Abstract
Disorders of the erythrocyte membrane, including hereditary spherocytosis, hereditary elliptocytosis, hereditary pyropoikilocytosis, and hereditary stomatocytosis, comprise an important group of inherited hemolytic anemias. These syndromes are characterized by marked clinical and laboratory heterogeneity. Recent molecular studies have revealed that there is also significant genetic heterogeneity in these disorders. This is particularly true for the spherocytosis syndromes where each kindred has a private mutation in one of the spherocytosis genes.
Treatment with splenectomy is curative in most patients. Splenectomy via a laparoscopic approach has become the surgical method of choice. Growing recognition and understanding of the long-term risks and complications of splenectomy, including cardiovascular disease, thrombotic disorders, and pulmonary hypertension, and the emergence of penicillin-resistant pneumococci, a concern for infection in overwhelming postsplenectomy infection, have led to reevaluation of the role of splenectomy. Recent management guidelines acknowledge these important considerations when entertaining splenectomy and recommend detailed discussion between health care providers, patient, and family.
Hemolytic anemias due to abnormalities of the erythrocyte membrane comprise an important group of inherited disorders.1,2 These include hereditary spherocytosis (HS), hereditary elliptocytosis (HE), hereditary pyropoikilocytosis (HPP), and the hereditary stomatocytosis (HSt) syndromes. These disorders are characterized by clinical and laboratory heterogeneity and, as evidenced by recent molecular studies, genetic heterogeneity.2
Hereditary Spherocytosis
The HS syndromes are a group of inherited disorders characterized by the presence of spherical-shaped erythrocytes on the peripheral blood smear.3 HS is found worldwide. It is the most common inherited anemia in individuals of northern European descent, affecting approximately 1 in 1000–2500 individuals depending on the diagnostic criteria.
Pathobiology
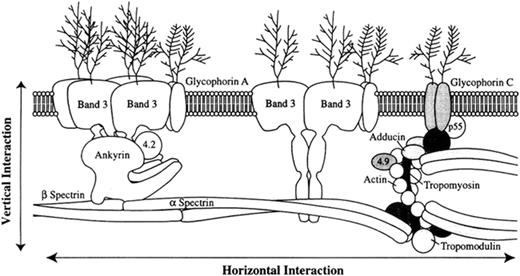
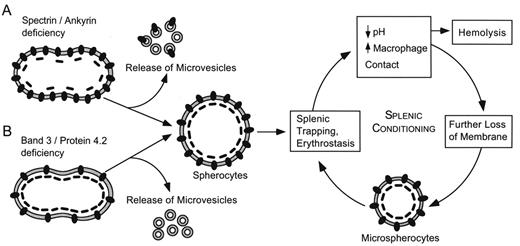
The primary cellular defect is loss of membrane surface area relative to intracellular volume, leading to spheroidal shape and decreased deformability.3 The loss of surface area is caused by increased membrane fragility due to defects in proteins of the erythrocyte membrane (Figure 1 ). Increased fragility leads to membrane vesiculation and membrane loss. Destruction of these abnormal erythrocytes in the spleen is the principal cause of hemolysis (Figure 2 ). Entrapment of abnormal erythrocytes in the splenic microcirculation followed by phagocyte ingestion is one proposed mechanism of cellular destruction. The splenic environment is hostile to erythrocytes. Low pH, low levels of glucose and adenosine triphosphate (ATP), and high local concentrations of toxic free radicals produced by adjacent phagocytes all contribute to membrane damage.
Membrane loss is due to defects in one of several membrane proteins, including ankyrin, band 3, α spectrin, β spectrin, and protein 4.2 (Table 1 ). Erythrocyte membranes from HS patients demonstrate qualitative and/or quantitative abnormalities of these proteins, most commonly combined spectrin and ankyrin deficiency, followed by band 3 deficiency, isolated spectrin deficiency, and protein 4.2 deficiency.
The genes responsible for HS include ankyrin, β spectrin, band 3 protein, α spectrin, and protein 4.2.4 In approximately two thirds of HS patients, inheritance is autosomal dominant. In this group of “typical” HS patients, ankyrin mutations are the most common cause of HS, followed by mutations in band 3 and β spectrin. Over the past few years, numerous point mutations, defects in mRNA processing, and gene deletions have been described in these genes. Except for a few rare exceptions, HS mutations are private, i.e., each individual kindred has a unique mutation.
In the remaining patients, inheritance is nondominant. Cases with autosomal recessive inheritance are due to defects in either α-spectrin or protein 4.2. In other non-dominant cases, genetic studies have revealed that an unexpectedly large number of nondominant cases are due to de novo mutations in the HS genes.4 A few cases of “double dominant” HS due to defects in band 3 or spectrin that result in fetal death or severe hemolytic anemia presenting in the neonatal period have been reported.
Clinical manifestations
Clinical manifestations of the spherocytosis syndromes vary widely. “Typical” HS is marked by evidence of hemolysis with anemia, reticulocytosis, splenomegaly, jaundice, and gallstones, evidence of spherocytosis with spherocytes on peripheral smear and increased erythrocyte osmotic fragility, and a positive family history. The degree of hemolysis varies widely, from asymptomatic patients who are diagnosed incidentally to severe, transfusion-dependent patients. Most HS patients have incompletely compensated hemolysis and mild to moderate anemia. The anemia is often asymptomatic except for pallor and/or fatigue. Jaundice is seen at some time in about half of patients, usually in association with viral infections. Splenomegaly is detectable in most older children and adults. About a quarter of HS patients have compensated hemolysis and little or no anemia. Most of these patients are asymptomatic except during times of increased erythroid stress, compensating for their hemolysis with increased erythropoiesis. Finally, a small group of HS patients have poorly compensated hemolysis and severe anemia. These patients may develop complications of severe uncompensated anemia including growth retardation, delayed sexual maturation, and extramedullary “tumors,” skin ulcers, or thalassemic facies. Many of these patients are transfusion dependent.
Complications of HS include cholelithiasis and associated problems including biliary obstruction, cholecystitis and cholangitis.5 As in other inherited hemolytic anemias, recent studies have shown that co-inheritance of Gilbert syndrome increases the risk of neonatal jaundice and cholelithiasis in HS patients.6 Hemolytic, aplastic, and megaloblastic crises may occur. Hemolytic crises are characterized by anemia, jaundice, increased splenomegaly, and reticulocytosis. Common in children, they are typically associated with viral illness and rarely require intervention. Aplastic crises occur after virally induced bone marrow suppression, most commonly parvovirus B19. In patients with severe HS, aplastic crisis may lead to severe anemia with serious complications including congestive heart failure or even death. Aplastic crisis may also be the event that brings an HS individual to medical attention, particularly the asymptomatic one with normally compensated hemolysis. Megaloblastic crisis occurs in patients with increased folate demands, e.g., the pregnant patient, growing children, or the elderly.
Initial assessment of a patient with suspected HS should include a family history and questions about history of anemia, jaundice, gallstones and splenectomy. Physical examination should seek signs such as scleral icterus, jaundice, and splenomegaly. After diagnosing a patient with HS, family members should be examined for the presence of HS.
Laboratory findings
Typical HS patients have obvious spherocytes lacking central pallor on peripheral blood smear (Figure 3A ). Less commonly, only a few spherocytes are present or, in severe cases, there are numerous small, dense spherocytes and bizarre erythrocyte morphology with anisocytosis and poikilocytosis. Molecular studies have shown that specific morphologic findings are associated with certain membrane protein defects such as pincered erythrocytes (band 3), spherocytic acanthocytes (β spectrin), or spherostomatocytes (protein 4.2).
Most patients have a mild to moderate anemia. The mean corpuscular hemoglobin concentration (MCHC) is increased (between 35% and 38%) due to relative cellular dehydration in approximately 50% of patients, but all HS patients have some dehydrated cells.
Incubated osmotic fragility (OF) testing is considered the gold standard in diagnosing HS in a patient with Coombs-negative spherocytic hemolytic anemia, particularly one of Northern European descent or with a positive family history of undiagnosed anemia. After incubation at 37°C for 24 hours, HS red cells lose membrane surface area more readily than normal cells because their membranes are leaky and unstable. This exposes the membrane defect upon OF testing. When the spleen is present, a subpopulation of fragile erythrocytes that have been conditioned by the spleen form the “tail” of the OF curve that disappears after splenectomy. OF testing suffers from poor sensitivity as ~20% of mild cases of HS are missed after incubation.
Other analyses such as the autohemolysis test, the hypertonic cryohemolysis test, and the acidified glycerol test suffer from lack of specificity and are not widely used. Flow cytometric analysis of eosin-5-maleimide binding to erythrocytes, a reflector of relative amounts of Rh-related integral membrane proteins and band 3, has recently been explored as a screening test for HS diagnosis.7 Specialized testing, such as membrane protein quantitation, ektacytometry, and genetic analyses, are available for studying difficult cases or when additional information is desired.
Other laboratory manifestations in HS are markers of ongoing hemolysis. Reticulocytosis, increased bilirubin, increased lactate dehydrogenase, increased urinary and fecal urobilinogen, and decreased haptoglobin reflect increased erythrocyte production or destruction.
Treatment and outcome
Splenectomy cures or markedly improves the anemia in most HS patients. Even patients with severe HS exhibit significant clinical improvement. Postsplenectomy, erythrocyte life-span normalizes, transfusion requirements abate, and the incidence of cholelithiasis is reduced. Reticulocyte counts fall to normal or almost normal levels, spherocytosis and altered osmotic fragility persist, but the “tail” of the osmotic fragility curve, created by conditioning of a subpopulation of spherocytes by the spleen, disappears.8
Laparoscopic splenectomy is now the surgical method of choice, resulting in less postoperative discomfort, quicker recovery time, shorter hospitalization, and decreased costs.9,10 Even huge spleens can be removed laparoscopically. Recently, partial splenectomy via laparotomy has been advocated for infants and young children with severe, usually transfusion-dependent, anemia.11,12 The goal is to palliate the hemolysis and anemia while maintaining residual splenic immune function. Long-term data for this procedure are lacking, but rapid regrowth of the spleen may limit its effectiveness in some cases.13
Operative complications of splenectomy include local infection, bleeding, and pancreatitis. Overwhelming postsplenectomy infection (OPSI), typically from encapsulated organisms, is an uncommon but significant late complication of splenectomy especially in the first few years of life. The introduction of pneumococcal vaccines and the promotion of early antibiotic therapy for febrile children who have had a splenectomy have led to decreases in the incidence of OPSI.
Previously, splenectomy was considered “routine” in HS patients. However, the risk of OPSI, the emergence of penicillin-resistant pneumococci, and growing recognition of increased risk of cardiovascular disease, particularly thrombosis and pulmonary hypertension, have led to reevaluation of the role of splenectomy in HS.14,15 Recent HS management guidelines acknowledge these important considerations when entertaining splenectomy and recommend detailed discussion between health care providers, patient, and family.16 One approach is to splenectomize all patients with severe spherocytosis and all patients who suffer from significant signs or symptoms of anemia including growth failure, skeletal changes, leg ulcers, and extramedullary hematopoietic tumors. Other candidates for splenectomy are older HS patients who suffer vascular compromise of vital organs.
Whether patients with moderate HS and compensated, asymptomatic anemia should have a splenectomy remains controversial. Patients with mild HS and compensated hemolysis can be followed carefully and referred for splenectomy if clinically indicated. The treatment of patients with mild to moderate HS and gallstones is also debatable, particularly since new treatments for cholelithiasis including laparoscopic cholecystectomy, endoscopic sphincterotomy, and extracorporal choletripsy, lower the risk of this complication.
Hereditary Elliptocytosis and Related Disorders
Hereditary elliptocytosis (HE) is characterized by the presence of elliptical, cigar-shaped erythrocytes on peripheral blood smear (Figure 3B ).17 It is common in individuals of African and Mediterranean descent, presumably because elliptocytes confer some resistance to malaria. Worldwide, the incidence of HE is estimated at 1:2000–4000, approaching 1:100 in parts of Africa. The actual incidence is unknown, as most patients are asymptomatic.
Hereditary pyropoikilocytosis (HPP) is a rare cause of severe hemolytic anemia characterized by erythrocyte morphology reminiscent of that seen in patients after a thermal burn (Figure 3C ). There is a strong association between HE and HPP. Up to a third of family members of HPP patients have HE. Many HPP patients experience severe hemolytic anemia in childhood that evolves into typical HE later in life. Finally, in many cases of HE and HPP, a defect of the erythrocyte membrane protein spectrin has been identified.
Pathobiology
The principal defect in HE/HPP erythrocytes is mechanical weakness or fragility of the erythrocyte membrane skeleton.2 Similar to HS, study of erythrocyte membrane proteins in these disorders has identified qualitative and/or quantitative abnormalities of various erythrocyte membrane proteins.1,4,18 These include α and β spectrin, protein 4.1 and glycophorin C. The majority of defects occur in spectrin, the principal structural protein of the erythrocyte membrane skeleton (Figure 1 ). Spectrin is composed of heterodimers of the related but nonidentical proteins α and β spectrin that self-associate into tetramers and higher-order oligomers. Spectrin integrity is critical for erythrocyte membrane stability and erythrocyte shape and function. Structural and functional defects of protein 4.1 appear to disrupt spectrin-actin contacts in the membrane skeleton. Glycophorin variants are also deficient in protein 4.1. The pathogenesis of the formation of elliptocytes in these syndromes is unknown.
HE is inherited in an autosomal dominant pattern with rare cases of de novo mutations.4,17 Abnormalities of either α or β spectrin associated with the majority of cases of HE/ HPP are due to mutations in the spectrin heterodimer self-association site.17,19 Most of these mutations are missense mutations at, or very near, highly conserved residues of spectrin. In contrast to HS, the HE/HPP syndromes, while also heterogeneous, have been associated with distinct spectrin mutations in persons of similar genetic backgrounds, suggesting a “founder effect” for these mutations. There is great clinical phenotypic heterogeneity in individuals with the same spectrin mutation, even in individuals from the same kindred. Some of this variability is attributable to modifier alleles, such as αLELY (Low Expression Lyon).
Clinical manifestations
The clinical presentation of HE is heterogeneous, ranging from asymptomatic carriers to patients with severe, life-threatening anemia. Most patients with “typical” HE are asymptomatic and are diagnosed incidentally during testing for unrelated conditions. Erythrocyte life span is normal in most patients, decreased in only ~ 10% of patients. It is this subset of HE patients with decreased red cell lifespan who experience hemolysis, anemia, splenomegaly and intermittent jaundice. Many of these patients have parents with typical HE and thus are homozygotes or compound heterozygotes for defects inherited from each of the parents. Interestingly, symptomatology may vary between members of the same family; indeed, it may vary in the same individual at different times.
Laboratory findings
The hallmark of HE is the presence of cigar-shaped elliptocytes on peripheral blood smear (Figure 3B ). These normochromic, normocytic elliptocytes may number from few to 100%; the degree of hemolysis does not correlate with the number of elliptocytes present. Spherocytes, stomatocytes and fragmented cells may also be seen. Elliptocytes may be seen in association with several disorders including megaloblastic anemias, hypochromic microcytic anemias, myleodysplastic syndromes and myelofibrosis. History and additional laboratory testing usually clarify the diagnosis of these disorders. The osmotic fragility is abnormal in severe HE and HPP. Other laboratory findings in HE are similar to those found in other hemolytic anemias and are nonspecific markers of increased erythrocyte production and destruction. In difficult cases or cases desiring a molecular diagnosis, specialized testing such as erythrocyte membrane protein quantitation and genetic testing are available.
Treatment and outcome
Treatment is rarely required in HE.17 In a few cases, occasional red blood cell transfusions are required. Similar to HS, in severe cases of HE and HPP splenectomy is essentially curative. The indications for splenectomy in HS are viewed as applicable for HE and HPP. Complications in severe cases, e.g., cholelithiasis, hemolytic, aplastic, or megaloblastic crises, are similar to those in HS.
Southeast Asian ovalocytosis
Southeast Asian ovalocytosis (SAO) is an unusual, dominantly inherited HE variant found in Malaysia, Papua New Guinea, the Philippines, and other parts of Southeast Asia. Rounded elliptocytes, or ovalocytes, and characteristic stomatocytes with longitudinal slits are found on peripheral blood smear. Most SAO patients are asymptomatic, although a few experience mild hemolysis. Compared to other membrane defects, SAO red cells are rigid and hyperstable, rather than unstable. The cause of SAO is a 27 bp genomic deletion leading to in-frame deletion of 9 amino acids in band 3. SAO is most prevalent in areas endemic for malaria and various studies have demonstrated that the condition confers some protection against malaria, particularly cerebral malaria.
Hereditary Stomatocytosis Syndromes
The hereditary stomatocytosis syndromes are a group of inherited disorders characterized by erythrocytes with a mouth-shaped (stoma) area of central pallor on peripheral blood smear (Figure 3D ).20 Stomatocytosis is associated with abnormalities in red cell cation permeability that lead to changes in red cell volume, which may be either increased (hydrocytosis) or decreased (xerocytosis), or, in some cases, near normal. The pathobiology of the stomatocytic shape is poorly understood and the molecular basis (or bases) of this group of disorders is unknown.
The hydrocytosis syndromes, also known as over-hydrated hereditary stomatocytosis, are characterized by significant stomatocytosis (Figure 3D ), severe hemolysis, macrocytosis (110–150 fL), elevated erythrocyte sodium concentration, reduced potassium concentration, and increased total sodium plus potassium content.20 The excess cations elevate cell water, producing large, osmotically fragile cells with a low MCHC (24%–30%). The clinical severity of overhydrated HSt (OHSt) is variable; some patients experience hemolysis and anemia while others are asymptomatic. Stomatin, an integral membrane protein, is decreased or absent from the erythrocyte membranes of affected patients, due to maturational loss in the bone marrow and in the circulation.21 Stomatin gene mutations have not been found in unrelated stomatocytosis patients deficient in this protein.
The dehydrated stomatocytosis syndromes, also known as xerocytosis, are characterized by contracted and spiculated red cells, variable numbers of stomatocytes, and target cells on peripheral blood smear.20,22 Most patients have nearly normal erythrocyte morphology, with only a few target cells and an occasional echinocyte or stomatocyte. The MCHC and MCV (95–115 fL) are increased and the osmotic fragility is decreased. Erythrocyte potassium concentration and total monovalent cation content are decreased. The gene for xerocytosis has been mapped to 16q23–q24.23 A clinical syndrome of xerocytosis, perinatal ascites, and pseudohyperkalemia has been described.24 Genetic studies of patients with this constellation of disorders and with isolated pseudohyperkalemia also shows linkage to this region.25
Hydrocytosis and xerocytosis represent the extremes of a spectrum of red cell permeability defects. Patients with features of both conditions have been reported with variability in the severity of permeability defects, stomatin deficiency, hemolysis, anemia, and numbers of stomatocytes.26,27 This suggests that hereditary stomatocytosis is a complex collection of syndromes caused by various molecular defects.
An unusual and important characteristic of the stomatocytosis syndromes is a marked predisposition to thrombosis after splenectomy.28 Stomatocytosis patients, both over-hydrated and dehydrated, have developed hypercoagulability after splenectomy, leading to catastrophic thrombotic episodes or chronic pulmonary hypertension. In some cases, this has been attributed to increased erythrocyte-endothelial cell adhesion due to erythrocyte phosphatidylserine exposure.29,30 Fortunately, the majority of patients are able to maintain an adequate hemoglobin level, so that splenectomy is not required.
Stomatocytosis is also present on peripheral blood smears of patients with Rh deficiency syndrome.31 Erythrocytes from these rare individuals have either absent (Rhnull) or markedly reduced (Rhmod) Rh antigen expression. There is mild to moderate hemolytic anemia. Mutations in the Rh30 and RhAG genes have been associated with this syndrome.
Sitosterolemia is a recessively inherited condition associated with early-onset xanthomatomatosis and atherosclerosis due to mutation of ABCG5/ABCG8 co-transporters, which leads to increased intestinal absorption and decreased biliary elimination of all sterols, particularly plant sterols. Patients may present with a stomatocytic anemia and macrothrombocytopenia.32
Erythrocyte membrane gene defects in disorders of the erythrocyte membrane.
| Gene . | Disorder1 . | Comment . |
|---|---|---|
| Abbreviations: HS, hereditary spherocytosis; HE, hereditary elliptocytosis; HPP, hereditary pyropoikilocytosis; NIHF, nonimmune hydrops fetalis; SAO, Southeast Asian ovalocytosis. | ||
| Reprinted with permission from Gallagher PG. Disorders of erythrocyte metabolism and shape. In Hematologic Problems in the Neonate. Christensen RD (ed). WB Saunders, Philadelphia, 1999. | ||
| Ankyrin | HS | Most common cause of typical dominant HS. |
| Band 3 | HS, SAO, NIHF | “Pincered” spherocytes seen on smear presplenectomy. SAO due to 9 AA deletion. |
| α Spectrin | HS, HE, HPP, NIHF | Location of mutation in spectrin determines clinical phenotype. α spectrin mutations are most common cause of typical HE. |
| β Spectrin | HS, HE, HPP, NIHF | “Acanthocytic” spherocytes seen on smear presplenectomy. Location of mutation in spectrin determines clinical phenotype. |
| Protein 4.2 | HS | Common in recessively inherited, HS in Japan. |
| Protein 4.1 | HE | An uncommon cause of HE. |
| Glycophorin C | HE | Concomitant protein 4.1 deficiency is basis of HE in glycophorin C defects. |
| Gene . | Disorder1 . | Comment . |
|---|---|---|
| Abbreviations: HS, hereditary spherocytosis; HE, hereditary elliptocytosis; HPP, hereditary pyropoikilocytosis; NIHF, nonimmune hydrops fetalis; SAO, Southeast Asian ovalocytosis. | ||
| Reprinted with permission from Gallagher PG. Disorders of erythrocyte metabolism and shape. In Hematologic Problems in the Neonate. Christensen RD (ed). WB Saunders, Philadelphia, 1999. | ||
| Ankyrin | HS | Most common cause of typical dominant HS. |
| Band 3 | HS, SAO, NIHF | “Pincered” spherocytes seen on smear presplenectomy. SAO due to 9 AA deletion. |
| α Spectrin | HS, HE, HPP, NIHF | Location of mutation in spectrin determines clinical phenotype. α spectrin mutations are most common cause of typical HE. |
| β Spectrin | HS, HE, HPP, NIHF | “Acanthocytic” spherocytes seen on smear presplenectomy. Location of mutation in spectrin determines clinical phenotype. |
| Protein 4.2 | HS | Common in recessively inherited, HS in Japan. |
| Protein 4.1 | HE | An uncommon cause of HE. |
| Glycophorin C | HE | Concomitant protein 4.1 deficiency is basis of HE in glycophorin C defects. |
The erythrocyte membrane. A model depicting the major proteins of the erythrocyte membrane is shown: α and β spectrin, ankyrin, band 3, 4.1 (protein 4.1), 4.2 (protein 4.2), actin, and GP (glycophorin).
Reprinted with permission from
The erythrocyte membrane. A model depicting the major proteins of the erythrocyte membrane is shown: α and β spectrin, ankyrin, band 3, 4.1 (protein 4.1), 4.2 (protein 4.2), actin, and GP (glycophorin).
Reprinted with permission from
Pathophysiology of hereditary spherocytosis. The primary defect in hereditary spherocytosis is a deficiency of membrane surface area. Decreased surface area may produced by two different mechanisms: 1) Defects of spectrin, ankyrin, or protein 4.2 lead to reduced density of the membrane skeleton, destabilizing the overlying lipid bilayer and releasing band 3-containing microvesicles. 2) Defects of band 3 lead to band 3 deficiency and loss of its lipid-stabilizing effect. This results in the loss of band 3-free microvesicles. Both pathways result in membrane loss, decreased surface area, and formation of spherocytes with decreased deformability. These deformed erythrocytes become trapped in the hostile environment of the spleen where splenic conditioning inflicts further membrane damage, amplifying the cycle of membrane injury.
Reprinted with permission from Gallagher PG, Jarolim P: Red cell membrane disorders. In Hematology: Basic Principles and Practice. Hoffman R, Benz EJ Jr, Shattil SJ, et al (eds). 4th ed, WB Saunders, Philadelphia, 2005.
Pathophysiology of hereditary spherocytosis. The primary defect in hereditary spherocytosis is a deficiency of membrane surface area. Decreased surface area may produced by two different mechanisms: 1) Defects of spectrin, ankyrin, or protein 4.2 lead to reduced density of the membrane skeleton, destabilizing the overlying lipid bilayer and releasing band 3-containing microvesicles. 2) Defects of band 3 lead to band 3 deficiency and loss of its lipid-stabilizing effect. This results in the loss of band 3-free microvesicles. Both pathways result in membrane loss, decreased surface area, and formation of spherocytes with decreased deformability. These deformed erythrocytes become trapped in the hostile environment of the spleen where splenic conditioning inflicts further membrane damage, amplifying the cycle of membrane injury.
Reprinted with permission from Gallagher PG, Jarolim P: Red cell membrane disorders. In Hematology: Basic Principles and Practice. Hoffman R, Benz EJ Jr, Shattil SJ, et al (eds). 4th ed, WB Saunders, Philadelphia, 2005.
Peripheral blood smears in disorders of the erythrocyte membrane. A. Hereditary spherocytosis. Characteristic spherocytes lacking central pallor are seen. B. Hereditary elliptocytosis. Smooth, cigar-shaped elliptocytes are seen. C. Hereditary pyropoikilocytosis. Pronounced microcytosis, poikilocytosis, fragmentation of erythrocytes and elliptocytes are seen. D. Hereditary stomatocytosis. Erythrocytes with slit-like, stomata are seen. Reprinted with permission from Gallagher PG. Disorders of erythrocyte metabolism and shape. In Hematologic Problems in the Neonate. Christensen RD (ed). WB Saunders, Philadelphia, 1999.
Peripheral blood smears in disorders of the erythrocyte membrane. A. Hereditary spherocytosis. Characteristic spherocytes lacking central pallor are seen. B. Hereditary elliptocytosis. Smooth, cigar-shaped elliptocytes are seen. C. Hereditary pyropoikilocytosis. Pronounced microcytosis, poikilocytosis, fragmentation of erythrocytes and elliptocytes are seen. D. Hereditary stomatocytosis. Erythrocytes with slit-like, stomata are seen. Reprinted with permission from Gallagher PG. Disorders of erythrocyte metabolism and shape. In Hematologic Problems in the Neonate. Christensen RD (ed). WB Saunders, Philadelphia, 1999.
Acknowledgments: Supported in part by grants from the National Institutes of Health, NIDDK and NHLBI.