Abstract
Despite recent improvements in the treatment of early-stage disease, the blastic phase of chronic myeloid leukemia (CML) remains a therapeutic challenge. For imatinib-naïve patients, imatinib provided encouraging hematologic and cytogenetic benefits; however, the vast majority of CML blast crisis cases today arise in patients already on imatinib-based therapy. Clonal evolution and duplication of the Philadelphia chromosome continue to be associated with blastic phase transformation, but recent studies have identified BCR/ABL kinase domain mutations in 30%–40% of blast crisis patients. This implies that BCR-ABL–targeted therapy might have influenced the molecular road map to blastic transformation. In this review, we will examine the effect of imatinib on primitive CML progenitors and how this might influence the pathophysiology of blast crisis. A rational framework for deciding how best to integrate stem cell transplantation, traditional chemotherapy, imatinib, and other BCR-ABL kinase inhibitors in the care of blast crisis patients will also be discussed.
Statement of the Problem: What Is CML Blast Crisis?
Chronic myeloid leukemia (CML) in blastic phase is the transition of CML in chronic or accelerated phase to an acute leukemia, characterized by ≥ 30% blasts in the bone marrow or peripheral blood, or the development of extramedullary disease outside of the spleen. In light of recent changes in the World Health Organization definition of acute leukemia, the percentage of blasts required for CML in blastic phase may someday be reduced to 20%. Consistent with the early stem cell nature of CML,1 blastic transformation may be myeloid, lymphoid, or undifferentiated/mixed, with myeloid blast crisis being about two times more common than lymphoid. Although the definition of CML in blastic phase has not changed from the pre- to post-imatinib era, the treatment history of our current CML patient population has: an ever-increasing proportion of patients with CML will have been managed exclusively with imatinib or other ABL kinase inhibitor therapy. Such a significant change in therapeutic approach will almost certainly affect the kinetics and molecular phenotype of CML blast crisis.
Pathophysiology of CML Blast Crisis—Why Does It Occur?
The pathophysiology of CML blast crisis is incompletely understood. However, recent advances in our understanding of cancer and leukemia stem cell biology and emerging data about imatinib-resistant CML and BCR-ABL-related genomic instability have led to the formulation of a general framework. This working mechanistic model of CML blast crisis is based on a few assumptions. The first is that BCR-ABL is directly or indirectly responsible for progressive genomic instability or epigenetic changes, which occur at the CML stem cell level and/or in later CML progenitor cells. The second is that the degree of genomic instability is proportional to the level of BCR-ABL kinase activity. The third is that CML stem cells are the least vulnerable to ABL-targeted therapy and may serve as reservoirs for occult CML progression. Together, these phenomena conspire to bring about an acquired loss of hematopoietic cell differentiation, resulting in a highly aggressive, acute leukemia.
Numerous studies have implicated BCR-ABL in providing a milieu favorable for the generation and maintenance of secondary DNA alterations.2,–9 The findings of these and other studies are consistent with clinical studies linking clonal evolution to CML progression, including the development of blast crisis. The most common gross cytogenetic abnormalities associated with CML blast crisis include duplication of the Ph chromosome, trisomy 8, and isochromosome 17; however, these abnormalities may also be observed in patients with earlier stages of CML10,11 Although more prevalent in blastic phase than other CML stages, alterations in p53 have been found in only a minority (24%) of cases.12 Loss of p16INK4A/ARF has been reported in up to half of patients with CML in lymphoid blast crisis but is rare in the myeloid form.13 Thus, it is reasonable to hypothesize that clonal evolution plays a role in blastic progression,10 likely facilitated by the dysregulation of normal apoptotic pathways by BCR-ABL.14,–17 Although clonal evolution implies some degree of BCR-ABL independence, some common cytogenetic changes observed in advanced CML may be surrogate markers of genomic instability rather than direct clues to which genes are pivotal to CML progression. Recent studies have implicated activation of the LYN, AKT, and STAT5 signaling pathways in the development of BCR-ABL-independent leukemogenesis,18,19 but additional studies will be required to determine if specific blockade of these pathways will have therapeutic benefit in CML.
How might the degree of residual BCR-ABL kinase activity present in CML progenitors, which appear to survive imatinib treatment,20 influence CML progression to blast crisis? The essential role of BCR-ABL tyrosine kinase activity in cellular transformation is well established21 and played a central role in the decision to pursue ABL-targeted therapy for CML. Thus, it is reasonable to conclude that CML progenitors surviving imatinib therapy would still be vulnerable to BCR-ABL–dependent influences underlying CML progression. Prior to imatinib, progression to CML blast crisis was virtually inevitable in the absence of a suitable allogeneic bone marrow transplant donor (Figure 1 ; dark circles). Although the length of follow up is still relatively short, it appears that imatinib will increase the latency of blastic transformation compared to interferon-α–based therapy;22 however, what proportion of patients with CML will avoid blastic transformation is unknown. If the amount of residual BCR-ABL kinase activity in CML progenitors were reduced to negligible levels, then imatinib would achieve durable CML control, perhaps preventing the secondary changes requisite for blastic phase (Figure 1 ; open circles). Alternatively, imatinib-mediated BCR-ABL inhibition might only delay the inevitable progression of CML from chronic to blastic phase (Figure 1 ; light gray circles). Whether any level of BCR-ABL tyrosine kinase activity is safe, that is, will not carry a risk for leukemic progression, is unknown. Studies using high-dose imatinib23 or more potent ABL kinase inhibitors24,25 at diagnosis will provide important opportunities to determine if more potent BCR-ABL suppression will lessen the chances of blastic transformation.
What about CML progenitors that harbor BCR/ABL kinase domain mutations? If the risk for acquiring secondary events correlates with the degree of BCR-ABL kinase activity, then CML progenitors with imatinib-resistant forms of BCR-ABL would be predicted to foster CML progression.26 Presumably, in these cells BCR-ABL tyrosine kinase activity is only modestly suppressed by imatinib, and this might shorten the interval between chronic and blastic phase (Figure 1 ; dark gray circles). Indeed, in imatinib-naïve CML patients screened by allele specific oligonucleotide PCR, the presence of BCR/ABL kinase domain mutations correlated with clonal evolution and advanced stage, but interestingly not with response to imatinib or survival.27 These findings suggest that clinically meaningful BCR/ABL mutations must occur in the “appropriate” hematopoietic progenitor and survive competition with wild-type BCR-ABL progenitors and normal hematopoietic elements. Further, since almost two-dozen different BCR-ABL mutants have been reported, it is unlikely that all will have equal biological potency.28 BCR/ABL kinase domain mutations appear to be rare at CML chronic phase diagnosis,29 but detection of rare mutant clones can be technically challenging. So, while it appears that most BCR/ABL kinase domain mutations may arise due to wild-type BCR-ABL-induced genomic instability, further research will be required to more precisely define cause versus effect. Perhaps a retrospective analysis of CML cohorts treated entirely in the pre-imatinib era will help answer these questions.
Imatinib and the CML Stem Cell
The limited ability of conventional chemotherapeutic drugs to eradicate leukemia stem cells has recently been indicted as the cause of most cases of leukemic relapse.30,31 So, the activity of imatinib or other ABL-targeted therapies against CML stem cells has critical implications for long-term disease control and even cure.32 The mechanisms responsible for the persistence of BCR/ABL-positive progenitors in most imatinib-treated CML patients have not been fully elucidated. Cell-extrinsic factors, such as the levels of the plasma protein α-1-acid glycoprotein which could enhance imatinib catabolism, have been implicated in imatinib-resistance,33,34 but most studies suggest cell-intrinsic factors may play a greater role in protecting CML progenitors from imatinib-induced cytotoxicity. Imatinib interacts with the multidrug resistance (MDR) ATP-binding cassette (ABC) proteins MDR1 (P-glycoprotein) and ABCG2, suggesting these drug efflux pumps may play a role in protecting CML progenitors from therapeutic concentrations of imatinib.35,–40 ABCG2 is downregulated during hematopoietic cell differentiation,41 perhaps explaining why imatinib achieves rapid hematological responses, while elimination of the earliest hematopoietic progenitors proves more challenging. Besides mechanisms involving imatinib efflux from cells, a recent study reported that imatinib is a substrate for organic cation transporter (OCT)1, a protein involved in cellular drug uptake, suggesting another possible variable in maintaining effective imatinib concentrations in CML progenitors.42
Studies in primary CML patient samples have shown that BCR-ABL–positive progenitors are resistant to a variety of apoptotic stimuli, particularly the infrequently dividing, or quiescent subset.43,44 In CML progenitors from chronic phase CML, imatinib had marked activity against the proliferative fraction but tended to spare non-dividing cells.44 Consistent with these findings, BCR/ABL-positive cells have been detected in CD34+ cells from imatinib-treated patients with chronic phase CML in complete cytogenetic response.20 This phenomenon is not unique to imatinib, as BCR-ABL–positive hematopoietic colonies were also obtained from interferon-α–treated CML patients in long-term complete cytogenetic remission.45 Does this mean imatinib and interferon-α have the same biologic limitations in the CML stem cell compartment? Longer follow-up studies will be required to compare the clinical fate of CML progenitors rendered dormant by imatinib- or interferon-α–based therapy.
Irrespective of issues of drug delivery to CML-reconstituting cells, does imatinib achieve effective kinase inhibition within this early progenitor compartment? In a recent study of purified CML CD34+ cells, imatinib was effective in suppressing the constitutive phosphorylation of Crk-L, a commonly used surrogate marker of BCR-ABL kinase activity.46,47 However, the effect of imatinib on signaling within the CML-reconstituting or quiescent cell fraction, which comprises only a small proportion of total CML CD34+ cells, is unknown.32,48 Further, it is conceivable that CML stem cells are less dependent on BCR-ABL kinase activity than later progenitors and may be able to persist despite effective kinase inhibition.49 In P210BCR/ABL-transduced human CD34+ cells, imatinib blocked proliferation but did not completely restore altered adhesion and cell motility.50 Whether this phenomenon indicates a tyrosine kinase independent function of BCR-ABL or is a consequence of imatinib-mediated inhibition of other signaling pathways such as c-KIT will require further study.51 It will also be informative to study the effect of other ABL kinase inhibitors on CML progenitor populations, since these compounds will likely have distinct patterns of tyrosine kinase inhibition.24,25,52 Although CML stem cells are unlikely to be BCR-ABL independent in early stage disease, disruption of constitutive tyrosine kinase activity in these cells may rely more on winning a war of attrition rather than a quick victory, and may even warrant consideration of an intermittent and/or combination approach to BCR-ABL–targeted therapy. Carefully designed clinical studies will be required to determine the most effective strategy to pharmacologically eradicate all CML progenitors.
The Kinetics of CML Blast Crisis in the Pre- and Post-imatinib Era
Ideally CML blastic phase can be prevented with BCR-ABL inhibition, stem cell transplantation or other means. For those patients who progress despite such maneuvers, the establishment of an early warning system for CML progression could allow initiation of more aggressive or alternative therapies at a stage where interventions have the optimal chance of being clinically meaningful. Because of the well-established cure rate of allogeneic transplantation in CML, patients in the appropriate age range should be HLA typed at diagnosis so that the options for related or unrelated stem cell transplantation are known early on. The principal fear of most CML caregivers is that patients will progress to blast crisis too rapidly to allow sufficient time for stem cell transplantation or other investigative strategies to be implemented. To determine the frequency of rapid progression to CML blast crisis, investigators at MD Anderson retrospectively analyzed 1093 CML patients who had received a variety of non-transplant treatments (only about 10% had received imatinib) and found that 17% had developed blast crisis during the course of study.53 Of patients with blast crisis, one-quarter had experienced sudden onset, defined as the development of CML in blastic phase within 3 months of a documented complete hematologic response. Interestingly, the survival of patients with sudden-onset blast crisis was slightly better than those whose blastic phase was more gradual in onset, perhaps because lymphoid blast crisis (which carried a more favorable prognosis) was three times more common in patients with sudden-onset blastic phase. Fifty-seven percent of sudden-onset patients were able to proceed to transplantation, but only one quarter of the transplanted patients achieved durable disease-free survival. With the availability of more sensitive molecular monitoring techniques, it is likely our definition of rapid-onset blast crisis may change, but thus far the prognosis of blast crisis appears ominous independent of its rate of onset, at least in the pre-imatinib era.
Because most de novo imatinib-treated CML patients achieve a complete cytogenetic response,22 quantitative assessment of BCR-ABL transcript levels has become a sensitive and clinically useful tool in CML management. Although patients may abruptly lose their response to imatinib,54,55 there is no evidence that imatinib-treated patients progress to blastic phase more rapidly than those treated with other modalities, including bone marrow transplantation,56 but formal comparative studies are lacking. Nonetheless, regular monitoring of imatinib-treated CML patients will maximize the opportunity for implementing changes in therapy. A recent study found that a greater than twofold rise in BCR-ABL transcript levels identified patients at high risk for harboring imatinib-resistant BCR/ABL mutations.57 In another study of 103 CML patients who had achieved a complete cytogenetic response with imatinib (two-thirds had previously received interferon-α), 26 suffered cytogenetic relapse at 48 months that was presaged by increasing BCR-ABL transcript levels.58 Four of the 26 patients progressed to accelerated or blast crisis during the study period. In contrast, patients who achieve undetectable BCR-ABL transcript levels or major molecular responses with imatinib appear at quite low risk for progression to blastic phase.59
Therapeutic Options for Patients Developing/Evolving into CML Blast Crisis
Historically, therapy for patients with CML in blastic phase has been disappointing because response rates have been far lower than those achieved with standard induction regimens for de novo acute myelogenous leukemia (AML) or acute lymphoblastic leukemia (ALL). The introduction of imatinib led to improved hematologic response rates in blastic phase CML (52%–70%), including some complete hematologic and major cytogenetic responses, leading to a median survival of 7–10 months.60,61 The relatively favorable results of imatinib in blast crisis CML represented a significant breakthrough. Imatinib allowed more durable disease control with less toxicity than traditional chemo-therapeutic regimens and provided some patients a bridge to stem cell transplantation. However, management of blast crisis CML in the present and near future may not be much more favorable than in the pre-imatinib era because almost all patients will have progressed to this stage despite imatinib. Thus, the establishment of treatment algorithms for patients with CML blast crisis is truly a work in progress (Figure 2 ).
In patients progressing to blastic phase while on imatinib, it is reasonable to discontinue imatinib while alternative treatment plans are being formulated. In some cases, improvements in molecular markers or clinical status have been observed after cessation of imatinib.62,63 In contrast, CML blast crisis associated with specific BCR/ABL mutations might temporarily respond to imatinib dose escalation, providing time to pursue additional therapeutic options.63 The initial choice of therapy for CML in blastic phase may depend in part on the kinetics of onset and the clinical status of the patient. Although it is highly desirable to enroll such patients on clinical trials, some may require traditional chemotherapy to allow time to assess trial eligibility. The recent availability of the novel ABL tyrosine kinase inhibitors AMN107 and dasatinib (BMS-354825) for clinical study broadens the options for patients developing blastic transformation on imatinib.24,25,64 It is anticipated that these agents will be most effective in patients whose blast crisis is driven by imatinib-resistant BCR-ABL mutants or increased levels of BCR-ABL, which may comprise 30%–40% of cases.65 However, dasatinib also has activity against SRC family members, which have recently been implicated in imatinib-resistant CML.18 Whether these novel ABL tyrosine kinase inhibitors will achieve durable remissions in blastic phase patients is unknown, but updated results of ongoing trials will be available soon. Ideally, choice of therapy for CML blast crisis patients should be based on screening for imatinib-resistant BCR/ABL mutations, since dasatinib and AMN107 are active against most imatinib-resistant BCR/ABL mutants, except for T315I.64 As BCR/ABL mutation screening is not yet widely available, other factors, such as patient stability, the presence of clonal evolution, and other clinical features must be considered when developing treatment plans.
Regarding more traditional chemotherapeutic agents, high-dose Ara-C–based regimens have often been used for the treatment of myeloid/mixed/undifferentiated blast crisis, which in the pre-imatinib era had a worse prognosis than the lymphoid form.11 Complete remission rates have ranged from 20% to 30%.66 Thus far, no high-intensity chemotherapy regimen has shown clear superiority, and durability has been poor, with median survivals of only a few months. Single agents are generally ineffective, although in one study high- to intermediate-dose decitabine had activity similar to more intensive regimens.67 Recently, the FLAG-Ida regimen (fludarabine, Ara-C, granulocyte colony-stimulating factor [G-CSF], idarubicin), with or without gemtuzumab ozogamicin,68 has shown encouraging activity in CML in myeloid blast crisis.55 Although active in early-stage disease, interferon-α–mediated responses are too gradual to be effective alone in blastic phase disease. For CML in lymphoid blastic phase, ALL-like induction regimens have been most commonly used, including the hyper-CVAD regimen.69 Because the response rate and durability of most traditional salvage chemotherapy regimens have been disappointing, a variety of new agents are being investigated for activity in CML blast crisis. They include inhibitors of the mammalian target of rapamycin (MTOR) pathway; anti-vascular endothelial growth factor (VEGF)–based agents; histone deacetylase inhibitors; and other novel small molecule inhibitors.66 The proportion of CML patients progressing to blastic phase is still relatively small, so further studies will be required to determine which agents show the most activity. Given the molecular heterogeneity of blastic phase CML, it is likely that combinations of novel targeted agents, or targeted agents plus traditional chemo-therapeutic agents, will be required for maximum effect. Because imatinib penetrates poorly into the cerebrospinal fluid,70 –72 evaluation or prophylactic treatment of the central nervous system (CNS) should be strongly considered while treatment plans are being formulated, particularly in lymphoid or mixed lineage blast crisis.
Because it is the only established curative modality, allogeneic bone marrow transplantation remains the ultimate salvage therapy for patients with imatinib-resistant CML. Long-term disease-free survival/cure rates range from 50% to 70% in patients in early chronic phase, but outcomes decline with advancing disease stage, nadiring at about 10% for patients transplanted while still in blast crisis.73,74 Outcome is somewhat better for CML blast crisis patients transplanted after achieving a second chronic phase with salvage therapy. With the availability of imatinib, fewer CML patients are being transplanted in the early stages of their disease, suggesting future challenges for stem cell transplantation. Perhaps further advances in targeted anti-CML drugs and non-myeloablative transplantation will counterbalance the risks inherent in transplanting older CML patients with more advanced disease. Unfortunately, only about one-third of CML patients have a suitable HLA-compatible donor and are within an appropriate age range, making allogeneic transplantation unavailable to most blast crisis patients. For these patients, autologous stem cell transplant might be considered, but advances in purging CML progenitors from both patient and autograft will be required. Effective autologous stem cell transplant strategies may also benefit from the availability of cryopreserved autologous product obtained during maximal molecular response, but this procedure is not in routine practice and may not be covered by third-party payers.
In summary, the last 5 years have seen important advances for the treatment of early-stage CML, but improvement in the care of blastic phase CML has been more modest. New ABL tyrosine kinase inhibitors will broaden the options for patients with CML in blast crisis, particularly those with leukemia still highly dependent on BCR-ABL. However, the molecular heterogeneity of blastic phase CML may limit the durability of treatment regimens based solely on ABL-targeted therapy. Allogeneic stem cell transplantation remains an important option for some patients but will be most successful when carefully integrated into a sensitive and rigorous monitoring plan based on validated molecular markers of CML progression. The limited number of patients with CML eligible for allogeneic transplantation underscores the importance of further research to improve allogeneic and autologous transplant technologies and to identify the mechanisms by which CML stem cells evade imatinib-mediated cytotoxicity.
A working model depicting a link between BCR-ABL tyrosine kinase activity and the accumulation of secondary genetic and epigenetic changes in CML progenitors over time. Once a threshold (at top) of secondary molecular events is reached, hematopoietic differentiation is lost, resulting in blastic transformation. Drugs with no effect on BCR-ABL tyrosine kinase activity would be associated with an inevitable progression to blast crisis (black circles). Complete suppression of BCR-ABL (white circles) in CML stem cells might prevent blastic progression indefinitely, while incomplete suppression (gray shaded circles) due to ineffective drug delivery or BCR/ABL kinase domain mutations would only delay the accumulation of secondary events.
A working model depicting a link between BCR-ABL tyrosine kinase activity and the accumulation of secondary genetic and epigenetic changes in CML progenitors over time. Once a threshold (at top) of secondary molecular events is reached, hematopoietic differentiation is lost, resulting in blastic transformation. Drugs with no effect on BCR-ABL tyrosine kinase activity would be associated with an inevitable progression to blast crisis (black circles). Complete suppression of BCR-ABL (white circles) in CML stem cells might prevent blastic progression indefinitely, while incomplete suppression (gray shaded circles) due to ineffective drug delivery or BCR/ABL kinase domain mutations would only delay the accumulation of secondary events.
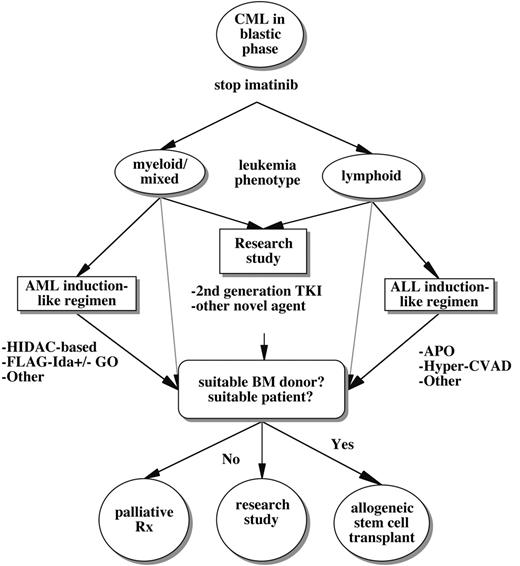
A general treatment algorithm for CML blast crisis occurring during imatinib therapy. Options include clinical trials studying alternate ABL tyrosine kinase inhibitors (TKI) or other novel agents, more traditional chemotherapeutic regimens tailored to the lineage of acute leukemic transformation, and/or allogeneic stem cell transplantation. Some potential chemotherapeutic regimens include HIDAC (high-dose Ara-C); FLAG-Ida (fludarabine, Ara-C, G-CSF, idarubicin) with or without gemtuzumab ozogamicin (GO); APO (adriamycin, prednisone, vincristine)-like regimens; or Hyper-CVAD (hyperfractionated cytoxan, vincristine, adriamycin, decadron). Because of the limited penetration of imatinib into the central nervous system, the risk of concomitant leukemic meningitis should be strongly considered, particularly in lymphoid blast crisis. (As discussed in the text, some patients with BCR-ABL mutations might respond temporarily to imatinib dose escalation.)
A general treatment algorithm for CML blast crisis occurring during imatinib therapy. Options include clinical trials studying alternate ABL tyrosine kinase inhibitors (TKI) or other novel agents, more traditional chemotherapeutic regimens tailored to the lineage of acute leukemic transformation, and/or allogeneic stem cell transplantation. Some potential chemotherapeutic regimens include HIDAC (high-dose Ara-C); FLAG-Ida (fludarabine, Ara-C, G-CSF, idarubicin) with or without gemtuzumab ozogamicin (GO); APO (adriamycin, prednisone, vincristine)-like regimens; or Hyper-CVAD (hyperfractionated cytoxan, vincristine, adriamycin, decadron). Because of the limited penetration of imatinib into the central nervous system, the risk of concomitant leukemic meningitis should be strongly considered, particularly in lymphoid blast crisis. (As discussed in the text, some patients with BCR-ABL mutations might respond temporarily to imatinib dose escalation.)
University of Texas Southwestern Medical Center