Abstract
Hemolytic anemia due to immune function is one of the major causes of acquired hemolytic anemia. In recent years, as more is known about the immune system, these entities have become better understood and their treatment improved. In this section, we will discuss three areas in which this progress has been apparent.
In Section I, Dr. Peter Hillmen outlines the recent findings in the pathogenesis of paroxysmal nocturnal hemoglobinuria (PNH), relating the biochemical defect (the lack of glycosylphosphatidylinositol [GPI]-linked proteins on the cell surface) to the clinical manifestations, particularly hemolysis (and its effects) and thrombosis. He discusses the pathogenesis of the disorder in the face of marrow dysfunction insofar as it is known. His major emphasis is on innovative therapies that are designed to decrease the effectiveness of complement activation, since the lack of cellular modulation of this system is the primary cause of the pathology of the disease. He recounts his considerable experience with a humanized monoclonal antibody against C5, which has a remarkable effect in controlling the manifestations of the disease. Other means of controlling the action of complement include replacing the missing modulatory proteins on the cell surface; these studies are not as developed as the former agent.
In Section II, Dr. Alan Schreiber describes the biochemistry, genetics, and function of the Fcγ receptors and their role in the pathobiology of autoimmune hemolytic anemia and idiopathic thrombocytopenic purpura due to IgG antibodies. He outlines the complex varieties of these molecules, showing how they vary in genetic origin and in function. These variations can be related to three-dimensional topography, which is known in some detail. Liganding IgG results in the transduction of a signal through the tyrosine-based activation motif and Syk signaling. The role of these receptors in the pathogenesis of hematological diseases due to IgG antibodies is outlined and the potential of therapy of these diseases by regulation of these receptors is discussed.
In Section III, Dr. Wendell Rosse discusses the forms of autoimmune hemolytic anemia characterized by antibodies that react preferentially in the cold–cold agglutinin disease and paroxysmal cold hemoglobinuria (PCH). The former is due to IgM antibodies with a common but particular structure that reacts primarily with carbohydrate or carbohydrate-containing antigens, an interaction that is diminished at body temperature. PCH is a less common but probably underdiagnosed illness due to an IgG antibody reacting with a carbohydrate antigen; improved techniques for the diagnosis of PCH are described. Therapy for the two disorders differs somewhat because of the differences in isotype of the antibody. Since the hemolysis in both is primarily due to complement activation, the potential role of its control, as by the monoclonal antibody described by Dr. Hillmen, is discussed.
I. Paroxysmal Nocturnal Hemoglobinuria: Current Understanding of the Biology of PNH
Peter Hillmen, PhD*
Leeds General Infirmary, Great George Street, Leeds LS1 3EX, UK
Paroxysmal nocturnal hemoglobinuria (PNH)1,2 is characterized by chronic intravascular hemolysis that is punctuated by episodes, or paroxysms, during which there is a marked increase in the intensity of hemolysis with macroscopic hemoglobinuria. The other elements of the clinical triad of PNH are venous thrombosis and an association with aplastic anemia. Recent therapeutic developments promise to radically alter the natural history of hemolytic PNH.
PNH is unique because it is an acquired hemolytic anemia due to an intrinsic red cell defect. In 1970 Oni et al first demonstrated that PNH red cells are monoclonal and that the remaining normal red cells are polyclonal. It was clear by the 1980s that the PNH cells were deficient in a large number of cell surface antigens but it was unclear how a single mutation could lead to the deficiency of such a variety of antigens nor how this resulted in the hemolysis characteristic of PNH.
Glycosylphosphatidylinositol-Linked Antigens and PNH
In the 1980s it became clear that a variety of antigens were attached to the cell membrane by a glycolipid structure. This structure is highly preserved throughout evolution with the same basic “backbone” consisting of a phosphatidylinositol, a single glucosamine, three mannoses and an ethanolamine—a glycosylphosphatidylinositol (GPI) structure. The antigens missing from PNH cells are all GPI-anchored to the cell membrane and thus a single mutation disrupting GPI biosynthesis would result in the PNH phenotype. The biosynthetic defect in PNH always affects the transfer of glucosamine from UDP-N-acetyl glucosamine onto phosphatidyl inositol. Kinoshita and his group first cloned the pig-a gene, which was subsequently found to be mutated in all cases of PNH reported to date.3– 5
Intravascular Hemolysis in PNH
The work of both Ham and Dacie in the 1930s first revealed that the hemolysis in PNH was due to the effect of a serum factor on abnormal PNH red cells. Rosse proceeded to show that this factor was complement that when activated led to the intravascular hemolysis of PNH red cells. The characteristic symptoms of PNH—abdominal pain, dysphagia, erectile failure and severe lethargy—can be attributed to the intense intravascular hemolysis and the resulting free plasma hemoglobin. This appears to be due to the absorption of nitric oxide by free hemoglobin and since nitric oxide is critical for smooth muscle function this results in the symptoms described (see below).
The functions of the GPI-linked antigens are extremely varied. At least two are important in the control of complement. Decay accelerating factor (DAF or CD55) controls the early part of the complement cascade by regulating the activity of the C3 and C5 convertases. Thus CD55 deficiency initially appeared to explain the sensitivity of PNH red cells to complement. However, the observation that individuals with inherited CD55 deficiency (Inab- phenotype) did not suffer from hemolysis proved that deficiency of CD55 does not cause the hemolysis in PNH. Membrane inhibitor of reactive lysis (MIRL or CD59) was identified in 1989 and is also GPI-linked. CD59 inhibits terminal complement by preventing the incorporation of C9 onto C5b-8 and therefore preventing the formation of the membrane attack complex (MAC). In 1990 an individual with inherited isolated deficiency of CD59 was described with many features similar to classic PNH, such as intravascular hemolysis with hemoglobinuria and thrombosis of the cerebral veins.6 Thus CD59 deficiency is the abnormality that is responsible for the hemolysis and thrombosis classic of PNH.
Venous thrombosis in PNH
The most feared complication of PNH is venous thrombosis, which has a predilection for the intra-abdominal and cerebral veins. In two historical series of patients, approximately 50% experienced venous thrombosis at some time during their disease and a third of patients died as a result of thrombosis.7,8 The risk of thrombosis is greater in patients from Europe and the United States than in patients from the Far East.9 A possible explanation is that aplastic anemia (AA) is more prevalent in the Far East and therefore patients are more likely to have PNH/AA rather than hemolytic disease. Alternatively patients from different ethnic groups may have different additional inherited prothrombotic traits—however, no correlation between the inherited thrombophilia and thrombosis in PNH has been demonstrated.10 The cause of the thrombotic tendency in PNH is not entirely clear. The overwhelming evidence implicates the GPI-deficient platelets, which are more easily activated by complement than normal platelets. Thus PNH platelets, which comprise the vast majority of platelets in almost all hemolytic patients, similar to the proportion of PNH neutrophils, undergo microvesiculation at far lower concentrations of activated complement leading to greater prothrombinase activity and to thrombus formation.11 Alternative mechanisms have been suggested, such as deficiency of urokinase plasminogen activator receptor from PNH neutrophils or directly due to intravascular hemolysis, but the platelet abnormalities appear to be the most important factor.11,12
Nitric Oxide and the Symptoms of PNH
It is likely that the symptoms of PNH during a paroxysm, such as esophageal spasm and abdominal pain, are caused by smooth muscle dysfunction due to disturbances in the metabolism of nitric oxide (NO). These symptoms are identical to those observed when free hemoglobin was given to normal individuals during the early attempts to produce artificial blood and can be induced by the inhibition of NO synthetase in normal individuals. Haptoglobin efficiently removes free hemoglobin and is necessary because the function of hemoglobin depends upon its correct compartmentalization in red cells. Free hemoglobin, as is seen in hemolytic PNH, is an extremely efficient scavenger of NO with 106 times greater affinity of the heme moiety for NO than that for oxygen. Thus intravascular hemolysis absorbs NO, disturbs smooth muscle function and causes the symptoms seen during a paroxysm.
Conventional Therapeutic Strategies in PNH
The principal conventional therapy for PNH is supportive care with transfusions as required and the treatment of complications, such as thrombosis, when they occur. The only curative strategy is allogeneic stem cell transplantation but this carries a considerable risk of mortality from the reported series13 and, in view of the fact that a proportion of patients will eventually experience a spontaneous remission of PNH (occurs in approximately 15% of hemolytic patients)7 and with the advent of potentially effective novel therapies, this should only be considered in selected cases, such as those with a syngeneic donor or with associated bone marrow failure. In these patients the indications for transplantation are similar to those for AA. Patients with cytopenias due to associated AA will often respond to immunosuppressive therapy with antilymphocyte globulin and/or cyclosporine. The occurrence of venous thrombosis affecting a major vessel is a life-threatening occurrence and the advent of reduced-intensity conditioning allogeneic stem cell transplantation, which carries a lower risk than conventional bone marrow transplantation (BMT), has led to this approach being used in some patients with life-threatening thromboses.
Diagnosis of PNH
Flow cytometry
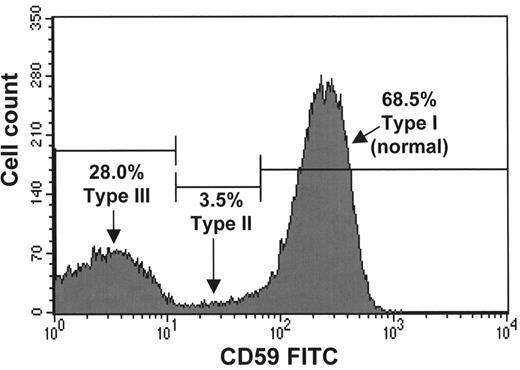
The gold standard diagnostic test for PNH is the analysis of GPI-linked molecules on the surface of hematopoietic cells by flow cytometry (see Figure 1 ).14 An unusual feature of flow cytometry for PNH diagnosis is that a positive test is defined by an absence of antigens rather than the aberrant expression of antigens as seen in most other flow cytometric applications. However in almost all PNH patients there is at least a small residuum of normal cells, which acts as an internal control to demonstrate that the staining and gating strategy are correct. The minimum requirement to diagnose PNH is the demonstration that a proportion of red cells are deficient in at least two different GPI-linked molecules. The proportion of GPI-linked deficient granulocytes is not affected by either hemolysis or transfusions and therefore gives the best estimate of the actual proportion of PNH hematopoiesis. In addition to establishing the diagnosis of PNH, flow cytometry also provides information regarding the severity of deficiency of the antigens from PNH red cells. PNH Type III red cells are completely deficient in GPI-linked molecules and are 15 to 25 times more sensitive to activated complement (compared to normal) whereas PNH Type II red cells have a partial deficiency (PNH Type II cells) and are only 3 to 5 times as sensitive. The size of the PNH clone and type of PNH cells are important determinants of the clinical presentation. For example, patients with hemolytic anemia and macroscopic hemoglobinuria will almost always have over 10% PNH Type III red cells and a majority, often over 95%, of PNH neutrophils. The risk of thrombosis is directly related to the proportion of PNH neutrophils, and this information has been used to stratify patients when deciding who should receive warfarin as primary prophylaxis against venous thrombosis.
Tests of complement sensitivity
Other tests, such as the demonstration that PNH red cells have an increased sensitivity to complement (the Ham test), are less sensitive than flow cytometry. These may be useful screening tests but can no longer be relied on to establish the diagnosis. The diagnosis should always be confirmed by flow cytometry. An alternative diagnostic approach is to utilize the toxin Aerolysin, which is produced by the bacteria Aeromonas hydrophila and binds to the GPI structure.15 Aerolysin has been fluorescently labeled (FLAER), and therefore any nucleated cell that expresses GPI-anchored antigens is positive, whereas the GPI-deficient cells remain negative. The FLAER test is currently under investigation as a diagnostic test for PNH.
PNH Pathophysiology: Relative Growth Advantage of the PNH Clone
PNH is a unique disorder in which an abnormal clone or small number of clones expand to replace almost the entire hematopoietic stem cell pool, but these clones do not have any “malignant” tendency in that they appear to be regulated in a normal manner with no tendency to metastasize beyond the normal hematopoietic compartment. Also GPI-deficient cells with pig-a mutations occur very frequently at low levels in normal individuals but do not expand in competition with the normal hematopoietic cells. Dacie first proposed that in order to develop PNH two things were required: first, the occurrence of a GPI-deficient clone arising in a multipotent hematopoietic stem cell; second, a second event that encourages the expansion of the PNH clone over the residual normal hematopoiesis—a relative growth advantage for the PNH cells.16 The clue to this second event is the close relationship between aplastic anemia and PNH. It appears that normal hematopoiesis is suppressed by the immune system, presumably either directly or indirectly through one or more GPI-linked antigens, and therefore this attack spares the GPI-deficient PNH clone. Thus, in an environment where there is intense pressure for hematopoiesis (aplastic anemia), the PNH clone is driven to produce mature hematopoietic cells and expands to fill the void left by the aplastic process. The exact mechanism for the relative growth advantage of PNH cells remains unclear but is the subject of considerable scientific activity.
Novel Therapeutic Strategies in PNH
Prevention and treatment of thrombosis
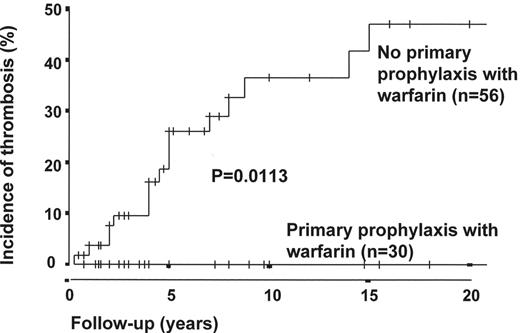
The first thrombosis in a patient with PNH heralds a significant deterioration in the patient’s health and a worsening prognosis. Reports of tissue plasminogen activator (tPA) for intra-abdominal venous thrombosis in PNH show that the thrombus can be cleared effectively in a proportion of patients.17 The standard of care after established venous thrombosis in PNH is life-long full anticoagulation. In view of the high risk of thrombosis and the fact that patients have decreased quality of life following the first thrombosis, selected patients could be considered for warfarin as primary prophylaxis prior to the thrombosis. Hall et al18 recently reported a retrospective analysis comparing 39 patients at high risk of venous thrombosis treated with warfarin as primary prophylaxis to 56 patients with similar sized clones who were not treated with warfarin. The incidence of thrombosis at 10 years in the group of patients not on warfarin was 36.5%, which was statistically significantly higher than the warfarin group in whom no patients had a thrombosis. However, 2 patients in the warfarin group had major hemorrhages, with 1 dying as a direct result of an intracranial bleed. Despite this being a retrospective analysis and the fact that the patients were not randomized the results suggest that primary prophylaxis may have a role (Figures 2 and 3 ). The data are compelling, particularly in view of the fact that because the patients in the warfarin group were selected for anticoagulation they would be expected to have at least as high a risk of thrombosis as the non-warfarin group. PNH patients with neutrophil clone sizes of over 50%, platelet counts above 100 × 109/L and no other contraindication for warfarin therapy should be considered for primary prophylaxis. There are no studies of antiplatelet drugs, such as aspirin or clopidogrel, in PNH.
Inhibition of the complement cascade
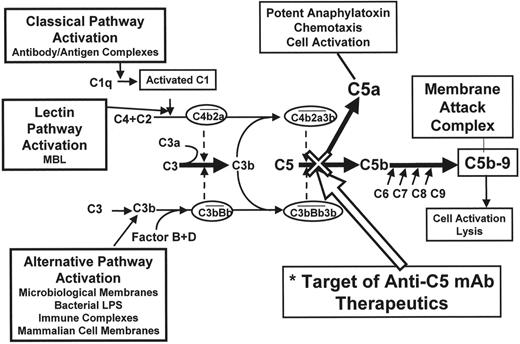
Complement is the principal effector of the innate immune system. Complement activation is a complex cascade leading to the production of anaphylatoxins, chemotaxins and the membrane attack complex (MAC). Three pathways activate the cascade (see Figure 4 ): the classical (initiated by antigen/antibody complex), alternative (initiated by microbial membranes or immune complexes), and lectin pathways. These pathways all converge to cleave C5 into C5a, a potent anaphylatoxin, and C5b. C5b is the initial molecule of terminal complement and binds C6, then C7, and then C8. C5b-8 forms the scaffold for C9 molecules, which bind to each other to form the MAC. The MAC forms a pore in the cell membrane, which results in cell lysis. CD59 prevents the incorporation of C9 onto C5b-8. A remarkable feature of the complement cascade is that the MAC appears to be largely redundant in normal adults. Individuals with inherited deficiency of any of the complement molecules prior to C5 are vulnerable to both pyogenic organisms and to autoimmune disorders. In contrast, deficiency of any of the molecules after C5 remarkably has little phenotype. The only apparent sequela is an increased risk of infection by encapsulated organisms, namely Haemophilus influenzae, Neisseria meningitides and N gonorrhoea. In the case of N meningitides, although the deficient individuals have an increased risk of suffering infection they also have a significantly lower mortality from infection when compared to complement-replete individuals. C5 is a good therapeutic target, because blockade here would not only prevent the creation of MAC but would also prevent the release of the potent anaphylatoxin C5a. Eculizumab is a humanized chimeric antibody against C5 with a completely nonfunctional Fc domain. Eculizumab has a high affinity for C5 and thus when bound remains so until the complex is removed from the circulation. Recently a pilot study of eculizumab in 11 patients with transfusion-dependent PNH was reported.19 The antibody was given at a dose of 600 mg every week for 4 weeks and then at a dose of 900 mg every other week. The drug is given by intravenous infusion over 30 minutes and was extremely well tolerated. The parameters of intravascular hemolysis, namely lactate dehydrogenase (LDH) and aspartate transaminase (AST), immediately fell to normal or near normal levels. The mean LDH prior to eculizumab was 3111 ± 598 IU/L (normal range 150–480) and this fell by the first week to 594 ± 32 IU/L. There was a significant improvement in the quality of life when comparing before the start of eculizumab and at week 12. There was also a significant reduction in mean transfusion requirement for the whole group from 2.1 units per month to 0.6 units per month. The benefit was most evident in patients with normal or near normal platelet counts and therefore no evidence of co-existent clinically significant marrow failure. There was also a complete cessation of hemoglobinuria immediately after commencing eculizumab. The proportion of PNH red cells in patients not on eculizumab is usually significantly lower than the proportion of PNH neutrophils because of the effect of transfusions and also the selective hemolysis of PNH red cells compared to their normal counterparts. The reduction or cessation of transfusions and control of hemolysis in patients on eculizumab leads to an increase in the proportion of PNH red cells toward the level of the PNH neutrophils. The median proportion of PNH type III red cells increased from 36.7% ± 5.9% prior to eculizumab to 59.2% ± 8.2% by week 12 of therapy. This raises the concern that stopping eculizumab for any reason might render the patient susceptible to a massive hemolytic attack and profound anemia. Two of the patients on eculizumab have had a breakthrough from complement blockade immediately prior to the next dose of eculizumab with a return of hemoglobinuria. Although both suffered hemolysis, the severity of the attack was not too extreme and in both the complement was reblocked immediately after the next dose of eculizumab and remained so on a slightly more frequent dosing regimen. Although eculizumab appears to almost completely stop the intravascular hemolysis, the patients’ hemoglobin does not return to normal (it usually plateaus between 10 and 12 g/dL), the bilirubin and reticulocytes remain elevated and the haptoglobin initially becomes detectable but reverts to undetectable within a few weeks of the start of therapy in almost all patients. Thus a continuing low level of well-compensated extravascular hemolysis persists, suggesting that the hemolysis in PNH is not only due to terminal complement activity but that there is also a component of extravascular hemolysis due to complement activity prior to C5. Eculizumab appears to be a highly promising therapy for PNH and is now the subject of a Phase III randomized clinical trial.
Replacement of complement regulatory proteins on PNH cells
An alternative therapeutic strategy in PNH could be to replace CD59, the deficient complement regulatory protein. In the first instance a gene therapeutic20 approach might appear attractive. However, introducing the pig-a gene into the PNH hematopoietic stem cell is far from trivial and would simply render the “corrected” cell as a target for the aplastic process that was the reason for the proliferation of the PNH clone. An alternative strategy would be to simply replace CD59 on the PNH red cell surface. It is impractical to extract GPI-linked CD59 from cell membranes as it is extremely tightly bound and does not re-attach well to cells in the presence of albumin. CD59 is functional when bound to the cell by alternative mechanisms, such as a transmembrane linkage, but again this approach is impractical.21 Sah et al22 have recently reported the use of an alternative artificial glycolipid anchor (Prodaptin) to anchor CD59 into the cell. Prodaptin-CD59 has been shown to effectively coat PNH cells in vitro and murine cells in vivo and to restore the cell’s resistance to complement. This approach is attractive because the complement system is not blocked and the increased risk of N meningitides, which is seen in complement deficient individuals, is no longer an issue.
Global PNH Registry
In view of the infrequency of PNH it is extremely difficult to perform large or randomized trials of therapeutic interventions. In addition, the advent of complement inhibitors, such as eculizumab, or the routine use of prophylactic anticoagulation promises to alter the natural history of PNH. In fact, the approval process of eculizumab as a therapeutic agent will be on the basis of relatively small and short clinical trials. Thus few patients are likely to have received more than 3 years of eculizumab. It is possible that any complications of such a novel agent might take several years to develop. These factors have led to the recent development of a Global Registry for PNH. It is hoped that a large number of PNH patients will be registered on the Global PNH Registry in order that a more thorough insight into the effect of these changes on the natural history of PNH can be established (www.PNHregistry.org).
Conclusion
The understanding of the biology and natural history of PNH has altered dramatically over the last 10 years. This has facilitated major steps forward in our ability to diagnose the disorder and in our therapeutic strategies for PNH. There is now initial evidence that we may be able to prevent the most feared complication of PNH, namely thrombosis, with the use of primary prophylaxis with anticoagulants for selected patients. The recent description of a small pilot study of the complement inhibitor eculizumab offers the first promise that we may have a “targeted” therapy capable of controlling the hemolysis of PNH. Other novel approaches to therapy in PNH are also being explored. The development of the Global PNH Registry offers the potential to further our understanding and therefore therapy of PNH.
II. Fcγ receptor Structure/Function and Role in Immune Complex–Mediated Autoimmune Disease
Randall G. Worth, PhD, Brian A. Jones, PhD, and Alan D. Schreiber, MD*
University of Pennsylvania, 421 Currie Blvd., BRB II and III Bldg., Philadelphia PA 19104
Antigen recognition by cells of the immune system occurs via several mechanisms. One important family of receptors involved in the recognition of immunoglobulin (Ig)-coated particles and complexes are Fc receptors. Fc receptors recognize the Fc portion of Ig and are accordingly grouped into subfamilies. They are named depending upon which class of Ig they bind. The major Fc receptors are Fcγ receptors that bind IgG, FcαR that bind IgA, and FcεR that bind IgE.1 Fc receptors are responsible for such functions as endocytosis, phagocytosis, granule release, reactive mediator release and cell activation/cytotoxicity. Fc receptors are found on specific cell types corresponding to their ability to recognize Ig. As such, Fcγ receptors are found primarily on neutrophils, macrophages and monocytes where they can detect and phagocytose IgG-coated particles, and on B lymphocytes.
Fc Receptor Subtypes and Expression Patterns
Fc receptors can be divided into two groups based on their signaling capability and structure. The first and most common type of Fc receptor is a multichain heterocomplex composed of a ligand-binding α-chain and one or more signal-transducing γ-chains. The second type of Fc receptor is a single-chain transmembrane receptor containing a signal-generating motif(s) in the cytoplasmic domain and, thus, not requiring another signal-transducing subunit.
Receptors for a specific isotype of IgG can vary in structure and can differ in ligand affinity and signaling ability.1 Fcγ receptors are categorized into three classes: FcγRI, FcγRII, and FcγRIII (Figure 5; see Color Figures, page 514). FcγRI is a high affinity receptor that can be subdivided into three groups (A, B and C) that are encoded by three different genes. FcγRI is expressed on monocytes, macrophages, neutrophils and some dendritic cells. The receptor can be upregulated upon stimulation with interferon (IFN)-γ, tumor necrosis factor (TNF)-α or granulocyte colony-stimulating factor (G-CSF).2,3 Similar to FcγRI, FcγRII has been divided into three families encoded by three genes, named A, B and C, which are of relatively low avidity for monomeric IgG but of high avidity for complexed IgG. FcγRII is expressed on various cell types and contributes to cell function based on the subclass of FcγRII. FcγRIIA is expressed on neutrophils, monocytes, macrophages, natural killer cells and platelets. FcγRIIB is expressed in two forms following alternative splicing, forming the FcγRIIB1 and FcγRIIB2 isoforms, and is limited in expression to B cells, neutrophils, macrophages and monocytes. FcγRIIC is expressed on human natural killer cells. FcγRIII is divided into two families, the transmembrane form FcγRIIIa and the GPI-linked FcγRIIIB found on neutrophils and natural killer cells. FcγRIIIA is found on monocytes and macrophages, and has also been shown to be expressed and upregulated on eosinophils.4
Crystal Structure/Affinity for IgG
Both recent immunotherapy using IgG antibodies as well as certain antibody-mediated autoimmune diseases utilize Fcγ receptors for the clearance of IgG-coated cells from circulation. While this may be beneficial in immunotherapy, it is detrimental in autoimmune disease. Understanding the interaction between the Fc domain of IgG and the Fcγ receptors is necessary in order to develop better therapy. There are two extracellular immunoglobulin-like domains for FcγRII and FcγRIII, while FcγRI has three immunoglobulin-like domains. The third domain of FcγRI has been attributed to the high affinity binding of IgG by this receptor; however, since the extracellular region of FcγRI has not been crystallized this has not been confirmed experimentally. The crystal structures of the extracellular region of FcγRIIA, FcγRIIB, FcγRIIIA, and FcγRIIIB have been determined.5 The two immunoglobulin-like domains of these receptors, D1 (membrane distal) and D2 (membrane proximal), are bent at an approximately 50–70° angle relative to each other depending on the algorithm used. All Fcγ receptors are structurally very homologous, with their domains oriented in a steep angle to each other. While most studies have shown Fcγ receptors existing in the membrane as a monomer, some conflicting reports have suggested that FcγRIIa forms a homodimer, with a 2:1 stoichiometry of receptor to IgG.6 However, the crystallization of FcγRIIIa in complex with the Fc region of IgG1 suggests a 1:1 stoichiometry of receptor to IgG.7 It is believed that the other Fcγ receptors display similar binding stoichiometry due to their high degree of homology.8 Also, as a dimer is able to crosslink Fc receptors to induce signaling, it may not be efficient for cells to be able to induce signaling by binding a single IgG molecule. However, the conformation of both bound and unbound FcγRIIa remains a matter of debate.
The binding sites of the Fcγ receptors for IgG are fairly well established. From the crystallization studies as well as some mutational studies, the D2, or membrane proximal, domain of the Fcγ receptor is important for the binding of IgG. The binding of an IgG by the receptor induces approximately a 10° shift in the angle between the D1 and D2 domains.7 The Fcγ receptor binds asymmetrically to an IgG molecule in the lower hinge region of the Cγ2 domains of an IgG heavy chain. The asymmetrical binding may result from a conformational change in one of the Cγ2 domains of IgG in relation to the other. A factor that could affect the IgG binding ability of the Fcγ receptor is the glycosylation state of the receptor and IgG. One example of this is found in the crystal structure of FcγRIIIa/IgG-Fc, whereby the glycosylation of the IgG1 at 297N was not involved in the binding site. However, two groups have shown that glycosylation of an IgG may be involved with the formation of a stable Cγ2-Cγ2 interaction of the two heavy chains of IgG1. When the carbohydrates are removed, there is decreased binding of IgG1 to both FcγRIII and FcγRIIb (Table 1 ).9,10 The glycosylation of Fcγ receptors can also affect binding of IgG. Expression of FcγRIIIa on different cell types leads to different glycosylation patterns. The differences in the ability to bind monomeric IgG by FcγRIIIa have been attributed to the cell type specific glycosylation of the receptor.11 Also, one particular glycosylation site in the D2 domain of FcγRIIIa and FcγRIIIb, 163N, is located in the IgG-binding site. Receptors lacking glycosylation at this amino acid have an increased affinity for IgG.12 Thus, the glycosylation state of the receptor, which may vary in cases of inflammation, could affect the binding of IgG.
Another example of affinity modulation is through polymorphism. As such, a polymorphism in FcγRIIa (131R or 131H) affects the binding affinity of FcγRIIa for human IgG2; FcγRIIa (131H) binds IgG2 but FcγRIIa (131R) does not.13 Results of a recent meta-analysis show that the FcγRIIa polymorphism is important in genetic susceptibility for systemic lupus erythematosus (SLE).14 There is also a polymorphism in FcγRIIIa (158V or 158F) that affects binding to human IgG1 and human IgG3. NK cells from patients with FcγRIIIa (158F) are unable to bind IgG3 and bind IgG1 with lower affinity, whereas NK cells from patients with FcγRIIIa (158V) are able to do so.15 In addition, a triallelic polymorphism in FcγRIIIa (48 H, R, or L) was originally reported to affect the binding affinity for human IgG. Subsequently, this difference in binding affinity was determined to be due to the polymorphism at amino acid 158.16 This is consistent with structural studies as 48 H/R/L is located in the D1 immunoglobulin-like domain whereas 158 V/F is located in the D2, IgG-binding domain. Finally, in FcγRIIIb, the GPI-linked Fcγ receptor expressed on neutrophils, there are two alleles (NA1 and NA2). These two alleles have minimal differences in binding affinity for IgG, but patients homozygous for the NA1 allele have a higher level of phagocytosis of IgG-coated particles.17
Signal Transduction from Fcγ Receptors
Given that most Fcγ receptors are transmembrane proteins, the first step of receptor activation and subsequent phagocytosis is binding of IgG containing complexes to the receptor extracellular domain. Binding and phagocytosis by human Fcγ receptors has been observed both in normal human leukocytes and in model systems such as transfected COS-1 cells.18 Initial Fcγ receptor activation takes place upon ligand binding to the extracellular domain. Since each Fcγ receptor has a structurally distinct extracellular domain, the receptor binds to IgG with varying affinity, as shown in Table 1 .
Fcγ receptors traditionally signal through an immunoreceptor tyrosine–based activation motif (ITAM) or through inhibitory residues found in an immunoreceptor tyrosine–based inhibitory motif (ITIM).19,20 ITIMs are composed of an I/VxYxxL/I sequence that recruits tyrosine phosphatases to the signaling complex. Therefore, the presence of ITIM-bearing receptors imposes a negative effect. As such, the presence of FcγRIIB, an ITIM-bearing receptor, inhibits phagocytic signaling mediated by activating Fcγ receptors.21
The classic ITAM motif consists of two YxxL sequences separated by 7 amino acids.22 Fcγ receptor ITAM sequences in the Fc receptor associated γ-chain abide by this structure. However, the cytoplasmic domain of FcγRIIa contains an ITAM-like domain. This ITAM-like domain contains the two YxxL motifs but a spacer sequence containing 12 amino acids instead of the usual 7.20 ITAM tyrosine residues have been shown to be crucial for mediating the phagocytic response. When either of the ITAM tyrosines are mutated to phenylalanine, phagocytosis is inhibited by ~70%–80%. However, if both tyrosine residues are mutated phagocytosis is abolished.23– 25
It has been proposed that ITAM tyrosines are phosphorylated by Src family kinases after crosslinking. Several members of the Src family have been shown to associate with specific Fcγ receptors. However, which Src kinase is responsible for phosphorylation of a specific Fc receptor is not established. Studies have been somewhat inconclusive in elucidating which kinase is responsible for phagocytic signaling through each Fcγ receptor. An example of these observations can be found in knockout experiments where phagocytosis is not abolished in Hck, Lyn, or Fgr single knockouts.26 In addition, in triple knockout mice, phagocytosis by macrophages and neutrophils is still partially intact, suggesting other kinases may play a redundant role in phagocytosis.27
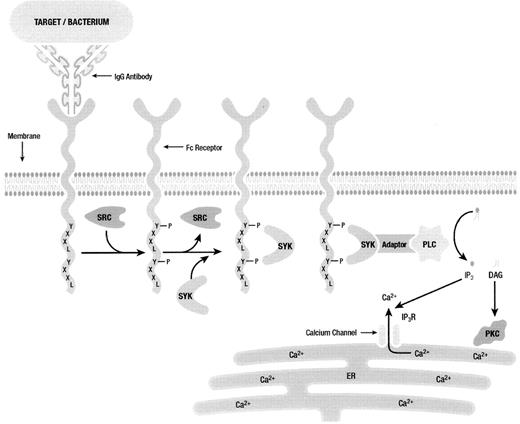
Phosphorylation of ITAM tyrosines creates Src homology 2 (SH2)-binding sites required for signal transduction involving other members of the tyrosine kinase family.28–,30 Most importantly, Syk tyrosine kinase, which contains two SH2-binding sites, is recruited to phosphorylated ITAM residues (Figure 6 ). FcγR phagocytosis is dependent upon Syk signaling, and has been observed to be enhanced upon the overexpression of Syk in model systems.31 In addition, when Syk kinase expression is inhibited with antisense oligonucleotides both in vitro and in vivo, phagocytosis and inflammation are abolished.32,33
SH2-containing proteins are important in signaling complexes. For example, recent studies have emphasized the role of adaptor proteins in phagocytic signaling. Adaptor molecules such as SLP-76, LAT, Cbl and others have the ability to recruit SH2-containing proteins to signaling complexes, notably in lipid rafts (Figure 6 ). These adaptor proteins play a significant role in recruiting such secondary signaling molecules as phospholipase C (PLC), Grb2, Shc and others. The ability for adaptor molecules to recruit proteins to the site of signal propagation by Fcγ receptors is important for efficiently triggering the downstream signaling leading to target internalization and mediator release.
Fcγ Receptor–Associated Diseases
As described above, the isotype of IgG determines the class of Fcγ receptor ligated and the nature of the signal propagated. Of particular interest are the myriad of diseases based upon IgG-Fcγ receptor interaction leading to inflammation, phagocytosis and endocytosis. Two particular diseases mediated by Fcγ receptors are immune thrombocytopenic purpura (ITP) and autoimmune hemolytic anemia (AIHA). Both of these diseases are caused by production of self-reactive antibodies against either platelet antigens or erythrocyte antigens. ITP is mediated by production of IgG against one or more antigens exposed on platelets. Specifically, antibodies reactive to platelet GPIIb-IIIa, GPIb-IX, GPIb and GPIIIa among others have been shown to be potentiators of ITP.34 AIHA has been shown to be due in large part to self-reactive antibodies against erythrocyte Band 3, an ion transporter found in erythrocyte membranes also shown to be involved in erythrocyte senescence. Both of these diseases are mediated by Fcγ receptors found on phagocytes (macrophages) as part of the reticuloendothelial system (RES) in the spleen and liver and may lead to thrombocytopenia or anemia, respectively.34
Various observations by multiple laboratories have determined the role of Fcγ receptors in these diseases most conclusively in murine models lacking the common γ-chain (γKO). Experimentally induced AIHA and ITP are markedly decreased in mice lacking the γ-chain.35 Additionally, administration of monoclonal antibody 2.4G2, which binds to and blocks mouse FcγRII and FcγRIII, allows a rapid recovery after induction of AIHA or ITP. Similarily, administration of GM-CSF has been shown by various groups to accelerate AIHA potentially due to upregulation of FcγRI.36 These reports show that altering the balance of stimulatory to inhibitory Fcγ receptors has a marked effect on disease progression and susceptibility. As such, viral infection has been shown to stimulate or increase susceptibility to both AIHA and ITP. However, the mechanism(s) by which viral infection does so has yet to be fully elucidated. Potential scenarios of viral infection that could lead to this outcome include stimulated production of IFNγ during viral infection that causes upregulation of FcγRI. Alternatively, viral infection may cause a change in the expression pattern of Fcγ receptors due to transcriptional activation or other mechanisms. For example, childhood ITP has been described after infection with varicella zoster virus (VZV), causing molecular mimicry of antibodies against VZV to crossreact with platelet antigens, thus causing ITP.37 ITP in some patients has been shown to be due to production of predominantly self-reactive IgG1.38
Therapy for ITP or AIHA
Treatment for ITP and AIHA has recently been reviewed elsewhere.39,40 Many of the therapies used involve altering Fcγ receptor expression or phagocytosis. One of the first treatments is the administration of glucocorticoids. Prednisone and dexamethasone have been shown to downregulate Fcγ receptor expression, thus potentially decreasing the phagocytosis of IgG-coated particles. Other treatments involve administration of anti-D IgG to Rh-D positive patients. The rationale behind this treatment is that the IgG will coat erythrocytes with subsequent binding by Fcγ receptors. Binding of the IgG-coated red blood cells will prevent the binding and phagocytosis of the IgG-coated platelets. However, self-limited hemolytic anemia is to be expected after anti-D treatment.
High-dose intravenous immunoglobulin (IVIG) is also used as a treatment for both ITP and AIHA. However, the mechanism by which this inhibits the clearance of the IgG-coated platelets or IgG-coated red blood cells is still unknown. It is believed to work through a number of different mechanisms, which may include anti-idiotypic antibodies and decreased autoantibody production,41 and, of interest here, its effects on Fcγ receptor function. One principal theory for a mechanism of IVIG’s function is through a blockade of Fcγ receptors. At high local concentrations of IgG, the IVIG may either bind to the low-affinity Fcγ receptors, preventing the binding of IgG-coated cells, or outcompete binding of the high avidity immune complexes. Also, the possibility of IgG dimers existing in IVIG preparations would even further enhance this blockade.42 Another possibility involves the inhibitory FcγRIIb, which is able to inhibit phagocytosis mediated by activating Fcγ receptors.21 Injection of IVIG into a mouse model of ITP increased circulating platelet counts.43 Also in this model was an increased percentage of non B cell splenocytes expressing FcγRIIb. Increased expression of this receptor relative to the expression of activating receptors could decrease the phagocytosis of IgG-coated platelets. The mechanism for the IVIG-mediated increase in Fcγ receptor–expressing cells is still unknown.
Summary
Fcγ receptors are integral for the removal of IgG-coated cells from the circulation. In autoimmune diseases such as autoimmune hemolytic anemia and immune thrombocytopenia the removal of the IgG-coated erythrocytes or IgG-coated platelets is detrimental to a patient. Much is known regarding the structure and signaling properties of the Fcγ receptor family. As the two autoimmune diseases mentioned involve phagocytosis of IgG-coated cells, interference at either the Fcγ receptor binding of these complexes or interference of the phagocytosis process could possibly improve on the current treatments available for these disorders. Understanding the crystal structure of the Fcγ receptors may lead to the development of molecules that inhibit the binding to an IgG-coated cell. Small molecules might inhibit Fcγ receptor signaling at many different steps leading to phagocytosis. Finally, treatment to decrease expression of the activating Fcγ receptor or increasing expression of the inhibitory Fcγ receptor may also prove to be an effective therapy.
III. Cold-Induced Immune Hemolytic Anemia
Wendell F. Rosse, MD*
Duke University, Department of Medicine, 4605 Timberly Drive, Durham NC 27707
The antibodies that cause immune hemolytic anemia can be classified by isotype (IgG, IgM, or IgA) and by the temperature at which they react maximally with the antigen on the red cell: warm reacting if that temperature is 37º, cold reacting if that temperature is less. Much of the pathophysiology and many of the clinical manifestations are determined by these characteristics. For the most part, the isotype and the temperature of reaction coincide: most frequently, cold-reacting antibodies are IgM and warm-reacting antibodies are IgG, but important exceptions exist. Two clinical entities due to cold-reacting antibodies are defined by differences in isotype: cold agglutinin disease is caused by IgM antibodies and paroxysmal cold hemoglobinuria by IgG antibodies.
The isotype is important because the isotypic characteristics of the antibody direct the mechanisms of destruction. As described in Section II, phagocytic cells have receptors specific for IgG molecules of specific subtypes (IgG1, IgG2, and IgG3) but not for IgM molecules; therefore, Fc-receptor–induced destructive processes apply only when IgG molecules are attached to the red cell in the presence of the phagocytic cells. On the other hand, IgM antibodies fix complement much more readily than IgG molecules because the two necessary attachment sites for C1q are present on a single molecule; therefore, complement plays a primary role in the destruction of the red cells by these antibodies.1,2
Why Are Cold-Reacting Antibodies Cold Reacting?
The interaction of antigen and antibody is reversible and the degree of attachment depends upon the forces interacting between the two. For all cold-reacting antibodies, the antigen with which they react is polysaccharide or the polysaccharide parts of glycoproteins. In the case of cold agglutlinins, the antigen is one of the following: the straight-chain paragloboside basic to the expression of the ABH antigens (the i antigen), a branched version of that molecule (the I antigen), polysaccharides resident on glycoproteins (Pr antigens), and rare sialylated polysaccharides. Since the branching enzyme responsible for the I antigen is not activated until after birth, cord cells (and the cells of individuals genetically lacking the branching enzyme) express the i antigen whereas the red cells of adult express the I antigen.
The interaction of antibody and polysaccharide is dependent upon weak forces that are easily disrupted by molecular activity that occurs at higher temperatures. The cold agglutinins of anti-I or anti-i specificity are strikingly similar to one another in the structure of the antigen binding site. These antibodies all react with a monoclonal antibody that identifies the product of the VH4-34 (VH4-21) gene segment.3 Other antibodies (monoclonal anti-Rh system antibodies, etc.) have been shown to use this same gene segment for the variable portion of the heavy chain and many of them also have cold agglutinin activity against the I/i antigens.4 The characteristic that leads to such activity is a relative hydrophobicity, and it has been shown by detailed studies that a hydrophobic patch in framework region 1 contributed to by two β-strands is important in binding to the polysaccharide antigen and that this binding is modified by sequences in the complementarity-determining region H 3.5 (This specific structure does not pertain to cold agglutinins of other specificity, and the reason for their specificity and reactivity, while probably similar, is unknown.)
How Do Cold-Reacting Antibodies Arise?
Cold agglutinins arise in two settings, both perhaps due in part to the fact that B cells utilizing the VH4-34 gene segment normally represent a relatively large percentage of the population (6%–13% of all mature B cells). These cells probably account for the low titers (< 1/10) of cold agglutinin that can be found in the serum of normal individuals. Oligoclonal antibodies, usually in elevated titers, appear routinely in moderate titers that are not sufficient to cause significant hemolysis in infectious mononucleosis (with anti-i specificity) and mycoplasma (with anti-I specificity) infections and less commonly in cases of cytomegalovirus, chicken pox, etc. Monoclonal cold-reacting antibodies are an expression of paraneoplasia (benign monoclonal gammopathy) or immunocyte neoplasia including chronic lymphocytic leukemia (CLL) and a variety of lymphomas.6 Trisomy (complete or partial) of chromosome 3 has been recorded in patients in whom cold agglutinin disease progressed to a lymphoproliferative disorder.7 The titer of the antibody is, in all cases, a relative measure of the number of abnormal cells.
The Donath-Landsteiner antibody of paroxysmal cold hemoglobinuria (PCH) formerly arose as a cross-reacting antibody to an antigen on Treponema pallidum (or so it is thought) and was frequently seen in secondary and tertiary syphilis; this is now rare. It is most commonly encountered in children as a response to viral illness or immunization, much like immune thrombocytopenia in this group; in these cases, it does not persist, but the clinical syndrome it produces may be severe enough to cause death. In adults, it is usually an autoimmune antibody and may be associated with other evidences of autoimmunity.8
Diagnosis of Diseases Due to Cold-Reacting Antibodies
Cold agglutinins are readily detected as, being IgM, they agglutinate red cells directly. Three characteristics should be determined: specificity, titer, and thermal amplitude (the highest temperature at which the antibody reacts with red cells). These are readily done with standard techniques.
The detection of Donath-Landsteiner antibodies is much more problematical. Direct agglutination is often present but in relatively low titers (usually less than 1/64). The direct antiglobulin test (direct Coombs’ test) with anti-IgG is negative as the antibody elutes from the cells during their preparation. The indirect antiglobulin test is usually ineffective as well but occasionally is positive.9 The test attributed to Donath and Landsteiner (the bithermic hemolysis of normal red cells) is positive only when the titer in the serum is relatively high. The sensitivity of the test can be increased greatly by the use of red cells from patients with PNH, which are more sensitive to the hemolytic action of complement. The best technique, not generally available, is the use of radiolabeled anti-IgG, which is affixed at 4°C but removed by raising the temperature to 37°C.10 By using this technique, we were able to identify two patients not detected by the PNH variant of the Donath-Landsteiner test. Many patients with unexplained immune hemolytic anemia, often with a negative direct antiglobulin test, probably have undiagnosed PCH.
Why Is the Degree of Hemolysis So Different Among Patients?
The degree of hemolysis varies among patients because the characteristics of the antibodies responsible for it are so highly variable.11 In the case of cold agglutinins, the amount of antibody (roughly measured by the titer) and the affinity of the antibody (roughly measured by its thermal amplitude) are the most important determinants of hemolysis. These should be measured both in saline and with albumin added. Some antibodies are less hemolytic because they fix complement inefficiently; these antibodies are usually cryoprecipitable and the effect may have to do with structural changes that result in that characteristic.12 Some antibodies are not inhibited by the residual C3 on the red cell surface, as most are, and this results in increased hemolysis. Although most antibodies are pentameric IgM, some patients produce considerable quantities of hexameric IgM, which is more efficient in fixing complement.13
The same general principles of titer and thermal amplitude apply to Donath-Landsteiner antibodies. Several explanations have been offered to explain why these IgG antibodies result in intravascular hemolysis when the supposedly more efficient IgM antibodies generally do not. Certainly, the agglutination effected by IgM antibodies inhibits the fixation of complement components in vitro, but how much of a role this plays in vivo is unclear. D-L antibodies are able to fix C4 (but not C3) in the cold phase, but again the in vivo importance of this is questionable. The antigens of the D-L antibodies are said to lie closer to the membrane surface, thus facilitating the fixation of complement. In fact, the reason for the greater lysis of D-L antibodies compared to cold agglutinins is not clear.
What Is the Treatment of Diseases Due to Cold-Reacting Antibodies?
A primary treatment of any syndrome of cold-reacting antibodies is keeping the patient warm. This sometimes involves discomfort and is difficult for many patients to maintain. Where possible, avoidance of winter by migration is advisable.
In the case of IgM cold agglutinins, antibody production is not suppressed by prednisone and it should be used only when the simultaneous presence of IgG antibodies is found. Antibody production can sometimes be suppressed by chemotherapy, but the rate of proliferation of benign clones is so small that this is often ineffective; obviously, if malignancy is present, its treatment will treat the cold agglutinin problem. More recently, the use of anti-CD20 (rituximab) has found some success14 as has the use of fludarabine.15 Some patients appear to benefit by small to moderate doses of erythropoietin (personal observation). Plasmapheresis can be used to remove antibody temporarily but is difficult to maintain for chronic treatment; great caution is required to be sure that the blood does not get cold during the procedure.16
The treatment of PCH is like that of any other autoimmune hemolytic anemia due to IgG, except that splenectomy has no place in the regimen as the spleen is not involved in hemolysis. Prednisone, chemotherapy, and anti-CD20 treatment have all been used.
The hemolysis in the hemolytic anemia of cold-reacting antibodies is entirely dependent upon the activation and fixation of complement. In the future, the use of anti-C5 reagents, as described in Section I, or other complement modifying measures should provide relief of the most taxing symptom.
Relative affinities of IgG subclasses for various Fcγreceptors.
| . | FcγRI . | FcγRIIa . | FcγRIIb . | FcγRIII . |
|---|---|---|---|---|
| Molecular Mass | 70 kDa | 40 kDa | 40 kDa | 50–80 kDa |
| IgG Subclass Specificity | 1 ≥ 3 > 4 > 2 | 3 > 1 > 2 > 4 | 3 ≥ 1 > 4 > 2 | 1 = 3 > 2 = 4 |
| IgG Affinity | 10−7–10−9 M | > 10−7 M | > 10−7 M | > 2 × 10−7 M |
| . | FcγRI . | FcγRIIa . | FcγRIIb . | FcγRIII . |
|---|---|---|---|---|
| Molecular Mass | 70 kDa | 40 kDa | 40 kDa | 50–80 kDa |
| IgG Subclass Specificity | 1 ≥ 3 > 4 > 2 | 3 > 1 > 2 > 4 | 3 ≥ 1 > 4 > 2 | 1 = 3 > 2 = 4 |
| IgG Affinity | 10−7–10−9 M | > 10−7 M | > 10−7 M | > 2 × 10−7 M |
Flow cytometry to diagnose paroxysmal nocturnal hemoglobinuria (PNH) and to quantitate the clone.
A. Demonstrates a population comprising 28% of the red cells that are completely deficient in CD59 (and other glycosylphosphatidylinositol (GPI)–linked antigens) and a second abnormal population of partially deficient red cells comprising 3.5% of the total.
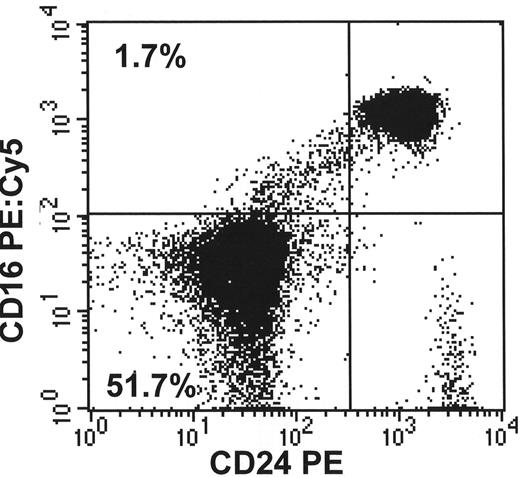
B. Demonstrates the lack of more than one GPI-linked antigen (CD16 and CD24) from the same patient’s granulocytes.
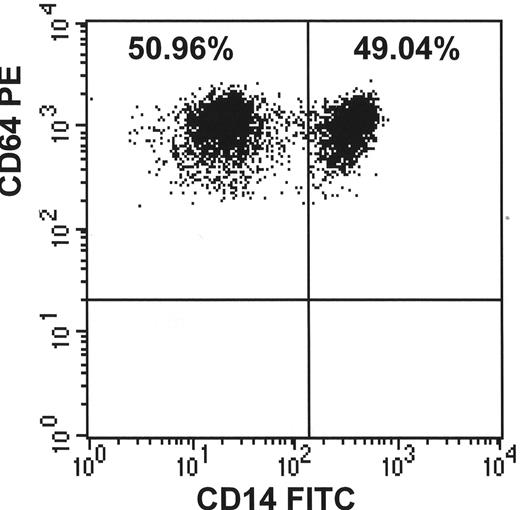
C. Demonstrates that the proportion of PNH monocytes usually closely corresponds to the granulocyte analysis. (Courtesy of Dr. S.J. Richards)
Flow cytometry to diagnose paroxysmal nocturnal hemoglobinuria (PNH) and to quantitate the clone.
A. Demonstrates a population comprising 28% of the red cells that are completely deficient in CD59 (and other glycosylphosphatidylinositol (GPI)–linked antigens) and a second abnormal population of partially deficient red cells comprising 3.5% of the total.
B. Demonstrates the lack of more than one GPI-linked antigen (CD16 and CD24) from the same patient’s granulocytes.
C. Demonstrates that the proportion of PNH monocytes usually closely corresponds to the granulocyte analysis. (Courtesy of Dr. S.J. Richards)
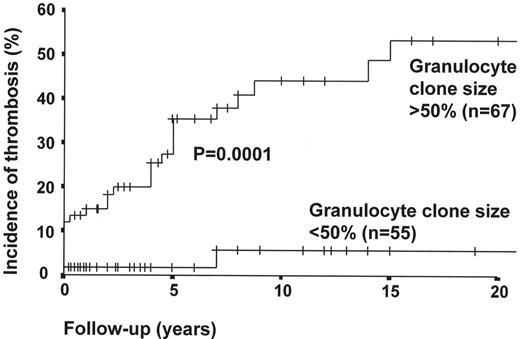
Effect of glycosylphosphatidylinositol (GPI)-deficient granulocyte clone size on incidence of venous thrombosis (primary prophylaxis patients excluded).
When primary prophylaxis patients are excluded, the 10 year cumulative incidence rate of thrombosis in patients with PNH granulocyte clone size of > 50% is 44%, compared with a thrombosis rate of 5.8% in those with clone size of < 50% (P < 0.01*).
* P value calculated with use of the log-rank test;
Effect of glycosylphosphatidylinositol (GPI)-deficient granulocyte clone size on incidence of venous thrombosis (primary prophylaxis patients excluded).
When primary prophylaxis patients are excluded, the 10 year cumulative incidence rate of thrombosis in patients with PNH granulocyte clone size of > 50% is 44%, compared with a thrombosis rate of 5.8% in those with clone size of < 50% (P < 0.01*).
* P value calculated with use of the log-rank test;
Effect of warfarin prophylaxis on venous thrombosis in patients with paroxysmal nocturnal hemoglobinuria (PNH) granulocyte clone sizes of > 50% (patients presenting with thrombosis excluded).
The 10-year cumulative incidence rate of venous thrombosis in patients with PNH granulocyte clones of > 50%, not presenting with thrombosis and not taking warfarin is 36.5%. In comparison, the current thrombosis rate is 0% in patients taking primary prophylaxis (P = 0.01*).
Thirty-two of the 39 patients on primary prophylaxis had granulocyte clone sizes > 50% and could therefore be included in this analysis. A further 2 of these patients were excluded because, having stopped warfarin (one through personal choice and one because of warfarin-associated hemorrhage), they went on to suffer venous thrombosis.
Time 0 was the time of presentation with PNH.
* P value calculated with use of the log-rank test;
Effect of warfarin prophylaxis on venous thrombosis in patients with paroxysmal nocturnal hemoglobinuria (PNH) granulocyte clone sizes of > 50% (patients presenting with thrombosis excluded).
The 10-year cumulative incidence rate of venous thrombosis in patients with PNH granulocyte clones of > 50%, not presenting with thrombosis and not taking warfarin is 36.5%. In comparison, the current thrombosis rate is 0% in patients taking primary prophylaxis (P = 0.01*).
Thirty-two of the 39 patients on primary prophylaxis had granulocyte clone sizes > 50% and could therefore be included in this analysis. A further 2 of these patients were excluded because, having stopped warfarin (one through personal choice and one because of warfarin-associated hemorrhage), they went on to suffer venous thrombosis.
Time 0 was the time of presentation with PNH.
* P value calculated with use of the log-rank test;
The complement cascade and eculizumab.
The complement cascade culminating in the production of the membrane attack complex that results in the lysis of the cell. The cleavage of C5 is the pivotal point of the pathway. Inherited deficiencies prior to C5 result in recurrent pyogenic infections and autoimmune disorders, whereas deficiencies after C5 have remarkably little effect except for an increased risk of infection by encapsulated organisms. The site of blockage of eculizumab is demonstrated.
The complement cascade and eculizumab.
The complement cascade culminating in the production of the membrane attack complex that results in the lysis of the cell. The cleavage of C5 is the pivotal point of the pathway. Inherited deficiencies prior to C5 result in recurrent pyogenic infections and autoimmune disorders, whereas deficiencies after C5 have remarkably little effect except for an increased risk of infection by encapsulated organisms. The site of blockage of eculizumab is demonstrated.