Abstract
Selective inhibitors of cyclooxygenase (COX)-2, the coxibs, were developed to inhibit inflammatory prostaglandins derived from COX-2, while sparing gastroprotective prostaglandins primarily formed by COX-1. However, COX-2-derived prostaglandins mediate not only pain and inflammation but also affect vascular function, the regulation of hemostasis/ thrombosis, and blood pressure control. All coxibs depress COX-2-dependent prostacyclin (PGI2) biosynthesis without effective suppression of platelet COX-1-derived thromboxane (Tx) A2, unlike aspirin or traditional nonsteroidal anti-inflammatory drugs, which inhibit both COX-1 and COX-2. The actions of PGI2 oppose mediators, which stimulate platelets, elevate blood pressure, and accelerate atherogenesis, including TxA2. Indeed, structurally distinct inhibitors of COX-2 have increased the likelihood of hypertension, myocardial infarction and stroke in controlled clinical trials. The detection of these events in patients is related to the duration of exposure and to their baseline risk of cardiovascular disease. Thus, coxibs should be withheld from patients with preexisting cardiovascular risk factors, and exposed patients at low cardiovascular baseline risk should be monitored for changes in their risk factor profile, such as increases in arterial blood pressure.
Traditional nonsteroidal anti-inflammatory drugs (tNSAIDs) and selective inhibitors of COX-2, the coxibs, are a chemically heterogeneous group of compounds, characterized by varying degrees of anti-inflammatory, analgesic and antipyretic activity. Their unifying therapeutic effect is the inhibition of prostaglandin (PG) biosynthesis. PGs are lipid mediators formed from arachidonic acid by the PG G/H synthases 1 and 2, commonly termed cyclooxygenases (COX). Recruitment of leukocytes and induction of COX-2 expression by inflammatory stimuli account for the high levels of PGs found in inflammatory lesions. PGE2, which sensitizes nociceptors, prostacyclin (PGI2) and perhaps thromboxane (Tx) A2 mediate pain and inflammation.1 Both PGI2 and PGE2 contribute to the vasodilation at sites of injury. In addition to such peripheral mechanisms, COX-1 and COX-2 products modulate nociception centrally. tNSAIDs such as ibuprofen, naproxen or indomethacin inhibit both COX isoforms reversibly. Aspirin is distinct, as it blocks the COXs irreversibly, which explains the cardio-protective effect of low-dose aspirin; it inactivates platelet COX-1 irreversibly and new platelets have to be formed to restore function.
The discovery of the second, rapidly inducible COX isoform in the early 1990s afforded an opportunity to suppress PG formation in a more targeted fashion by designing inhibitors with higher affinity for COX-2 than for COX-1, the coxibs. The underlying hypothesis—sometimes referred to as the ‘COX-2 hypothesis’—was that inflammatory prostaglandins are primarily derived from COX-2, while prostaglandins formed by COX-1 have generally homeostatic roles, including the protection of the gastrointestinal mucosa. Thus, selective inhibitors of COX-2 were expected to be as efficacious as non-selective tNSAIDs and cause less serious gastrointestinal side effects, which are attributed to inhibition of COX-1. Although biological reality turned out to be more complex—COX-2 has also important physiological functions in the absence of inflammation2 and COX-1 plays also a role in nociception3—all coxibs were indeed shown to cause less gastroduodenal ulcerations than comparator tNSAIDs, as visualized by endoscopy.2 Celecoxib (Celebrex, Pfizer) and rofecoxib (Vioxx, Merck) were approved by the FDA in 1999, valdecoxib (Bextra, Pfizer) in 2001. Other compounds (etoricoxib/Arcoxia, Merck; lumiracoxib/Prexige, Novartis; parecoxib/Dynastat, Pfizer) are pending approval. Boosted by the attraction of the ‘COX-2 hypothesis’—and by aggressive marketing campaigns—the coxibs achieved sales of about $9 billion by the time of the withdrawal of rofecoxib in September 2004. Interestingly, the majority of patients prescribed with a coxib were not at particularly high risk for serious tNSAID related gastrointestinal complications and the incidence of uncomplicated gastric upset on a coxib turned out to be no different from that of tNSAIDs. Only three randomized, controlled outcome trials have studied whether the coxibs actually reduce the incidence of serious gastrointestinal complications in larger populations. The Vioxx Gastrointestinal Outcomes Research (VIGOR) Study and the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET) have shown that rofecoxib and lumiracoxib, cause less serious gastrointestinal adverse events than non-isoform selective tNSAIDs.4,5 The trial studying the oldest marketed COX-2 inhibitor, the Celecoxib Long-term Arthritis Safety Study (CLASS) study,6 failed to support the hypothesis, as its gastrointestinal endpoint did not differentiate this coxib from the comparator tNSAIDs.6 Nevertheless, celecoxib became the best selling coxib and, indeed, remains the only such drug on the market.
While no randomized controlled outcome trial of a coxib has sought to demonstrate superiority of pain relief over comparator tNSAIDs, some patients report a unique quality of pain relief by COX-2 inhibitors, which they had not experienced on tNSAIDs. It has been long recognized, but never systematically studied, that the clinical response to a particular tNSAID—or its toxicity—may vary substantially from patient to patient. Interestingly, the pharmacological response within the group of selective COX-2 inhibitors is similarly variable,7 and detailed analyses of factors that condition such diverse responses may lead to a more individualized therapy with both, tNSAIDs and coxibs.7
Concurrent with the clinical development of the coxibs, it was recognized that—in contrast to the initial hypothesis—COX-2-derived prostaglandins mediate not only pain and inflammation, but also affect vascular function, thrombosis and blood pressure.8,9 Thus, COX-2 inhibitors depress the vascular biosynthesis of PGI2, leaving platelet COX-1-derived TxA2 unaffected—in contrast to tNSAIDs or aspirin, which inhibit both COX-1 and COX-2.8,9 The actions of PGI2 oppose all mediators that stimulate platelets, elevate blood pressure, and accelerate atherogenesis, including TxA2.10–12 Thus, drug selectivity for inhibition of COX-2 may increase the likelihood of hypertension, myocardial infarction and stroke.1 Three structurally distinct compounds, rofecoxib, valdecoxib, and celecoxib, have increased the incidence of these cardiovascular complications significantly in controlled trials,4,13–15 suggesting that the entire class of COX-2 inhibitors confers a cardiovascular hazard. Rofecoxib and valdecoxib have been withdrawn from the market. This paper reviews the pharmacology of COX inhibition, discusses mechanisms by which coxibs may promote thrombosis, atherosclerosis and hypertension, summarizes results of clinical trials that detected a cardiovascular risk and provides perspective on how this may inform clinical decisions regarding coxib and tNSAID use.
Pharmacology of COX Inhibition
Arachidonic acid, released from cell membranes by the activity of phospholipases, is the main COX substrate. Both, COX-1 and COX-2 synthesize PGH2, a labile intermediate that is further metabolized by downstream isomerases to form the prostaglandins, PGE2, PGF2α, PGD2, PGI2 and TxA2 (Figure 1 ), which activate specific transmembrane receptors coupled to G proteins. COX-1 is expressed constitutively in most tissues. It is the only form of the enzyme in mature platelets and is expressed in vascular endothelium, gastrointestinal epithelium, spinal cord, brain and kidney, amongst other tissues. COX-2 is highly inducible by cytokines, tumor promoters and mitogens. In addition to its role in prostaglandin formation in inflammation it has been implicated in carcinogenesis. The strict distinction between ‘inducible’ and ‘constitutive’ forms of the enzyme, however, is an oversimplification;2 COX-2 is constitutively expressed in several tissues, such as spinal cord, brain, kidney and COX-1 can be upregulated to a certain degree in inflammation. COX-2 is also induced in vascular endothelium under physiological conditions of flow16 and both isoforms both are developmentally regulated.17
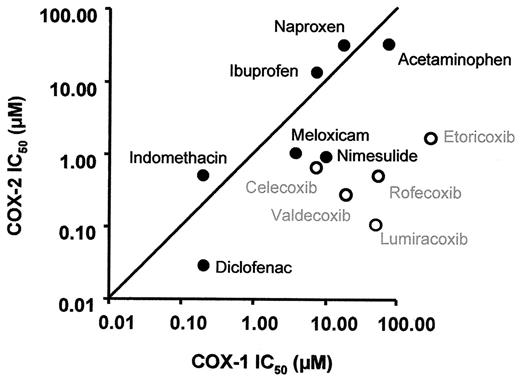
While COX-1 and COX-2 are structurally similar, the substrate-binding channel of COX-2 contains a side pocket, which is absent in COX-1. This allowed for the design of inhibitors with side chains that fit within the COX-2 channel, but are too large to block COX-1 with equally high affinity. Thus, the ratio of the affinities to COX-1 and COX-2 determines how ‘selective’ a compound is and represents a continuous, rather than a discrete, variable. The second generation coxibs, etoricoxib and lumiracoxib, were developed to be more selective for COX-2 than rofecoxib and valdecoxib, which are roughly similar in their degree of selectivity but more selective than celecoxib (Figure 2 ).2 In human studies, the degree of COX-2 selectivity is commonly determined by whole blood assays that measure the ratio of COX-1 and COX-2 inhibition ex vivo.18 As ‘selectivity’ is conditioned by differences in the affinity to the COX isoforms, the concentration of the compound has an effect on ‘selectivity.’ Thus, high drug concentrations will also inhibit COX-1 to a certain degree and very high, supratherapeutic concentrations may not be COX-2 selective at all. Thus ‘selectivity’ achieved in humans is not a purely structural property of a compounds but may also be influenced by pharmacokinetic (e.g., plasma concentration) and pharmacodynamic factors (e.g., genetic variations of the target enzymes).7 tNSAIDs can also be ranked on the selectivity scale using the same criteria. Naproxen or ibuprofen are slightly more potent inhibitors of COX-1 than of COX-2, while the degrees of selectivity of diclofenac, nimesulide and meloxicam are—perhaps surprisingly—comparable to that of celecoxib.2 One would expect that these characteristics relate to the safety profile of both tNSAIDs and coxibs. For example, the similarity of diclofenac and celecoxib may be one reason why the CLASS trial failed to detected difference in the gastrointestinal endpoint between the groups.6 Very little information exists about the cardiovascular safety of tNSAIDs 19 and prospective, placebo-controlled outcome studies have not been performed.
Impact of COX Inhibition on Thrombogenesis, Atherogenesis and Blood Pressure Regulation
Products of the COX pathway have established relevance in clinical syndromes of vascular occlusion. Thus, aspirin reduces the secondary incidence of myocardial infarction, stroke and important vascular events by roughly 25%.20 Suppression of the major product of platelet COX-1, TxA2, which is a potent platelet activator, vasoconstrictor, and mitogen,21 is the basis of cardioprotection by aspirin. TXA2 biosynthesis is increased in conditions of acute or chronic platelet activation, such as coronary thrombosis and diabetes, and augmented TxA2 biosynthesis has been linked to the risk of future events.22 Low-dose aspirin suppresses platelet TxA2 formation by acetylation of a serine residue within the substrate binding channel of COX-1 obstructing access of arachidonic acid to the active site. This modification results in the irreversible inhibition of platelet function, due to the limited capacity of the anucleate platelets to form new proteins. tNSAIDs other than aspirin attain maximal inhibition of platelet COX-1 only transiently during the dosing interval, due to their reversible interaction with the enzyme. Thus, tNSAIDs are not cardioprotective. COX-2 is not expressed in mature platelets, although small quantities of COX-2 protein are detectable in immature platelet forms.23,24 Thus, the coxibs depress platelet TxA2 formation only marginally at high concentrations and have no effect on platelet function.8,9
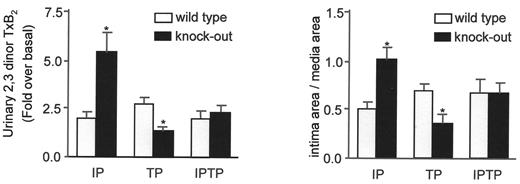
The possibility that selective inhibitors of COX-2 may increase cardiovascular risk was raised in 1999 based on observations that both rofecoxib and celecoxib reduced PGI2 formation in healthy individuals by about 70% without concurrent inhibition of platelet function.8,9 Biosynthesis of prostacyclin, a potent inhibitor of platelet aggregation, is increased in syndromes of platelet activation, such as severe atherosclerosis and unstable angina probably as a homeostatic response to accelerated platelet-vascular interactions.10 Thus, deletion of the PGI2 receptor (IP) in mice augmented the cardiovascular effects of TxA2 in vivo.10 The mice responded with an exaggerated formation of TxA2 to vascular injury followed by more pronounced neointimal proliferation (Figure 3 ). Both responses were reverted by simultaneous deletion of the TxA2 receptor (TP).10 Thus, depression of COX-2 dependent PGI2 biosynthesis, without concomitant inhibition of platelet function provides a mechanism for a prothrombotic effect of COX-2 inhibitors. However, the impact of PGI2 depression on thrombotic events would be expected to pertain only when the baseline risk of thrombosis is augmented, as mice deficient in the prostacyclin receptor do not develop thrombosis spontaneously.
Depression of COX-2-derived PGI2 formation may also impact atherosclerotic plaque development, as deletion of the IP accelerates the initiation and early development of atherosclerosis in mice (Figure 3 ).12,25 In this context, PGI2 acts to limit the interactions of platelets and leukocytes with the vasculature and the attendant oxidant stress.12,25 Deletion of the IP increases the biosynthesis of TxA2 in hypercholesterolemic mice, as an index of increased platelet activation in vivo.25 Platelet activation contributes to atherogenesis and suppression of TxA2 activity retards atherogenesis (Figure 4 ).12,26,27 These observations suggest that COX-1-derived TxA2 and COX-2-derived PGI2 may have opposing activities in atherogenesis,12,28 although this has not yet been addressed directly in humans. This implies that an increasing degree of selectivity for COX-2 may proportionately accelerate progression of atherogenesis.
The elevation of blood pressure by the COX-inhibitors is a more indirect mechanism by which they may accelerate atherogenesis and increase cardiovascular risk. It is well recognized that tNSAIDs and coxibs can induce sodium retention, edema and hypertension.29,30 However, it remains unclear whether the coxibs differ from tNSAIDs in their effects on blood pressure regulation. Very little data exist on the relative roles of COX isoforms in the human kidney. Some information, however, can be derived from experiments in mice. Interestingly, these show—just like in the case of atherogenesis—that COX-1 and COX-2 may have opposing functions in the kidney.11 In mice, COX-1 products, likely TxA2 and perhaps PGF2α, contribute to blood pressure homeostasis by the renin angiontensin system and increase blood pressure.31 Conversely, the vasodilator COX-2 products, PGI2 and PGE2, increase renal medullary blood flow, which drives pressure natriuresis and diuresis.29 COX-2 inhibition, just like nonselective COX-inhibition, reduces acutely medullary blood flow, sodium excretion and urine volume.11 However, this results only in a hypertensive response when the autoregulatory mechanisms are already otherwise stressed.11,32 In humans, hypertensive responses to COX inhibition tend to occur when predisposing conditions exist, such as excessive salt intake, preexisting hypertension, heart disease and diabetes, or depletion of the effective circulating volume. This is consistent with clinical observations in young and healthy individuals, in which both nonselective COX inhibition and selective COX-2 inhibition have no effects on arterial pressure despite transient changes in sodium handling and glomerular filtration rate on initiation of dosing.33 The latter may be caused by the inhibition of dilator prostanoids that contribute to the patency of afferent arterioles and by a reduction of COX-2 dependent, cortical PGE2 formation, a component of the tubuloglomerular feedback mechanism.31,34 Thus, given the role of COX-1 in elevating blood pressure, one would expect that the degree of selectivity of COX-2 inhibition could affect blood pressure control in susceptible individuals. Indeed, a recent meta-analysis of approximately 45,000 patients in 19 clinical trials suggests that selective inhibition of COX-2 elevates blood pressure more than non-selective inhibition.30
Detection of a Cardiovascular Risk of the Coxibs
Epidemiological approaches and clinical trials have relatively poor precision for the detection of uncommon but serious adverse effects, such as clinical events that are prevalent in the relevant populations. The first clinical evidence of a cardiovascular hazard of the coxibs arose when a 5-fold higher incidence of myocardial infarction was observed in the rofecoxib group as compared to the naproxen comparator group of the VIGOR study, the prospective trial designed to assess rofecoxib’s gastrointestinal safety.4 Approximately 8000 rheumatoid arthritis patients were treated either with rofecoxib or naproxen with a median follow-up of 9 months. The gastrointestinal event rate was reduced from 4.5 to 2.1 per 100 patient-years by rofecoxib. The study population would probably be considered medium cardiovascular risk at baseline—with chronic rheumatoid inflammation as an independent risk factor. Two explanations for the adverse cardiovascular outcome were discussed at the time—aside from the possibility that this was due to chance:2 (i) a reduction of the cardiovascular event rate in the control group by a cardioprotective effect of naproxen; and/or (ii) a cardiovascular hazard of rofecoxib. Naproxen, unlike other tNSAIDs, has a long elimination half-life and causes an extended inhibition of platelet function and might provide some degree of cardioprotection.2 However, the substantial interindividual variability of its activity suggests that much fewer patients would benefit from naproxen than from aspirin.35 This is consistent with observational studies that detected a small cardioprotective benefit from naproxen—approximately half that of aspirin.36 While the absolute number of cardiovascular events in VIGOR was too small to estimate accurately the size of the effect, the potential cardioprotective effect of naproxen alone is unlikely to account for the 5-fold difference between the groups. It seems more plausible that this coincided with a cardiovascular hazard of rofecoxib. In contrast to rofecoxib, no excess cardiovascular risk was initially associated with celecoxib therapy in its gastrointestinal safety study, the CLASS trial.6 However, in this study the use of low-dose aspirin was allowed and one of the tNSAID comparators was diclofenac, which is similar to celecoxib in its degree of COX-2 selectivity. Additionally, the patient population in the CLASS trial was probably at lower baseline risk for cardiovascular events as compared to the VIGOR trial. The third large gastrointestinal safety study, the TARGET trial, assessed also the cardiovascular safety profile of lumiracoxib in comparison to ibuprofen and naproxen in approximately 18,000 osteoarthritis patients, of which a quarter took also low-dose aspirin. An important finding of this study was that the favorable gastrointestinal safety profile of the COX-2 inhibitor was offset by concurrent low-dose aspirin therapy and the advantage over a tNSAID was lost.5 Lumiracoxib was not associated with a statistically significant increase in cardiovascular risk in this population of apparently low baseline risk exposed for only 12 months.37
The trial that led to the withdrawal of rofecoxib—the Adenomatous Polyp Prevention on Vioxx (APPROVe) study, a placebo controlled cancer prevention trial of approximately 2600 patients—detected the cardiovascular risk only after a much longer time of drug exposure.13 It was stopped at 36 months of treatment when a twofold increase in the cardiovascular event rate (rofecoxib: 1.5% vs placebo: 0.78%) was noticed. This suggests that a small minority of the patients, apparently of initial low risk of cardiovascular disease, might have proceeded to increase that risk to a point that culminated in clinical events. A similar pattern, again, implying a time-dependent change of cardiovascular risk, was observed in a long-term cancer prevention trials of celecoxib in patients of initially low apparent cardiovascular risk. The Adenoma Prevention with Celecoxib (APC) study detected a dose-dependent increase in the cardiovascular event rate (placebo, 1%; celecoxib 200 mg/bid, 2.3%; celecoxib 400 mg/bid, 3.4%) in a study population of 2035 patients after 36 months of exposure.14 An unpublished, smaller trial (“preSAP Trial”) that included approximately 1500 adenoma patients receiving a single daily dose of 400 mg celecoxib has shown a non-significant increase in cardiovascular events (placebo: 0.91% vs celecoxib: 1.23%; FDA Advisory Committee Meeting, Gaithersburg, Maryland, February 16–18, 2005). This may relate to the difference in dosing (once daily vs twice daily in the APC study) and to the lower power of the preSAP trial.
The cardiovascular signal was detected very early in two postoperative pain management trials of high risk patients, referred to as ‘CABG-1’ and ‘CABG-2.’15,38 Patients undergoing coronary bypass surgery were initially treated intravenously with the prodrug of valdecoxib, parecoxib, or placebo and then switched to oral valdecoxib or placebo. The majority of cardiovascular events occurred within days of treatment initiation in these populations, who were subject to intense hemostatic activation after bypass surgery. The outcomes of the CABG studies are compatible with a prothrombotic effect of the coxibs in situations of elevated thrombotic risk. In summary, the combined clinical experience of trials that detected cardiovascular complications attributable to the coxibs suggests that the emergence of events relates to baseline cardiovascular risk and to the duration of drug exposure, which accords remarkably with the biological observations discussed above.
Conclusions and Future Directions
The distinct roles of the COX isoforms in platelet function, atherogenesis and blood pressure control provide a unitary, plausible mechanism—depression of vascular PGI2 biosynthesis—by which selective inhibition of COX-2 may increase the likelihood of myocardial infarction and stroke in at-risk patients and in patients who are exposed for extended periods of time. Mouse biology, human pharmacology, and clinical evidence suggest that the cardiovascular hazard pertains to all coxibs. However, it seems likely that differences in the degree of selectivity attained by various drugs may translate into quantitative differences in clinical outcomes. Both the US Food and Drug Administration (FDA) and the European Agency for the Evaluation of Medicinal Products (EMEA) concluded that rofecoxib, valdecoxib and celecoxib conveyed a small (1%–2%), but absolute risk of cardiovascular adverse events. Rofecoxib and valdecoxib have been withdrawn from the market in the US and Europe. The FDA applied a “black box” warning to celecoxib, which remains on the market, and the EMEA imposed restrictions on celecoxib (and on etoricoxib, which is on the market in some European countries), and it is possible that new coxibs will be introduced to the market probably with similar restrictions.
What role, then, can the coxibs play in the treatment of chronic inflammatory conditions in the future?
They should be reserved for patients who are most likely to benefit from them, for example those who develop gastrointestinal complications on tNSAIDs in combination with a gastroprotective medication, such as a proton pump inhibitor. The dilemma is that the only drug presently on the US market, celecoxib, has not convincingly demonstrated a gastrointestinal benefit in its outcome trial even if this may relate in part to a suboptimal study design.6
Coxibs should be avoided when patients have preexisting risk factors for cardiovascular events.
The cardiovascular roles of the COX products affected by the coxibs are not meant to be viewed as an imbalance of PGI2 and TxA2, which might imply that suppression of TxA2 by low-dose aspirin would be completely protective against a cardiovascular hazard. PGI2 acts as a constraint on the biological activity of all agonists, which stimulate platelets, elevate blood pressure, and accelerate atherogenesis, of which TxA2 is only one mediator. Thus, low dose aspirin would be expected to attenuate, rather than abolish the cardiovascular hazard deriving from selective inhibition of COX-2.
We know very little about the cardiovascular safety profile of individual tNSAIDs. The FDA applied a “black box” warning of potential cardiovascular risks also to all tNSAIDs, while the EMEA concluded that there was no evidence to prompt a change in their advice about tNSAIDs. Clearly, prospective studies are warranted to determine if patients are at elevated cardiovascular risk with long-term use of specific tNSAIDs. Pharmacologic studies have already found that ibuprofen and naproxen can undermine the anti-platelet effectiveness of low-dose aspirin, but not diclofenac.39,40 This indicates that tNSAIDs are not a homogenous group of compounds and it is likely that differences in their cardiovascular safety profiles will be revealed, just like the differences in their gastrointestinal safety profiles.
Finally, as we move towards an individualization of therapy, we should identify paradigms within which these useful drugs can be administered safely for extended periods in individuals at low cardiovascular risk. The detectable variability between individuals in their response to the coxibs7 and the existence of clinical conditions that can modulate the response may be exploited to identify the small number of patients at emerging cardiovascular risk. Regular monitoring of risk factors during the treatment, including frequent blood pressure measurement, is a first step.
The cyclooxygenase (COX)-1 and -2 pathway. Prostaglandins (PG) are formed by specific isomerases from the COX product PGH2. They act through G protein transmembrane receptors.
Abbreviations: IP, prostacyclin receptor; TP, thromboxane receptor; DP, PGD2 receptor, EP, PGE2 receptor, FP, PGF2α receptor.
The cyclooxygenase (COX)-1 and -2 pathway. Prostaglandins (PG) are formed by specific isomerases from the COX product PGH2. They act through G protein transmembrane receptors.
Abbreviations: IP, prostacyclin receptor; TP, thromboxane receptor; DP, PGD2 receptor, EP, PGE2 receptor, FP, PGF2α receptor.
The degrees of cyclooxygenase (COX)-selectivity of various traditional non-steroidal anti-infammatory drugs (tNSAIDs) and coxibs (open circles). The concentrations required to inhibit COX-1 and COX-2 by 50% (IC50) have been measured using whole blood assays of COX-1 and COX-2 activity in vitro.18 The line indicates equivalent COX-1 and COX-2 inhibition. Drugs plotted below the line are more potent inhibitors of COX-2 than drugs plotted above the line. The distance to the line is a measure of selectivity. Lumiracoxib is the compound with the highest degree of selectivity for COX-2 as its distance to the line is the largest. Celecoxib and diclofenac have similar degrees of COX-2 selectivity, as their distances to the line are similar; however, diclofenac is active at lower concentrations and, thus, located more to the left. (Updated from FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2.
The degrees of cyclooxygenase (COX)-selectivity of various traditional non-steroidal anti-infammatory drugs (tNSAIDs) and coxibs (open circles). The concentrations required to inhibit COX-1 and COX-2 by 50% (IC50) have been measured using whole blood assays of COX-1 and COX-2 activity in vitro.18 The line indicates equivalent COX-1 and COX-2 inhibition. Drugs plotted below the line are more potent inhibitors of COX-2 than drugs plotted above the line. The distance to the line is a measure of selectivity. Lumiracoxib is the compound with the highest degree of selectivity for COX-2 as its distance to the line is the largest. Celecoxib and diclofenac have similar degrees of COX-2 selectivity, as their distances to the line are similar; however, diclofenac is active at lower concentrations and, thus, located more to the left. (Updated from FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2.
The impact of deletion of the prostacyclin receptor on the thrombotic (left panel) and proliferative (right panel) response to catheter-induced carotid vascular injury in mice. The endothelium of the common carotid artery was denuded in this model using a fine wire. This activates platelets, as reflected by an enhanced biosynthesis of thromboxane (Tx) A2 (left panel), and causes neointimal formation, which can be quantified on histological crossections two weeks after the procedure (right panel). The procedure-related platelet activation and thromboxane biosynthesis, as reflected by urinary excretion of its metabolite 2,3 dinor TxB2, was augmented in mice deficient for the prostacyclin receptor (IP). Deletion of the thromboxane receptor (TP) reduced the procedure-related increment in thromboxane biosynthesis. Simultaneous deletion of both receptors (IPTP) compensated for the effects of the individual receptors.
Redrawn with permission from Cheng Y, Austin SC, Rocca B, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2.
The impact of deletion of the prostacyclin receptor on the thrombotic (left panel) and proliferative (right panel) response to catheter-induced carotid vascular injury in mice. The endothelium of the common carotid artery was denuded in this model using a fine wire. This activates platelets, as reflected by an enhanced biosynthesis of thromboxane (Tx) A2 (left panel), and causes neointimal formation, which can be quantified on histological crossections two weeks after the procedure (right panel). The procedure-related platelet activation and thromboxane biosynthesis, as reflected by urinary excretion of its metabolite 2,3 dinor TxB2, was augmented in mice deficient for the prostacyclin receptor (IP). Deletion of the thromboxane receptor (TP) reduced the procedure-related increment in thromboxane biosynthesis. Simultaneous deletion of both receptors (IPTP) compensated for the effects of the individual receptors.
Redrawn with permission from Cheng Y, Austin SC, Rocca B, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2.
Quantification of atherosclerotic lesions area in aortas of wildtype (+/+), TP deficient (TP−/ −) and IP deficient (IP −/ −) mice on an apoE deficient background at 20 weeks of age. TP deficiency retarded and IP deficiency accelerated atherogenesis, suggesting that these mediators have opposing roles in atherosclerotic lesion development. Data are means ± SEM (n = 5 each).
(Redrawn with permission from Kobayashi T, Tahara Y, Matsumoto M, et al. Roles of thromboxane A2 and prostacyclin in the development of atherosclerosis in apoE-deficient mice.
Quantification of atherosclerotic lesions area in aortas of wildtype (+/+), TP deficient (TP−/ −) and IP deficient (IP −/ −) mice on an apoE deficient background at 20 weeks of age. TP deficiency retarded and IP deficiency accelerated atherogenesis, suggesting that these mediators have opposing roles in atherosclerotic lesion development. Data are means ± SEM (n = 5 each).
(Redrawn with permission from Kobayashi T, Tahara Y, Matsumoto M, et al. Roles of thromboxane A2 and prostacyclin in the development of atherosclerosis in apoE-deficient mice.
Acknowledgments: Supported by grants from the American Heart Association (S.F.: Pennsylvania-Delaware Affiliate Postdoctoral Fellowship; T.G.: Scientist Development Grant 0430148N).