression is lost as hematopoietic cells differentiate. A large body of work has shown that it plays roles in survival, proliferation and differentiation.1–4
Its ligand, FLT3 ligand (FL), causes synergistic expansion of HSPC when combined with other growth factors acting on HSPCs in vitro (e.g., kit ligand, thrombopoietin, interleukin-3). Additional information on the role of FLT3 signaling in normal hematopoiesis has come from murine gene knockout models targeting either FLT3 or FL.5,6 Both mouse genotypes result in viable mice that are born with normal litter sizes and in the expected Mendelian ratio. The mice have normal life spans with no hematologic diseases noted. However, these mice do show reduced numbers of B progenitor cells, NK cells and DCs, and while bone marrow (BM) cells from these mice successfully reconstitute lethally irradiated recipient mice and provide long-term engraftment, they are only 25% as effective as BM from wild-type mice in competitive repopulation assays. Thus, FLT3 or FL knockout mice have a subtle HSPC deficit.
Although FLT3 expression is usually lost upon HSC differentiation, dendritic cells (DCs) are an exception, as mature DCs display persistent FLT3 expression. Furthermore, FL treatment greatly expands DCs in the spleen, GI tract, skin, and lymph nodes in vivo and also expands DCs in vitro. For this reason it has been proposed as an adjuvant in anti-tumor and other types of vaccines to boost antigen presentation to T cells.7
FLT3 Mutations in Leukemia
FLT3 is overexpressed at the level of RNA and protein in most B lineage and acute myeloid leukemias (AML).8 It is also overexpressed in a smaller subset of T-acute lymphocytic leukemia (ALL) and chronic myeloid leukemia (CML) in blast crisis. Studies have shown that the leukemic cells of B lineage ALL and AML frequently co-express FL, setting up autocrine or paracrine signaling loops that result in the constitutive activation of FLT3.9 Added FL stimulates proliferation of many leukemia-derived cell lines as well as primary AML samples in vitro.
A seminal discovery that confirmed the importance of FLT3 in leukemia was the finding of FLT3 mutations.10 FLT3 mutations are one of the most frequent somatic alterations in AML, occurring in approximately 1/3 of patients. FLT3 mutations consist of two major types: internal tandem duplication (ITD) mutations of 3-400 bp (always in-frame) that map to the juxtamembrane region (23% of AML patients) and point mutations that most frequently involve aspartic acid 835 of the kinase domain (KD) but have also been found less frequently in several other sites (8–12% of AML patients) (see Figure 1 and Table 1 ).11 Deletion and deletion/insertion mutations affecting the juxtamembrane region have also been reported, so the mutations mapping to the juxtamembrane region (comprising ITD and these deletion mutations) are sometimes referred to as length mutations (LM) in the literature. We will use the term ITD to refer to all juxtamembrane mutations. The overall incidence of ITD mutations is lower in children with AML (approx. 15%), but this is mostly due to very low rates of FLT3 mutations in children under age 10. However, children have about the same rate of KD point mutations as adults. FLT3/ITD mutations occur much less frequently in ALL (< 1%), primarily in cases that are biphenotypic leukemia. FLT3 activating mutations do occur more frequently in ALL in MLL-rearranged and hyperdiploid patients (5–22%).12 Because ITD mutations interfere with the negative regulatory function of the juxtamembrane region and KD point mutations most frequently involve the activation loop, both types of mutations result in constitutive activation of the receptor’s tyrosine kinase activity in the absence of ligand.13
Many studies in AML have shown that the presence of ITD mutations portends a poor prognosis for both pediatric and adult patients (see Table 1 ). The difference in outcome is often substantial: for example, in one study event-free survival was 44% for patients without a FLT3/ITD mutation compared to only 7% for those with a FLT3/ITD mutation.14 A particularly bad prognostic group consists of those patients who have ITD mutations and have lost the wild-type copy of FLT3. FLT3/ITD mutations in adults older than 60 were not found to be prognostic, although this is likely due to the fact that this group already has such abysmal outcomes that detecting any negative influence would be difficult. Interestingly, in most studies the KD point mutants do not seem to have the same unfavorable prognostic effect though one recent meta-analysis has challenged this conclusion. This may be reflective of signaling differences between the different types of mutations. Not all patients with FLT3/ITD mutations have a bad prognosis. In cases where the allelic ratio (ratio of mutant to wild-type FLT3 as determined by PCR amplification) is low, patients appear to have survival comparable to AML patients without FLT3 mutations. There is also some evidence that size does matter in that patients with larger ITDs fare worse than those with smaller ITDs.
AML patients who do not have FLT3 mutations at diagnosis have been found to occasionally acquire them at the time of relapse.15 FLT3 mutations have also been seen in myelodysplastic syndrome in about 3–5% of newly diagnosed patients. In MDS patients without FLT3 mutations, they sometimes appear when these patients progress to AML.16 Thus, at least for MDS transforming to AML, they can be an early or a late “hit” in the process of leukemogenesis.
Signaling through FLT3
Activation of FLT3 by mutation results in autophosphorylation as well as phosphorylation of a number of proteins, either directly or indirectly. A partial list of the proteins reported to be phosphorylated downstream of activated FLT3 includes: GAB1, GAB2, SHP-2, AKT, FOXO3a, CBL, STAT5a, ERK1/2, GRB2, SHC, VAV, LYN, GAP, p90RSK, BAD, C/EBPα, and SHIP, among others. Examination of these proteins makes it clear that the major signal transduction pathways leading from FLT3 include PI-3-kinase/AKT, RAS/MAPK, and STAT5 (see Figure 2; Color Figures, page 509).
Constitutive activation of FLT3 ultimately results in alterations in gene expression that have been uncovered through a number of microarray and other expression studies of leukemic cell lines and primary leukemia samples.17 Genes that are reported to be upregulated by constitutively activated FLT3 signaling include: Pim-1, Pim-2, SOCS-2, SOCS-3, GP49B, PGE-R, p21, MYC, CCND3, DUSP6, and IL2Rα .
Genes for which expression is reported as being suppressed by constitutively activated FLT3 signaling include: C/EBPa, Pu.1, Bim, p27kip, GADD45, BTG-2, AATYK, maf-B, RARγ , MAID, RB2/p130, p16ink4A, Frat1, LSC, RGS2, Gα 15, TISS11, Evi-2, Xbp1, and TNF2.
These changes reflect a number of targets that would be expected because of activation of the Ras/MAPK, PI-3-kinase/AKT and JAK/STAT pathways through phosphorylation downstream of FLT3 activation, as mentioned above. Specific interrogation of some of these targets (e.g., Pim-1 and Pim-2) have confirmed their role in mutant FLT3 signaling. More work remains to elucidate the complete signaling pathway altered by FLT3 in leukemia.
There is an almost mutually exclusive occurrence of FLT3 and RAS mutations in AML samples. This is added evidence that the RAS pathway is one of the major ways FLT3 signals because if either gene is mutated, there is little additional advantage to the other being mutated.
Models of Leukemogenesis Involving FLT3
A number of models have investigated the potential role of FLT3 signaling in leukemogenesis. Transfection of non-malignant factor dependent cell lines with constitutively activated forms of FLT3 results in factor independent growth and leukemia when these cells are injected into syngeneic mice.18 However, retroviral transfection of primary murine bone marrow cells with FLT3 mutations does not cause leukemia, but rather results in an oligoclonal fatal myeloproliferative disease (MPD) with a mean time to development of 40–60 days.19 The lack of polyclonality could be a clue that the retroviral insertion sites might be activating other genes which could be contributing to the MPD phenotype. When FLT3 mutations are combined with other genetic alterations, full transformation to leukemia does occur. For example, when FLT3/ITD mutations are used to retrovirally infect BM from MRP8 PML-RARα transgenic mice that model APL, full transformation to acute leukemia occurs with a reduced lag time compared to the disease that occurs in the mice without FLT3/ITD infection (from a median of 250 days without to 105 days with a FLT3/ITD).20 In addition, these mice tend to have the hyper-leukocytosis that characterizes M3 AML in patients with FLT3/ITD mutations. Again the disease is oligoclonal, implying that retroviral insertion sites could also be contributing. Other genetic alterations that result in leukemia when combined with FLT3 mutations through retroviral infection of murine bone marrow include: NUP98-Hox, AML1-ETO, MLL-ENL, and MLL-SEPT6.
Transgenic mouse models expressing FLT3 mutations have also been reported. When a FLT3/ITD is expressed transgenically under control of the vav promoter it results in MPD with a disease latency of 6–12 months in most founder lines as well as B or T-lymphoid disease in two other founder lines.21 The large lag time to development of MPD could imply that other alterations are necessary to cooperate with FLT3/ITD signaling to give MPD or lymphoid malignancies.
There is also some evidence from a murine model that constitutive activation of wild-type FLT3 can contribute to leukemogenesis. This comes from an experiment in which transplantation of lethally irradiated mice with murine bone marrow stem/progenitor cells transduced with a construct expressing FL developed either lymphoid or myeloid leukemias after a long lag period.22
FLT3 Mutations and Leukemia Stem Cells
One concept that is beginning to bring about a better understanding of cancer in general and of leukemia in particular is that a subset of the malignant cells within a tumor is responsible for the propagation of the disease. In the case of leukemia, these cells have been termed “leukemia stem cells” (LSCs). LSCs often share many of the characteristics of normal hematopoietic stem cells (HSCs). Even within LSCs there appear to be hierarchies, with some LSCs only able to propagate disease for short periods of time, while others are able to sustain long-term disease. While they share many of the characteristics of HSCs, LSCs are not necessarily derived from them.
There are at least some data supporting the idea that FLT3 mutations occur in LSCs. In 84% of patients with an FLT3/ITD mutation the FLT3 mutation is still present when relapse occurs.15 On the other hand, in 16% of cases, the FLT3 mutation is no longer present at relapse, implying it occurred in a subclone and not in the LSC leading to the relapse. The situation is very different for FLT3/KD mutations, which are lost at relapse in more than 50% of cases.23 Further evidence for FLT3 mutations occurring in subclones is the finding discussed earlier of low mutant allelic ratios at diagnosis in some patients. If the mutation is in the LSC from which all of the cells are derived, one expects to find an allelic ratio of 1. If the leukemic sample consists of a mixture of normal cells with the leukemic cells this will artificially depress the ratio. However, even when the percentage of blasts in the sample is taken into consideration and used to adjust the ratio, a fraction of patients still show a low allelic ratio. This again implies that the mutation occurred in a subclone and not in the LSC. However, most of the time (approx. 2/3 of patients) the allelic ratio approaches 1. The allelic ratio has been examined after purifying the CD34+/38− LSC-enriched fraction away from bulk leukemic cells and no change was observed in 8 samples.24 When these purified cells were injected into NOD/SCID mice, they engrafted with a high frequency. Most importantly, when the allelic ratio was tested in the leukemia that developed from these cells at 14 weeks after injection (at which time the cells are derived from the injected LSCs), the ratio again did not change. This implies that at least in these samples, the FLT3 mutation was in the functional LSC.
Discovery and Preclinical Study of FLT3 Inhibitors
The findings that FLT3 is frequently activated by somatic mutations in AML, and that a poor prognosis is associated with these mutations, have made it an attractive molecular target for the development of novel therapeutics to inhibit its kinase domain, much as had been done for BCR-ABL fusions in CML. These small molecule tyrosine kinase inhibitors (TKI) work, for the most part, as competitive inhibitors of ATP binding to its pocket in the kinase domain. The first FLT3 TKI was discovered by screening compounds that were known to be inhibitors of PDGFR, a closely related family member. AG1295 and AG1296 were shown to inhibit the autophosphorylation of FLT3 with an IC50 of approximately 300 nM and to induce apoptosis in cell lines transformed with FLT3 mutations.25 As an important proof of principle, treatment of primary AML samples with AG1296 resulted in preferential cytotoxicity in the samples expressing FLT3 mutations. This is likely due to the postulated phenomenon of “oncogene addiction,” in which cells become very dependent on signaling pathways activated by mutated proto-oncogenes.
Since that time a large number of FLT3 inhibitors have been discovered and investigated, in large part through collaborations between academia and industry.26 Some of the best characterized include CEP-701 (Lestaurtinib), PKC412, MLN518, and SU11248 (Sunitinib).27–30 These FLT3 TKI all have activity at the nM level and have been shown to kill leukemic cell lines and primary AML samples expressing FLT3 mutations. They also improve survival and reduce engraftment of these same samples when used in vivo in a variety of murine leukemia models. Many other FLT3 inhibitors are at various stages of preclinical testing or early clinical trials and include: BAY43-9006, KRN383, KP372-1, CHIR-258, SU11657, GTP-14564, CEP-5214, Ki23819, and D-65476 (see Table 2 ). The potency for FLT3 inhibition differs between these drugs and some inhibit the ITD mutations but not certain KD mutations. In addition, their spectra of off-target kinase activity and level of protein binding differ as well. The spectrum of activity is important because inhibition of certain kinases is likely to lead to additional toxicities, particularly when the drugs are combined with chemotherapy. The protein binding is an issue because most of these drugs are very hydrophobic and are bound tightly by plasma proteins (> 99% for some drugs), including α 1-acid glycoprotein. Only free drug is available to bind to FLT3 and inhibit its kinase activity. Thus, measurement of each drug’s activity in human plasma can help to predict the levels that will be needed to attain adequate levels of FLT3 inhibition in patients.
Perhaps because the mutated forms of FLT3 are more energetically difficult to fold, they appear to be more dependent on chaperone proteins including Hsp90 for their proper folding. Thus, one way to preferentially target mutant FLT3 is to use an inhibitor of Hsp90. A number of Hsp90 inhibitors have been developed and some have already entered clinical trials against a variety of solid tumors. Other studies have shown synergy between FLT3 inhibitors and Hsp90 inhibitors. Synergies between FLT3 TKI and rapamycin have also been demonstrated.
Another way to target FLT3 specifically is through anti-FLT3 antibodies. Fully human phage display antibodies were generated and selected for binding to human FLT3 and the ability to block FL binding to FLT3 in an ELISA assay.31,32 They have the ability to block signaling through wild-type FLT3 and also through some mutated forms of FLT3. Most importantly, they have been shown to induce antibody dependent cellular-mediated cytotoxicity (ADCC) and mediate killing of some, but not all, cell lines and primary samples of both AML and ALL when used in vivo in NOD/SCID mice engrafted with these cells.
FLT3 Inhibitors in Clinical Trials
A number of FLT3 inhibitors have reached clinical trials as monotherapy in relapsed or refractory AML patients, some or all of whom had FLT3 mutations.33–35 The results of these trials have been informative. In most studies, the drugs have been well-tolerated as outpatient oral medications. When patients’ blasts have a cytotoxic response to in vitro FLT3 inhibition and adequate levels of inhibition are attained in these patients in vivo, the patients have demonstrated a clinical response. In trials that have treated both FLT3 mutant and wild-type FLT3 AML patients, there is more frequent response to FLT3 TKI in the mutant FLT3 patients, paralleling the increased in vitro response of mutant cells previously observed. The responses in all patients, however, are usually restricted to a clearing of peripheral leukemia cells. Major bone marrow responses are uncommon. In most patients, the clinical response is short-lived with most patients’ peripheral blasts returning within weeks to months. This is in sharp contrast to the results in chronic phase CML where Gleevec results in responses that last a number of years in most patients. Part of the difference in the response to inhibitors of mutated kinases in these diseases is that FLT3 mutations occur in the setting of acute leukemia, where it is but one of several somatic genetic alterations required to fully transform cells. In contrast, CML in chronic phase represents a myeloproliferative disorder in which the BCR-ABL fusion may be the only molecular abnormality. In patients relapsing on Gleevec the most frequent cause appears to be the selection of mutations within BCR-ABL that make it resistant to inhibition by Gleevec. In contrast, only 1 patient thus far on FLT3 inhibitor trials has been shown to have acquired a FLT3 resistance mutation.36 This again points to other mechanisms outside FLT3 signaling as the reason for the limited duration of response.
Preclinical studies demonstrated synergistic killing of leukemic cells in vitro when FLT3 TKI are combined with conventional chemotherapy in a sequence-specific order.37 Synergism was seen when the two classes of agents were used simultaneously or the FLT3 TKI followed chemotherapy. Antagonism due to cell-cycle blockade was observed when chemotherapy followed FLT3 TKI treatment. Several current trials of FLT3 TKI are using this approach of combination treatment in either relapsed or newly diagnosed adult AML studies. CEP-701 (Lestaurtinib) combined with chemotherapy is in a phase III trial in relapsed FLT3 mutant AML patients in many institutions in the US, Italy, Israel, and Spain. Another trial of CEP-701 with chemotherapy in newly diagnosed AML patients is now underway through the Medical Research Council (MRC) in Great Britain. A study of PKC412 combined with chemotherapy in newly diagnosed AML patients is planned through a combination of cooperative groups in the US. Presentations at the 2005 American Society of Hematology annual meeting on preliminary results and/or correlative data from both the CEP-701 and PKC412 trials generated enthusiasm for their combination with chemotherapy.38,39
Pediatric trials utilizing combinations of CEP-701 with chemotherapy should be shortly underway in the Children’s Oncology Group in both relapsed FLT3-mutant AML patients and in infants with MLL-rearranged ALL. Preclinical work has shown important roles for FLT3 signaling in both of these groups and preferential sensitivity to induction of cytotoxic responses by FLT3 TKI.40–43
Anti-FLT3 antibody trials are also being contemplated in AML and ALL because of the frequent expression of FLT3, either mutant or wild-type, in these diseases. Besides the potential for interfering with FLT3 signaling, anti-FLT3 antibodies can also induce ADCC as an additional mechanism for inducing cytotoxicity.
Conclusions
FLT3 mutations remain an important molecular target for the development of therapeutics that could be used to treat AML and other forms of leukemia. When used as mono-therapy in phase I and II trials, FLT3 inhibitors have shown limited clinical responses. This could be a result of genomic instability leading to multiple pathways of escape from dependence on FLT3 signaling. Current trials are combining FLT3 inhibitors with conventional chemotherapy in an attempt to realize synergistic cytotoxicity against leukemia cells. It is hoped that such trials will begin to reverse the poor prognosis for AML patients with FLT3/ITD mutations. Combinations of FLT3 TKI with other molecularly targeted agents affecting the other pathways that, together with FLT3 mutations, cooperate to fully transform hematopoietic stem/progenitor cells is the ultimate goal. Successful achievement of this goal may improve the outcome for FLT3-mutant leukemia patients by preventing relapse and reducing the toxicities associated with nonselective cytotoxics.
Incidence and outcome of FLT3/ITD mutations in acute myeloid leukemia (AML) grouped according to AML subtype. Incidences are listed as percentages within a given subtype, with the actual patient numbers below in parentheses.
| Study (No. Patients) . | Adult AML . | M3 AML . | Normal/Int Cytogenetics* . | 8:21 or INV16 . | Poor-risk Cytogenetics** AML† . | 20 . | Elderly AML‡ . | Pediatric AML . | Survival . |
|---|---|---|---|---|---|---|---|---|---|
| * Normal and/or intermediate cytogenetics. This included patients with normal cytogenetics (studies 3, 8, 15, 16, and 19) and those lacking 15:17, 8:21, INV16, and poor-risk features. | |||||||||
| ** Poor-risk cytogenetics. This included patients with monosomy 5 or 7, 5q–, 3q–, or complex karyotype. | |||||||||
| † Secondary AML. This was defined as AML arising out of a myelodysplastic syndrome (MDS) and AML with trilineage myelodysplasia. Three of the studies listed (studies 1, 4 and 7) also included treatment-related AML in this group. | |||||||||
| ‡ Elderly AML patients were defined as being > 55 (study 6) or > 60 (study 4) years of age. | |||||||||
| § All 3 patients with high mutant-to-wild-type ratio in this study died. | |||||||||
| 1. Horiike et al (30) | 14.7% (5/34) | ND | |||||||
| 2. Kiyoi et al (74) | 20.3% (15/74) | Decreased | |||||||
| 3. Yamamoto et al (429) | 8.9% (81/429) | 28.0% (23/82) | 11.8% (4/34) | 18.2% (2/11) | Decreased | ||||
| 4. Rombouts et al (75) | 23.7% (14/59) | 24.2% (16/66) | 0.0% (0/6) | 18.2% (2/11) | 22.9% (8/35) | Decreased | |||
| 5. Abu-Duhier et al (106) | 10.0% (1/10) | 22.7% (10/44) | 5.8% (3/52) | Decreased | |||||
| 6. Stirewalt et al (140) | 33.6% (47/140) | No Effect | |||||||
| 7. Kottaridis et al (854) | 26.6% (227/854) | 36.8% (49/133) | 34.2% (96/281) | 8.3% (9/109) | 3.4% (5/147) | 27.4% (17/62) | Decreased | ||
| 8. Whitman et al (82) | 28.0% (23/82) | No Effect | |||||||
| 9. Iwai et al (94) | 5.3% (5/94) | Decreased | |||||||
| 10. Xu et al (87) | 13.8% (12/87) | Decreased | |||||||
| 11. Kondo et al (64) | 10.9% (7/64) | Decreased | |||||||
| 12. Meshinchi et al (91) | 16.5% (15/91) | Decreased | |||||||
| 13. Liang et al (80) | 11.3% (9/80) | No Effect§ | |||||||
| 14. Thiede et al (979) | 22.2% (217/979) | 33.3% (13/39) | 29.7% (134/451) | 4.5% (4/88) | 1.7% (5/298) | 8.8% (3/34) | Decreased | ||
| 15. Schnittger et al (1003) | 23.3.% (234/1003) | 35.8% 24/67) | 39.3% (149/379) | 5.4% (6/11) | 2.7% (3/110) | 15.6% (12/77) | No Effect | ||
| 16. Boissel et al (159) | 25.2% (40/159) | 35.4% (28/79) | 0.0% (0/23) | No Effect | |||||
| 17. Noguera et al (90) | 36.7% (33/90) | No Effect | |||||||
| 18. Frohling et al (523) | 22.8% (119/523) | 39.2% (20/51) | 31.7% (71/224) | 5.7% (4/70) | 2.2% (1/45) | 9.2% (7/76) | Decreased | ||
| 19. Kainz et al (100) | 38.1% (8/21) | 30.2% (16/53) | 7.7% (2/26) | Decreased | |||||
| 20. Moreno et al (208) | 17.3% (36/208) | 0.0% (0.14) | Decreased | ||||||
| 21. Jilani et al (85) | 21.2% (18/85) | Decreased | |||||||
| 22. Arrigoni et al (45) | 22.2% (10/45) | Decreased | |||||||
| 23. Zwaan et al (234) | 11.5% (27/234) | Decreased | |||||||
| TOTAL (4613 adult, 695 pediatric) | 22.9% (986/4299) | 33.6% (163/485) | 32.5% (566/1741) | 6.1% (32/527) | 2.6% (7/617) | 15.6% (46/294) | 31.4% (55/175) | 12.2% (85/695) | |
| Study (No. Patients) . | Adult AML . | M3 AML . | Normal/Int Cytogenetics* . | 8:21 or INV16 . | Poor-risk Cytogenetics** AML† . | 20 . | Elderly AML‡ . | Pediatric AML . | Survival . |
|---|---|---|---|---|---|---|---|---|---|
| * Normal and/or intermediate cytogenetics. This included patients with normal cytogenetics (studies 3, 8, 15, 16, and 19) and those lacking 15:17, 8:21, INV16, and poor-risk features. | |||||||||
| ** Poor-risk cytogenetics. This included patients with monosomy 5 or 7, 5q–, 3q–, or complex karyotype. | |||||||||
| † Secondary AML. This was defined as AML arising out of a myelodysplastic syndrome (MDS) and AML with trilineage myelodysplasia. Three of the studies listed (studies 1, 4 and 7) also included treatment-related AML in this group. | |||||||||
| ‡ Elderly AML patients were defined as being > 55 (study 6) or > 60 (study 4) years of age. | |||||||||
| § All 3 patients with high mutant-to-wild-type ratio in this study died. | |||||||||
| 1. Horiike et al (30) | 14.7% (5/34) | ND | |||||||
| 2. Kiyoi et al (74) | 20.3% (15/74) | Decreased | |||||||
| 3. Yamamoto et al (429) | 8.9% (81/429) | 28.0% (23/82) | 11.8% (4/34) | 18.2% (2/11) | Decreased | ||||
| 4. Rombouts et al (75) | 23.7% (14/59) | 24.2% (16/66) | 0.0% (0/6) | 18.2% (2/11) | 22.9% (8/35) | Decreased | |||
| 5. Abu-Duhier et al (106) | 10.0% (1/10) | 22.7% (10/44) | 5.8% (3/52) | Decreased | |||||
| 6. Stirewalt et al (140) | 33.6% (47/140) | No Effect | |||||||
| 7. Kottaridis et al (854) | 26.6% (227/854) | 36.8% (49/133) | 34.2% (96/281) | 8.3% (9/109) | 3.4% (5/147) | 27.4% (17/62) | Decreased | ||
| 8. Whitman et al (82) | 28.0% (23/82) | No Effect | |||||||
| 9. Iwai et al (94) | 5.3% (5/94) | Decreased | |||||||
| 10. Xu et al (87) | 13.8% (12/87) | Decreased | |||||||
| 11. Kondo et al (64) | 10.9% (7/64) | Decreased | |||||||
| 12. Meshinchi et al (91) | 16.5% (15/91) | Decreased | |||||||
| 13. Liang et al (80) | 11.3% (9/80) | No Effect§ | |||||||
| 14. Thiede et al (979) | 22.2% (217/979) | 33.3% (13/39) | 29.7% (134/451) | 4.5% (4/88) | 1.7% (5/298) | 8.8% (3/34) | Decreased | ||
| 15. Schnittger et al (1003) | 23.3.% (234/1003) | 35.8% 24/67) | 39.3% (149/379) | 5.4% (6/11) | 2.7% (3/110) | 15.6% (12/77) | No Effect | ||
| 16. Boissel et al (159) | 25.2% (40/159) | 35.4% (28/79) | 0.0% (0/23) | No Effect | |||||
| 17. Noguera et al (90) | 36.7% (33/90) | No Effect | |||||||
| 18. Frohling et al (523) | 22.8% (119/523) | 39.2% (20/51) | 31.7% (71/224) | 5.7% (4/70) | 2.2% (1/45) | 9.2% (7/76) | Decreased | ||
| 19. Kainz et al (100) | 38.1% (8/21) | 30.2% (16/53) | 7.7% (2/26) | Decreased | |||||
| 20. Moreno et al (208) | 17.3% (36/208) | 0.0% (0.14) | Decreased | ||||||
| 21. Jilani et al (85) | 21.2% (18/85) | Decreased | |||||||
| 22. Arrigoni et al (45) | 22.2% (10/45) | Decreased | |||||||
| 23. Zwaan et al (234) | 11.5% (27/234) | Decreased | |||||||
| TOTAL (4613 adult, 695 pediatric) | 22.9% (986/4299) | 33.6% (163/485) | 32.5% (566/1741) | 6.1% (32/527) | 2.6% (7/617) | 15.6% (46/294) | 31.4% (55/175) | 12.2% (85/695) | |
List of some FLT3 inhibitors. IC50 refers to the concentration required for the inhibition of FLT3 autophosphorylation. “Other receptors” refers to other receptors known to be inhibited by the compound with an IC50 similar to that for FLT3.
| Compound . | Chemical Class . | FLT3 IC50 . | Other receptors . | Clinical Trial . |
|---|---|---|---|---|
| AG1295 | Quinoxaline | 300 nM | PDGFR, KIT | |
| AG1296 | Quinoxaline | 300 nM | PDGFR, KIT | |
| AGL2043 | Quinoxaline | 100 nM | PDGFR, KIT | |
| D64406 | Bis-indlyl- methanone | 300 nM | PDGFR, KIT | |
| SU5416 | 3-Substituted indolinone | 100 nM | KIT, VEGFR | Phase I |
| SU5614 | 3-Substituted indolinone | 10 nM | KIT, FMS | |
| SU11248 | 3-Substituted indolinoneindolinone | 50 nM | KIT, PDGFR, VEGFR | Phase I |
| MLN518 | Quinazoline | 30 nM | KIT, PDGFR, | Phase II |
| CEP-701 | Indolocarbazole | 2 nM | TRKA | Phase III |
| PKC412 | Indolocarbazole | 10 nM | KIT | Phase III planned |
| GTP-14564 | Cyclopenta-[a]- inden | 300 nM | KIT, FMS, PDGFR | |
| Ki23819 | Quinoline-urea | 1 nM | NR | |
| CHIR-258 | Benzimidalzole- quinoline | 10 nM | KIT, FMS, FGFR, VEGFR | Phase 1 |
| KW-2449 | NR | 6 nM | FLT3, KIT, Aurora | Phase 1 planned |
| Compound . | Chemical Class . | FLT3 IC50 . | Other receptors . | Clinical Trial . |
|---|---|---|---|---|
| AG1295 | Quinoxaline | 300 nM | PDGFR, KIT | |
| AG1296 | Quinoxaline | 300 nM | PDGFR, KIT | |
| AGL2043 | Quinoxaline | 100 nM | PDGFR, KIT | |
| D64406 | Bis-indlyl- methanone | 300 nM | PDGFR, KIT | |
| SU5416 | 3-Substituted indolinone | 100 nM | KIT, VEGFR | Phase I |
| SU5614 | 3-Substituted indolinone | 10 nM | KIT, FMS | |
| SU11248 | 3-Substituted indolinoneindolinone | 50 nM | KIT, PDGFR, VEGFR | Phase I |
| MLN518 | Quinazoline | 30 nM | KIT, PDGFR, | Phase II |
| CEP-701 | Indolocarbazole | 2 nM | TRKA | Phase III |
| PKC412 | Indolocarbazole | 10 nM | KIT | Phase III planned |
| GTP-14564 | Cyclopenta-[a]- inden | 300 nM | KIT, FMS, PDGFR | |
| Ki23819 | Quinoline-urea | 1 nM | NR | |
| CHIR-258 | Benzimidalzole- quinoline | 10 nM | KIT, FMS, FGFR, VEGFR | Phase 1 |
| KW-2449 | NR | 6 nM | FLT3, KIT, Aurora | Phase 1 planned |
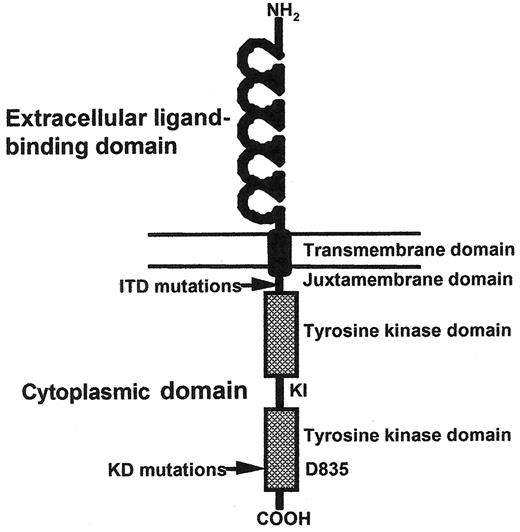
Cartoon of FLT3 structure.
Shown in schematic fashion are the 5 immunoglobulin-like folds that make up the ligand-binding extracellular domain, single transmembrane domain, and cytoplasmic domain made up of a kinase domain interrupted by a kinase insert. The juxtamembrane domain where internal tandem duplications (ITDs) occur, and aspartic acid 835 where most kinase domain mutations occur are indicated by arrows.
Cartoon of FLT3 structure.
Shown in schematic fashion are the 5 immunoglobulin-like folds that make up the ligand-binding extracellular domain, single transmembrane domain, and cytoplasmic domain made up of a kinase domain interrupted by a kinase insert. The juxtamembrane domain where internal tandem duplications (ITDs) occur, and aspartic acid 835 where most kinase domain mutations occur are indicated by arrows.
Sidney Kimmel Cancer Center at Johns Hopkins, Johns Hopkins University School of Medicine
Acknowledgments: The author would like to thank all members of the laboratory for their contributions and Drs. Mark Levis and Patrick Brown for helpful suggestions regarding the manuscript and the NCI, Burroughs Wellcome Fund, Leukemia & Lymphoma Society, and Kyle Haydock Professorship for support.