Abstract
Members of the BCL-2 family of proteins regulate and execute many cell intrinsic apoptosis pathways, including those arising from dysregulated expression of cellular oncogenes. Since pro-survival members of the family are often strongly elevated in diverse cancers, with the potential to confer resistance to both endogenous cell death stimuli and many cancer treatments, there has been intense interest to develop strategies to therapeutically modulate their activity. Although encouraging genetic and pharmacological preclinical proof of concept has been obtained, the challenge for clinical development will be to devise strategies that address the fact that multiple pro-survival members are typically up-regulated in a given cancer and the family operates primarily through protein-protein interactions. Moreover, since several current therapies themselves are known to stimulate the levels of one or more family members, there will be additional challenges (and opportunities) in exploiting this target in the clinic. In this review, we describe the rationale for targeting the BCL-2 family of apoptosis suppressors in cancer and the progress that has been made in modulating the family by small molecule antagonists.
Apoptosis is believed to have evolved in metazoans to regulate tissue homeostasis and to eliminate individual cells that have become superfluous, have become dysfunctional due to infections, or sustained chromosomal alterations that could subvert normal growth control. Apoptosis therefore provides a defense against numerous assaults that could otherwise inflict damage or kill the organism. During on-cogenesis the cell must bypass these inherent apoptosis mechanisms if the cancer cell is to survive because apoptosis is otherwise triggered by both the aberrant growth pattern of these cells and the application of many cancer therapies. Cancer cells can evade apoptosis in either of two ways: by inactivation of genes or gene products that promote apoptosis (e.g., the p53 tumor suppressor gene) or by activation of inhibitors of cell death pathways (e.g., the BCL-2 family of apoptosis suppressors). Therapies that target these regulators and re-instate the normal apoptotic mechanisms in cancer cells hold significant promise.1–3
Oncogenes as Inducers of Apoptosis Pathways
Unrestricted mitogenic stimuli arising from dysregulated oncoprotein signaling is an early step in conferring a predisposition to malignant transformation, a condition that is normally held in check by interlocking tumor suppressor mechanisms, usually resulting in apoptosis.4,5 The concept that transforming oncogenes can stimulate apoptosis mechanisms is now well established in many contexts, and includes cell membrane signaling by Ras6 or transcriptional changes effected by Myc.7,8 Animal models have been engineered for pancreatic beta cell oncogenesis in which a combination of c-Myc expression and upregulation of a suppressor of apoptosis, BCL-XL, is both necessary and sufficient to permit c-Myc-induced initiation and progression of cells into angiogenic, invasive tumors.9 Conditional activation of c-Myc in adult, mature beta cells in this transgenic mouse model in the absence of an apoptosis suppressor, on the other hand, induced uniform beta cell proliferation but was accompanied by massive apoptosis, which rapidly degraded the beta cell mass. Conversely, in a mouse lymphoblastic leukemia model driven by constitutive c-Myc10 and conditional BCL-2 expression, subsequent elimination of BCL-2 yielded rapid loss of leukemic cells and significantly prolonged survival. After turning off BCL-2, the oncogenic potential of c-Myc was overcome by apoptosis, formally validating inhibitors of BCL-2 as a rational strategy for therapeutic development. Based on these and related findings it has been proposed that a combination of dysregulated cell proliferation and reduction in apoptosis is key for the development of cancer, with the secondary traits of diverse neoplasms resulting as outcomes of this platform.9,11
BCL-2 Family of Death Regulators
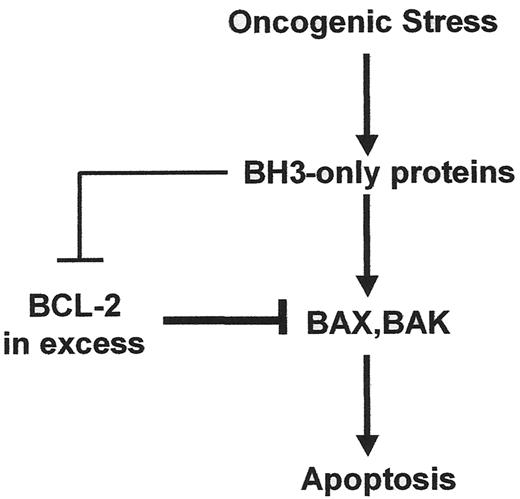
The BCL-2 family is central to both the regulation and execution of most intrinsic apoptotic pathways.12 The family is comprised of 3 groups, classified according to their content of BCL-2 homology (BH) domains (for a recent detailed review, see 12). Anti-apoptotic members (e.g., BCL-2, BCL-XL, BCL-w, MCL-1, and A1) contain four BH domains defined by their similarity among the members of the family; the multi-BH domain pro-apoptotic members BAX and BAK contain BH domains 1–3; and a diverse group of loosely related pro-apoptotic proteins (e.g., BID, BAD, BIM, BIK, PUMA, NOXA, etc.) contain only BH domain 3 (BH3). All anti-apoptotic members as well as BAX and BAK contain a hydrophobic transmembrane (TM) domain located at their extreme C-terminus, whereas among BH3-only members BIK, BIM, and PUMA contain a C-terminal TM. Anti-apoptotic members have the potential to hetero-dimerize with pro-apoptotic members through binding of the exposed BH3 helix on the surface of pro-apoptotic members into a deep groove on the surface of anti-apoptotic members, formed by helices 1 and 2.13 BAX and BAK, as well as certain BH3 only proteins, undergo a conformational change in response to upstream death signaling pathways, resulting in exposure or availability of the BH3 domain. Heterodimerization with anti-apoptotic BCL-2 members, therefore, typically occurs with activated pro-apoptotic conformers.14–16 Of note, a number of BH3 only proteins, including PUMA, NOXA and BIK exist as constitutively active conformers and therefore their contribution to death signaling necessarily involves new protein synthesis. In addition, certain BH3-only proteins can interact, at least transiently with BAX or BAK.14–16 The outcome of death signals that are regulated by the BCL-2 family, therefore, depends upon a complex three-way ratio of the multi-domain anti-apoptotic, multi-domain pro-apoptotic, and BH3-only members (Figure 1 ).
Studies employing double gene deletions of murine Bax and Bak have shown that these two proteins function as essential effector molecules in many death pathways17 and that the anti-apoptotic BCL-2 and pro-apoptotic BH3-only members operate both upstream and through these effector molecules.18 The BH3-only members function as proximal sensors of apoptotic stimuli and in their active conformers can bind and inhibit BCL-2 members (e.g., BAD and NOXA) or they can both inhibit BCL-2 members as well as activate BAX and BAK by a “hit-and-run” mechanism (e.g., tBID and BIM). The former act as sensitizers of stimuli that activate BAX and BAK, whereas the latter are both sensitizers and activators.15
As depicted in Figure 1 , the ratio between pro-survival BCL members and pro-death members dictates the outcome of many death-initiating signalling pathways. To achieve this, the BCL-2 family functions at two sites within the cell: mitochondria, where the BCL members regulate the release of factors from the organelle that activate caspases and remodel chromatin; and endoplasmic reticulum (ER), where the BCL members regulate ER Ca2+ homeostasis and release.19 The BCL-regulated ER Ca2+ pathway is linked to the mitochondria, causing morphological and structural transitions that allow mitochondria to respond to pro-apoptotic stimuli.20 Of note, pro-survival BCL members are differentially enriched at mitochondria and ER.21Figure 2 (see Color Figures, page 552) illustrates the pathway at mitochondria, in which an oncogenic stimulus results in activation of one or more BH3-only members, which can target and antagonize pro-survival members. Productive antagonism of pro-survival members either alone or coupled with stimuli to directly activate BAX and BAK, results in the oligomerization of BAX or BAK. This allows the formation of a predicted conduit for release of pro-apoptotic factors such as cytochrome c, a co-factor that results in activation of the apoptosome, which in turn activates effector caspases −3 and −7.12
Not All BH3 Domains Are Created Equal
Individual BH3-only BCL-2 members appear to have evolved both to link specific upstream signals to downstream activation of the mitochondrial apoptosis pathway and to selectively target preferred BCL-2 binding partners. For example a recent study of the affinity of 8 BH3 peptides for the soluble forms of 5 pro-survival BCL-2 proteins, employing a Biacore Biosensor, revealed a 10,000-fold range in binding affinity.22 BIM and PUMA, for example, exhibited similar affinities for all pro-survival members whereas NOXA bound only to MCL-1 and A1. BH3-only BIK, which can be induced by oncogenic stress, preferentially targets the ER where it binds pro-survival members and initiates Ca2+-mediated remodelling of mitochondrial cristae, mobilizing mitochondrial stores of cytochrome c as a prerequisite for its release to the cytosol.20 Interestingly, BIK and NOXA cooperate to release cytochrome c. Likewise, BAD and NOXA, which have non-overlapping binding preferences for pro-survival members, cooperate to induce cell killing.22 From a therapeutic perspective, it might be expected that mimetics of the BH3 domains of BIM and PUMA exhibit pan-BCL inhibition, whereas more selective antagonists might be generated from other BH3 mimetics, such as those derived from BAD or NOXA.
Pro-survival BCL-2 Proteins: Therapeutic Targets
Since pro-apoptotic BCL family proteins dock into the BH1-BH2 groove of pro-survival members via their BH3 domain, it has been proposed that BH3 mimetics that antagonize the pro-survival members could be used to alter the ratio between pro-survival and pro-apoptotic members, allowing apoptosis to proceed in cancer cells. Strong support for such a strategy has come from the findings that BH3 peptides themselves can inhibit BCL members, induce apoptosis in cancer cell lines, and in one case where the pharmacological properties of the peptide were improved, induce apoptosis in a mouse xenograft tumor model.23 In this latter case, a chemical strategy termed hydrocarbon stapling was used to stabilize the alpha-helical BH3 peptide derived from the BH3-only protein BID. BID is strongly pro-apoptotic and belongs to the class that activates BAX and BAK as well as antagonizes pro-survival BCL members.23 The stapled peptide proved to be cell permeant and protease-resistant, and interacted with pro-survival members with increased affinity. It was also effective in inhibiting the growth of human leukemia xenografts in SCID mice. Thus, a BH3 peptide, and therefore small molecules that mimic this domain, has the potential to therapeutically modulate BCL-2 family proteins.
Therapeutic Small Molecule Discovery Strategies
The challenge of this strategy is to discover corresponding small molecule BCL antagonists with drug-like properties. Moreover, because of the complexity of BCL proteins in cancer cell biology, including upregulation of multiple family members in a single cell and the contribution of different family members to mitochondria- and ER-regulated pathways, a small molecule antagonist of multiple pro-survival members is likely required, i.e., a pan-BCL-2 inhibitor, at least for initial proof of concept studies in the clinic. Although a number of such antagonists are currently at various stages of development, we focus below on two examples representing distinct discovery strategies: rational design and functional screening.
ABT-737
One approach is based on rational design and high throughput SAR by NMR.23 Utilizing the high resolution structure of the BH3 docking groove on the surface of the pro-survival BCL member, BCL-XL,13 inhibitors of BCL-XL, BCL-2, and BCL-w were generated by covalently bridging chemical entities that bind to separate regions of the groove. One, ABT-737, exhibits an affinity for these targets in vitro 2- to 3-orders of magnitude more potent than the multiple small molecule antagonists that have previously been reported in the literature (24 and references cited therein). Of note, however, it exhibits significantly reduced affinity for MCL-1, a BCL member whose structure is intermediate between the “closed” conformation of unliganded BCL-2/BCL-XL and their more “open” BH3-complexed conformers.25 A number of amino acids in the binding groove also distinguish MCL-1 from other members. Nevertheless, ABT-737 demonstrated potent single-agent killing of select cell lines from small cell lung carcinoma and lymphoma, and against peripheral blood mononuclear cells (PBMNCs) derived from 7 of 13 patients with chronic lymphocytic leukemia (CLL). From a mechanistic perspective, ABT-737 appears to fall into the class of BH3 “sensitizers,” since it fails to directly activate BAX or BAK and release cytochrome c from mitochondria in vitro.24 Despite the relatively large size of the compound, ABT-737 achieved potent anti-tumor activity in mouse H146 and H1963 small cell lung carcinoma (SCLC) mouse xenograft models when administered i.p. at 75–100 mg/kg daily for 3 weeks.
GX15-070
An alternative discovery approach is based on functional outcomes and seeks small molecules that inhibit BCL protein-protein interactions. Since BCL members have the potential to undergo conformational changes,14,16,27 these assays accommodate the possibility of dynamic changes in protein structure contributing to these interactions. Thus, a high throughput protein-protein interaction discovery screen was used to interrogate natural compound libraries, which identified a chemotype that falls within the polypyrrole class of molecules28 as a starting point for optimization. Further development resulted in the compound GX15-070, a non-prodigiosin, which is currently in clinical development.
[3H]-labelled GX15-070 was found to bind to BCL-w, BCL-XL, and MCL-1 with KD values in the 0.5 μM range. In contrast to ABT-737, therefore, GX15-070 appears to bind pro-survival members as purified entities in vitro with apparent reduced affinity. However it also targets MCL-1. After exposure of sk-MEL5 melanoma cells to GX15-070 for 5 hours and detergent extraction, interaction between MCL-1 and BAK was inhibited relative to vehicle controls, as judged by co-immunoprecipitation, with an apparent IC50 of about 1.5 μM.
To formally prove that GX15-070 can antagonize pro-survival BCL members, resulting in activation of BAX or BAK, the BCL pathway was engineered into yeast cells. S cerivisiae does not express BCL-related proteins and is not sensitive to GX15-070-mediated cytotoxicity. In contrast, overexpression of BAK in these yeast is cytotoxic, but can be countered by pro-survival members BCL-w, MCL-1, or BCL-XL. However, treatment of the yeast cells with GX15-070 was toxic, an effect dependent on the presence of BAK suggesting that GX15-070 can antagonize the pro-survival BCL proteins, overcoming their ability to inhibit BAK. Consistent with this mechanism, when transformed baby mouse kidney epithelial cells expressing adenovirus E1A and dominant-negative p53 and derived from either wt mice or mice doubly deleted of Bax, Bak, the double knock out cells resisted the activation of caspases by GX15-070. As expected, treatment of cancer cell lines with GX15-070 resulted in oligomerization of mitochondrial BAK, release of cytochrome c, and activation of caspases. Collectively, the results suggest the mechanism illustrated in Figure 2 (lower panel; see Color Figures, page 552) for the activation of caspases by GX15-070.
Further testing showed that GX15-070 exhibits single agent cytotoxicity against a broad range of cell lines and ex vivo against PBMNCs from patients (n > 30) with CLL. Delivery of formulated drug by intravenous bolus injection into the tail veins of Balb/c or CB17 SCID/SCID mice daily for 5 consecutive days was well tolerated, and in animals pre-implanted subcutaneously with cell lines derived from cervical (C33A), colon (SW480), prostate (PC3), or mammary (4T1) carcinomas and allowed to form palpable tumors, administration of GX15-070 on this schedule resulted in inhibition of tumor growth relative to vehicle alone. For example, at 2 mg drug/kg body weight given daily for 5 days, inhibition of growth of these tumors ranged from 60%–85% 14 days after initiating the administration of drug, with no weight loss observed in the animal cohorts. Thus, as predicted from the mechanism of action of BCL proteins and their ability to antagonize oncogenic apoptotic pathways, GX15-070 exhibits antitumor activity as a single agent across diverse cancer cell types.
Phase I evaluation of GX15-070, administered by intravenous infusion on an every 3 week schedule in patients with refractory CLL and weekly in patients with refractory solid tumors, is in progress.
Pharmacodynamic Markers for BCL-2 Mechanism-Based Cancer Treatments
Evidence of mechanistic and biological activities of BCL mechanism-based therapies can be obtained by measuring these activities directly in cancer cells isolated from the patient as well as by measuring surrogate markers released into the circulation. As indicated, PBMNCs isolated from patients with CLL and incubated with GX15-070 or ABT-737 ex vivo underwent apoptosis. In the case of GX15-070, evidence of disruption of interactions between MCL-1 and BAK was observed following cell extraction in detergent and co-immunoprecipitation. Similar protein-protein interaction assays can be performed on circulating leukemia cells isolated from patients at timed intervals after receiving the drug by intravenous administration. Additionally, end products of apoptosis such as chromatin fragments can be detected following their release into the circulation,29 thereby serving as a surrogate of tumor cell death. Collectively the results of such biological measurements can be exploited to optimize dose and schedule of drug administration.
Rational Combination Treatments
Since BCL-2 proteins confer resistance to most cell death stimuli that operate through the mitochondria apoptosis pathway, it is predicted that a number of current cytotoxic cancer treatments might benefit from combination therapy with BCL-2 antagonists. For example, among others, ABT-737 has been reported to enhance the cytotoxicity of paclitaxel in A549 NSCLC cells.26 Additionally, however, certain current therapies themselves can directly influence the expression of BCL-2 family proteins. The proteasome inhibitor Velcade® (Bortezomib, PS-341; Millennium Pharmaceuticals) is currently approved for the treatment of multiple myeloma and is in development for other indications.30 By blocking ubiquitin-mediated protein degradation, Bortezomib is predicted to interfere with, among others, survival mechanisms associated with nuclear factor (NF)-κB pathways. It has also been shown to cause elevation of BH3-only NOXA,31,32 a preferred binding partner for MCL-1.22 However, at steady state the turnover of MCL-1 is rapid, and this protein also is subject to ubiquitin-mediated degradation via the proteasome.33 Indeed, proteasome inhibitors can lead to a rapid increase in MCL-1 protein levels in various cell lines within several hours of treatment. If the rise in anti-apoptotic MCL-1 is not offset by pro-apoptotic NOXA (or other BH3 ligand), then a combination of Bortezomib and an effective small molecule antagonist of MCL-1 may prove beneficial.
Conclusions
The complex interplay between multiple BH3-only proteins and their pro-survival binding partners raises both challenges and opportunities in devising small molecule BH3 therapeutic mimetics for the treatment of cancer. Since more than one pro-survival member is typically over-expressed in a given cancer, early exploration of preclinical and clinical proof of concept is focusing on small molecule antagonists that target multiple pro-survival members. As our understanding of the role of individual pro-survival members in cancer signalling improves, it may prove desirable in certain contexts to design more selective antagonists. The fact that different BH3-only proteins have evolved to accomplish such selectivity suggests that this may indeed be feasible. Coupled with this, however, will be the need to better understand the differential contributions that individual pro-survival members make to cancer-related apoptosis pathways, and to devise the pharmacodynamic and biomarker tools necessary to exploit these opportunities clinically. The clinical development of first generation BCL-2 family antagonists may teach us valuable information about the most effective way to modulate this important family of apoptosis suppressors.
Model for regulation of oncogene-driven apoptosis by BCL-2 family proteins. Oncogenic stress pathways lead to activation of several BH3-only proteins. They all antagonize pro-survival BCL-2 members but in addition certain of these BH3-only members also activate BAX and BAK. When in excess, pro-survival members prevent apoptosis by antagonizing the activated conformers of BAX and BAK. Therapeutics that shift the balance in favor of excess antagonists of pro-survival members should permit oncogenic stress pathways to productively engage the apoptosis mechanism. Details are given in the text.
Model for regulation of oncogene-driven apoptosis by BCL-2 family proteins. Oncogenic stress pathways lead to activation of several BH3-only proteins. They all antagonize pro-survival BCL-2 members but in addition certain of these BH3-only members also activate BAX and BAK. When in excess, pro-survival members prevent apoptosis by antagonizing the activated conformers of BAX and BAK. Therapeutics that shift the balance in favor of excess antagonists of pro-survival members should permit oncogenic stress pathways to productively engage the apoptosis mechanism. Details are given in the text.
GCS: Gemin X Biotechnologies Inc. and Department of Biochemistry and McGill Cancer Center, McGill University, Montréal, Canada; JV: Gemin X Inc., Malvern, PA
Acknowledgments: We thank Pierre Beauparlant for reviewing the manuscript and providing input.