Abstract
Our understanding of the pathogenesis of congenital and acquired neutropenia is rapidly evolving. New ground-breaking observations have identified the genes responsible for many of the congenital neutropenia syndromes and are also providing new insights into normal neutrophil commitment and differentiation. Acquired neutropenia remains a poorly understood syndrome, although new insights into its pathogenesis are also emerging, especially with regard to subsets of immune neutropenia.
In Section I, Dr. Marshall Horwitz reviews the current understanding of the genetic basis, molecular pathology, and approaches to treatment of congenital neutropenia and cyclic hematopoiesis. Mutations in the ELA2 gene, which encodes for neutrophil elastase, cause cyclic hematopoiesis. ELA2 mutations are also the most common cause of congenital neutropenia, where their presence may equate with a more severe clinical course and higher frequency of leukemic progression. Emerging evidence indicates interrelatedness with Hermansky Pudlak syndrome and other disorders of neutrophil and platelet granules.
In Section II, Dr. Nancy Berliner presents an overview of the clinical approach to the evaluation and treatment of acquired neutropenia. This includes a review of the pathogenesis of primary and secondary immune neutropenia, drug-induced neutropenia, and non-immune chronic idiopathic neutropenia of adults. Studies used to evaluate patients for potential immune neutropenia are reviewed. Management issues, especially the use of granulocyte colony-stimulating factor (G-CSF), are discussed.
In Section III, Dr. Thomas Loughran, Jr., reviews the pathogenesis and clinical manifestations of large granular lymphocyte (LGL) leukemia. Possible mechanisms of neutropenia are discussed. In particular, discussion focuses on the relationship between LGL leukemia, rheumatoid disease, and Felty’s syndrome, and the complex interplay of defects in neutrophil production, distribution, destruction, and apoptosis that underly the development of neutropenia in those syndromes.
I. Congenital and Cyclic Neutropenia
Marshall Horwitz, MD, PhD*
University of Washington, Dept. of Medicine, Div. of Medical Genetics, Box 357720, Seattle WA 98195
The two mains forms of hereditary neutropenia are cyclic neutropenia, also known as cyclic hematopoiesis, and severe congenital neutropenia (SCN), sometimes referred to as Kostmann syndrome. Other syndromes can feature neutropenia as a component (Table 1 ).
Cyclic Neutropenia
In cyclic neutropenia, peripheral blood neutrophil counts oscillate with approximately 21 day frequency, with a nadir approaching zero and a peak near normal (as reviewed in 1). Monocytes cycle but do so in a phase opposite to that of neutrophils. Similar periodicity may occur in acquired diseases, including chronic myelogenous leukemia (CML), large granular lymphocytosis (LGL), and hypereosinophilic syndrome. Life-threatening infections can accompany the 3- to 4-day neutropenic nadir of the cycle, with frequent aphthous stomatitis, periodontitis, typhlitis, and occasional sepsis. There may be particular vulnerability to infection with anaerobic bacteria, suggesting that the deficiency in neutrophils is not merely one of low numbers. Most cases respond to granulocyte colony-stimulating factor (G-CSF) treatment, administered at about 2–3 μg/kg at 1- to 2-day intervals.1 G-CSF does not abrogate cycling, but instead reduces infectious complications by shortening the cycle period and increasing the amplitude of the waves. Genetic transmission is autosomal dominant. As with other dominant disorders, sporadic cases commonly arise from new mutations.
ELA2 Mutations in Cyclic Neutropenia
Genetic linkage analysis and positional cloning demonstrated that heterozygous, germline mutations of the ELA2 gene, encoding neutrophil elastase, explain many cases of cyclic neutropenia.2 There are many different alleles, but the most common are intronic substitutions that destroy a splice donor site in intron 4. This forces the utilization of an upstream, cryptic splice donor site resulting in an internal deletion of ten amino acid residues from the protein (ΔV161-F170).
Severe Congenital Neutropenia
Kostmann was among the first to describe congenital agranulocytosis3 when he reported static neutropenia accompanied by a promyelocytic maturation arrest in the bone marrow in a consanguineous family in Sweden, with apparent autosomal recessive inheritance. However, SCN is genetically heterogeneous, and most cases seem to arise sporadically, consistent with its transmission as an often lethal, autosomal dominant disorder. A rare case of sex-linked recessive inheritance has been described and constitutes an allelic variant of the Wiskott-Aldrich syndrome.4 SCN usually responds to G-CSF but requires higher doses than those used to treat cyclic neutropenia.3 Addition of corticosteroids may benefit cases otherwise not responding to G-CSF.5 In the past, most patients died from infections (and many still do). With improved survival because of G-CSF, myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) have emerged as significant complications, occurring in about 10% of all SCN patients.6 In general, patients with cyclic neutropenia do not develop leukemia, although there are a few exceptions.7
G-CSF Receptor Mutations in SCN
Mutations of G-CSFR, the gene encoding the G-CSF receptor, were initially reported as causative of some SCN cases (as reviewed in 7,8). Later, it was appreciated that G-CSFR mutations represent acquired, nonheritable, somatic events in the bone marrow, accumulating as SCN progresses to MDS and AML, although the mutations do not invariantly occur in AML and may also appear in the absence of neoplasia. Leukemia in SCN may also show acquired monosomy 7, trisomy 21, and ras mutations. Subsequently, there have been reports of 2 sporadic SCN patients, resistant to G-CSF therapy, with constitutional heterozygous G-CSFR mutations that do appear to represent new pathogenetic germline mutations.
ELA2 Mutations in SCN
A candidate gene study determined that constitutional heterozygous ELA2 mutations are present in DNA extracted from the peripheral blood of 35%–84% of SCN cases.9 Most cases are sporadic, but ELA2 mutations segregate with multigenerational transmission of the disease in several pedigrees where there are multiple affected family members, thus indicating that the mutations, at least in familial cases, occur constitutionally in the germline. The possibility of somatic ELA2 mutations, similar to the case for G-CSFR, remains unexplored. Mutations causing SCN are generally distinct from those responsible for cyclic neutropenia. The genotypes and phenotypes can overlap,7,8 however, and the mutation P110L appears roughly equally among both cyclic neutropenia and SCN patient populations. Chain terminating nonsense and frameshift mutations near the carboxyl terminus are the most common SCN mutations. SCN patients whose disease is the result of ELA2 mutations represent a subset with worse disease: lower neutrophil counts, requirement for higher doses of G-CSF to achieve a response, and higher rate of neoplastic progression.10 Some ELA2 mutations are particularly severe. G185R occurs in 4 SCN patients known in the French neutropenia registry10 and among our samples, each of whom has failed G-CSF treatment and has developed MDS or AML. Homozygous mutation of ELA2 is unknown, and this gene would be an unlikely candidate for the recessive syndrome first reported by Kostmann, where the responsible gene remains unidentified. The possibility of somatic ELA2 mutations in MDS or AML arising in the absence of hereditary neutropenia has not been addressed.
Proof of Causality of ELA2 Mutations
Genetic data supporting the role of ELA2 mutation in the pathogenesis of cyclic neutropenia and SCN have been confirmed independently in several laboratories (as reviewed in 8). Clinical Laboratory Improvement Amendment (CLIA)-certified tests to detect ELA2 mutation are commercially available. Nevertheless, the finding that such a pedestrian enzyme as neutrophil elastase causes hereditary neutropenia was greeted with skepticism. At least two further observations unambiguously establish causality. First, the probability of identifying by chance a new mutation—an extremely rare occurrence—when screening a single gene, from among > 20,000 human genes, in a sporadic case whose unaffected parents lack the illness, is infinitesimal. Yet, for sporadic cases, new mutation of ELA2 occurs invariantly in cyclic neutropenia and commonly in SCN. Second, germline mosaic individuals11 who have fathered children with SCN demonstrate ELA2 mutations in myeloid progenitors, but not neutrophils, indicating that the mutation alone is sufficient to prevent the maturation of stem cells into neutrophils (or to direct them to an alternate cell fate, such as monocytes).
ELA2 as a Cancer Gene
A debate focusing on the origins of MDS and AML in SCN has drawn two sides. One argues that malignant evolution is a consequence of bone marrow failure per se, noting that clinically and genetically distinct cytopenic disorders, such as Shwachman-Diamond syndrome, also undergo such transformation. The other centers on the possible contributions of G-CSF treatment. In fact, neither argument may be correct. Epidemiological data do not reveal an association between G-CSF dose or duration of treatment and neoplasia.6 Recent observations indicate that even though SCN patients without ELA2 mutations generally have clinically indistinguishable disease, MDS or AML arises almost exclusively in the subset of SCN patients whose illness is caused by ELA2 mutations.10 Neutrophil elastase could thus be the first protease known to act as an oncoprotein. In fact, it may have a role in other malignancies. Genetic deficiency of α1-antitrypsin, the major inhibitor of neutrophil elastase, is associated with an increased risk of (in addition to pulmonary emphysema) lymphoma and carcinoma of the lung, liver, gall bladder, and bladder (as reviewed in 12). Common sequence variants of the ELA2 promoter that cause its overexpression are found at higher frequency in lung cancer patients. The beige mouse, a genetic model of the human neutropenic Chediak-Higashi syndrome resulting from LYST mutation and leading to secondary deficiency of neutrophil elastase, is resistant to UV- and benzopyrene-induced skin cancer.
Varying Biochemical Effects of ELA2 Mutations
ELA2 is expressed only in promyelocytes and promonocytes, but the neutrophil elastase protein persists through the cell divisions of terminal differentiation to neutrophils and monocytes, respectively (as reviewed in 8). The mature protein is 218 amino acids in length (following removal of pre-pro amino terminal sequences and a carboxyl tail). Neutrophil elastase predominately resides in granules but is also present in the plasma membrane and released into serum. As a chymotryptic protease, it is capable of digesting many substrates, including matrix components such as elastin, clotting factors, immunoglobulins, and complement. Intriguingly, with respect to disease pathogenesis, neutrophil elastase also cleaves G-CSF, the G-CSF receptor, the c-KIT receptor, and Notch family receptors (as reviewed in 8).
Nevertheless, in crude recombinant expression assays, the mutations have varying effects on catalytic activity, with some markedly reducing activity and others appearing to be largely inconsequential.13 Given this background, determining how the mutations cause disease has proven to be elusive. The proposal that these ELA2 mutations cause accelerated apoptosis has been called into question (as reviewed in 8).
Insight from Canine Neutropenia, a Model of Hermansky Pudlak Syndrome
Cyclic neutropenia in dogs, also known as the gray collie syndrome, differs from the human form of the illness because it features autosomal recessive inheritance, oculocutaneous albinism, cycling of all blood lineages, and a periodicity closer to 2, instead of 3, weeks. A candidate gene approach found that canine cyclic neutropenia is the equivalent of the rare human Hermansky Pudlak syndrome type 2 (HPS2), with both diseases resulting from homozygous inactivating mutations of AP3B1, encoding the beta subunit of the adaptor protein 3 (AP3) trafficking complex.14 There are just 4 patients from 3 families known to have HPS2; all are neutropenic, and, in the only patient in whom cycling was investigated, the neutropenia was severe and static.15
As recently reviewed,8 there are four heterotetrameric adapter protein complexes, and all are involved in the intracellular transport of luminal “cargo” proteins within membrane-bound organelles. AP3 specifically shuttles cargo proteins from the trans-Golgi network to lysosomes, which, in neutrophils, generally correspond to granules. The mu or beta subunits recognize tyrosine or dileucine based peptide motifs, respectively, within cargo proteins. In the absence of AP3, cargo proteins are routed to a default destination in the plasma membrane. Mutations yielding absence of beta subunits lead to disassembly and decay of the entire complex. Several lines of evidence suggest that neutrophil elastase is a cargo protein for AP3.14 First, its localization in granules is compatible with the distribution of other known AP3 cargo proteins, and mutations in either cause similar diseases. Furthermore, neutrophil elastase is deficient in canine cyclic neutropenia, even though the canine ELA2 gene is intact and appropriately expressed. Finally, neutrophil elastase (processed free of its carboxyl tail) and the AP3 mu subunit interact in a yeast two-hybrid assay, and a tyrosine residue in neutrophil elastase (NE) is required for their association. The most common category of SCN mutations—those that delete the carboxyl terminus—also remove the tyrosine residue required for association in vitro between neutrophil elastase and the mu subunit of AP3 and redirect neutrophil elastase to the plasma membrane in transfected cells.
Nevertheless, these observations raise a potential biological problem. AP3 coats the cytoplasmic (outer) surface of membrane-bound organelles, and cargo proteins, within the interior of such vesicles, must extrude through the membrane in order to interact with AP3. Thus, if neutrophil elastase is a genuine AP3 cargo protein, then it must have at least one transmembrane segment. Neutrophil elastase, a textbook serine protease, has been extremely well studied. Although routinely appearing on membranes, it had generally been regarded as a soluble protein.
Neutrophil Elastase as a Predicted Transmembrane Protein
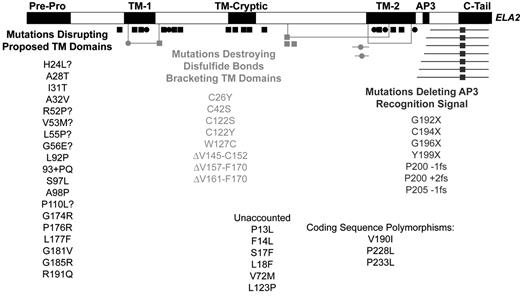
Somewhat surprisingly, several, though not all, computer algorithms designed to predict transmembrane domains detect two such segments in neutrophil elastase.14 Interestingly, when the location of mutations is superimposed on the predicted transmembrane domains, a striking pattern emerges (Figure 1 ): mutations capable of causing cyclic neutropenia approximately overlap with predicted transmembrane segments. Experimentally, expression of neutrophil elastase representing cyclic neutropenia mutations in proposed transmembrane segments appears to cause enhanced granular accumulation of the protein, whereas wild-type protein also shows some distribution in the plasma membrane.
Overexpression of ELA2 in Neutropenia Caused by Gfi1 Mutations
Gfi1 encodes a zinc finger transcriptional repressor oncoprotein identified in a retroviral screen for interleukin (IL)-2 growth-independence of lymphomas. It regulates a subset of genes governing myeloid differentiation, including ELA2.16 Gene targeting unexpectedly revealed neutropenia in Gfi1-deficient mice. Consequently, a screen of 105 neutropenic individuals (49 with SCN and 56 with “nonimmune chronic idiopathic neutropenia of adults” [NI-CINA], incorporating milder neutropenia diagnosed as an adult) lacking ELA2 mutations led to the identification of two different, heterozygous, autosomal dominant Gfi1 zinc finger missense mutations in a family of 3 SCN patients and in an NI-CINA patient.17 One mutation, N382S in the fifth zinc finger, disrupts DNA binding and another, K403R, perturbs a lysine residue that may serve as a site for posttranslational modification with the SUMO polypeptide. SUMO is involved in DNA replication and repair, nuclear-cytoplasmic transport, and subnuclear localization. The clinical features of human Gfi1 mutation resemble the mouse knock-out and, in addition to neutropenia, consist of circulating primitive myeloid cells and B cell and CD4 T cell lymphopenia.
Mistrafficking of Neutrophil Elastase as a Hypothesis for Hereditary Neutropenia
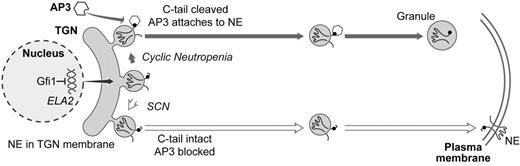
Our group has proposed a somewhat speculative model (Figure 2 ) for how mutations in neutrophil elastase, adaptor protein 3, and Gfi1 cause cyclic and congenital neutropenia and HPS2.8 Neutrophil elastase can exist in both soluble and transmembrane conformations. (In the soluble isoform, the transmembrane segments are folded into disulfide-bonded loops.) Processing of the carboxyl tail exposes a tyrosine-based signal permitting its recognition and transport to granules as an AP3 cargo protein. There are four categories of mutations (Figure 2 ):
The most commonly occurring ELA2 mutations in SCN prematurely terminate neutrophil elastase and delete the AP3 recognition signal, thereby sending neutrophil elastase to the plasma membrane, the default destination for cargo proteins in the absence of AP3.
In HPS2, the absence of AP3 similarly redirects neutrophil elastase to the plasma membrane.
Mutations of Gfi1 lead to overexpression of neutrophil elastase, which overwhelms normal AP3-based granular transport pathways and leads to excessive accumulation in the plasma membrane. Upregulating ELA2 promoter variants may act similarly to cause SCN.8
Mutations that can produce cyclic neutropenia tend to disrupt transmembrane segments, thus favoring a shift toward a soluble form of neutrophil elastase, predominately localizing intraluminally within granules.
It is possible that mistrafficking of neutrophil elastase causes neutropenia in other syndromes in which neutropenia is a component feature (Table 1 ). For example, in the beige mouse model of the human neutropenic disorder Chediak-Higashi syndrome, posttranslational processing and trafficking of neutrophil elastase is disturbed (as reviewed in 8). The autosomal recessive Cohen syndrome of mental retardation, dysmorphic features, and neutropenia results from mutation of COH1, encoding a protein with homology to the yeast protein VPS13, involved in vesicle sorting and intracellular protein transport.18
Finally, as outlandish as it may be to propose that neutrophil elastase leads a secret double life as a transmembrane protein, it may have even more tricks up its sleeve. Neutrophil elastase appears to cleave the PML/RARα fusion gene, the product of the t(15;17) translocation in FAB M3 AML, and ELA2-deficient mice expressing a PML/RARα transgene are resistant to the leukemia that otherwise would develop.19 Even more bizarre, a recent report suggests that neutrophil elastase and chromatin are expulsed together from neutrophils to form net-like, extracellular traps for bacteria.20 Neutrophil elastase is turning up in some surprising places.
II. Acquired Neutropenia
Nancy Berliner, MD*
Yale University School of Medicine, Section of Hematology, WWW428, 333 Cedar Street, New Haven CT 06510
Acquired neutropenia is a relatively rare disorder. This section focuses on primary and secondary immune neutropenia, drug-induced neutropenia, and nonimmune chronic idiopathic neutropenia of adults.
Immune Neutropenia
Immune neutropenia is caused by antibodies directed against neutrophil-specific antigens.1 Antineutrophil antibodies are detected by several methods (Table 2 ) and are directed against a defined group of neutrophil-specific glycoproteins (Table 3 ).2– 5 Immune neutropenia can be alloimmune or autoimmune.
Tests for anti-neutrophil antibodies parallel tests that have been done to detect antibody against red cells. The most widely performed assays are the granulocyte agglutination test (GAT) and the granulocyte immunofluorescence test (GIFT). The GAT was one of the first tests to be developed and depends on the detection of the functional effect of antibody binding resulting in visible agglutination of neutrophils. Results with this assay are reported to have widely varied rates of sensitivity, depending on the procedures used. The GIFT detects neutrophil-bound antibody by binding of glutaraldehyde-fixed neutrophils to fluorescently labeled anti-human IgG. Glutaraldehyde fixation prevents spontaneous fluorescence of neutrophils, which can confound interpretation of the test. The assay can be performed directly on patient neutrophils, although this may be difficult if the patient is profoundly neutropenic. Alternatively, detection of circulating antibodies in serum can be detected by incubating glutaraldehyde-fixed heterologous neutrophils with patient serum and then assaying with fluorescently labeled antihuman IgG. This can be further adapted by using typed neutrophils homozygous for known neutrophil antigens, allowing identification of the target antigen of the patient’s antibody. Fluorescence can be detected by inspection under a microscope or by flow cytometry. More refined tests have been developed, including enzyme-linked immunoassays (ELISA), in which glutaraldehyde-fixed normal neutrophils are fixed onto microtiter plates, incubated in serum, and antibody binding detected with conjugated anti-human IgG. Perhaps the most specific test employs monoclonal antibody-specific immobilization of granulocyte antigens (MAIGA). In this assay, neutrophils with defined antigen specificity are incubated with patient serum and monoclonal antibodies directed against an antigen on the neutrophil surface. Antigens are then isolated on an affinity column coated with anti-mouse IgG and assayed for the presence of human antibody.6 This allows for the direct and simultaneous determination of antibody specificity directed at a variety of antigens.
Tests of neutrophil antibodies are less widely performed and are more difficult to interpret than comparable tests on erythrocytes. Direct assay for the presence of antibody bound to neutrophils, either on patient neutrophils or on heterologous neutrophils incubated with patient serum or plasma, is fraught with difficulty.1 Because neutrophils have abundant Fc receptors, false positive results may complicate interpretation of any of these studies, especially in the presence of high levels of circulating antibodies (as in myeloma or HIV infection) or in the setting of immune complex disease. Furthermore, neutrophils are fragile, tend to aggregate spontaneously in vitro, and often lyse upon manipulation, complicating interpretation of direct assays further. The degree to which these difficulties are estimated to complicate the interpretation of the assays varies among investigators. However, it may explain the presence of detectable antibodies in certain nonneutropenic study populations as well as the lack of correlation between the level of detected antibody and the degree of neutropenia reported by most investigators.
The most common antigens defined to cause immune neutropenia were defined in 1998 by the Granulocyte Antigen Working Party of the International Society of Blood Transfusions5 (Table 3 ). They include the glycoprotein modifications of FcγRIIIb, termed human neutrophil antigen 1 (HNA-1) with three defined alleles (a, b, and c), as well as determinants on gp50-64, 70-95, and CD11a and b, for which no allelic diversity has been defined. In addition, investigators have sought potential nuclear antigens that can be expressed on the cell surface that may play a role in the secondary autoimmune neutropenia seen in patients with systemic lupus erythematosus (SLE). One such antigen that may play an important role in SLE-associated neutropenia is SSB/La, as discussed below with secondary autoimmune neutropenia.
Neonatal Alloimmune Neutropenia
Alloimmune neonatal neutropenia (AINN) is caused by maternal sensitization to paternal neutrophil antigens, resulting in IgG antibodies that are passed via the placenta to the fetus. Newborns develop transient neutropenia that recovers spontaneously after an average of 11 weeks. In general, infectious complications are minor, and most series report no septic deaths.4 When necessary, patients respond well to G-CSF.7
The majority of patients with AINN develop neutropenia in response to antibodies directed against antigens of HNA-1. The HNA antigens are located on the IgG-Fc receptor type IIIb (FcγRIIIb, CD16). Pan-FcγRIIIb antibodies can arise in individuals who lack the receptor altogether as the result of a gene deletion; this is a rare cause of AINN.8 Although FcγRIII deficiency was first discovered in the evaluation of patients who had newborns with AINN, the incidence of the development of antineutrophil antibodies was actually quite low. For example, in a study of 21 patients with FcγRIIIb deficiency, among 3 patients with 10 at-risk pregnancies, there were no newborns with neutropenia.8
Interestingly, the incidence of AINN appears to be lower than the incidence of detectable granulocyte-specific antibodies in the population. In one survey of over 1000 postpartum women, 1.1% demonstrated granulocyte-specific antibodies, but no newborns had neutropenia.4 Whether the detected antibodies were false positives inherent in the test or whether relative clinical silence reflects the biology of antineutrophil antibodies is unknown.
Primary Autoimmune Neutropenia
Primary autoimmune neutropenia (AIN) is a rare disorder. It occurs predominantly in early childhood: one study of 143 patients with AIN demonstrated that of 101 patients with primary AIN, 76 patients were under age 3.2 The average age of onset is 6–12 months, and patients develop a moderate to severe chronic neutropenia. Infections are usually mild to moderate, and serious infections are unusual. Spontaneous remission occurs in 95% of childhood AIN patients over the course of 2 years,9 with one group suggesting that the level of detected antibody is predictive of both infectious complications and time to remission.10 Treatment with prophylactic antibiotics ameliorates infectious complications. Although patients are almost uniformly responsive to G-CSF, chronic administration is usually unnecessary and should be reserved for recurrent or severe infections.11
Tests for antineutrophil antibodies in AIN are nearly always detected by GIFT, but in about 3% of cases may be positive only by GAT. Antibodies are uniformly IgG, and are directed primarily against HNA1 and 2, with rarer cases associated with antibodies to CD11b (HNA-5a) or pan-FCγRIIIb.2,12 This is in contrast to secondary AIN, where pan-FcγRIIIb antibodies are frequently detected, as discussed below.
Primary AIN is rare in adults, where autoimmune neutropenia is more often secondary to underlying rheumatologic syndromes. In adults, infectious complications are also frequently absent or mild, although the disease is usually chronic and spontaneous recovery is unusual.13 Again, since symptoms may be minimal, treatment should be based on the patient’s clinical course rather than on the absolute level of the neutrophil count.
Secondary Autoimmune Neutropenia
Secondary AIN in adults is usually associated with systemic autoimmune disease, predominantly rheumatoid arthritis (RA) and SLE.14 Neutropenia in RA is usually attributable either to Felty’s syndrome (FS) or to LGL leukemia. LGL is discussed in detail in Section III.
Felty’s syndrome
FS typically occurs in patients with longstanding RA associated with end-organ manifestations of RA, including pulmonary fibrosis, vasculitis, rheumatoid nodules, and splenomegaly. Patients may also have Sjögren’s syndrome. Patients may have considerable morbidity from bacterial infection and may in rare cases succumb to overwhelming sepsis.14
Laboratory evaluation of patients with FS demonstrates high levels of rheumatoid factor, circulating immune complexes, and hypergammaglobulinemia. In addition, many patients may be antinuclear antibody (ANA) positive. Ninety percent of patients with FS are HLA-DR4+. Interestingly, patients with LGL leukemia share this incidence of HLA-DR4. This and other pathophysiologic features of the disease have prompted some investigators to suggest that FS and LGL leukemia represent a spectrum of the same disease process.15 This and the pathophysiology of the disease are discussed in detail in Section III.
SLE-associated neutropenia
Neutropenia occurs in approximately half of patients with SLE. It is rarely severe and serves more as a marker of disease activity than as a clinically important complication. Neutropenia has little impact on the course of the disease and does not appear to predispose to an increase in infectious complications. The incidence of infectious complications is more reflective of immunosuppressive therapy than the height of the neutrophil count.14
Neutropenia in SLE has been attributed to neutrophil-specific antibodies, to increased apoptosis of neutrophils, and to decreased marrow neutrophil production. All of these effects appear to be antibody-mediated. Increased neutrophil-associated IgG has been detected in half of patients diagnosed with SLE, but not all patients are neutropenic.16 This further supports the observation that interpretation of increased neutrophil-associated IgG is especially difficult in the presence of immune complex disease. Both immune complexes and neutrophil antigen-specific antibodies have been implicated in the pathogenesis of SLE-associated neutropenia, but the correlation between laboratory testing and clinical neutropenia is poor.
Some investigators have hypothesized that antinuclear antibodies may crossreact with neutrophil surface antigens, either because of crossreactive epitopes or because the nuclear antigens themselves are expressed on the cell surface. Neutropenia in SLE has been hypothesized to be pathogenetically related to the presence of anti-SSA (Ro) and anti-SSB (La) antibodies. Anti-Ro antibodies have been shown to bind a crossreacting antigen on the neutrophil cell surface and to fix complement. In another study,17 immunoscreening of a leukocyte expression library identified La as an antigen bound by antineutrophil-positive sera from patients with SLE; this too was demonstrated to increase neutrophil apoptosis, as well as decreasing phagocytosis and increasing IL-8 production.18 Finally, some antibodies have been demonstrated to be reactive against early myeloid progenitors, leading to decreased neutrophil production.19
Secondary AIN in childhood
Secondary AIN in children is rare and may be associated with autoimmune lymphoproliferative syndrome (ALPS). This disorder is caused by heterozygous mutations in the fas gene, leading to abnormalities of lymphoid apoptosis. ALPS is associated with autoimmune cytopenias in association with adenopathy and splenomegaly. Patients have increased numbers of circulating double negative (CD4−, CD8−) T cells. ALPS is associated with a markedly increased incidence of non-Hodgkin’s lymphoma.20
Drug-Induced Neutropenia
Drug-induced neutropenia is an idiosyncratic reaction that results in profound neutropenia or agranulocytosis.21 Unlike the chronic immune neutropenias, which have a surprisingly low rate of morbidity and mortality, drug-induced neutropenia is associated with a high rate of infectious complications and has a mortality rate of approximately 10%.22,23 The most common drugs associated with agranulocytosis are antithyroid medications and sulfonamides (Table 4 ).
The pathogenesis of drug-induced neutropenia is poorly understood. Investigation is limited because cases are rare, sporadic, and transient. In some cases, anti-neutrophil antibodies are detected and have been characterized as both autoantibodies and drug-dependent antibodies detectable only in the presence of the offending drug.24 Some of these antibodies have been demonstrated to bind complement. In the setting of Graves’ disease, antineutrophil antibodies have been associated with an antigen that is crossreactive with thyroid-stimulating hormones (TSH)25 as well as with antigens related to antineutrophil cytoplasm antibodies (ANCA).26
Clozapine-induced agranulocytosis has a unique etiology that appears to be genetically determined. Clozapine is associated with a high rate of agranulocytosis, which can be seen in 1% of patients receiving the drug. There is no evidence for an immune etiology. It is thought to be caused by accumulation of nitrenium ion, a metabolite of clozapine, which in turn causes depletion of ATP and reduced glutathione, rendering the neutrophils highly susceptible to oxidant-induced apoptosis.27 The reaction is linked to the MHC locus and has been most closely associated with polymorphism of the tumor necrosis factor (TNF) genes, which are in linkage disequilibrium with human leukocyte antigen (HLA) alleles.28,29
Nonimmune Chronic Idiopathic Neutropenia
A subset of patients with chronic neutropenia has no evidence of immune-mediated disease. NI-CINA is an acquired syndrome associated with chronic neutropenia, normal marrow cytogenetics, and no evidence for underlying autoimmune disease, nutritional deficiency, or myelodysplasia.30 The marrow findings are variable, ranging from a hypoplastic to a hyperplastic myeloid series. The clinical course is usually quite benign, and many of these patients are diagnosed by examination of routine laboratory tests in the absence of any history of infection or other symptoms.13
The pathogenesis of NI-CINA is poorly understood. The pathophysiology of the disease has been hypothesized to reflect decreased neutrophil production, excessive neutrophil margination, and increased peripheral neutrophil destruction. There has been a suggestion that patients with this syndrome have an undiagnosed underlying inflammatory illness, with increased production of inflammatory cytokines. A recent study by Papadaki et al concentrated on those patients with myeloid hypoplasia on marrow examination. Investigation of marrow progenitors and colony forming unit– granulocyte macrophage (CFU-GM) production documented a selective decrease in CD34+/CD33− myeloid progenitors, with evidence for increased fas-mediated apoptosis as the cause for the reduction in CFU-GM. In addition, they demonstrated that stromal cell layers produced increased amounts of TNF.31,32 Finally, the same group has previously demonstrated that increased risk of developing NI-CINA may be related to HLA phenotype.33 It should be noted that the TNF locus lies within the HLA cluster, and the apparent HLA predilection for the development of clonazapine-induced agranulocytosis actually was more tightly linked to TNF microsatellite polymorphisms.28 Hence, it is tempting to speculate that perhaps a predisposition to NI-CINA is also linked to polymorphisms of the same locus. Finally, two patients with NI-CINA have been found to have heterozygous mutations in Gfi-1, as described in Section I.34
Treatment of Neutropenia
The main issue in the treatment of acquired neutropenia focuses on the use of G-CSF. Nearly all studies demonstrate that in primary and secondary immune neutropenia and in NI-CINA, response to G-CSF is rapid and occurs in nearly all patients.11,21 Treatment is frequently unnecessary, however, and is usually reserved for recurrent or serious infections. In drug-induced neutropenia, most studies have shown that G-CSF shortens the time to neutrophil recovery, although several authors have commented that evidence-based data are lacking to justify its use.11,35 One study suggested that drug-induced neutropenia induced by antithyroid medication does not improve time-to-neutrophil recovery, but the dose used was low and the study size was small.36 Given the rarity and heterogeneity of drug-induced neutropenia, it seems unlikely that an evidence-based algorithm for G-CSF use will ever be validated. However, because drug-induced neutropenia is an acute, life-threatening complication of therapy, with a mortality of 10%, the demonstrated safety and apparent effectiveness of G-CSF in hastening neutrophil recovery justifies its use in this setting.
III. Large Granular Lymphocyte Leukemia and Neutropenia
Eric J. Burks, MD, Gordon Starkebaum, MD, and Thomas P. Loughran, Jr, MD*
Pennsylvania State Cancer Institute, H072, 500 University Drive, PO Box 850, Hershey PA 17033
T cell large granular lymphocyte leukemia (T-LGL) is a clonal disorder of cytotoxic T lymphocytes (CTL).1,2 This disorder manifests itself most commonly with severe chronic neutropenia, although mild to moderate anemia is not an infrequent finding. Less commonly, adult onset cyclic neutropenia, pure red cell aplasia, and immune-mediated thrombocytopenia may be the presenting hematologic feature. The establishment of T-LGL as a leukemia rather than a reactive disorder was based on nonrecurring chromosomal abnormalities observed in some patients with T-LGL in conjunction with the presence of tissue infiltration in the bone marrow, spleen, and liver.2 Despite these findings, T-LGL is an indolent disorder that responds well to immunosuppressive therapies.
Clinical Features and Diagnosis
T-LGL represents only 4% of the chronic lymphoproliferative disorders3; however, it represents the most frequent T cell malignancy.4 Patients tend to be older, with a median age of 60; there is an equal male/female distribution.5 Infection and fever are the presenting features in 20%–40% of patients. As the infection is related to neutropenia, common locations of infection are the skin, oropharynx, and perirectal regions. B symptoms (fever, night sweats, and weight loss) occur in 20%–30% of patients, while about 1/3 are asymptomatic. Organomegaly involving the spleen (20%–50%) and liver (10%–20%) is typical, while lymphadenopathy and skin involvement are uncharacteristic. Typical bone marrow findings demonstrate increased numbers of cytotoxic T cells in an interstitial and sinusoidal distribution. The diagnosis should be suspected in patients with cytopenia and blood smears should be carefully examined for LGL. All patients suspected of having T-LGL should have peripheral blood flow cytometry and PCR for T cell receptor (TCR) gene rearrangement. The diagnosis of T-LGL is confirmed by demonstrating the characteristic immunophenotype of CD3+, CD8+, CD16+, and CD57+ and finding a clonal TCR gene rearrangement.
Pathogenesis
Central to the understanding of T-LGL leukemogenesis is the notion that T-LGL cells are antigen activated in vivo.6 Multiple levels of evidence support this hypothesis, including immunophenotypic data demonstrating CD45RO, HLA-DR, perforin, and CD57 expression. Functionally, T-LGL display non-MHC–restricted cytotoxicity after anti-CD3 monoclonal antibody stimulation. Molecular evidence of common usage of TCR-β junctional motifs suggests antigenic pressure in the clonal evolution of the disease.7,8 Finally, DNA microarray analysis has demonstrated upregulated proteases while protease inhibitors are downregulated. While the source of the antigenic activation is still uncertain, some patients with T-LGL show serum reactivity to gag p24 and env p21e of HTLV-I virus but do not have prototypical HTLV infection. These findings suggest a possible infection with a retrovirus with homology to HTLV-I as an underlying drive for the leukemic process.
In contrast to normal CTL, T-LGL cells are resistant to Fas-mediated apoptosis. Unlike the mechanism of autoimmune lymphoproliferative disease, no mutation in the death domain of Fas in T-LGL has been observed. Alternatively, constitutive activation of STAT3 with upregulation of mcl-1 has been implicated in apoptotic resistance. Furthermore, novel splice variants of soluble Fas molecules resulting in “decoy receptors” allow T-LGL cells to circumvent immunosurveillance. A more detailed discussion of the leukemogenesis of T-LGL is provided in a recent review.6
Chronic Neutropenia in Rheumatoid Arthritis: Are T-LGL/RA and Felty’s the Same Disease?
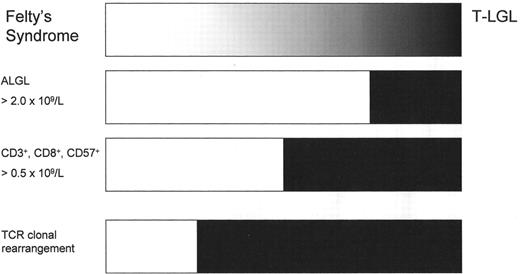
T-LGL is frequently associated with autoimmune disorders. RA is the most common associated disorder, with T-LGL occurring in 25%–33% of patients.9 Similar to T-LGL, FS is likewise characterized by neutropenia with variable splenomegaly in conjunction with RA. Indeed it is our opinion that these disorders are part of a single disease process. The original diagnostic criteria for lymphoproliferative disorder of granular lymphocytes (including both T and NK forms of LGL) required an absolute large granular lymphocytosis in excess of 2.0 × 109/L. Since that time, patients with lower numbers of LGLs have been shown to have similar clinical courses and response to therapy as those meeting the former diagnostic criteria.10 Previously published historical studies had reported a low occurrence (8%) of LGL counts < 1.0 × 109/L, while more recent studies have reported patients with T-LGL having LGL counts < 0.5 × 109/L in 25%–30% of cases.11,12 In such studies, the diagnosis is based on multicolor flow cytometric immunophenotyping and molecular analysis of the TCR genes. As would be expected from this shift, disorders originally diagnosed as FS in the past may well be classified as T-LGL in the present (Figure 3 ).
Given the subtleties in diagnostic distinction, it is not surprising that these disorders share a common immunogenetic link. Patients with FS and T-LGL with RA have a similar frequency of the HLA-DR4 allele (80%–90%).13 However, T-LGL patients without RA lack the elevated allele frequency and are similar to normal racially matched controls (33%).14 More recently, the immunohistochemical evaluation of the T cell infiltrate within the bone marrow of patients with T-LGL have shown the quantity and pattern of infiltration to be specific to the diagnosis11,12; however, we have observed similar patterns of T cell infiltration in patients with FS as those of T-LGL (unpublished observations). What can safely be said about FS and T-LGL with RA is that they appear to be related disorders whose distinction is somewhat arbitrary. These disorders can be separated by the presence of clonal TCR gene rearrangements in T-LGL, but not in FS, recognizing that clonally expanded CD3+, CD57+ lymphocytes (LGL) may be detected using sensitive techniques in nonneutropenic patients with RA as well as some normal elderly individuals. What is not understood yet is why patients with RA are especially prone to develop clonal expansions of T-LGL. Nevertheless, it is likely that the pathogenesis of neutropenia in these disorders overlaps as discussed below.
Pathogenesis of Neutropenia in Felty’s Syndrome and T-LGL
Early studies into the mechanisms of neutropenia in FS revealed two distinct groups of patients. One group exhibited evidence of humoral mediated destruction (60%).15 These patients had either monomeric (non-complexed) immunoglobulin, presumed to be neutrophil autoantibodies and/or immune complexes of varying sizes.16 Alternatively, about 40% of patients lacked neutrophil-bound immunoglobulin and were reported to have depressed granulocyte colony growth in bone marrow co-culture experiments using patient lymphocytes.15 It was these early studies of FS that led to a variety of published studies more specifically addressing each of these pathogenetic mechanisms.
Neutrophil autoantibodies
While neutrophil autoantibody-mediated autoimmune neutropenia is perhaps the easiest mechanism to conceptualize, it is the most difficult to evaluate in the laboratory. Unlike red cells in autoimmune hemolytic anemia, granulocytes are more difficult to isolate and therefore a direct “Coombs test” for neutrophils is not practical. As such, indirect assays using patient serum and banked normal neutrophils, namely the granulocyte immunofluorescent test (GIFT) and granulocyte agglutination test (GAT), represent the screening methods of choice. To add further complexity, neutrophils, unlike red cells, express HLA-antigens as well as Fc-receptors for immunoglobulin, which limits the ability of the test to distinguish between neutrophil autoantibodies, HLA alloantibodies, and immune complexes. The antibody-dependent lymphocyte-mediated granulocytotoxicity test (ADLG) can detect neutrophil-bound monomeric immunoglobulin and, with prior absorption using random pooled platelets, can distinguish between confounding HLA alloantibodies and neutrophil autoantibodies. When a battery of such tests is performed with FS sera, 77% have bound immunoglobulin via either the GIFT or GAT tests. Based on the results of the ADLG test, showing positivity on only 14%, the bound antibody appears to be made of predominantly immune complexes. Of those with positive ADLG test, all were inhibited by platelet absorption indicative of HLA alloantibodies, which implies that true neutrophil autoantibodies are not present.17 A similar analysis of 5 patients with T-LGL without RA revealed low levels of immune complexes in only 1 patient and no definitive neutrophil autoantibodies in the remaining 4 patients.18 Testing for antineutrophil antibodies is not recommended for routine clinical practice.
Because of the complexity of this approach, newer techniques have been employed to assess neutrophil-specific autoantibodies. Using an antibody phage display library made from the RNA of FS bone marrow lymphocytes, a putative neutrophil autoantibody was discovered. Panning against a myeloid antigen expression library with this antibody revealed a protein identified as eukaryotic elongation factor-1A-1 (eEF1A-1). An ELISA test, using this factor to screen sera of patients with FS, revealed positivity in 66% of 62 patients. The antibody does not appear to be specific to FS, as positive results were also seen in RA without neutropenia (23%) and SLE (9%), but not normal controls.19 Similarly, adults with atopic dermatitis without neutropenia also express antibody to eEFA1-1.20 The frequency of eEFA1-1 autoantibodies has yet to be tested in patients with T-LGL.
Immune complexes and neutropenia
Immune complexes represent another humoral mechanism by which neutropenia may occur. Using column separation, ultracentrifugation, and C1q binding assays, patients with FA have increased numbers of immune complexes as compared to normal controls and patients with RA without neutropenia. Furthermore, the neutrophil-bound immune complexes were of varying sizes and were at least partially composed of rheumatoid factor.16 The binding of immune complexes to neutrophils leads to several important physiologic sequelae. First, neutrophils become activated after binding immune complexes, and the degree of activation appears to be dependent on the type of immune complex encountered. When neutrophil activation is measured by chemiluminescence, activation is most intense after incubation with precipitated immune complex followed by soluble immune complex with monomeric (non-complexed) IgG providing the least activation. Not surprisingly then, neutrophils incubated with sera from patients with FS showed greater activation than with sera from patients with RA and normal controls.21
Neutrophil activation results in cellular changes that account for neutropenia. Neutrophils incubated with sera from patients with FS, but not from patients with RA, display increased adherence to endothelial cells in vitro.22 This mechanism of increased endothelial adherence may explain the occurrence of transient neutropenia in mice after injection with sera from patients with FS and to a lesser degree with sera from RA patients, while injection of sera from normal controls results in transient elevations of the neutrophil counts. Necropsy of mice following injection with FS sera confirmed that the increased endothelial adherence occurs in vivo, showing increased neutrophil margination with deposition of IgG, IgA, and IgM within vascular beds.23 These observations suggest that immune complexes may induce neutropenia by altering the distribution of neutrophils into the marginating pool.
Finally, some types of immune complexes appear to induce neutrophil apoptosis. The characteristics of the immune complexes appear to be critical in this role, as precipitated immune complexes induce apoptosis while soluble immune complexes decrease apoptosis. Neutrophils can bind immune complexes by means of Fc-receptors, of which FcRγ-II (CD32) but not FcRγ-III (CD16) appears critical in the pathway of neutrophil apoptosis. Surprisingly, CCR3 (CD11b/CD18) is not essential in precipitated immune complex-induced apoptosis. Moreover, precipitated immune complex-induced apoptosis is independent of the Fas/Fas-L pathway and is instead dependent upon reactive oxygen intermediates, in particular hydrogen peroxide.24
Cell-mediated mechanisms of neutropenia
Cell-mediated mechanisms are of critical importance in the pathogenesis of neutropenia in T-LGL. While the mechanisms responsible for T-LGL cell survival result from inhibition of signaling pathways leading to Fas/FasL-induced apoptosis, the Fas/FasL system is also intimately related to neutropenia in T-LGL. As previously discussed, T-LGL cells constitutively express Fas-ligand (FasL) on their cell surface, whereas normal T and NK cells express FasL only after activation. Moreover, the FasL expressed on T-LGL cells can produce cytotoxicity against W4 cells in vitro, and this cytotoxicity appears to be independent of the perforin pathway of cytotoxicity.25 The FasL receptor, Fas (CD95), is expressed ubiquitously on a variety of cells including normal granulocytes. Neutrophils express higher levels of Fas than eosinophils or monocytes. It therefore follows that neutrophils are more susceptible to Fas-mediated apoptosis than eosinophils or monocytes when treated with Fas-activating antibody (CH-11).26 Others have noted that proinflammatory cytokines such as FasL and INF-γ may inhibit myeloid progenitors in patients with idiopathic chronic neutropenia.27
While FasL is constitutively expressed on the surface of T-LGL cells, matrix metalloproteinases can cleave this protein and generate soluble FasL (sFasL). In support of this process is marked elevation of sFasL in the sera of patients with T-LGL, NK-LGL, and NK-lymphomas but not other leukemias.25 Indeed, sera from patients with T-LGL with elevated sFasL induce neutrophil apoptosis in vitro in a similar fashion to Fas-activating antibody (CH-11). Furthermore, clinical response to therapy in patients with T-LGL is associated with a decrease in serum sFasL.28 It remains unclear, however, what significance sFasL plays in the mechanism of neutropenia in vivo as disorders such as NK-LGL and NK-lymphomas also exhibit high levels of sFasL but are characterized by severe pancytopenia and hepatic dysfunction rather than the isolated neutropenia observed in T-LGL. Furthermore, mice injected with high levels of sFasL experience hepatic necrosis, which is uncharacteristic of T-LGL.29 As such, the pattern of neoplastic cell infiltrate better correlates with the distribution of tissue dysfunction in these disorders, suggesting bound FasL and perhaps the local paracrine effects of sFasL contribute to neutropenia in T-LGL and FS, while the diluted serum sFasL represents a useful test in monitoring disease activity.
Summary of neutropenic mechanisms in T-LGL and Felty’s syndrome
In summary, the mechanisms of neutropenia can be attributed to problems with production, distribution, and destruction. Unlike many of the congenital neutropenias described elsewhere in this section, neutrophil production defects are not typical in T-LGL/FS. Examination of the bone marrow myeloid elements in patients with T-LGL usually reveals mild hypercellularity with left-shifted myeloid maturation.11,12 This assessment would suggest that neutropenia is at least in part a result of peripheral destruction. However, bone marrow coculture experiments with the lymphocytes from some patients with FS results in diminished granulocyte colony growth.15 It appears likely then that both peripheral and intramedullary destruction of neutrophils are responsible for neutropenia in T-LGL. Neutrophil destruction may occur by means of immune complex induction via FcRγ-II (CD32) activation leading to death by reactive oxygen intermediates. Fas-mediated apoptosis resulting from direct contact of T-LGL cells expressing FasL or local paracrine effects of sFasL released by the functions of matrix metalloproteinases also are important in pathogenesis of neutropenia. Finally, measured neutropenia may result from increased margination as a result of precipitated immune complex activation of neutrophils (Figure 4; see Color Figures, page 508).
While these studies have approached the mechanisms of neutropenia in T-LGL and FS independently, it should be clear that the variability in diagnostic criteria utilized renders these distinctions meaningless. Furthermore, it is likely that any or all the above mechanisms are involved in the pathogenesis of neutropenia in a given patient with T-LGL. In patients with active rheumatologic disease, immune complexes may play a dominant role in inducing neutrophil margination and apoptosis via reactive oxygen intermediates. In such patients, only small populations of clonal cytotoxic T cells may exist and their contribution to neutropenia may be less pronounced. Alternatively, patients with marked expansions of clonal FasL-bearing T-LGL cells may experience neutropenia as a result of Fas-mediated apoptosis in the absence of active rheumatological disease. Still, patients with relatively inactive RA with only moderate numbers of clonal T-LGL cells may experience severe neutropenia, and the mechanism of this cytopenia is probably a result of the combined mechanisms described herein. The mechanism of adult onset cyclic neutropenia in T-LGL remains unknown.
Management
Indications for therapy of T-LGL include severe neutropenia < 500/mL or recurrent infections due to chronic, less severe neutropenia. Symptomatic or transfusion-dependent anemia are other reasons for initiating treatment.5 The majority of patients with T-LGL (75%) will require therapy over the course of their disease, although rare cases will spontaneously remit. Methotrexate 10 mg/m2/week orally induces complete remission in 50% of patients; however, it is likely that indefinite treatment is required to prevent relapse. It is important to note that several months of therapy are generally required before counts improve. Cyclosporine A represents an alternative therapy to methotrexate. In a study of 25 patients, 50% had response to therapy, with 24% gaining complete remission. It is interesting to note that therapeutic response to cyclosporine A is related to HLA-DR4 haplotype, which is common in patients with FS and T-LGL with RA.30 In addition, patients with concurrent T-LGL and myelodysplasia have a lower response to cyclosporine A than patients with T-LGL alone.31 Cyclophosphamide has been used orally to treat T-LGL with good response.32 Prednisone in combination with cyclophosphamide appears to increase the duration of response compared to prednisone alone. Overall response to therapy with prednisone and cyclophosphamide is 66% with a median duration of 32 months. Two prospective therapeutic trials are currently underway. The Eastern Cooperative Oncology Group (ECOG) is evaluating the efficacy of oral methotrexate 10 mg/m2/week with crossover to cyclophosphamide in non-responders. The Cancer and Leukemia Group B (CALGB) is testing cyclosporine A orally 2 mg/kg/q12 hours as front-line therapy. Indications for both trials are neutropenia or symptomatic or transfusion-dependent anemia. Because immunosuppressive therapies slowly correct neutropenia, hematopoietic growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) or G-CSF may induce more rapid correction of the neutrophil count in patients with severe neutropenia. A national registry for LGL leukemia has been established at the Penn State Cancer Institute in order to define the natural history and prognosis of this disease. Clinicians with patients suspected to have this disorder are encouraged to contact us (tloughran@psu.edu).
Selected neutropenic syndromes.
| Syndrome . | Inheritance . | Gene . | Clinical Features . | Animal Model . | Animal Model Phenotype . |
|---|---|---|---|---|---|
| Abbreviations: SCN, severe congenital neutropenia; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; G-CSF, granulocyte colony-stimulating factor; COPD, chronic obstructive pulmonary disease | |||||
| Cyclic neutropenia | Autosomal Dominant | ELA2 | Alternate 21 day cycling of neutrophils and monocytes | Mouse knock-out |
|
| Autosomal Dominant | ELA2 (35–84%) |
| Mouse knock-in (V72M) | No obvious phenotype | |
| SCN | Autosomal Dominant | Gfi1 (rare) |
| Mouse knock-out | Resembles human Gfi1 deficiency |
| Sex-linked | wASP (rare) | Neutropenic variant of Wiskott-Aldrich syndrome | Mouse knock-out |
| |
| Autosomal Dominant | G-CSFR (rare) |
| Mouse knock-out |
| |
| Kostmann syndrome | Autosomal Recessive | Unknown | Static neutropenia without MDS or AML | ||
| Hermansky Pudlak syndrome, type 2 | Autosomal Recessive | AP3B1 |
| Gray collie syndrome of dogs |
|
|
| ||||
| Chediak-Higashi syndrome | Autosomal Recessive | LYST |
| Blue-smoke Persian cat |
|
|
| ||||
| Barth syndrome | Sex-linked | TAZ |
| ||
| Cohen syndrome | Autosomal Recessive | COH1 |
| ||
| Syndrome . | Inheritance . | Gene . | Clinical Features . | Animal Model . | Animal Model Phenotype . |
|---|---|---|---|---|---|
| Abbreviations: SCN, severe congenital neutropenia; MDS, myelodysplastic syndrome; AML, acute myeloid leukemia; G-CSF, granulocyte colony-stimulating factor; COPD, chronic obstructive pulmonary disease | |||||
| Cyclic neutropenia | Autosomal Dominant | ELA2 | Alternate 21 day cycling of neutrophils and monocytes | Mouse knock-out |
|
| Autosomal Dominant | ELA2 (35–84%) |
| Mouse knock-in (V72M) | No obvious phenotype | |
| SCN | Autosomal Dominant | Gfi1 (rare) |
| Mouse knock-out | Resembles human Gfi1 deficiency |
| Sex-linked | wASP (rare) | Neutropenic variant of Wiskott-Aldrich syndrome | Mouse knock-out |
| |
| Autosomal Dominant | G-CSFR (rare) |
| Mouse knock-out |
| |
| Kostmann syndrome | Autosomal Recessive | Unknown | Static neutropenia without MDS or AML | ||
| Hermansky Pudlak syndrome, type 2 | Autosomal Recessive | AP3B1 |
| Gray collie syndrome of dogs |
|
|
| ||||
| Chediak-Higashi syndrome | Autosomal Recessive | LYST |
| Blue-smoke Persian cat |
|
|
| ||||
| Barth syndrome | Sex-linked | TAZ |
| ||
| Cohen syndrome | Autosomal Recessive | COH1 |
| ||
Detection of antineutrophil antibodies.
| Granulocyte immunofluorescence test (GIFT) | Detection of neutrophil-bound antibody by binding of glutaraldehyde- fixed patient neutrophils to fluorescently labeled anti-human IgG |
| Granulocyte indirect immunofluorescence test (GIIFT) | Detection of serum antibody by incubation with normal neutrophils, or specifically phenotyped neutrophils, with subsequent staining with fluorescently labeled anti-human IgG |
| Granulocyte agglutination test (GAT) | Incubation of granulocytes with patient sera and microscopic evaluation of agglutination |
| Enzyme linked immunoassays (ELISA) | Detection of antibody in serum by binding to glutaraldehyde-fixed normal neutrophils on microtiter plates with detection by conjugated anti-human IgG |
| Monoclonal antibody-specific immobilization of granulocyte antigens (MAIGA) | Simultaneous incubation of defined neutrophils with patient serum and monoclonal antibodies directed against neutrophil-specific antigens. Antigens are then immobilized on column coated with anti-mouse ab, and assayed for presence of human antibody |
| Granulocyte immunofluorescence test (GIFT) | Detection of neutrophil-bound antibody by binding of glutaraldehyde- fixed patient neutrophils to fluorescently labeled anti-human IgG |
| Granulocyte indirect immunofluorescence test (GIIFT) | Detection of serum antibody by incubation with normal neutrophils, or specifically phenotyped neutrophils, with subsequent staining with fluorescently labeled anti-human IgG |
| Granulocyte agglutination test (GAT) | Incubation of granulocytes with patient sera and microscopic evaluation of agglutination |
| Enzyme linked immunoassays (ELISA) | Detection of antibody in serum by binding to glutaraldehyde-fixed normal neutrophils on microtiter plates with detection by conjugated anti-human IgG |
| Monoclonal antibody-specific immobilization of granulocyte antigens (MAIGA) | Simultaneous incubation of defined neutrophils with patient serum and monoclonal antibodies directed against neutrophil-specific antigens. Antigens are then immobilized on column coated with anti-mouse ab, and assayed for presence of human antibody |
Antineutrophil antibodies.
| Antigens . | Previous Nomenclature . | Glycoprotein . | Allele Frequency (%) . |
|---|---|---|---|
| HNA-1a | NA1 | FcγIIIb (CD16) | 58 |
| HNA-1b | NA2 | FcγIIIb (CD16) | 88 |
| HNA-1c | SH, NA3 | FcγIIIb (CD16) | 5–38 |
| HNA-2a | NB1 | CD177(gp50-64) | 94 |
| HNA-3a | 5b | Gp70-95 | 97 |
| HNA-4a | MART | CD11a | 99 |
| HNA-5a | OND | CD11b | 96 |
| Antigens . | Previous Nomenclature . | Glycoprotein . | Allele Frequency (%) . |
|---|---|---|---|
| HNA-1a | NA1 | FcγIIIb (CD16) | 58 |
| HNA-1b | NA2 | FcγIIIb (CD16) | 88 |
| HNA-1c | SH, NA3 | FcγIIIb (CD16) | 5–38 |
| HNA-2a | NB1 | CD177(gp50-64) | 94 |
| HNA-3a | 5b | Gp70-95 | 97 |
| HNA-4a | MART | CD11a | 99 |
| HNA-5a | OND | CD11b | 96 |
Drugs most commonly causing agranulocytosis.
| Antithyroid medications | Carbamizole Methimazole Thiouracil |
| Antibiotics | Cephalosporins Penicillins Sulfonamides Chloramphenicol |
| Anticonvulsants | Carbamazapine Valproic acid |
| Antithyroid medications | Carbamizole Methimazole Thiouracil |
| Antibiotics | Cephalosporins Penicillins Sulfonamides Chloramphenicol |
| Anticonvulsants | Carbamazapine Valproic acid |
Fit ofELA2mutations into one of three proposed functional categories.
The linear sequence of the protein is marked with respect to the processed pre-pro amino and carboxyl termini, predicted transmembrane domains (TM-1 and TM-2), cryptic transmembrane (TM-cryptic) domain predicted as a result of some mutations, and proposed recognition site of the AP3 mu subunit. Squares represent mutations exclusively causing severe congenital neutropenia (SCN). Circles indicate mutations found in cyclic neutropenia patients (but that may also appear in SCN patients). Horizontal lines depict deletions. Lines connected by right angles reveal disulfide bonds that generally bracket predicted transmembrane domains, with mutated cysteine residues at their corners. Missense mutations generally aligning with transmembrane domains are colored black. Mutations destroying disulfide bonds are shaded light gray. Chain terminating nonsense and frameshift mutations that delete the AP3 mu recognition signal are shaded dark gray. Each mutation, compiled from reference 10 and the author’s unpublished data, is shown once. Mutations unaccounted for by this classification scheme are listed in the text, but not charted.
Fit ofELA2mutations into one of three proposed functional categories.
The linear sequence of the protein is marked with respect to the processed pre-pro amino and carboxyl termini, predicted transmembrane domains (TM-1 and TM-2), cryptic transmembrane (TM-cryptic) domain predicted as a result of some mutations, and proposed recognition site of the AP3 mu subunit. Squares represent mutations exclusively causing severe congenital neutropenia (SCN). Circles indicate mutations found in cyclic neutropenia patients (but that may also appear in SCN patients). Horizontal lines depict deletions. Lines connected by right angles reveal disulfide bonds that generally bracket predicted transmembrane domains, with mutated cysteine residues at their corners. Missense mutations generally aligning with transmembrane domains are colored black. Mutations destroying disulfide bonds are shaded light gray. Chain terminating nonsense and frameshift mutations that delete the AP3 mu recognition signal are shaded dark gray. Each mutation, compiled from reference 10 and the author’s unpublished data, is shown once. Mutations unaccounted for by this classification scheme are listed in the text, but not charted.
Proposed model of normal and pathological processing and transport of neutrophil elastase.
The product of the ELA2 gene, neutrophil elastase (NE), is shown in the membrane of the trans Golgi network (TGN). If the C-terminus is cleaved, then NE normally interacts with AP3 (via the mu subunit of AP3 recognizing a tyrosine residue, depicted by a black dot, in the cytoplasmic tail of NE), which transports it to granules, where NE re-equilibrates into a soluble form. If the C-terminus remains intact, then interaction with AP3 is blocked and NE is routed to a default destination in the plasma and other membranes. In SCN, deletions of the AP3 recognition signal or missense mutations that favor a transmembrane configuration of NE increase trafficking through the membrane pathway. Mutations of AP3 itself, as in canine cyclic neutropenia and HPS2, act similarly. In cyclic neutropenia, mutations disrupting the transmembrane segments favor a shift in equilibrium to soluble forms accumulating in granules. Mutations of Gfi1 lead to overexpression of ELA2, overwhelming normal AP3-mediated trafficking and diverting excess NE to membranes.
Proposed model of normal and pathological processing and transport of neutrophil elastase.
The product of the ELA2 gene, neutrophil elastase (NE), is shown in the membrane of the trans Golgi network (TGN). If the C-terminus is cleaved, then NE normally interacts with AP3 (via the mu subunit of AP3 recognizing a tyrosine residue, depicted by a black dot, in the cytoplasmic tail of NE), which transports it to granules, where NE re-equilibrates into a soluble form. If the C-terminus remains intact, then interaction with AP3 is blocked and NE is routed to a default destination in the plasma and other membranes. In SCN, deletions of the AP3 recognition signal or missense mutations that favor a transmembrane configuration of NE increase trafficking through the membrane pathway. Mutations of AP3 itself, as in canine cyclic neutropenia and HPS2, act similarly. In cyclic neutropenia, mutations disrupting the transmembrane segments favor a shift in equilibrium to soluble forms accumulating in granules. Mutations of Gfi1 lead to overexpression of ELA2, overwhelming normal AP3-mediated trafficking and diverting excess NE to membranes.
Relative frequencies of Felty’s syndrome and T cell large granular lymphocyte leukemia (T-LGL) based on selected diagnostic criteria.
The number of CD3+, CD8+, CD57+ cells is measured by flow cytometry. TCR clonal rearrangement is assessed by PCR.
Abbreviations: ALGL, absolute large granular lymphocytosis
Relative frequencies of Felty’s syndrome and T cell large granular lymphocyte leukemia (T-LGL) based on selected diagnostic criteria.
The number of CD3+, CD8+, CD57+ cells is measured by flow cytometry. TCR clonal rearrangement is assessed by PCR.
Abbreviations: ALGL, absolute large granular lymphocytosis