Abstract
The primary therapeutic approach to acquired aplastic anemia (AA) in older adults differs from the primary approach used in children and younger adults because in the former group, the results of allogeneic bone marrow transplantation (BMT) are less favorable. With increasing age of the patients, immunosuppressive therapy with antithymocyte globulin (ATG) and cyclosporine (CsA) constitutes the primary treatment option and may be better than BMT. There are very few clinical clues as to the selection of patients likely to respond to immunosuppression. Repeated ATG/CsA cycles are often used as salvage regimens, but in refractory patients BMT may be the best treatment option, as the prognosis for non-responders is poor without definitive treatment. Conservative therapy such as intense immunosuppression is associated with a high relapse rate but does not impact the survival and overall prognosis. The inability to eliminate autoimmune T cell clones using current therapeutic strategies suggests that prolonged immunosuppressive maintenance therapy may be needed for a substantial proportion of patients. Late clonal complications of conservatively treated patients include evolution to myelodysplasia and paroxysmal nocturnal hemoglobinuria and may develop in 20% of the patients. However, BMT also has several sequelae including an increased frequency of solid tumors. Novel immunosuppressive and immunomodulatory agents and constantly improving results of allogeneic BMT will further improve the survival rate of adult patients with AA.
Clinical Features of Aplastic Anemia in Adults
Clinical presentation
Typical acquired aplastic anemia (AA) is a disease of young adults, but a second peak in incidence has been reported in the fifth or sixth decade of life. In older adults the differential diagnosis of AA includes hypocellular myelodysplastic syndrome (MDS), which may be difficult to distinguish due to the insufficient marrow cellularity often precluding morphologic evaluation and successful chromosome analysis. As a normal karyotype common in MDS and some elderly cases of AA may represent misdiagnosed MDS, clues to the recognition of MDS include micromegakaryocytes, myeloid dysplasia and residual blasts.
According to the current definition of AA, a severely depressed marrow cellularity (usually < 25%) must be accompanied by a decrease in 2 out of 3 blood lineages. Some conditions may mimic AA in all or some of its features. Depending on the clinical circumstances, some of the alternate diagnoses associated with cytopenias have to be excluded. A history of previous chemotherapy agents is not compatible with the diagnosis of idiopathic AA.
The requirement of normal cytogenetics for the diagnosis of AA is a subject of controversy; in a proportion of patients, cytogenetic analysis may be not informative. Most experts believe that the presence of karyotypic abnormalities at presentation is only consistent with the diagnosis of MDS. However, in many reports, cases of “AA with abnormal cytogenetics” have often been included. Certain karyotypic abnormalities such as trisomy 8 may be more common in these cases, and cytogenetic evaluation may show only a portion of affected metaphases and likely may just reflect oligoclonal hematopoiesis. Therapeutically, this distinction may not be essential, as responses to immunosuppression (IS) have been reported in patients with abnormal cytogenetics in the context of MDS as well as AA.
Several rare inherited syndromes can present as AA or evolve to AA. They include Fanconi anemia, dyskeratosis congenita and the newly described mutations of the telomerase gene (TERT). This latter condition may not become clinically obvious until adulthood and shows a variable penetrance. Analysis of a large cohort of AA patients showed that such a mutation is a very rare cause of what appeared to be idiopathic AA.1 All were cases of familial AA characterized by excessive telomere shortening, but only a minority of patients with AA and short telomeres had germline mutations in TERT. Routine testing is not available and suspected cases should be referred to specialized centers. Clearly, the diagnosis of inherited bone marrow (BM) failure is of most significance in pediatric AA, but appropriate testing may also be indicated in younger adults, given that genetic factors may constitute a propensity to develop the disease even in non-pediatric patients. Besides the TERT mutations and the HLA-typing (see below), among the most recently described immunogenetic factors, polymorphisms of the interferon-γ (IFN-γ) and transforming growth factor-β1 (TGF-β1) genes were associated with an increased risk of AA.2 A proper diagnosis of Fanconi anemia or other inherited bone marrow failure syndromes has major therapeutic implications; unnecessary therapy with antithymocyte globulin (ATG) can be avoided, and, should BM transplant (BMT) be considered, special conditioning regimens are necessary.
Diagnostic considerations
Blood counts provide a distinction between severe and moderate AA and, consequently, the assessment of the urgency of therapy (Table 1 ). Bone marrow aspiration and biopsy are needed for the determination of cellularity and exclusion of other diseases. The presence of blasts or abundant megakaryocytes is not compatible with the diagnosis of AA. Elevation of transaminases may point towards AA/hepatitis syndrome. Flow cytometry should be used to rule out lymphoproliferative syndromes such as large granular lymphocytic (LGL) leukemia as well as occult lymphoid malignancies, especially hairy cell leukemia, which can mimic AA. HLA-typing is performed if the patient could be considered a candidate for allogeneic bone marrow transplantation. HLA-DR*15 has been found at increased frequency in AA and paroxysmal nocturnal hemoglobinuria (PNH) and may constitute a positive prognostic factor with regard to IS therapy.
Flow cytometric analysis of red cells and granulocytes should be performed to establish the presence of a PNH clone. Of importance is that proper testing be performed using multi-color flow cytometry with staining for CD55 (e.g., CD66b) and CD59 as well as a lineage-specific antigen (glycophorin for erythrocytes or CD15 for granulocytes). Recently, fluorescein-labeled aerolysin, a bacterial toxin that selectively binds to the glycosyl phosphatidyl inositol (GPI)-anchor, was used for precise flow cytometric distinction between normal and PNH phenotypes. At least one third of patients with AA will harbor PNH clones of various sizes.3 It is likely that some of these patients may develop clinically significant PNH in the course of their disease, but the factors determining this complication remain unknown. PNH has been described in children, but childhood AA is less likely associated with the presence of PNH clones. The presence of PNH clones has been associated with a good response to IS. There is controversy as to the cut-off values used for the flow cytometric diagnosis of the PNH clones, and some investigators believe that, by using the proper technology, even very tiny PNH clones can be identified and have prognostic value.4 Of note is that PNH clones have been found also in apparently healthy individuals.5
Several novel tests may be helpful in assessment of immune responsiveness. For example, flow cytometric determination of IFN-γ expression, as well as serum levels of these cytokines, are indicative of a reversed TH1/TH2 ratio and correlate with response to IS (for review see 6). Activated cytotoxic T lymphocytes (CTL) and a reversed CD4/CD8 ratio have often been described in AA, but correlation with the activity of the disease was poor. More recently, T cell receptor (TCR) variable beta chain (VB) genotyping has been used to identify oligoclonal skewing of the TCR repertoire within cytotoxic T cells. Overexpansion of individual VB families, for example as detected by flow cytometry, may be present in AA and, if determined to be oligoclonal by genotyping, may indicate the presence of immunodominant clones involved in the autoimmune attack on hematopoietic stem cells. The TCR VB CDR3 regions can be used as a marker of the autoimmune process and their levels correlate with the hematologic response and relapse.7
Clinical Subentities
Most cases of idiopathic AA are due to immune-mediated mechanisms. However, within this rather broad category several distinct subentities can be distinguished.
Moderate chronic AA.
In contrast to severe AA (as defined by blood counts), AA with moderately depressed counts has a favorable prognosis and often does not require therapy. The definition of moderate AA is difficult as it may represent a transition stage to severe AA. Only a sufficient observation period (> 3 months) with chronically and not progressively depressed counts warrants the diagnosis of moderate AA. Over time the blood counts may decline, thus evolving to a severe AA. It remains unclear whether moderate AA represents a separate entity, a number of nosologic entities such as familial bone marrow failure syndromes, or a stage of typical AA. Various therapeutic approaches can be selected for moderate AA, including observation or aggressive therapy similar to that applied for severe AA. Due to often lesser urgency, less intense IS with ATG or cyclosporine (CsA) alone or with anti-interleukin (IL)-2R monoclonal antibody can be implemented.8;9 The decision to treat may be based on the presence of one severely affected hematopoietic lineage such as platelets or transfusion-dependent anemia. A theoretical argument can be made for early therapy as a measure to prevent progressive stem cell loss due to an unopposed autoimmune process. The response rates to IS may be lower than those seen in severe AA. However, it has to be noted that response criteria used for severe AA cannot be directly adopted.
AA/PNH syndrome.
It has been hypothesized that the autoimmune attack responsible for the stem cell depletion in AA generates permissive conditions under which an otherwise dormant PNH clone can evolve, as the stem cells may show differential insensitivity to T cell-mediated inhibition of stem cell function.10 Patients with AA in whom a PNH clone has been identified can be classified as having AA/PNH syndrome. These patients, unlike those with a primary hemolytic form of PNH, have hypocellular BM and low reticulocytes. In addition to the possibility of clonal evolution and progression to significant hemolytic disease, the finding of a large proportion of PNH cells complicates administration of ATG, which may precipitate a major hemolytic episode. During the course of disease, the fate of PNH is erratic. Some patients may evolve into a manifest form of PNH while in others the size of the PNH clone remains stable.3 IS therapy does not appear to influence the pace of PNH clonal expansion.
Hepatitis-associated AA.
AA/hepatitis syndrome has been described as a rare but very instructive variant of this disease, clearly pointing to the viral etiology of some cases of AA.11 Despite extensive laboratory investigation such a virus has not been identified, but a non-A, non-B, non-C hepatitis virus is suspected. Hepatitis is associated with jaundice. There is often a pronounced rise in transaminases and there may even be fulminant liver failure. In patients who survive the hepatic phase, transaminases decrease followed by a latency interval. After a variable time period, pancytopenia develops with a clinical picture typical of severe AA. ATG therapy is effective and can often result in complete remission. For selected patients BMT may be a viable treatment option.
AA in pregnancy.
Pregnancy seems to predispose to AA but this issue remains controversial. The mechanism that triggers AA in pregnancy remains unclear, but AA often resolves with the termination of pregnancy and can recur during subsequent pregnancies. Even if the initial presentation of AA was not associated with pregnancy, women with a recent history of successfully treated AA should be counseled to not get pregnant. However, successful pregnancies have been described and in the majority of case series most of the women had positive outcomes.12 The therapy of pregnancy-associated AA depends on the gestational age of the fetus. The baby of a mother with severe AA may delivered, if it is close to term, a measure which may result in improvement. Earlier in pregnancy, supportive measures are most commonly used, but ATG has been also administered to women with severely depressed counts, especially low ANC.
Therapy
Conservative therapies
Immunosuppression.
In general, IS therapy remains the most important treatment modality for the major portion of patients affected by AA. History consistent with drug-induced AA (e.g., gold) or infection-associated AA (hepatitis-associated AA) does not preclude response to IS treatments.
The most common IS regimens combine horse (ATGam at 20 mg/kg per day for 4 days) or rabbit ATG (Thymoglobulin at 3.5 mg/kg per day for 5 days) with CsA (12–15 mg/kg in a divided dose bid) given usually for 6 months. Steroids are usually added to counteract the serum sickness intrinsic to ATG therapy. Based on results obtained in a salvage trial of patients who did not respond to horse ATG, rabbit ATG is likely as effective as horse ATG, but their relative efficacy has not been compared in a randomized trial.13 The response rate to horse ATG ranges from 70% to 80% with a 5-year survival of 80%–90%.14 ATG appears to be superior to CsA8,15 and the combination of ATG and CsA provides better results than ATG or CsA alone.16 The results of the most important trials are summarized in Table 2 .14,17–19 Intense IS with ATG/CsA has been also administered with good success to elderly patients.20 Addition of granulocyte colony-stimulating factor (G-CSF) may improve neutropenia but does not increase survival, but early response to G-CSF following a course of ATG is a good prognostic factor for overall response.21 Overall, AA patients who respond to combination ATG/CsA have excellent survival while those who are refractory have less favorable survival. Counts at 3 months post-ATG therapy have good correlation with long-term prognosis.14 Newer IS regimens may employ other agents such as mycophenolate mofetil and, in the context of CsA toxicity, Zenapax (anti-IL-2 receptor [CD25] monoclonal antibody [mAb])9 may be helpful but the efficacy of these agents is not known. Similarly, Campath-1H is currently being tested in a refractory setting to assess its potential usefulness as an IS agent (Table 3 ).
Current regimens are mostly empirically established. Consequently, treatment failures may reflect under-dosing and there is little guidance as to rational dose adjustment and modification. CsA levels should be monitored but no rational justification exists as to targeted levels and the impact of the CsA levels on the therapy success. Experiences with IS in solid organ transplant suggest that CsA levels do not correlate well with the depth of IS and risk of rejection, and specific functional tests can be applied to determine the level of IS. So far such assays have not been used to guide IS treatment in AA.
IS therapy failures may represent under-treatment (as suggested by a high salvage rate with ATG13;22) or exhaustion of stem cell reserves precluding hematopoietic recovery. In addition, lack of response may be due to misdiagnosis or may suggest a non-immune pathogenesis such as familial AA (Table 4 ). Relapses can be due to early termination of IS, and patients’ blood counts may often remain CsA-dependent. Similarly, induction therapy with current regimens of ATG or even cyclophosphamide may not always be sufficient to eliminate autoimmune T cells.23
Refractory patients may be retreated with multiple courses of ATG, which may result in salvage of a significant proportion of patients. In one study of patients refractory to horse ATG, rabbit ATG resulted in a 50% response rate and excellent long-term survival.13 No good prognostic factors are available with regard to the response to ATG with the exception of the presence of HLA-DR*15 alleles and PNH clones, which both correlated with responsiveness to IS4 but the correlation was not absolute.
High-dose cyclophosphamide has been advocated as an effective first-line therapy in AA.24 High response rates were associated with prevention of relapse and also clonal disease. However, prolonged cytopenia resulted in excessive toxicity related to neutropenic complications in randomized trials between ATG/CsA and cyclophosphamide/CsA, resulting in a termination of the trials.23 Long-term follow-up of patients treated with cyclophosphamide showed that relapse and clonal disease can occur after this type of therapy.23 It seems that high-dose cyclophosphamide does not constitute advancement over ATG/CsA and should be used only in very selected cases or as a part of a controlled experimental trial with a narrowly defined indication spectrum.
Relapse
Conceptually, in analogy to the therapy of malignant disorders, intense IS with ATG may be viewed as induction treatment, which may require a prolonged maintenance period with CsA or even the reinduction. The relapse rate following IS therapy is as high as 35% in 7 years.14 In general, relapse has a good prognosis and survival of relapsed patients is not significantly shortened.14 Patients with falling blood counts can first receive a trial of CsA and, if unsuccessful in rescuing the counts, a repeated course of ATG should be given. The response rates are likely comparable to those seen with an initial course of ATG. In some instances, rabbit ATG can be used instead of horse ATG, but it is unclear whether this measure helps to avoid more violent allergic reactions. High-dose cyclophosphamide has been suggested to provide an IS modality that prevents subsequent relapses. However, this notion has not been confirmed. Of note is that in studies of cyclophosphamide the time to response was more than 1 year. In contrast, 75% of the responses to ATG are within the first 3 months, and relapses occur within 1 year following ATG therapy.24
Salvage therapies
Repeated cycles of immunosuppression.
Patients refractory to an initial course of ATG can respond to repeated cycles of ATG; in one study, a significant salvage rate of patients refractory to horse ATG was achieved with a second cycle of rabbit ATG.13 However, the third cycle was unlikely to induce response in patients who did not respond to repeated therapy.22 Attempts at salvage therapy may delay BMT; the impact of this delay is a subject of controversy. Patients who have a matched sibling donor and did not respond to ATG/CsA therapy should undergo BMT. In children, a better outcome was reported for those patients who were transplanted following the initial ATG failure.25 In addition to repeated ATG courses, new agents such as Campath-1H or anti-CD3 mAb could be used in the context of a clinical trial.
Hematopoietic growth factors and anabolic steroids.
Hematopoietic growth factors should not be used as a sole treatment modality for AA in the primary setting. Some patients will show an improvement of neutropenia with G-CSF, but severe neutropenia due to typical AA is mostly refractory. In combination with an ATG/CsA regimen, G-CSF can improve neutropenia and response to this therapy constitutes an early positive prognostic factor with regard to the future response.21 Dose escalation of G-CSF does not appear to be beneficial. G-CSF in combination with other agents has been used as salvage therapy in the refractory setting and their prolonged administration has been associated with recovery of counts in some patients. However, some reports implicated prolonged therapy with G-CSF as a cause of clonal evolution, especially monosomy-7 (see below).
Anabolic steroids were widely used to treat AA prior to the advent of IS therapy. Currently androgens are only used as salvage therapy for IS-refractory patients but constituted a main pillar of the therapy in the past. The currently available androgens include oxymethylone and danazol.
Bone marrow transplantation
The most common conditioning regimen includes cyclophosphamide and ATG and has been shown to be superior to the historical cyclophosphamide with total thoracoabdominal irradiation.26 Improvement in the general care and treatment of graft-versus-host disease (GVHD) has rendered BMT a much safer procedure and made transplantation an option for more AA patients. BMT offers a truly curative treatment alternative in contrast to the long-term complications of conservative IS therapy, including evolution to MDS and a high relapse rate.
Matched allogeneic BMT.
Allogeneic BMT is available for only a minority of patients (only approximately 30% have HLA-matched siblings). With the general improvement in the outcomes of BMT, the overall survival for matched sibling donor transplantation has been as good as 94%. Even better results were reported in children, in whom BMT appears to be more effective in improving survival than IS.27Table 5 summarizes recently reported results.18,26,28–30 However, a typical decrease in the overall survival is observed with increasing age of the recipient, making the therapeutic decision for older patients a challenge. Clearly, children and young adults with a matched sibling donor should be offered BMT as a first therapeutic option. The age limit for the primary choice of BMT has not been fully established, and in patients older than 30–35 years, intense IS may be selected as a first attempt, with BMT used as salvage therapy for non-responders. Delaying BMT may decrease the chance of its success, but this concern is not well supported in adults,26 and high treatment-related mortality of BMT in older patients may justify all attempts at remission induction.
In general, the survival rates for matched unrelated BMT are by far less impressive than those performed from sibling donors, but overall progress in transplantation techniques, molecular HLA-typing, matching, and supportive care render the survival curves of sibling and unrelated transplants similar. Various methods, including modified conditioning regimens and T cell depletion, have been used to improve the results. In studies with adults the results were less favorable than in children, with around one third of patients surviving, with deaths due to GVHD, graft failure and opportunistic infections (5-year survival 44% and 35% for those ≤ 20 years and 21–40 years, respectively).28 In an analysis of 141 patients from the National Marrow Donor Program, 3-year survival was 36%. Recent results in children are more favorable.25 Perhaps due to the poor prognosis, unrelated BMT has been performed mostly in patients refractory to IS, raising the question whether early transplantation would result in better outcomes. For example, children who received repeated cycles of IS followed by BMT fared less well than those who received BMT following one cycle of failed IS.25
Nonmyeloablative stem cell transplantation has been developed to improve the treatment-related mortality through decreased intensity conditioning. Such an approach, if successful in AA, would extend the indication spectrum of BMT for older patients. So far a systematic experience in AA has not been published; however, historically conditioning regimens utilized for AA patients undergoing BMT have been less intense than those adopted for patients with malignancy. Several conditioning regimens have been proposed including low-dose irradiation, fludarabine, cyclophosphamide and ATG. Excellent results from nonmyeloablative transplantation have been described for PNH31 and a small series of patients with AA who received grafts from siblings and unrelated donors. Although the observation intervals were relatively short, the results were encouraging given the high-risk patient groups transplanted.
A randomized trial comparing IS with BMT has not been performed, but it appears that younger patients benefited more from transplantation while the results in older patients may show an advantage for those who were treated with ATG/CsA. In one non-randomized study 6-year survival was 69% and 79% for IS and BMT, respectively.18 Comparable survival was obtained for older adults when the data from the European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia (WPSAA) were analyzed.19
Late Complications
Complications of BMT
Chronic GVHD is a common complication of allogeneic BMT. In addition, after a long latency period an increased frequency (12%) of solid tumors has been observed.26,30 Other complications include lung disease, cataracts, and bone/joint problems.30 With the introduction of IS therapy, the survival of AA patients improved, allowing for long-term follow-up. Evolution of clonal hematopoietic diseases such as PNH and MDS has been recognized as a serious late complication in conservatively treated patients. Although the appearance of PNH clones is often already observed at first presentation of BM failure,3 manifest PNH develops in a much smaller but significant proportion of patients. Graft failure has also been described, and in some cases such patients may benefit from autologous reconstitution of hematopoiesis.
MDS evolution.
Aberrant differentiation of hematopoietic precursor cells, increased numbers of myeloblasts, and marrow hypercellularity are all characteristic of MDS, but persistent BM hypocellularity in AA may preclude reliable morphological analysis. The finding of a cytogenetic defect is considered to be objective evidence of clonal evolution to MDS.32,33
The development of MDS in the setting of AA has been described in several studies, but these vary significantly in design and especially in case definition,32 exemplifying diverse views with regard to the criteria required for the diagnosis of both MDS and AA. In historical studies of AA, patients with abnormal cytogenetics and hypoplastic marrows at presentation were often included, and in some institutions, abnormal cytogenetic studies are compatible with a primary diagnosis of AA (for example see 34). In a series involving 122 patients treated with intensive IS consisting of ATG and CsA, the risk of MDS evolution was about 21% at 10 years.33 In 100 patients from the GITMO (Gruppo Italiano Trapianto di Midollo Osseo) and EBMT study involving antilymphocyte globulin (ALG), CsA, prednisone and G-CSF, 11 patients developed cytogenetic abnormalities during a median follow-up of 5 years.35 The differences in the diagnostic criteria are obvious, such as in a recent analysis by the EBMT AA Working Party, in which karyotypic abnormalities occurred in 23 of 170 patients, but in 4 cases chromosomal changes were present at first diagnosis36 and would be classified as MDS at other institutions.
After clonal evolution, marrow morphology was characterized by predominance of hypercellularity (41%) and patchy biopsy cellularity (27%), while continued hypocellularity was found in 33% of the patients. Frank dysplasia was observed in a large proportion of patients, but in many patients there were no morphologic changes suggestive of MDS.33 While the entity of AA with cytogenetic abnormalities may exist, the new appearance of an abnormal clone in the course of AA warrants the change of diagnosis from AA to MDS. Because the detection of a new cytogenetic abnormality is a stringent diagnostic sign, it may not reflect the total rate of MDS evolution in AA. In primary MDS, the proportion of patients with a normal karyotype is 40%–60%, and by analogy, it is possible that also in post-AA, MDS can evolve without an overt chromosomal change. The most commonly found cytogenetic abnormalities following AA were aberrations of chromosome 7 and trisomy 8.33 In a recent report from Japan, a series of 9 patients with 13q– following otherwise typical AA were reported;37 in the NIH experience, 13q– was also reported in several of the 29 patients who developed an abnormal karyotype.33 In both studies, patients showed stable counts and a good response to IS. While prolonged G-CSF treatment was linked by Japanese investigators to the evolution of monosomy 7,38 there was no increased risk observed in a randomized study of ATG and CsA with and without G-CSF39 or in the analysis of EBMT data.19
There are no predictive factors to identify patients at risk for clonal evolution to MDS. Good response to IS does not correlate with a smaller risk of cytogenetic evolution; out of 29 patients who developed a clonal abnormality (28 treated with IS), there were 12 patients who did not respond to IS and 16 responders.33
Prognosis and therapy
Clearly, the diagnosis of MDS in the course of AA has prognostic significance. Most obvious modifiers include the presence of blasts, hypercellular bone marrow, certain types of defects (e.g., monosomy-7 and complex karyotypes), and recurrence or persistence of profound cytopenia, all constituting unfavorable prognostic markers. In one report AA patients who developed secondary chromosomal abnormalities had a mortality rate of about 27%. All but 2 deaths were related to AML.33 Response to IS in patients with aplasia and an abnormal karyotype may be as high as 50%,34 and certain karyotypic abnormalities (Trisomy 8, 13q–) may favorably respond to IS. While the low numbers of reported patients preclude generalization, no individual abnormality predicted unresponsiveness. However, certain types of chromosomal defects are less likely to benefit from IS, including monosomy 7 or complex karyotypes, and BMT may be the only therapeutic option. The benefit of hypomethylating agents such as 5-azacytidine or lenalidomide, is unclear but some responsiveness may be inferred from the effects of this drug in primary MDS.
Evolution of PNH
A PNH clone can be found in a significant proportion of patients with AA already at presentation, but most of these patients harbor small clones without clinical significance. Their presence constitutes a positive prognostic factor for the response to IS.4,40 The behavior of the PNH clone in the course of the disease and following therapy is erratic. In some patients the clonal size does not change, while clinical PNH can evolve in up to 10% of AA patients over a period of 10 years. Currently there are no good predictive factors and most of the current data is derived from an older cohort of patients. It remains unclear whether AA patients who developed PNH did have minor PNH clones detectable at presentation or whether their PNH developed truly de novo. PNH can be a very disabling chronic complication of AA and may be associated with hemolysis, transfusion dependence and thrombotic complications. In some patients PNH may have a very indolent course. The effectiveness of the anti-complement antibody eculizumab for PNH is currently being investigated.
Summary
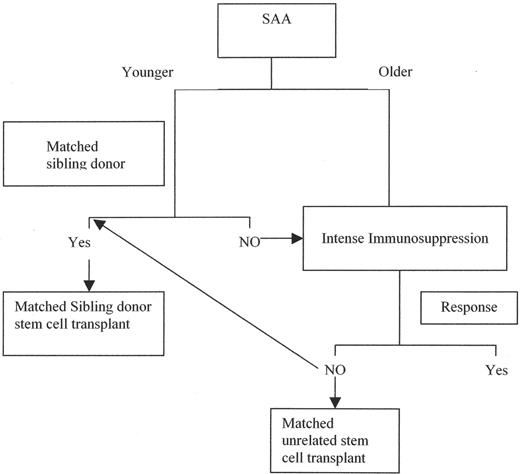
The currently established therapeutic algorithm of acquired adult AA is structured according to the age of patients; with increasing age IS may provide more favorable survival results than BMT (Figure 1 ). However, even very intense IS may not be sufficient to eradicate the autoimmune process, and prolonged maintenance therapy may be needed for the prevention of relapses. Refractory patients constitute a significant challenge and their prognosis is poor. With increasing survival, evolution of clonal disease is a serious complication of AA for which only BMT constitutes a curative option. The progress in the therapy of AA is highly influenced by the general improvement of BMT techniques, especially in the matched unrelated setting, as well as by the introduction of novel more specific IS agents that could allow for the induction of permanent tolerance to the offending antigen.
Classification of aplastic anemia by counts.
| Severe AA . | Moderate AA . |
|---|---|
| Abbreviations: ANC, absolute neutrophil count; ARC, absolute reticulocyte count; MAA, moderate AA | |
| •ANC < 500/μL | AA not fulfilling severity criteria |
| •ARC < 40,000/μL in anemic/tranfusion-dependent patients | Diagnosis of chronic MAA requires persistent moderately depressed counts > 3 months |
| •Platelets < 20 x 103 /μL | |
| 2 out of 3 criteria | |
| Severe AA . | Moderate AA . |
|---|---|
| Abbreviations: ANC, absolute neutrophil count; ARC, absolute reticulocyte count; MAA, moderate AA | |
| •ANC < 500/μL | AA not fulfilling severity criteria |
| •ARC < 40,000/μL in anemic/tranfusion-dependent patients | Diagnosis of chronic MAA requires persistent moderately depressed counts > 3 months |
| •Platelets < 20 x 103 /μL | |
| 2 out of 3 criteria | |
| N . | Dx . | Ages (years) . | Response (%) . | Survival (%) . | Relapse (%) . | Study . |
|---|---|---|---|---|---|---|
| Abbreviations: Dx, diagnosis; SAA, severe AA; MAA, moderate AA; ALG, antilymphocyte globulin; CsA, cyclosporine; ATG, antithymocyte globulin; G-CSF, granulocyte colony-stimulating factor | ||||||
| 122 | SAA | <18 N = 31, >18 N = 91 | 61 at 6 mo 58 at 1 yr | 55 at 7 y | 35 at 5 y | NIH |
| 182 | SAA | Mean 25 | ALG+CsA 83 ATG+CsA+G-CSF 85 | NA | NA | EBMT/GITMO |
| 51 | SAA | Mean 43 | 70 at 6 mo | 64 at 3.5 y | 11 | Germany |
| 46 | MAA | Mean 29 | 74 at 6 mo | 93 at 4 y | NA | EBMT |
| 83 | SAA | 14–40 | 47 at 6 mo | 69 at 6 y | 7.1 | Korea |
| N . | Dx . | Ages (years) . | Response (%) . | Survival (%) . | Relapse (%) . | Study . |
|---|---|---|---|---|---|---|
| Abbreviations: Dx, diagnosis; SAA, severe AA; MAA, moderate AA; ALG, antilymphocyte globulin; CsA, cyclosporine; ATG, antithymocyte globulin; G-CSF, granulocyte colony-stimulating factor | ||||||
| 122 | SAA | <18 N = 31, >18 N = 91 | 61 at 6 mo 58 at 1 yr | 55 at 7 y | 35 at 5 y | NIH |
| 182 | SAA | Mean 25 | ALG+CsA 83 ATG+CsA+G-CSF 85 | NA | NA | EBMT/GITMO |
| 51 | SAA | Mean 43 | 70 at 6 mo | 64 at 3.5 y | 11 | Germany |
| 46 | MAA | Mean 29 | 74 at 6 mo | 93 at 4 y | NA | EBMT |
| 83 | SAA | 14–40 | 47 at 6 mo | 69 at 6 y | 7.1 | Korea |
Novel immunosuppressive agents with potential utility in aplastic anemia (AA).
| . | Currently Available . | In Clinical Trials for Other Indications . |
|---|---|---|
| Abbreviations: mAb, monoclonal antibody; TNF, tumor necrosis factor; IFN, interferon | ||
| Immunosuppressive agents | Anti-IL-2 mAb (Zenapax) | Anti-CD3 mAb (Nuvion) |
| Campath-1H | New mTOR inhibitors (RAD001) | |
| Rapamycin | Anti-CD11a mAb (Efalizumab) | |
| Anti-CD2 mAb (Alefacept) | ||
| Immunomodulation/tolerance | CTLA-4-Ig (Abatacept) | |
| Anti-CD154 (CD40L) mAb | ||
| Anticytokine therapy/immunomodulation | Anti-TNF-α mAb (Remicade) | Anti-IFN-γ mAb (HuZap) |
| TNF Rilg (Enbrel) | Anti-α4-integrin mAb (Natalizumab) | |
| . | Currently Available . | In Clinical Trials for Other Indications . |
|---|---|---|
| Abbreviations: mAb, monoclonal antibody; TNF, tumor necrosis factor; IFN, interferon | ||
| Immunosuppressive agents | Anti-IL-2 mAb (Zenapax) | Anti-CD3 mAb (Nuvion) |
| Campath-1H | New mTOR inhibitors (RAD001) | |
| Rapamycin | Anti-CD11a mAb (Efalizumab) | |
| Anti-CD2 mAb (Alefacept) | ||
| Immunomodulation/tolerance | CTLA-4-Ig (Abatacept) | |
| Anti-CD154 (CD40L) mAb | ||
| Anticytokine therapy/immunomodulation | Anti-TNF-α mAb (Remicade) | Anti-IFN-γ mAb (HuZap) |
| TNF Rilg (Enbrel) | Anti-α4-integrin mAb (Natalizumab) | |
Causes of treatment failure and relapse in aplastic anemia.
| Cause . | Possible Etiology . |
|---|---|
| Exhaustion of stem cell reserves | Immune mediated aplastic anemia |
| Insufficient immunosuppression | Persistent immune attacks |
| Misdiagnosis | |
| Hereditary bone marrow failure | Non-immune pathogenesis |
| Cause . | Possible Etiology . |
|---|---|
| Exhaustion of stem cell reserves | Immune mediated aplastic anemia |
| Insufficient immunosuppression | Persistent immune attacks |
| Misdiagnosis | |
| Hereditary bone marrow failure | Non-immune pathogenesis |
| N . | Survival . | Age . | Source . |
|---|---|---|---|
| Abbreviations: TAI, thoracoabdominal irradiation; Cy, cyclophosphamide; ATG, antithymocyte globulin; GVHD, graft-versus-host disease; CsA, cyclosporine; MTX, methotrexate | |||
| 133 | 59% at 16 y for TAI/Cy 95% at 4.4 y for ATG/Cy | 55% < 20 y | France |
| 211 | 89% at 20 y without GVHD 69% at 20 y with GVHD | 18 y | FHCRC |
| 915 | Actuarial survival 77% for patients 68% for patients 17–40 y 54% for patients > 40 y | ≤ 16 y | EBMT |
| 61 | At 6 y 79% | 14–40 y | Korea |
| 71 | 94% at 8 y with CsA/MTX 78% at 7 y with CsA | 20 y | GITMO |
| 1699 | 5 y survival: 75% for patients ≤ 20 y 68% for patients 20–40 y 35% for patients > 40 y | IBMTR | |
| N . | Survival . | Age . | Source . |
|---|---|---|---|
| Abbreviations: TAI, thoracoabdominal irradiation; Cy, cyclophosphamide; ATG, antithymocyte globulin; GVHD, graft-versus-host disease; CsA, cyclosporine; MTX, methotrexate | |||
| 133 | 59% at 16 y for TAI/Cy 95% at 4.4 y for ATG/Cy | 55% < 20 y | France |
| 211 | 89% at 20 y without GVHD 69% at 20 y with GVHD | 18 y | FHCRC |
| 915 | Actuarial survival 77% for patients 68% for patients 17–40 y 54% for patients > 40 y | ≤ 16 y | EBMT |
| 61 | At 6 y 79% | 14–40 y | Korea |
| 71 | 94% at 8 y with CsA/MTX 78% at 7 y with CsA | 20 y | GITMO |
| 1699 | 5 y survival: 75% for patients ≤ 20 y 68% for patients 20–40 y 35% for patients > 40 y | IBMTR | |
JP Maciejewski: The Cleveland Clinic Foundation, Taussig Cancer Center, Cleveland Clinic College of Medicine of the Case Western Reserve University, Cleveland, Ohio
AM Risitano: Division of Hematology, Federico II University of Naples, Via Pansini 5, 80131 Naples, Italy