Abstract
Rituximab was the first humanized antibody widely used on patients, so research on its optimal use was a clinical challenge. Many studies have been performed to optimize its dose and schedule, and more are ongoing. The dose of 375 mg/m2 has become standard, mainly because it shows activity and has little associated toxicity. The combination of rituximab with chemotherapy has been shown to prolong remission in all types of lymphomas, and in patients with diffuse large B-cell lymphoma it can improve survival. As a single agent, particularly when the treatment is prolonged over several months, results are similar to chemotherapy but with fewer side effects. Finally, used as maintenance therapy it can prolong the duration of chemotherapy-obtained remissions. Based on available data, the administration of 375 mg/m2 before each chemotherapy cycle can be recommended for first line treatment of patients with curable B-cell lymphomas and for patients with high-risk indolent lymphoma who are rituximab-naïve. Single-agent treatment at a prolonged schedule is recommended for cases of indolent disease not in need of urgent response and for patients who are unlikely to tolerate chemotherapy.
Since its first use in humans more than 10 years ago, rituximab has become an essential component of the treatment of all types of B-cell lymphoma. Its modulating effects on the normal immune system have also prompted its increasing use in non-neoplastic hematologic diseases (such as idiopathic thrombocytopenic purpura or cryoglobulinemia) and in non-hematologic diseases (such as rheumatoid arthritis or other autoimmune diseases). Despite its widespread use, the optimal schedule for drug administration, either alone or in combination with other modalities, is still not completely defined. This review focuses on the data available to define the most appropriate way of using rituximab in patients with lymphoma.
Rituximab as Single Agent
In 1997 rituximab at the schedule of 375 mg/m2 weekly for 4 consecutive weeks was approved by the US Food and Drug Administration (FDA) for the treatment of indolent lymphoma. This original schedule was developed based on two phase I studies demonstrating that this dose was safe and active.1 The rationale for this schedule was based mostly on empiric and logistic considerations. At the time of FDA approval, no other treatment regimen had been tested, but because of its efficacy and safety,2 it remained the standard drug schedule for several years. Nevertheless, several considerations led to the initiation of studies to improve the effect of rituximab. All aspects of the schedule could in fact be challenged: the unitary dose, the number of infusions, the duration of the treatment, the interval between administrations, and the speed of the infusion (Figures 1–3Figure 2,Figure 3 ).
Preclinical data on dose and schedule
Rituximab has many proposed mechanisms of action. Some of these actions depend on the activation of complement and of the host immune system, such that in vitro studies might not be representative of the drug’s in vivo efficacy. The majority of in vitro studies suggest that there is a dose-response relationship up to the 10–25 mg/mL threshold, after which the curve levels off, possibly due to a saturation of the receptors or of other effector mechanisms.3 This does not mean that 10–25 mg/mL should be the target concentration to be reached in humans: persistent blood levels of rituximab suggest saturation of all available CD20, so that any measurable rituximab level means that sufficient drug is present in the patient. Nevertheless, because pharmacokinetic (PK) data from the pivotal trial identified 25 mg/mL as the median 3-month level associated with response,4 a schedule maintaining this level in the blood could be considered as an empiric target.
Unitary dose
In the original phase I trials, no dose-limiting toxicity was seen and the dose of 375 mg/m2 was chosen based on the limited availability of the drug for the first phase II trial. Only a limited number of studies have since investigated the effect of higher or lower dosages. Coiffier et al, in a small randomized study comparing 375 mg/m2 with 500 mg/m2 in aggressive lymphomas, could establish no difference in the response rate and response duration of the two schedules.5 In patients with chronic lymphocytic leukemia (CLL), O’Brien et al escalated the dose of rituximab from 375 mg/m2 to 2250 mg/m2 weekly for 4 consecutive weeks.6 Even though the study was not randomized, a comparison among the small cohorts of the study suggest a dose-response relationship in this disease without any increase in toxicity. Despite these encouraging data, no similar trial was carried out in other B-lymphoproliferative diseases, possibly because of what has been called the “economical” dose-limiting toxicity. The data generated in CLL patients cannot be extrapolated to “solid” lymphoprolipherative diseases, both because CLL cells express low levels of CD20 on their surface and because the disease is associated with a uniquely high level of circulating CD20 antigen.7
Duration of the weekly treatment
Although the response rate with 4 weeks of treatment was satisfactory and comparable to other single-agent chemotherapies, it soon became evident that the duration of the responses and the proportion of complete responses (CR) were somehow lower than those observed with chemotherapy. In an effort to improve upon these results, investigators tried to extend the treatment to 6 or 8 weeks duration.8,9 Unfortunately this was done only in small pilot phase II trials, which suggested some benefit in CR rate (even leading to the registration of the 8-week “extended” schedule), but which were not further confirmed. Overall, we do not have sufficient evidence today to recommend an extension of the weekly treatment beyond the standard 4 weeks.
Optimal duration of rituximab treatment
The PK analysis in the first pivotal study of rituximab indicated that patients maintaining a higher and more prolonged blood level of rituximab had an increased chance of responding.4 This suggested a relationship between the duration of exposure of the tumor cells to the drug and response and led to investigation of extended treatment beyond the standard 4 weeks. Hainsworth et al piloted a strategy of scheduled re-treatment, consisting of a regular 4-week rituximab treatment every 6 months for a total of 4 courses. In two phase II studies (one in follicular and small lymphocytic lymphoma [FL], the other in CLL), they showed that the duration of response was much higher than expected using this strategy when compared to the standard 1-month treatment.10,11 In a subsequent randomized study in relapsed FL patients, in which patients could receive further rituximab courses at scheduled 6-month intervals or only when needed for relapse, they showed that, even though the duration of remission was significantly longer with scheduled re-treatment, the overall rituximab benefit was not different in the two arms.12 In deciding on the optimal approach, one must balance the financial advantage of less therapy (20% less rituximab used in the group treated when needed) against the advantage to the patient of fewer relapses and longer disease-free intervals. The ECOG is now running a trial to verify on a larger patient set the equivalence in the duration of rituximab benefit of a scheduled maintenance compared to re-treatment at relapse: the RESORT study randomizes newly diagnosed FL patients responding to rituximab to either one of these two schedules. A different approach was chosen by the Swiss Group for Clinical Cancer Research (SAKK) who randomized patients with FL or mantle cell lymphoma (MCL) to the standard 1-month treatment or to the same treatment (“induction”) followed by 1 infusion of rituximab every 2 months for 4 cycles; the latter prolonged schedule resulted in a treatment of 9 months and an approximate rituximab exposure of 1 year. In this study it was shown that for patients with FL responding to induction the duration of remission was doubled with the prolonged schedule,13 while in MCL the difference did not reach statistical significance.14 The SAKK is now conducting a trial comparing the 9-month schedule with a treatment of 5 years to investigate the benefit of prolonged rituximab treatment in maintaining long-term remission.
Interval between administrations
The half-life of rituximab is about 1 week, but the median duration of persistence in the blood at active levels is of about 3 months:4 the need to infuse the drug at 1-week intervals is therefore questionable. Even though some have claimed that the first doses of rituximab should be administered at short intervals to saturate all the CD20 receptors on malignant B-cells and render chemoresistant cells chemosensitive,15 this hypothesis has not been substantiated. Because the amount of rituximab given at the current schedule is probably in excess, a schedule of every 3 weeks or even longer intervals could possibly be as active. A prospective PK-based study by Gordan et al addressed the optimal interval between administrations. Having defined a serum concentration of 25 mg/mL as the target, they measured the rituximab blood level in treated patients monthly and infused 1 dose every time the concentration fell below the 25 mg/mL threshold.16 With this strategy the great majority of patients could maintain active levels of drug with an infusion interval of 3 months, and all of the patients could be kept with blood levels in this range if they were treated every 2 months. We can therefore assume that an infusion of rituximab every 2–3 months should be sufficient to maintain tumors constantly exposed to active concentrations of the drug. This is actually the schedule that was chosen by many cooperative study groups for their maintenance strategy.
Speed of administration
About 1 of 4 patients receiving rituximab for the first time experiences infusion-related side effects. This has led to a policy of slow and gradually increasing speed of infusion, resulting in the first infusion being given over approximately 4 hours. The reaction is mainly due to the acute lysis of circulating B-cells, which have almost all disappeared by the time the second infusion is due: from the second administration on, the infusion-related symptoms are therefore much milder or even absent.2 Despite this, and although other human immunoglobulins are administered at a much higher rate, a duration of infusion of about 3 hours was recommended for further drug administrations as a measure of caution. Investigators in British Columbia have experimented with infusing rituximab in 1½-hour intervals from the second infusion on, with steroid pre-medication, and have shown the feasibility of this approach.17 A phase I speed escalation study ongoing in Switzerland has shown that rituximab can be given in 1-hour intervals even without steroid pre-medication, provided the first 2 infusions were given without serious reactions (Ghielmini, abstract ASH 2005).
As discussed above, several schedules of rituximab administration have equivalent or better activity, but observations in a single disease can not necessarily be generalized to all lymphomas. For instance, prolonged treatment according to the SAKK schedule showed benefit in FL but not in MCL.13,14 Consequently, the effect of different dosage schedules of single-agent rituximab requires further study. Furthermore, while equivalent activity was demonstrated for several schedules, we are still not certain of their respective impact on side effects. For example, prolonged treatment causes a longer B-cell depletion and a significant reduction in IgM levels. Even though this has not yet resulted in increased morbidity,13,14 we don’t know the possible impact of this immunosuppression if the treatment is prolonged beyond 2 years, as is the case in ongoing clinical studies.
Response to rituximab can appear rapidly, but usually the time to maximum response is measured in months. These observations can be explained by the multiple mechanisms of action of the drug, which have potentially sequential effects.18
Rituximab in combination with chemotherapy
Rituximab and chemotherapy are both active against lymphomas: their different mechanisms of action and spectrum of toxicity made it natural to attempt to combine them. The combination of the two modalities could have additive, synergistic or antagonistic effects, and several trials have addressed the question of whether the two modalities should be given concomitantly or sequentially, in which sequence, and on what schedule.
Additive or synergistic effect
The in vitro combination of rituximab and chemotherapy could demonstrate additive or synergistic effects,19 while in patients the effect might differ depending on which drugs are combined and which disease is being treated (Table 1 ). In diffuse large B-cell lymphoma (DLBCL), a disease in which rituximab is not curative as single agent, the GELA and the MINT trials showed that the addition of rituximab to CHOP increases the proportion of cured patients:20,21 this suggests synergy. On the other hand, the ECOG trial in a comparable group of patients showed that rituximab obtains the same effect on time-to-treatment failure (TTF) if given together with chemotherapy or after chemotherapy in the form of maintenance:22 this suggests an additive effect. In previously untreated patients with FL, the duration of remission after prolonged single-agent rituximab is approximately 18 months;13 while the combination of prolonged rituximab with CVP (cyclophosphamide, vincristine, prednisone), CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), or FCM (fludarabine, cyclophosphamide, mitoxantrone) chemotherapy increases the event-free survival of respectively 20–30 months:23–25 this could suggest an additive effect with some chemotherapy combinations and a synergistic effect with others. On the other hand, the addition of rituximab to MCP (mitoxantrone, chlorambucil, predisolone) prolongs the median survival of first-line FL patients, suggesting again a synergistic effect.26 In MCL the median EFS of single-agent prolonged rituximab is 12 months,14 but rituximab added to chemotherapy improves EFS by only an additional 4–6 months, suggesting a lack of synergy.
Combined or sequential treatment
Because rituximab depends on the host immune system for part of its activity,18 and because chemotherapy is toxic to the immune system,13 it might be preferable to give rituximab first, then consolidate the result with chemotherapy. On the other hand, because rituximab is more active when the disease load is smaller,13 it could be better to give it after chemotherapy. Finally, because in vitro the two modalities have shown to be in part synergistic,3,19 it could be wiser to give them together. Using chemotherapy to consolidate a response to rituximab has not been tested clinically (and should in fact be tried), but using rituximab to consolidate a response to chemotherapy has been tested and shown to be effective. In a study by Zinzani et al patients with FL who were not in complete clinical or molecular response after chemotherapy, received a consolidation treatment with rituximab and obtained an improvement of the complete response and of the molecular response rate.28 The same observation was made by Brugger et al in first-line FL and MCL patients after autologous transplantation, 100% of whom were converted to molecular remission only after consolidating the remission with post-transplant rituximab.29 On the other hand similar clinical and molecular response rates are obtained when rituximab and chemotherapy (both at standard and at high doses) are administered concomitantly.15,30 In a randomized phase 2 trial of fludarabine combined with rituximab given either concomitantly or sequentially, the PFS was similar in both arms.38
Schedule with chemotherapy
What is the best dose, interval and sequence of rituximab when combined with chemotherapy? Because the dose of 325 mg/m2 allows a persistence of active levels in the blood for a much longer time than chemotherapy, a lower rituximab dose could possibly be sufficient for the synergistic effect to be exploited. On the other hand, due to this long persistence in the blood, administering rituximab with every chemotherapy cycle (i.e., every 3 weeks) could be excessive, and administration of a loading dose followed by 1 infusion every 2–3 months during chemotherapy, independently of the day of chemotherapy administration, could be another option. This was the schedule originally piloted by Czuczman et al.15 Nevertheless, in studies using rituximab in combination with CHOP in DLBCL, the clinical results of giving the combination on day 1 of each cycle produced better results than the administration according to the Czuczman schedule.20,22 This suggests again that the effect of the combination is more than additive and that it is higher when high concentrations of rituximab are present in the patients blood at the time of chemotherapy.
Irrespective of the additive or synergistic effect, many trials have shown that the addition of rituximab to chemotherapy improves both response rate and response duration (Table 1 ) and, in some studies, translates into an improvement in survival. Because practically nearly all the trials have shown a better activity of the combination compared to chemotherapy alone, it is difficult to assess which, if any, chemotherapy is more potentiated than another by rituximab. Therefore, since receiving rituximab together with chemotherapy is more practical for the patient than receiving it afterwards as a maintenance (shorter duration of treatment, same side-effects), it could be recommended to administer rituximab on the same day as chemotherapy.
Maintenance Treatment with Rituximab
Maintaining remission has been a long-standing goal in hematology-oncology: in ALL, in AML, in breast cancer and in many other tumors, investigators have tried for decades to prolong the disease-free interval by administering maintenance treatment. In only a few cases does this strategy appear worthwhile, due to the short- and long-term side effects of prolonged chemotherapy, such as fatigue or secondary leukemia. Rituximab on the other hand is almost devoid of acute side effects, making it an ideal candidate for a prolonged treatment without interference with quality of life. We should caution that data on long-term administration of rituximab are scarce and we need to await the results of prospective randomized trials of maintenance versus no maintenance before we can recommend this treatment as standard. The appropriateness of rituximab maintenance may depend on whether the antibody was or was not included in the remission induction regimen. The data on rituximab maintenance after rituximab monotherapy were described above, while below, we will consider the data of rituximab maintenance after standard chemotherapy, after high-dose chemotherapy and after combined rituximab-chemotherapy.
Maintenance after chemotherapy
Patients responding to chemotherapy benefit from rituximab maintenance, at least in terms of remission duration. This was demonstrated for patients with indolent and aggressive lymphomas. The ECOG conducted a trial in untreated FL patients treated with CVP: patients in remission were randomized to observation or maintenance with a 4-week course of rituximab every 6 months for 4 cycles. Patients in the latter arm experienced a median remission of 30 months compared to 18 months for controls.34 In an EORTC-HOVON study patients with relapsed or resistant FL were treated with CHOP, followed or not by 1 infusion of rituximab every 3 months for 2 years: again the maintenance arm experienced a significantly longer remission (31% vs 67% at 3 years, P < 0.0001) without excessive side effects.24 Finally the ECOG randomized trial in DLBCL patients responding to CHOP compared observation with rituximab maintenance: the latter cohort experienced a significantly longer remission.22 We can therefore affirm that in general, for patients with B-cell lymphomas experiencing remission after chemotherapy, maintenance with rituximab is likely to prolong remission duration. The cost-effectiveness of this strategy and its impact on survival still remain to be demonstrated.
Maintenance after high-dose chemotherapy
Because rituximab has been shown to be more active in patients with less disease burden, it is appealing to give maintenance in patients with minimal residual disease following high-dose chemotherapy and autologous stem cell transplantation (ASCT). A few pilot studies showed the feasibility of the treatment but were not designed to compare their efficacy with observation.29 An ongoing study by the EBMT is investigating the role of rituximab maintenance (1 infusion every 3 months for 2 years) after ASCT for relapsed FL. Another trial in Germany is investigating the role of maintenance after ASCT for MCL. Because these trials are still ongoing and patients treated with this modality usually experience long remissions, it will be several years until it can be demonstrated whether maintenance in this setting improves outcome.
Maintenance after combined rituximab-chemotherapy
The rationale behind using rituximab both during and after chemotherapy lies in the possibility that it first acts as a chemosensitizer, synergizing with chemotherapy, and later works on minimal residual disease through immunologically mediated mechanisms. In first-line FL the ongoing PRIMA study investigates the role of rituximab maintenance (once every 2 months for 2 years) after chemotherapy and rituximab, while an EORTC-HOVON trial studies the role of rituximab maintenance (once every 3 months for 2 years) in patients with relapsed FL responding to R-CHOP.
Schedule for maintenance
With regard to both monotherapy and combination treatment, we still do not know the best schedule for maintenance: dose, duration and interval between doses. The only prospectively studied options are planned re-treatment every 6 months (the Hainsworth schedule10) or a single dose every 2 to 3 months for 1 to 2 years.13,24 None of the ongoing studies is comparing different maintenance schedules after chemotherapy, but the data discussed above for single-agent rituximab suggest as the best choice the schedule of 375 mg/m2 every 3 months for at least 2 years.
To summarize the data to date, maintenance rituximab should be recommended for patients who were not exposed to rituximab during remission induction, but the cost-effectiveness of this approach must still be established.
Rituximab Retreatment
Patients relapsing after single-agent rituximab can be retreated at relapse and respond again in about 50% of the cases.39 These data were obtained in a small retrospective trial and have not been confirmed by other studies. We do not have consistent information on the efficacy of re-treatment with rituximab combined with chemotherapy in patients who were already exposed to rituximab. Because rituximab with chemotherapy improves response rate and survival in a number of B-cell neoplasias, this combination is rapidly becoming the standard first-line treatment. When these patients relapse, the usefulness of re-administering rituximab with further chemotherapy regimens must be addressed. Although many clinicians tend to add rituximab to further lines of chemotherapy, no studies have addressed this issue and data from retrospective observations are scanty and inconclusive.40 Does it make sense to re-challenge patients with rituximab if they relapse after a previous rituximab-containing regimen? Given the cost of the drug, the question is not trivial and should be addressed in a randomized trial.
Conclusion
In conclusion, 10 years after the appearance of rituximab in clinical practice, we can say that this antibody should be used in the following situations (see Table 2 for the suggested schedules):
With first-line chemotherapy for curable B-cell neoplasias
With the first chemotherapy in rituximab-naive indolent lymphomas requiring chemotherapy
As a single agent with a prolonged schedule for follicular lymphomas not in need of rapid response
As single agent for patients with B-cell neoplasias who are unsuitable for chemotherapy
Event-free survival in randomized trials of chemotherapy +/− rituximab.
| Disease . | First Author . | Ref. . | Chemotherapy . | n . | Line of Treatment . | No Rituximab . | + Rituximab . | P-value . |
|---|---|---|---|---|---|---|---|---|
| * Patients underwent a second randomization stratified by age to either HDCT/SCT versus IFN or between two doses of IFN | ||||||||
| ** Patients underwent a second randomization to +/− rituximab maintenance. | ||||||||
| † PFS from time of randomization (add approx 6 m for induction CVP). | ||||||||
| † responding patients received IFN maintenance. | ||||||||
| Abbreviations: DLBCL, diffuse large B cell lymphoma; CVP, cyclophosphamide, vincristine, prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; MCP, mitoxantrone, chlorambucil, predisolone; FCM, fludarabine, cyclophosphamide, mitoxantrone; HDCT, high-dose chemotherapy; SCT, stem cell transplantation; IFN, interferon; PFS, progression-free survival | ||||||||
| Follicular | Marcus | (23) | CVP | 322 | 1st | 7 m | 27 m | 0.0001 |
| Hiddemann | (32) | CHOP* | 557 | 1st | 30 m | Not reached | < 0.0001 | |
| Herold | (33) | MCP† | 201 | 1st | 19 m | Not reached | < 0.0001 | |
| Hagenbeck | (24) | CHOP** | 369 | Relapsed | 20 m | 47 m | 0.03 | |
| Dreyling | (25) | FCM | 67 | Relapsed | 20 m | 51 m | 0.00003 | |
| DLBCL | Feugier | (20) | CHOP | 399 | 1st | 13 m | 45 m | 0.00002 |
| Mantle cell | Lenz* | (31) | CHOP | 122 | 1st | 14 m | 21 m | 0.013 |
| Herold | (33) | MCP | 90 | 1st | 14 m | 20 m | 0.24 | |
| Forstpointner | (27) | FCM | 48 | Relapsed | 4 m | 8 m | 0.4 | |
| Disease . | First Author . | Ref. . | Chemotherapy . | n . | Line of Treatment . | No Rituximab . | + Rituximab . | P-value . |
|---|---|---|---|---|---|---|---|---|
| * Patients underwent a second randomization stratified by age to either HDCT/SCT versus IFN or between two doses of IFN | ||||||||
| ** Patients underwent a second randomization to +/− rituximab maintenance. | ||||||||
| † PFS from time of randomization (add approx 6 m for induction CVP). | ||||||||
| † responding patients received IFN maintenance. | ||||||||
| Abbreviations: DLBCL, diffuse large B cell lymphoma; CVP, cyclophosphamide, vincristine, prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; MCP, mitoxantrone, chlorambucil, predisolone; FCM, fludarabine, cyclophosphamide, mitoxantrone; HDCT, high-dose chemotherapy; SCT, stem cell transplantation; IFN, interferon; PFS, progression-free survival | ||||||||
| Follicular | Marcus | (23) | CVP | 322 | 1st | 7 m | 27 m | 0.0001 |
| Hiddemann | (32) | CHOP* | 557 | 1st | 30 m | Not reached | < 0.0001 | |
| Herold | (33) | MCP† | 201 | 1st | 19 m | Not reached | < 0.0001 | |
| Hagenbeck | (24) | CHOP** | 369 | Relapsed | 20 m | 47 m | 0.03 | |
| Dreyling | (25) | FCM | 67 | Relapsed | 20 m | 51 m | 0.00003 | |
| DLBCL | Feugier | (20) | CHOP | 399 | 1st | 13 m | 45 m | 0.00002 |
| Mantle cell | Lenz* | (31) | CHOP | 122 | 1st | 14 m | 21 m | 0.013 |
| Herold | (33) | MCP | 90 | 1st | 14 m | 20 m | 0.24 | |
| Forstpointner | (27) | FCM | 48 | Relapsed | 4 m | 8 m | 0.4 | |
When and how to use rituximab in B-cell lymphoproliferative diseases.
| When . | How . |
|---|---|
| With first line chemotherapy in curable B-cell lymphomas | 375 mg/m2 on d1 of each chemotherapy cycle |
| With chemotherapy in rituximab-naïve indolent lymphomas requiring rapid response | 375 mg/m2 on d1 of each chemotherapy cycle |
| As a single agent for grade I-II follicular lymphoma with FLIPI low-intermediate, not in need of a rapid response | 375 mg/m2 weekly x 4, then 375 mg/m2 every 3 months for 1–2 years |
| As a single agent for any type of lymphoma requiring therapy, but unsuitable for chemotherapy (e.g., age, co-morbidity, refusal) | 375 mg/m2 weekly x 4, then 375 mg/m2 every 3 months for 1–2 years |
| When . | How . |
|---|---|
| With first line chemotherapy in curable B-cell lymphomas | 375 mg/m2 on d1 of each chemotherapy cycle |
| With chemotherapy in rituximab-naïve indolent lymphomas requiring rapid response | 375 mg/m2 on d1 of each chemotherapy cycle |
| As a single agent for grade I-II follicular lymphoma with FLIPI low-intermediate, not in need of a rapid response | 375 mg/m2 weekly x 4, then 375 mg/m2 every 3 months for 1–2 years |
| As a single agent for any type of lymphoma requiring therapy, but unsuitable for chemotherapy (e.g., age, co-morbidity, refusal) | 375 mg/m2 weekly x 4, then 375 mg/m2 every 3 months for 1–2 years |
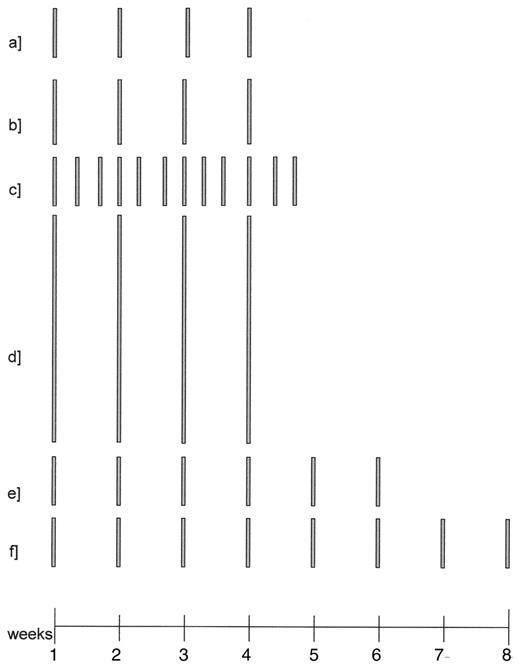
Schedules of rituximab used as single agent to induce remission.
375 mg/m2 weekly, wk 1–4 (1)
500 mg/m2 weekly, wk 1–4 (5)
375 mg/m2 3x/week, wk 1–4 (35)
2250 mg/m2 weekly, wk 1–4 (6)
375 mg/m2 weekly, wk 1–6 (9)
375 mg/m2 weekly, wk 1–8 (10)
The height of the bars is proportional to the dose administered.
Schedules of rituximab used as single agent to induce remission.
375 mg/m2 weekly, wk 1–4 (1)
500 mg/m2 weekly, wk 1–4 (5)
375 mg/m2 3x/week, wk 1–4 (35)
2250 mg/m2 weekly, wk 1–4 (6)
375 mg/m2 weekly, wk 1–6 (9)
375 mg/m2 weekly, wk 1–8 (10)
The height of the bars is proportional to the dose administered.
Schedules of rituximab in combination with chemotherapy.
375 mg/m2 on d1 of each chemotherapy cycle (20)
375 mg/m2 on d1 of chemotherapy, starting from cycle 2 (37)
375 mg/m2 on d-7 and -3 of first cycle, and -3 of cycle 2, 4, 6, and 2x on week 4 after the end of treatment (36)
375 mg/m2 on d-7 and -3 of first cycle, then on d1 of each cycle (38)
375mg/m2 twice between high-dose cycles, and twice after the last myeloablative chemotherapy (30)
Schedules of rituximab in combination with chemotherapy.
375 mg/m2 on d1 of each chemotherapy cycle (20)
375 mg/m2 on d1 of chemotherapy, starting from cycle 2 (37)
375 mg/m2 on d-7 and -3 of first cycle, and -3 of cycle 2, 4, 6, and 2x on week 4 after the end of treatment (36)
375 mg/m2 on d-7 and -3 of first cycle, then on d1 of each cycle (38)
375mg/m2 twice between high-dose cycles, and twice after the last myeloablative chemotherapy (30)
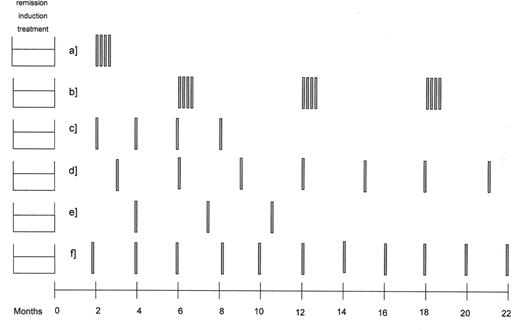
Schedules of rituximab used as single agent to consolidate or prolong remission.
375 mg/m2 weekly, wk 1–4, 2 months after end of induction (29)
375 mg/m2 weekly, wk 1–4, every 6 months for 3 times (10)
375 mg/m2 at month 2, 4, 6, 8 after end of induction (13)
375 mg/m2 every 3 months for 2 years (24)
375 mg/m2 every time the rituximab blood levels falls under 25 mg/mL (16)
375 mg/m2 every 2 months for 2 years (ongoing PRIMA study)
Schedules of rituximab used as single agent to consolidate or prolong remission.
375 mg/m2 weekly, wk 1–4, 2 months after end of induction (29)
375 mg/m2 weekly, wk 1–4, every 6 months for 3 times (10)
375 mg/m2 at month 2, 4, 6, 8 after end of induction (13)
375 mg/m2 every 3 months for 2 years (24)
375 mg/m2 every time the rituximab blood levels falls under 25 mg/mL (16)
375 mg/m2 every 2 months for 2 years (ongoing PRIMA study)
Michele Ghielmini, MD, Oncology Inst. of Southern Switzerland, Ospedale San Giovanni, Bellinzona 6500, Switzerland; Phone +41 (91) 8117915, Fax +41 (91) 8117916, mghielmini@ticino.com