Abstract
Acute promyelocytic leukemia (APL), a relatively rare hematologic malignancy, is highly curable with current treatment strategies. However, these strategies may be unavailable in countries with limited resources. A review of records in several Latin American countries revealed that approximately 30% of deaths among children and adults with APL were caused by early complications associated with the disease or its treatment. Further, APL accounts for 20% to 25% of cases of AML in these countries, consistent with the previous observation of increased incidence of APL in Latin Americans. The lack of population-based registries in developing countries has made it difficult to determine the real incidence of APL. Moreover, APL appears to have other unique epidemiologic characteristics, including association of primary APL with an increased body mass index at diagnosis and association of secondary APL with breast cancer. To facilitate the development of local capacity and implement effective treatment of APL in developing countries, the International Committee of the American Society of Hematology has assembled a working group to formulate treatment guidelines based on evidence from clinical trials results in the developed world but adapted to local resources. It is hoped that uniform treatment, careful documentation of specific outcome data, and ongoing monitoring of treatment efficacy and toxicity will improve the cure rate and provide biologic and epidemiologic information about APL in developing countries. This initial demonstration project may be joined by other countries, providing a framework for additional clinical investigation in this highly curable form of leukemia.
Acute promyelocytic leukemia (APL), once one of the most lethal subtypes of leukemia, is now curable in most cases.1,2 The key to this success is the availability of several effective agents, including all-trans retinoic acid (ATRA), arsenic trioxide (ATO), and anthracycline compounds.3 Moreover, information about the biology of APL has been incorporated into overall management to reduce early mortality and improve survival rates. APL is one of the first types of acute myeloid leukemia (AML) to be successfully treated with targeted therapy. (See M Sanz, “Treatment of Acute Promyelocytic Leukemia,” in this volume.)
Because APL predominantly affects young adults, more productive years are saved by curing this disease than by curing more common forms of AML, which typically affect individuals more than 55 years of age, who respond poorly to current therapies. Although effective management of APL has been available for more than 15 years and yields a cure rate in excess of 75%, the status of APL treatment in the developing countries is unknown. The limited available data suggest that the rate of early mortality is high and that long-term survival is poor in many developing countries. To facilitate the development of local capacity and implement effective treatment for APL in developing countries (essentially an educational endeavor), the International Committee of the American Society of Hematology has assembled a working group comprising experts in APL. This initiative, designated the International Consortium on APL (IC-APL), involves close collaboration between internationally recognized APL experts who are conducting clinical trials in the developed world and hematologist/oncologist colleagues working in developing countries.
Here we describe the epidemiology of APL and review the results of a survey of its clinical manifestations and outcome in children and adults in selected Central and South American countries. The transfer of optimal treatment of APL to developing countries in the context of building local capacity for collaborative clinical and epidemiological research will greatly improve the survival of children and adults with this disease and will yield insights into the biology of APL.
Epidemiology of APL
Demographics
The real incidence of APL is not known. Until recently, population-based registries did not distinguish APL from other subtypes of AML, and the incidence of APL was estimated on the basis of its relative frequency among other AML subtypes in large clinical trials. In 2005, the expected incidence of AML in the US was 11,930 cases (6350 men and 5580 women) per year.4 The Cancer Surveillance Program of Los Angeles County has provided specific information about APL.5 In this registry, 107 (4.8%) of the 2222 cases of AML registered between 1980 and 1995 were APL. This incidence is somewhat lower than the 5% to 13% reported by many large clinical trials and single institutions in the US. Given the current limitations of population-based registries, the true number of newly diagnosed cases of APL per year in the US is estimated to be 600 to 800.
Descriptive epidemiologic data have shown several differences between APL and other subtypes of AML. One of the most striking features of APL is its age-associated incidence rate. In general, the incidence of AML increases proportionally with age up to age 55 years, and it then increases exponentially. Conversely, the incidence of APL is very low in children under 10 years of age, increases steadily during the teen years, reaches a plateau during early adulthood, then remains constant until it decreases after age 60 years. The great majority of cases of APL are diagnosed between ages 20 and 50 years, although evidence of the t(15;17)(q22;q21) has been documented in neonates,6,7 suggesting that this rearrangement can occur during hematopoietic development in utero. This finding is not surprising in view of data demonstrating the crucial role of retinoids in embryonic and fetal hematopoiesis.8
The role of ethnic background
Another remarkable epidemiologic feature of APL is its high incidence in certain ethnic groups. Douer was the first to call attention to the increased incidence of APL among patients of Latin American descent.5 Subsequently reported data from hospital-based registries in Mexico,9 Peru,10 and Spain11 also showed a relatively high incidence of APL. Similarly, Estey et al12 reported 120 cases of APL (9.6%) among 1245 patients with newly diagnosed AML treated at the MD Anderson Cancer Center (Houston, Texas). Patients with a Latin American background were much more likely to have APL (18.2%; 95%CI, 15%–27.7%) than were white (7.7%; 95%CI, 6.2%–9.5%) or black (10.3%; 95%CI, 5.7%–18.7%) patients without a Latin American background. More recent studies have suggested a similarly increased incidence of APL in Latin American countries.13 In Costa Rica, APL accounted for 34% of 167 cases of de novo AML. Remarkably, none of the Costa Rican APL patients were of pure African, Oriental, or Amerindian descent. They were mainly Caucasian descendants of European immigrants whose ethnicity was less mixed than that of patients with other subtypes of AML.14
Although a large body of data suggests that the descendants of Spaniards who colonized Central and South America may have a genetic predisposition to APL, there are some contradictory data. For example, Hernández et al of the Instituto de Hematología e Inmunología (Havana, Cuba) reported that APL accounted for 31.3% of the pediatric (less than 15 years of age) cases of AML but for only 13% of the adult cases.15 Moreover, the incidence of APL varies within Spain. Recently, investigators in Navarra and Salamanca reported a study of 1129 successive patients with AML who had successful cytogenetic studies at institutions in three distinct regions of Spain. In the south, 21.6% of the cases of AML were APL, whereas the frequency of APL was 17% and 12.6% in the central and northern regions, respectively (P = 0.03).16 Finally, the distribution of breakpoints in the PML gene during formation of the APL-specific PML-RARα gene rearrangement differs between Latin Americans and other patients. In APL in patients of Latin American background residing in Los Angeles, Peru, and Mexico, 63%–75% of the breakpoints in the PML gene were reported to occur at intron 6 (breakpoint cluster region [bcr] 1).17,18 In contrast, this region was involved in only about 50% of APL cases in Europeans and in residents of the USA who did not have a Latin American background. Because many of the patients characterized as having a Latin American background in these studies were Mestizo (having both Caucasian and Amerindian forebears), it is hypothesized that the higher rate of bcr1 APL in Latin Americans may be genetically attributable to Amerindian forebears who immigrated into the Americas from eastern Asia approximately 12,000 years ago.19
Other factors
Other clinical observations add complexity to the descriptive epidemiology of APL. Estey et al12 observed that individuals with APL had a significantly greater body mass index than patients with non-APL AML. This observation has been corroborated by other investigators.20 Because the RARα gene is involved in the regulation of hematopoiesis and adipogenesis, constitutional or environmental factors that lead to dysregulation of RARα may increase adipogenesis and predispose RARα to illegitimate recombination during hematopoiesis. Environmental factors can affect retinoid pathway function, as shown by the fact that the composition of lipids in the diet of European sea bass can cause downregulation of RARα and skeletal malformation during larval development.21
APL presenting as a secondary or treatment-related malignancy also has unique clinical and epidemiological characteristics (see M Sanz, “Treatment of Acute Promyelocytic Leukemia,” in this volume). First, etoposide is clearly associated with treatment-related AML other than APL, but it accounts for only a minority of cases of treatment-related APL. Other topoisomerase II inhibitors, such as anthracycline and anthracenedione (mitoxantrone), are more often associated with treatment-related APL.22 Interestingly, in cases of mitoxantrone-related APL, the breakpoints in the PML gene are located within a short 8-bp region of intron 6, whereas in primary APL or APL related to other agents, the breakpoints are scattered within intron 6.23 Second, the incidence of treatment-related AML other than APL depends on the cumulative dose and schedule of epipodophyllotoxin, whereas the type of primary malignancy is a risk factor for APL. Women with cancer of the breast or reproductive organs have about 70% of all cases of secondary APL, although many have received only radiotherapy, hormone therapy, or surgery alone for their primary cancer. These observations suggest that breast cancer increases the predisposition to APL irrespective of treatment, although mitoxantrone may amplify the predisposition.24,25 It would be of interest to determine whether breast cancer in women who develop APL is associated with constitutional predisposition, including BRCA1, BRCA2, or P53 mutations.
In summary, there is evidence that the incidence of APL varies across ethnic groups and that genetic and environmental factors play a role in the etiology of APL. However, a pathogenetic model that accounts for all of the unique descriptive epidemiological data, including age and gender distribution, increased incidence in Latin American and Spaniard populations, and association of treatment-related APL with breast cancer, remains elusive. A synthesis of epidemiologic and molecular data from different clinical groups and diverse geographic regions might provide crucial information about the biology of this disease.
Outcome of APL in Selected Developing Countries
Optimal management of APL requires prompt diagnosis and immediate initiation of treatment.26,27 (See M Sanz, “Treatment of Acute Promyelocytic Leukemia,” in this volume.) Because of APL-associated coagulopathy, about 3% to 5% of patients die of hemorrhagic complications before treatment can be started, even in developed countries.2 Hence, the efficiency of access to care—the time span between the first signs and symptoms and initiation of specific therapy—in a given public health system determines the outcome of diseases such as APL that require multifaceted and multidisciplinary approaches. Access to care varies tremendously in developing countries. Eighteen sites were surveyed to determine the impact of modern treatment, including ATRA, on the outcome of childhood and adult APL in selected developing countries. Six of these sites (in San José, Costa Rica; San Salvador, El Salvador; Guatemala City, Guatemala; Tegucigalpa, Honduras; Recife, Brazil; and Culiacan, Mexico) have been partners of the St. Jude Children’s Research Hospital (St. Jude) International Outreach Program (IOP) for more than 5 years. Another pediatric hospital (Manuel de Jaesus Rivera Hospital, La Mascota, Managua, Nicaragua) has had a twinning program with Monza, Italy (Giuseppe Masera, Mario Negri Institute for Pharmacological Research, Milan, Italy) for more than a decade. The remaining 11 sites are university-based adult hematology-oncology centers in large Brazilian cities, some of which treated individuals less than 18 years of age. The inclusion criteria were morphological/cytochemical diagnosis of APL. Demographic and outcome data were obtained directly from the primary physicians by using a standardized questionnaire.
Table 1 lists selected clinical, laboratory, and outcome data for 121 children with APL who were admitted to pediatric cancer units (PCUs) in the seven resource-poor regions surveyed. ATRA was used in the management of most of these cases, but in Culiacan, 9 of the 10 patients received intensive chemotherapy without ATRA. The median age overall was 9 years (range, 0.1 to 17.7 years). There was a slight predominance of boys (M:F ratio, 1.2 ). APL represented 13.6% (in San Salvador) to 28.5% (in Managua) of cases of childhood AML (median, 16.6%). The median white blood cell (WBC) count at diagnosis varied from 8.5 × 109/L to 45.6 × 109/L, and the median platelet count from 15.5 × 109/L to 56.3 × 109/L. The frequency of laboratory evidence of DIC varied from 19.0% in Recife, Brazil to 50.0% in Tegucigalpa, Honduras. Death occurred within 14 days of diagnosis (early death) in 28 (23.1%) cases. Rates of complete remission (CR) ranged from 76.1% in Recife to 41.6 % in Tegucigalpa. Relapse was observed in 29 of the 69 patients who entered remission (42.0%). Death due to infectious complications was observed in 11 children. Abandonment or refusal of treatment was documented in 15 cases. At the time of the survey, only 37 of 122 (30.3%) children with APL remained alive in first remission. An additional 2 children treated in San José survived in second remission. One child in Guatemala City is receiving palliative care for relapsed APL.
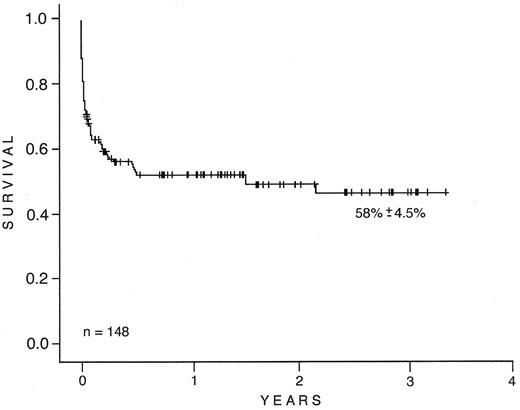
Table 2 lists similar information about 132 adults and 16 children with APL obtained from 11 Brazilian centers. ATRA was used in the management of all of these cases. The median age overall was 32 years (range, 5 to 79 years). There was a slight predominance of females (M:F ratio, 0.8). Only four centers reported the frequency of APL in relation to other subtypes of AML. APL accounted for more than 20% in all of them (range, 21.4% to 26.4%). The median WBC count at diagnosis varied from 2.9 × 109/L to 12.1 × 109/L, and the median platelet count from 19.0 × 109/L to 49.0 × 109/L. Laboratory evidence of DIC was noted in 68 patients (45.9%); its frequency among the 11 sites was 29.0% to 100.0%. Severe hemorrhagic complications were present in 26 patients (17.6%). Early death was observed in 42 (28.4%) cases. Bleeding was the most common cause of mortality (36 cases). The central nervous system (23 cases), lungs (10 cases), and gastrointestinal tract (4 cases) were the most common sites of bleeding. ATRA syndrome was reported as the cause of mortality in 11 patients (26.2%). Infectious complications (10%) and unknown causes accounted for the remaining 2% of early failures. Seventy-four patients (50%) achieved CR. CR rates ranged from 20% to 77.0% among the 11 institutions. Infectious complications (11 cases; 42%), ATRA syndrome (9 cases; 35%), and bleeding (6 cases; 23%) were the most common causes of treatment failure after the first 2 weeks of treatment. Relapse was observed in 4 (5.4%) of the 74 patients who entered remission. Abandonment or refusal of treatment was documented in 4 cases. Six patients are still receiving induction/consolidation therapy. At the time of this report, 68 of 148 (45.9%) patients with APL remained alive in first remission. The survival estimate is 58.2% ± 4.8% (95% CI 48.9% to 67.6%) (Figure 1 ).
These findings suggest that the outcome of children and adults with APL remains comparatively poor in these developing countries. The same is likely to be true in other developing countries in which health care expenditure is relatively small. Although most of the patients in this survey received ATRA remission induction therapy, the CR rates are much lower than the 90% overall rate of CR in developed countries. The main cause of failure during the remission induction phase was death due to bleeding. Retinoic acid syndrome was also an important cause of mortality. Early mortality remains very high, even in places such as the Recife PCU, where CR rates for ALL and Burkitt lymphoma exceed 90%.28 Advanced-stage disease, delay between diagnosis and the start of ATRA therapy, and lack of experience in the management of retinoic acid syndrome may partially account for fatalities during induction. At some of the sites where ATRA is not readily available, there is a delay between diagnosis and the start of treatment. Moreover, because APL is less common than other leukemias, health care providers in many of these centers may lack expertise in diagnosing it and in anticipating the complex clinical problems that may occur after treatment is started. In summary, the availability of ATRA and the knowledge and skills required to treat APL have not been fully transferred from developed countries to many developing Latin American countries, where the incidence of APL is high.
American Society of Hematology Initiative to Improve the Survival of Patients with APL in Developing Countries
One of the greatest challenges of modern medicine is to introduce proven curative procedures into developing countries to benefit patients of diverse socioeconomic classes. Although an increasing number of health care providers in the developing countries participate in educational initiatives, such as those provided by ASH and the American Society of Clinical Oncology (ASCO), the impact of these activities on the survival of patients with potentially curable diseases and on the promotion of sustainable clinical investigation in these countries is unknown. The International Consortium on APL (IC-APL) embarked on a demonstration project whose main goal is to improve the survival of patients with APL in selected developing countries and establish a network among hematologists/oncologists to facilitate collaborative, locally sustainable clinical investigations. Internationally recognized experts in APL volunteered their time to meet with hematologists/oncologists from developing countries on the day before the start of the annual ASH meeting for the first time in 2004. ASH provided financial support for the latter and subsequent workshops in 2004, 2005, and 2006, and covered the travel and lodging expenses of many participants. Because of its previous experience in establishing successful medical projects in developing countries, St. Jude IOP also participated in this initiative. The first step was to create a protocol that was feasible in these countries. Treatment guidelines were collaboratively developed by the participants, taking into account the resources of the different countries. The participants’ consensus was to adopt a slightly modified version (Table 3 ) of a protocol that has been extensively used in Spain. In addition, a laboratory manual was developed to provide technical guidelines for the diagnosis and follow-up of APL, including the immunofluorescence testing that allows the rapid and accurate diagnosis of this disease.29
It was anticipated that the protocol and the availability of drugs per se would not eliminate many of the causes of treatment failure. An APL study group including partner-site investigators and experts was formed to discuss all new patients in real time. The goal of these interactions is to reduce early mortality by considering APL a medical emergency, initiating ATRA as soon as the diagnosis is suspected, administering platelet transfusion prophylactically, and treating ATRA syndrome at the prodromal stage. Inexpensive computer-based technologies that support communication among clinical investigators are readily available. One of these that is used extensively by St. Jude IOP clinicians for live meetings(www.cure4kids.org) will provide a platform for the IC-APL group participants. Financial support by ASH and the Veronesi Foundation has allowed training and salary support for data managers and for transport of samples to referral laboratories for diagnostic testing. As the initial clinical management issues are addressed, relapse of APL is expected to become an important cause of failure. Intensified therapy for selected patients at high risk of relapse and introduction of new treatment strategies may increase overall survival, but further improvement of hospital supportive care and diagnostic infrastructure will be required. Our hope is that this project will identify and resolve the main obstacles to the transfer of effective curative APL therapy to countries with limited resources. Further, these collaborative interactions among investigators at different centers across the world may encourage and facilitate additional meaningful clinical and laboratory research.
Selected clinical, laboratory, and outcome features of 121 children with acute promyelocytic leukemia (APL).
| Features . | Culiacan, Mexico . | Guatemala City, Guatemala . | Managua, Nicaragua . | Recife, Brazil . | San Salvador, El Salvador . | San Jose, Costa Rica . | Tegucigalpa, Honduras . |
|---|---|---|---|---|---|---|---|
| Abbreviations: AML, acute myeloid leukemia; WBC, white blood cell count; DIC, disseminated intravascular coagulation; CR, complete remission. | |||||||
| Values shown are median (range). | |||||||
| Modified from Ribeiro RC and Razzouk B. (with permission). Haematologica Reports . 2005 ;1 :91 –93 | |||||||
| Period | 1989–2004 | 2000–2005 | 1995–2004 | 1997–2004 | 1998–2004 | 1996–2005 | 1998–2004 |
| Number of patients | 10 | 16 | 36 | 21 | 14 | 12 | 12 |
| Age, years (range) | 6.8 (11.1–16.0) | 8 (2.0–16.0) | 9 (2.7–16.4) | 11.4 (0.1–16.2) | 7.7 (0.8–17.7) | 7 (1.7–11.9) | 10.2 (2.6–16.1) |
| Sex (M/F ratio) | 2.3 | 1.0 | 1.0 | 1.6 | 2.5 | 0.5 | 1.4 |
| % of AML cases | 17.2 | 22.0 | 28.5 | 16.6 | 13.6 | 15.2 | 15.9 |
| WBC × 109/L | 45.6 (1.3–189.0) | 8.5 (1.1–143.0) | 10.0 (0.1–100.1) | 17.7 (1.1–121.3) | 11.9 (1.5–165.9) | 19.2 (0.8–124.0) | 17.7 (0.5–279.6) |
| Platelets × 109/L | 56.3 (22.0–100.0) | 30.0 (7.5–180.0) | 21.0 (1.3–54.0) | 18.0 (6.0–157.3) | 15.5 (1.3–143.4) | 32.5 (7.0–91.0) | 28.1 (4.0–160.0) |
| DIC (%) | 5 (50.0) | 7 (43.7) | 8 (22.2) | 4 (19.0) | 6 (42.8) | 4 (33.3) | 6 (50.0) |
| CR rate (%) | 6 (60.0) | 7 (43.7) | 20 (55.5) | 16 (76.1) | 7 (50.0) | 8 (66.6) | 5 (41.6) |
| Early death (%) | 1 (10.0) | 8 (50.0) | 6 (16.6) | 3 (14.2) | 4 (28.5) | 2 (16.6) | 4 (33.3) |
| Abandonment/refusal (%) | 2 (20.0) | 1 (6.2) | 4 (11.1) | 2 (9.5) | 1 (7.2) | 0 | 5 (41.6) |
| Relapse (%) | 2 (20.0) | 1 (6.2) | 14 (38.8) | 5 (23.8) | 2 (14.2) | 4 (33.3) | 0 |
| Other failures (%) | 1 (10.0) | 1 (6.25) | 6 (16.6) | 2 (9.5) | 1 (8.3) | 2 (16.6) | 2 (16.6) |
| Alive (%) | 4 (40.0) | 6 (37.5) | 6 (16.6) | 9 (42.8) | 6 (42.8) | 6 (50.0) | 1 (8.3) |
| Features . | Culiacan, Mexico . | Guatemala City, Guatemala . | Managua, Nicaragua . | Recife, Brazil . | San Salvador, El Salvador . | San Jose, Costa Rica . | Tegucigalpa, Honduras . |
|---|---|---|---|---|---|---|---|
| Abbreviations: AML, acute myeloid leukemia; WBC, white blood cell count; DIC, disseminated intravascular coagulation; CR, complete remission. | |||||||
| Values shown are median (range). | |||||||
| Modified from Ribeiro RC and Razzouk B. (with permission). Haematologica Reports . 2005 ;1 :91 –93 | |||||||
| Period | 1989–2004 | 2000–2005 | 1995–2004 | 1997–2004 | 1998–2004 | 1996–2005 | 1998–2004 |
| Number of patients | 10 | 16 | 36 | 21 | 14 | 12 | 12 |
| Age, years (range) | 6.8 (11.1–16.0) | 8 (2.0–16.0) | 9 (2.7–16.4) | 11.4 (0.1–16.2) | 7.7 (0.8–17.7) | 7 (1.7–11.9) | 10.2 (2.6–16.1) |
| Sex (M/F ratio) | 2.3 | 1.0 | 1.0 | 1.6 | 2.5 | 0.5 | 1.4 |
| % of AML cases | 17.2 | 22.0 | 28.5 | 16.6 | 13.6 | 15.2 | 15.9 |
| WBC × 109/L | 45.6 (1.3–189.0) | 8.5 (1.1–143.0) | 10.0 (0.1–100.1) | 17.7 (1.1–121.3) | 11.9 (1.5–165.9) | 19.2 (0.8–124.0) | 17.7 (0.5–279.6) |
| Platelets × 109/L | 56.3 (22.0–100.0) | 30.0 (7.5–180.0) | 21.0 (1.3–54.0) | 18.0 (6.0–157.3) | 15.5 (1.3–143.4) | 32.5 (7.0–91.0) | 28.1 (4.0–160.0) |
| DIC (%) | 5 (50.0) | 7 (43.7) | 8 (22.2) | 4 (19.0) | 6 (42.8) | 4 (33.3) | 6 (50.0) |
| CR rate (%) | 6 (60.0) | 7 (43.7) | 20 (55.5) | 16 (76.1) | 7 (50.0) | 8 (66.6) | 5 (41.6) |
| Early death (%) | 1 (10.0) | 8 (50.0) | 6 (16.6) | 3 (14.2) | 4 (28.5) | 2 (16.6) | 4 (33.3) |
| Abandonment/refusal (%) | 2 (20.0) | 1 (6.2) | 4 (11.1) | 2 (9.5) | 1 (7.2) | 0 | 5 (41.6) |
| Relapse (%) | 2 (20.0) | 1 (6.2) | 14 (38.8) | 5 (23.8) | 2 (14.2) | 4 (33.3) | 0 |
| Other failures (%) | 1 (10.0) | 1 (6.25) | 6 (16.6) | 2 (9.5) | 1 (8.3) | 2 (16.6) | 2 (16.6) |
| Alive (%) | 4 (40.0) | 6 (37.5) | 6 (16.6) | 9 (42.8) | 6 (42.8) | 6 (50.0) | 1 (8.3) |
Selected clinical, laboratory, and outcome features of 148 adults and children with acute promyelocytic leukemia at 11 centers in Brazil.
| Hospital . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: AML, acute myeloid leukemia; WBC, white blood cell count; DIC, disseminated intravascular coagulation; CR, complete remission. Values are median (range) except where otherwise noted. | |||||||||||
| “Other failures” included infection (14 patients), abandonment/refusal of treatment (4), bleeding (8), ATRA syndrome (9), and myocardial infarction (1). In 10 patients more than one cause was noted. | |||||||||||
| No. patients | 12 | 10 | 6 | 5 | 13 | 5 | 9 | 12 | 16 | 39 | 21 |
| Age, years | 33 (11–57) | 29 (19–70) | 49 (33–72) | 13 (9–29) | 34 (18–79) | 44 (17–51) | 30 (17–48) | 42 (16–79) | 39 (17–65) | 28 (7–67) | 30 (5–78) |
| Sex (M/F ratio) | 0.7 | 0.6 | 1 | 0.6 | 1.1 | 0.25 | 1.1 | 1.2 | 1 | 0.9 | 0.4 |
| WBC × 109/L | 6.5 (0.9–402) | 4.9 (0.4–193) | 10.3 (1.5–38) | 5.5 (1.7–44.1) | 5.8 (0.7–110) | 5.5 (2–12.2) | 10 (0.3–64.2) | 6.3 (0.7–85.3) | 12.1 (1.1–403) | 4.4 (0.4–128) | 2.9 (0.7–147) |
| Platelet count × 109/L | NA | 20.5 (7–161) | 19 (10–41) | 19 (9–43) | 20 (5–67) | 49 (24–140) | 21 (10–41) | 23 (15–93) | 25.5 (9–48) | 24 (5–92) | 32 (10–193) |
| DIC (%) | 12 (100) | 0 | 6 (100) | 3 (60) | 6 (46) | 0 | 9 (82) | 7 (58) | 11 (69) | 10 (26) | 6 (29) |
| CR rate (%) | 6 (50) | 2 (20) | 4 (67) | 1 (20) | 10 (77) | 2 (40) | 2 (22.2) | 4 (33.3) | 7 (43.7) | 21 (54) | 14 (67) |
| Early death (%) | 4 (33.3) | 5 (50) | 1 (17) | 3 (60) | 2 (15) | 0 | 4 (44.5) | 2 (16.7) | 7 (43.7) | 9 (23) | 5 (24) |
| Relapse (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (16.7) | 1 (6.2) | 1 (2.6) | 1 (4.8) |
| Other failures (%) | 1 (8.3) | 3 (30) | 1 (17) | 1 (20) | 2 (15) | 3 (60) | 2 (22.2) | 3 (25) | 2 (12.5) | 8 (21) | 2 (9.5) |
| Alive (%) | 7 (58.3) | 2 (20) | 4 (67) | 1 (20) | 9 (70) | 2 (40) | 3 (33.3) | 5 (41.6) | 6 (38) | 22 (56) | 14 (67) |
| Hospital . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: AML, acute myeloid leukemia; WBC, white blood cell count; DIC, disseminated intravascular coagulation; CR, complete remission. Values are median (range) except where otherwise noted. | |||||||||||
| “Other failures” included infection (14 patients), abandonment/refusal of treatment (4), bleeding (8), ATRA syndrome (9), and myocardial infarction (1). In 10 patients more than one cause was noted. | |||||||||||
| No. patients | 12 | 10 | 6 | 5 | 13 | 5 | 9 | 12 | 16 | 39 | 21 |
| Age, years | 33 (11–57) | 29 (19–70) | 49 (33–72) | 13 (9–29) | 34 (18–79) | 44 (17–51) | 30 (17–48) | 42 (16–79) | 39 (17–65) | 28 (7–67) | 30 (5–78) |
| Sex (M/F ratio) | 0.7 | 0.6 | 1 | 0.6 | 1.1 | 0.25 | 1.1 | 1.2 | 1 | 0.9 | 0.4 |
| WBC × 109/L | 6.5 (0.9–402) | 4.9 (0.4–193) | 10.3 (1.5–38) | 5.5 (1.7–44.1) | 5.8 (0.7–110) | 5.5 (2–12.2) | 10 (0.3–64.2) | 6.3 (0.7–85.3) | 12.1 (1.1–403) | 4.4 (0.4–128) | 2.9 (0.7–147) |
| Platelet count × 109/L | NA | 20.5 (7–161) | 19 (10–41) | 19 (9–43) | 20 (5–67) | 49 (24–140) | 21 (10–41) | 23 (15–93) | 25.5 (9–48) | 24 (5–92) | 32 (10–193) |
| DIC (%) | 12 (100) | 0 | 6 (100) | 3 (60) | 6 (46) | 0 | 9 (82) | 7 (58) | 11 (69) | 10 (26) | 6 (29) |
| CR rate (%) | 6 (50) | 2 (20) | 4 (67) | 1 (20) | 10 (77) | 2 (40) | 2 (22.2) | 4 (33.3) | 7 (43.7) | 21 (54) | 14 (67) |
| Early death (%) | 4 (33.3) | 5 (50) | 1 (17) | 3 (60) | 2 (15) | 0 | 4 (44.5) | 2 (16.7) | 7 (43.7) | 9 (23) | 5 (24) |
| Relapse (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (16.7) | 1 (6.2) | 1 (2.6) | 1 (4.8) |
| Other failures (%) | 1 (8.3) | 3 (30) | 1 (17) | 1 (20) | 2 (15) | 3 (60) | 2 (22.2) | 3 (25) | 2 (12.5) | 8 (21) | 2 (9.5) |
| Alive (%) | 7 (58.3) | 2 (20) | 4 (67) | 1 (20) | 9 (70) | 2 (40) | 3 (33.3) | 5 (41.6) | 6 (38) | 22 (56) | 14 (67) |
ASH International Committee Acute Promyelocytic Leukemia Protocol (IC-APL2006).
| Remission Induction (all patients) . | DNR 60 mg/m2/day (days 2,4,6 and 8)* ATRA 45 mg/m2/day§ (day 1 until CR) Dexamethasone 2.5 mg/m2/12h × 15 (if WBC > 5 × 109/L) . | |||
|---|---|---|---|---|
| . | . | . | High-Risk (WBC >10 × 109/L Platelets ≤ 40 × 109/L) . | |
| Consolidation (risk-adapted) . | Low-risk (WBC ≤ 10 × 109/L Platelets > 40 × 109/L) . | Intermediate-Risk (WBC ≤ 10 × 109/L Platelets ≤ 40 × 109/L) . | ≥ 60 years old . | < 60 years old . |
| Abbreviations: ATRA, all-trans retinoic Acid; DNR, daunorubicin; MTZ, mitoxantrone; Ara-C, cytarabine; CR, complete remission. | ||||
| *Patients > 70 years old receive only three doses of daunorubicin (days 2, 4, and 6) during induction. | ||||
| §In patients < 20 years old, the ATRA dose is reduced to 25 mg/m2/day given in 2 doses. | ||||
| DNR 25 mg/m2/day (days 1,2,3,4) | DNR 35 mg/m2/day (days 1,2,3,4) | DNR 35 mg/m2/day (days 1,2,3,4) | DNR 25 mg/m2/day (days 1,2,3,4) | |
| ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | Ara-C 1000 mg/m2/day (days 1,2,3,4) ATRA 45 mg/m2/day × 15 | |
| MTZ 10 mg/m2/day (days 1,2,3) | MTZ 10 mg/m2/day (days 1,2,3) | MTZ 10 mg/m2/day (days 1,2,3) | MTZ 10 mg/m2/day (days 1,2,3,4,5) | |
| ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | |
| DNR 60 mg/m2/day (day 1) | DNR 60 mg/m2/day (days 1,2) | DNR 60 mg/m2/day (days 1,2) | DNR 60 mg/m2/day (day 1) | |
| ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | Ara-C 150 mg/m2/8 h (days 1,2,3,4) ATRA 45 mg/m2/day × 15 | |
| Maintenance (all patients) | 2 years ATRA 45 mg/m2/day × 15 (every 3 months) Methotrexate 15 mg/m2/day (weekly) 6-Mercaptopurine 50 mg/m2/day | |||
| Remission Induction (all patients) . | DNR 60 mg/m2/day (days 2,4,6 and 8)* ATRA 45 mg/m2/day§ (day 1 until CR) Dexamethasone 2.5 mg/m2/12h × 15 (if WBC > 5 × 109/L) . | |||
|---|---|---|---|---|
| . | . | . | High-Risk (WBC >10 × 109/L Platelets ≤ 40 × 109/L) . | |
| Consolidation (risk-adapted) . | Low-risk (WBC ≤ 10 × 109/L Platelets > 40 × 109/L) . | Intermediate-Risk (WBC ≤ 10 × 109/L Platelets ≤ 40 × 109/L) . | ≥ 60 years old . | < 60 years old . |
| Abbreviations: ATRA, all-trans retinoic Acid; DNR, daunorubicin; MTZ, mitoxantrone; Ara-C, cytarabine; CR, complete remission. | ||||
| *Patients > 70 years old receive only three doses of daunorubicin (days 2, 4, and 6) during induction. | ||||
| §In patients < 20 years old, the ATRA dose is reduced to 25 mg/m2/day given in 2 doses. | ||||
| DNR 25 mg/m2/day (days 1,2,3,4) | DNR 35 mg/m2/day (days 1,2,3,4) | DNR 35 mg/m2/day (days 1,2,3,4) | DNR 25 mg/m2/day (days 1,2,3,4) | |
| ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | Ara-C 1000 mg/m2/day (days 1,2,3,4) ATRA 45 mg/m2/day × 15 | |
| MTZ 10 mg/m2/day (days 1,2,3) | MTZ 10 mg/m2/day (days 1,2,3) | MTZ 10 mg/m2/day (days 1,2,3) | MTZ 10 mg/m2/day (days 1,2,3,4,5) | |
| ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | |
| DNR 60 mg/m2/day (day 1) | DNR 60 mg/m2/day (days 1,2) | DNR 60 mg/m2/day (days 1,2) | DNR 60 mg/m2/day (day 1) | |
| ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | ATRA 45 mg/m2/day × 15 | Ara-C 150 mg/m2/8 h (days 1,2,3,4) ATRA 45 mg/m2/day × 15 | |
| Maintenance (all patients) | 2 years ATRA 45 mg/m2/day × 15 (every 3 months) Methotrexate 15 mg/m2/day (weekly) 6-Mercaptopurine 50 mg/m2/day | |||
Survival of 148 adults and children with acute prolymphocytic leukemia (APL) at 11 Brazilian centers. The survival estimate is 58.2% ± 4.8% (95% CI, 48.9%–67.6%).
Survival of 148 adults and children with acute prolymphocytic leukemia (APL) at 11 Brazilian centers. The survival estimate is 58.2% ± 4.8% (95% CI, 48.9%–67.6%).
Affiliations: RCR: Department of Hematology-Oncology and the International Outreach Program, St. Jude Children’s Research Hospital, Memphis, TN, USA and the Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN, USA. ER: Hematology Division and Center for Cell Based Therapy, Department of Internal Medicine, Medical School of Ribeirão Preto, University of São Paulo, Brazil
This work was supported in part by a Cancer Center Support Grant (CA21765) from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC).
Acknowledgments: The authors wish to thank Eduardo Altamirano Álvarez, Federico Antillon, Miguel Bonilla, Ligia Fu, Rafael Jácomo, Marta Navarete, Roberta Ortiz and Francisco Pedrosa for data collection and analysis and Sharon Naron for editing the article.