Abstract
In the vein-to-vein flow of blood from donor to patient, the role of the transfusion medicine specialist has become increasingly centered at the bedside. Three clinically centered issues in blood safety and in blood conservation are presented in this chapter.
In Section I, Dr. Patricia Hewitt presents the epidemiologic and clinical evidence regarding new variant Creutzfeldt-Jakob disease (nvCJD) in the UK and its relevance to transfusion medicine. Lessons learned from the responses by the National Blood Service to this crisis are discussed, particularly in the context of recent evidence of a case of vCJD transmission by blood transfusion and a second case of apparent transmission of abnormal prion protein without development of clinical illness.
In Section II, Dr. Christopher Silliman and his colleagues summarize recent knowledge gained regarding transfusion-related acute lung injury (TRALI), which is now the leading cause of transfusion-related mortality. Two different etiologies have been proposed: a single antibody-medicated event, involving anti-HLA Class I and Class II, or anti-granulocyte antibodies; and a two-event model, which includes the clinical condition of the patient resulting in pulmonary endothelial activation and neutrophil sequestration. The second event is the transfusion of a biologic response modifier (lipids or antibodies) in the blood component that activates primed neutrophils. Prevention, clinical treatment, and proposed definition of TRALI are discussed.
In Section III, Dr. Lawrence Goodnough and colleagues present a transfusion medicine service approach to the utilization of recombinant factor VIIa (rFVIIa) in non-approved clinical settings. rFVIIa has a potential role as a hemostatic intervention in a variety of clinical settings, yet few clinical trials have been completed to date to guide indications for its use. The policies presented here are those in place at the authors’ medical center, and will undergo periodic review and revision as relevant new information and data are generated.
I. vCJD and Blood Transfusion: How Real Is the Risk?
Patricia E. Hewitt, MD*
National Blood Service, Colindale Centre, Colindale Avenue, London NW9 5BG, UK The author wishes to acknowledge the following co-workers: Professor R G Will, UK National CJD Surveillance Unit, Edinburgh, UK; Jan Mackenzie, UK National CJD Surveillance Unit, Edinburgh, UK; Dr H J Ward, UK National CJD Surveillance Unit, Edinburgh, UK; Dr C Llewelyn, National Blood Service, Cambridge Centre, UK
The possibility that variant Creutzfeldt-Jakob disease (vCJD) might present a risk to the blood supply has been debated since the first description of cases by the UK National CJD Surveillance Unit (NCJDSU) in 1996. Risk assessments, experimental work, and expert committees all contribute to the debate. A disease that is essentially limited to one European country has evoked world-wide interest. The appearance of vCJD provoked renewed interest in sporadic (“classical”) CJD, never previously of significant concern within the blood transfusion community. The small number of cases of vCJD, and limited geographical distribution, mean that careful surveillance is crucial in acquiring knowledge about the disease and possible consequences. This section focuses on work over the last 8 years, with particular reference to CJD surveillance and blood transfusion issues in the UK, and highlights the joint work between the NCJDSU and UK Blood Services (UKBS), culminating in the identification of the first case of possible transfusion transmission of vCJD.
Epidemiology of CJD
CJD is a rare and fatal human neurodegenerative condition. In common with other transmissible spongiform encephalopathies (TSEs), CJD is experimentally transmissible to animals. Affected animals show characteristic spongiform change on microscopic examination of the brain.
There are a number of forms of CJD. Globally, over 80% of cases occur as sporadic disease (sCJD). Epidemiological studies indicate 1–2 cases of sCJD per million population per year, worldwide. Other forms of CJD include various genetic forms, iatrogenic forms, and vCJD. All these forms show much lower incidence than sporadic CJD. In addition, incidence varies between countries. For example, iatrogenic CJD has been associated with adminstration of human growth hormone (USA, UK and others between 1958 and 1985) human gonadotrophin hormones (Australia, UK) and use of dura mater (Germany and others before 1992).
The main features of the different forms of CJD are summarized in Table 1 .
Sporadic CJD remains of unknown cause. In particular, there is no evidence of a link with naturally occurring animal TSEs, such as scrapie, nor with bovine spongiform encephalopathy (BSE). Most cases occur between the ages of 60 and 80 years. Clinical and pathological features of sCJD are variable and influenced by a naturally occurring polymorphism at codon 129 of the gene encoding for the prion protein (PRNP gene).
The main type of genetic CJD is familial CJD. Other inherited forms of CJD, such as fatal familial insomnia, are recognized. All are rare. Genetic CJD is expressed as an autosomal dominant trait associated with an abnormality of the PRNP gene.
Iatrogenic CJD has followed treatment with contaminated human pituitary-derived growth hormone or gonadotrophin, as well as dura mater grafts, corneal transplants, neurosurgical instruments and electroencephalogram (EEG) electrodes. Although these potential sources of infection have been recognized, and measures taken to reduce (or abolish) the risk of such transmission, the number of cases continues to increase due to past exposure to these known risks.
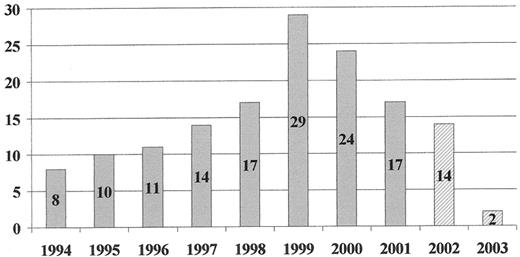
Workers at the NCJDSU first described 6 cases of vCJD in the UK in 1996.1 There have now been 146 cases of confirmed/probable vCJD in the UK2 (Figure 1 ) and small numbers in France (6 cases), Republic of Ireland, Italy, US and Canada. All except the French and Italian cases resided in the UK between 1980 and 1996. All tested cases have been homozygous for methionine at codon 129 of the PRNP gene.
What Is the Relationship Between BSE and vCJD?
The causal link between vCJD and BSE is based on epidemiological, biochemical and transmission studies. A scientific consensus that BSE-contaminated food was the main route of exposure of the human population was reached by a Joint World Health Organization/Food and Agriculture Organization/Office International des Epizooties Technical Consultation on BSE in 2001.3 Cattle and their products and by-products are traded worldwide, so that the risk of BSE (and therefore vCJD) is a global risk.
Clinical Features of vCJD
The median age of onset is 28 years (range 12–74). Whereas sCJD typically presents with rapidly progressive, clearly neurological symptoms, vCJD presentation tends toward behavioral/psychiatric symptoms and with slower progression. It may initially be difficult to determine that there is a neurological illness.3 Features of depression are common and patients may initially be referred to psychiatry services. Nearly half the patients are affected by sensory symptoms. At some point other neurological features appear, with definite neurological abnormality developing at around 6 months from first symptoms. Ataxia is often prominent. Eventually, the picture is of a dementia with multiple neurological features including myoclonus. The illness duration has a median of 14 months (range 6–40). The differential diagnosis of a progressive neuropsychiatric disorder in relative youth is potentially wide. It may be difficult or impossible to make a diagnosis of vCJD in the early stages.
Risk of Transmission of CJD by Blood Transfusion
Infectivity can be demonstrated in the blood of rodents experimentally infected with TSE agents, including the BSE agent. In these animal models, infectivity can be demonstrated in both cellular and plasma components.4 Infectivity has also been demonstrated in primates experimentally infected with BSE and in whole blood and buffy coat of sheep with scrapie.
A transfusion study in sheep has demonstrated transmission of experimentally produced BSE.5,6 Sheep from flocks free of scrapie and BSE were fed BSE-infected food. Blood from infected and control (non-infected) donor sheep was transfused to uninfected animals. Recipient sheep of infected donors have developed illness. Further work aimed at investigating how long during the incubation period infectivity is present, and the effect of leukodepletion, is awaited with interest.
There has been no evidence of transfusion transmission of sCJD (or familial CJD) despite epidemiological studies and case investigations.7 In particular, hemophiliacs exposed to plasma-derived coagulation factors have no increased prevalence of CJD, and studies involving examination of brain tissue post-mortem have not demonstrated any evidence of CJD.8 Experience with vCJD is much more limited, but there is now evidence of possible transmission of vCJD through blood transfusion.9
The Transfusion Medicine Epidemiology Review
The Transfusion Medicine Epidemiology Review (TMER) is a collaborative project between the UK NCJDSU and UKBS, investigating whether there is any evidence that CJD or vCJD have been transmitted by blood. Cases of CJD (all types) and controls who have acted as blood donors or have themselves received transfusion are identified by NCJDSU. Details are provided to the UKBS in a blinded study. The UKBS traces records relating to donors and looks back to identify recipient(s), whose details are provided to NCJDSU. For blood recipients who have developed CJD, and their controls, the UKBS obtains details of the transfused blood components from the hospital in which the transfusion took place. The UKBS identifies the donors, and forwards details to NCJDSU. Identified recipients and donors are checked with the CJD database; copies of death certificates are obtained.
Because of regulatory requirements relating to vCJD and plasma products, all vCJD cases (definite and probables) are notified to the UKBS by NCJDSU to establish whether any have acted as donors. To date, 27 of 146 vCJD cases were reported to have been blood donors. Records for 18 have been traced: 16 had donated blood, producing 57 blood components issued to hospitals. Of these, 7 components were not traced, and 50 recipients were identified. In addition, 23 plasma components were fractionated into plasma products. One of the blood component recipients was identified, in retrospect, as developing symptoms of vCJD 6½ years after receiving a transfusion of red cells donated 3½ years before the donor developed symptoms of vCJD. The donor was in the typical age range for vCJD; the recipient was the second oldest case to date. The older age may have accounted for the lack of a clinical diagnosis of vCJD before death. A second recipient, of a different donor who had died of vCJD, remained asymptomatic from the neurological viewpoint until death. Prion protein was detected in the spleen and in one cervical lymph node at autopsy, but not in the brain or gut-associated lymphoid tissue.10 The linking of these two cases, through the the TMER, to cases of vCJD in two different donors is the first evidence of possible transmission of vCJD through blood transfusion.
There is currently no evidence of vCJD in the other 18 surviving recipients in the TMER study. In the reverse study, 9 vCJD cases were reported to have themselves received blood transfusions, but only 5 had records of transfusion. These 5 individuals had received 122 components of blood (103 components to 1 recipient), which have been traced to 120 named donors (including the vCJD donor described above). The donors of 2 components are not traceable. None of the donors has been reported to develop vCJD.
Measures to Reduce the Risk of Transmission of vCJD Through Blood Components and Plasma Products
A number of donor selection criteria are aimed at excluding donors at risk of CJD.11 This includes a family history of CJD, treatment with pituitary-derived hormones and a history of neurosurgical procedures. Measures to protect against vCJD are more difficult. Many countries have introduced restrictions for donors who have lived in the UK between 1980 to 1996, when BSE was a concern through the food chain. Recall of plasma products produced from pools containing donations from donors who later developed vCJD were introduced in Europe in 1999.12
Risk reduction measures within the UK are more problematical. All adults (except strict vegetarians) are considered to have been at risk of vCJD through the food chain. An early risk assessment (February 1999) made two firm recommendations on risk reduction.13 The switch to non-UK plasma for fractionation was achieved over the second half of 1998. Leukodepletion was achieved in a progressive program completed by 1 November 1999, although the bulk of components were leukodepleted well before that date.
Other suggested risk reduction measures included the appropriate use of blood, consideration of blood conservation strategies including autologous donation and cell salvage, reduction in the volume of plasma in red cell and platelet components, use of imported (non-UK) plasma instead of fresh frozen plasma from UK donors, and exclusion of donors who have themselves received a blood transfusion. The 2004 report of possible transmission of vCJD by blood transfusion led to a further investigation of these possibilities. Exclusion of donors who had themselves received a blood transfusion in the UK since 1980 was implemented in April 2004. Approximately 2%–3% of donors have been excluded by this measure. The impact has been minimized by careful advance planning and recruitment campaigns, but places a further pressure on an already diminishing donor base.
Notification of Recipients of Affected Components and Products
The CJD Clinical Incident Panel was set up in 2000 to advise on management of incidents where patients might have been put at risk of CJD through clinical interventions. The UKBS asked for advice on the management of recipients of blood components donated by individuals who later developed vCJD. A further risk assessment was commissioned in 200213 and used to formulate the advice of the Panel and incorporated in a Framework Document.14 The advice indicated that these individuals should be notified, but this required a mechanism for notification and support. That work was progressing when the TMER demonstrated a link between a donor and recipient. As a result, the Secretary of State announced in December 2003 that other recipients of blood components donated by individuals who later developed vCJD would be notified (as already advised by the Clinical Incident Panel).
Notification was carried out through local Health Protection Teams (HPTs). Local Consultants in Communicable Disease Control (CsCDC) worked with others to satisfy themselves that the correct recipient had been identified (usually by reviewing medical records) before contacting General Practitioners with a view to notifying the recipients. Although the timing was less than ideal, notification of all living recipients was achieved within a short space of time.
The updated risk assessment has been applied to fractionated plasma products now known to have contained plasma from donors who later developed vCJD. As no UK-derived plasma has been used for manufacture since late 1998, these batches were transfused years ago. In general, “at risk” recipients have received multiple doses of certain products, in particular coagulation factors and intravenous immunoglobulin. The earliest cases of vCJD in former blood donors triggered notification exercises in 1998 and 2000. Some albumin products had been provided outside the UK and used as excipients (more or less inert substances used as a vehicle for medicinal agents) in other products. The notification therefore had repercussions outside the UK and led to many recipients, exposed to minute quantities of the affected product, being informed that they had been put at risk of vCJD. The current risk assessment would not put such individuals in a risk group. On the other hand, many hemophiliacs have been exposed to coagulation factor treatment from one or more affected batches. Others may have been exposed to batches not yet identified as a risk. Hemophiliacs, as a group, will be considered “at risk” and treated accordingly when requiring surgery. Individuals can elect to be told specifically if they have received treatment from affected batches.
The vCJD epidemic in the UK appears to be in decline.15 Nevertheless, experts warn against complacency, since cases seen between 1996 and 2004 might reflect only early onset cases. There could be later cases representing a longer incubation period of disease. The epidemic in cattle has not so far demonstrated any resurgence in the UK, but vCJD represents a TSE that has crossed the species barrier and may not behave in the same way as naturally occurring TSEs. UKBS are preparing themselves for the possibility of a blood test to detect individuals who might be infected with vCJD. The potential problems of such a test, such as sensitivity, specificity and predictive value, are already recognized. A facility to provide panels of blood samples for assessment of such tests is in progress. Other interventions, such as prion removal filters, are already on the horizon. For the United Kingdom, at least, the “precautionary principle” (application of proportionate measures to remove or reduce threats of serious harm, even in the absence of scientific proof) will operate for some time to come.
II. Transfusion-Related Acute Lung Injury (TRALI)
Christopher C. Silliman, MD, PhD*
University of Colorado School of Medicine, Bonfils Blood Center, 717 Yosemite Street, Denver CO 80230 This work was supported in part by Bonfils Blood Center, grant #HL59355 from NHLBI, and the Departments of Pediatrics and Surgery University of Colorado School of Medicine
Transfusion-related acute lung injury (TRALI) was not recognized as a distinct clinical entity until the 1980s with the report of a series of 36 patients.1 TRALI has since become recognized as a not uncommon clinical complication of transfusion and over the past two reporting years has become the leading cause of transfusion-related death in the US.2
Clinical Presentation and Treatment
While TRALI can occur within 6 hours of transfusion, the majority of cases present either during the transfusion or within the first 1–2 hours.1,3,4 TRALI is clinically identical to the acute respiratory distress syndrome (ARDS) with the insidious onset of acute pulmonary distress temporally related to transfusion.1,3,4 The clinical findings of TRALI consist of the rapid onset of tachypnea, cyanosis, dyspnea and fever (≥ 1°C).1,5 Auscultation of the lungs reveals diffuse crackles and decreased breath sounds, especially in dependent areas.1,5 The physiologic findings include acute hypoxemia, with PaO2/FiO2 < 300 mm Hg, and decreased pulmonary compliance despite normal cardiac function.1,4–,6 Radiographic examination reveals diffuse, fluffy infiltrates consistent with pulmonary edema.1,5 Treatment consists of aggressive respiratory support including supplemental oxygen and mechanical ventilation.1,4,5 Milder forms of TRALI have been described that require prompt delivery of supplemental oxygen alone.3,4,7,8 As with acute lung injury (ALI) and ARDS, there is no role for treatment with corticosteroids or diuretics, although one infant was successfully treated with extracorporeal membrane oxygenation (ECMO) for a particularly severe clinical presentation.5,7,9,10
The mortality from TRALI is 5%–25%, with lower rates being more common.3,5,10,11 Most patients recover within 72 hours; however, the data regarding TRALI are limited, and its attendant morbidity and mortality may be underappreciated due to lack of recognition and underreporting.3,5,10,11 In addition, in epidemiological studies of ARDS, blood transfusion was implicated as the most common risk factor for the genesis of ARDS, and a number of these cases may represent severe cases of TRALI.12
Differential Diagnosis
The differential diagnosis of patients who have pulmonary insufficiency following transfusion must include circulatory overload, anaphylactic transfusion reactions, and transfusion of blood products contaminated with bacteria.5,10,11 Transfusion-associated circulatory overload (TACO) presents within minutes to hours of the transfusion as respiratory distress with tachypnea, tachycardia, hypertension, and cyanosis.13 All blood components have been implicated in TACO, and it rapidly responds to aggressive diuresis and ventilatory support.14 Anaphylactic transfusion reactions involve respiratory distress related to bronchospasm manifested by tachypnea, wheezing, cyanosis, and severe hypotension.15 Facial and truncal erythema and edema are common, with urticaria characteristically involving the head, neck, and trunk.15 The respiratory distress from anaphylactic transfusion reactions is related to laryngeal edema rather than pulmonary edema as in TRALI.15 These reactions occur rapidly during the transfusion of any type of protein containing blood component and may occur following the transfusion of very small volumes of blood.15 Lastly, bacterial contamination manifests as fever, hypotension, and vascular collapse, which may include respiratory distress, although pulmonary involvement is not a consistent finding.16 Bacterial contamination is most frequent in platelet concentrates and packed red blood cells (PRBCs) and must be considered in patients who become acutely ill following transfusion of these components.16
Incidence and Patient Predisposition
In North America the reported incidence of TRALI is 1/5000–1/1323 transfusions.1,3,4 The incidence in Europe is markedly lower, 1/7980, but the true incidence of TRALI remains unknown, and it is unlikely to be determined until a consensus definition can be reached.11
Although no specific patient groups are predisposed to TRALI, Van Buren et al first postulated that the clinical status of the patient played a significant role in the genesis of TRALI.17 In a retrospective series of 10 TRALI patients compared to 10 patients with uncomplicated febrile or urticarial transfusion reactions, investigators hypothesized that TRALI was the result of two independent insults because every patient in the TRALI group (10/10) had an antecedent “first event” (such as recent major surgery, active infection, or massive transfusion); only 2/10 patients in the control group had such a predisposing clinical condition.3 These first events may have predisposed these patients to TRALI through activation of the pulmonary endothelium resulting in neutrophil (polymorphonuclear leukocyte [PMN]) sequestration in the lungs.3 Transfusion represented the second event that activated the PMNs in the lung causing endothelial damage and capillary leak, culminating in TRALI.3 A prospective, epidemiologic study demonstrated that two patient groups were at particular risk for developing TRALI, those patients in the induction phase of treatment for hematological malignancies and patients with cardiovascular disease who required bypass surgery.4 A recent report implicated massive transfusion as a risk factor for TRALI in patients receiving solid organ transplants.18 Moreover, follow-up information from the Mayo Clinic group in their series of 36 TRALI patients revealed that all of these patients had had recent surgery.1,10 In addition, two other patient groups appear to be at risk for TRALI, patients receiving fresh frozen plasma for coumadin reversal and patients with thrombotic thrombocytopenic purpura (TTP) who have widespread endothelial cell activation (personal communications from Patricia M. Kopko, MD and Richard Benjamin, MD).
Implicated Blood Components
In published series of TRALI, plasma-containing blood components are most commonly implicated, with whole blood–derived platelet concentrates (WB-PLTs) having caused the largest number of these reactions (Table 2 ).4,5,10 Although the plasma fraction of blood or blood components rather than the cellular constituents appears to be etiologic in TRALI, two of the most frequently implicated products (WB-PLTs and PRBCs) do not contain large amounts of plasma.
Pathogenesis
Two basic mechanisms have been proposed for the pathogenesis of TRALI. The first hypothesis is that TRALI is antibody-mediated and is caused by either the passive infusion of donor antibodies directed against recipient antigens or the infusion of donor leukocytes into a recipient who has antibodies directed against these donor leukocytes.5,7 The second hypothesis is that TRALI is caused by at least two independent events.3,4,10,19 The first event relates to the underlying clinical condition of the patient such that this individual has pulmonary endothelial activation resulting in pulmonary sequestration of neutrophils.3,4,10,19 The second event is the infusion of specific antibodies, directed against the adherent PMNs in the lung, or other biologic response modifiers (including lipophilic compounds) that cause activation of these primed, adherent PMNs resulting in activation of the microbicidal arsenal of PMNs leading to endothelial damage, capillary leak, and acute lung injury.3,4,10,19
Antibody-mediated TRALI due to HLA class I and antigranulocyte antibodies
In 1985, Popovsky and Moore proposed the infusion of donor antibodies to explain TRALI.1 They documented donor antibodies to granulocytes in 89% of these cases and antibodies to HLA antigens in 72% of cases examined.1 Most of the granulocyte antibodies did not exhibit specificity, but 59% of the HLA class I antibodies did.1 These findings have been confirmed by a number of other groups, and approximately 50% of donor antileukocyte antibodies display specific reactivity to recipient antigens.5,17,20,21 The infusion of leukoagglutinins is postulated to cause complement activation resulting in PMN influx into the lung followed by activation of these PMNs and release of cytotoxic agents, resulting in endothelial damage, capillary leak and pulmonary damage.1,5,21 In addition, TRALI can be caused by the binding of recipient antibodies to discrete antigens on transfused donor granulocytes; however, the number of viable PMNs is an issue and such a mechanism represents only 10% of TRALI cases.5,22 Importantly, this mechanism has particular relevance to patients receiving granulocyte transfusions and must be taken into account for these individuals.22
Animal models of antibody-mediated TRALI
The relevance of these observations was reported in an ex vivo rabbit model of TRALI in which lungs were isolated from rabbits and perfused with human PMNs, antibodies directed against specific PMN antigens or not, and rabbit plasma as a complement source. These experiments demonstrated that acute lung injury, characterized by severe pulmonary edema, resulted from the infusion of a mixture of human PMNs [HNA-3a+ (5b+)], human HNA-3a antibodies, and a complement source.23 In this ex vivo model pulmonary edema occurred 3–6 hours following the infusion of the admixture.23 However, if any one of the three components were deleted pulmonary edema did not occur.23 Furthermore, if immunoglobulins with indeterminate antigen specificity were infused together with complement and human PMNs, lung injury was not observed.23 Recently, Bux et al have demonstrated that employing anti-granulocyte antibodies and PMNs that have the cognate antigens may cause pulmonary edema without the addition of a complement source.24
Although antibodies to HLA class I or granulocyte antigens explain many TRALI cases, a number of problems with this mechanism remain. In the original description, only 59% of the immunoglobulins identified demonstrated antigen specificity and in published series of TRALI only about 50% of the implicated antibodies demonstrate specificity for recipient antigens.1,5 Since such “non-specific antibodies” did not cause TRALI in the ex vivo animal model, the significance of these immunoglobulins, especially in the context of TRALI, is undefined.23 The precise mechanism for antibody-mediated TRALI is not known; moreover, there are a number of cases of TRALI in which an antibody either in the donor or in the recipient is not present, and recently a case of autologous TRALI has been reported.1,3–,5,10
TRALI secondary to the infusion of class II HLA antibodies
Recently Kopko et al postulated that TRALI is due to the infusion of HLA class II antibodies with specificity for class II antigens in the recipient, and these findings have been confirmed.7,25,26 Furthermore, Kopko et al demonstrated in vitro that HLA class II antibodies implicated in TRALI could activate circulating monocytes that expressed these antigens causing synthesis of significant amounts of tumor necrosis factor alpha (TNFα), interleukin (IL)-1β and tissue factor over a 4-hour time period as compared to monocytes incubated with control sera.27 In addition, because HLA class II antigens also are expressed on endothelial cells, these investigators questioned whether infusion of class II antibodies into a recipient with cognate antigen expression on the pulmonary endothelium could manifest TRALI due to endothelial activation, changes in cellular shape, fenestration, and capillary leak.27
The infusion of class II HLA antibodies into patients who express the cognate antigens represents an attractive model for TRALI but raises a number of questions. First, although the synthesis of cytokines by circulating monocytes is interesting, there is a significant time delay for the production of these inflammatory mediators; moreover, in these studies these cytokines were intracellular and were not released into the supernatant.27 Second, this model has relevance only if the infused antibody specifically recognizes a recipient antigen.27
The two-event model of TRALI
All proposed models of TRALI implicate the PMN as the effector cell, and both TRALI and ARDS are clinically identical.1,3,4,7,10,19 Thus, it is important to understand PMN physiology, especially the interaction of PMNs with pulmonary vascular endothelium (Figure 2 ) and PMN-mediated cell damage leading to acute lung injury (Figure 3 ). Moreover, the underlying clinical condition of the patient is important as demonstrated in three “look back” studies; those of Van Buren with a donor with HNA-2b antibodies, Kopko with a donor with HNA-3a antibodies and Toy with a donor with multiple HLA class I and II antibodies8,17 (P. Toy, personal communication). These studies demonstrated that the majority of transfused patients did not develop TRALI even though their leukocytes contained the cognate antigens 8,17 (P. Toy, personal communication).
The accumulation of PMN priming activity in stored blood
During routine storage of cellular components, an effective PMN priming activity accumulates that is lipophilic as determined by its solubility in chloroform.28,29 Separation and characterization of this activity in WB, PRBCs and platelet concentrates demonstrated that this activity consisted of a mixture of lysophosphatidylcholines (lyso-PCs).28,29 These compounds effectively prime the PMN oxidative burst and can activate primed adherent PMNs both in vitro.28–,31 In addition an in vitro model of TRALI that employed human pulmonary microvascular endothelial cells (HMVECs) as targets demonstrated that two events were required for PMN cytotoxicity.31 The first was HMVEC activation, which demonstrated PMN adherence to the HMVEC surface that required chemokines for PMN attraction and firm adherence via the PMN β2-integrins and the ICAM-1 on HMVECs.31 The second event, introduction of lyso-PCs from stored blood, could then activate these PMNs, causing HMVEC death, and this PMN cytotoxicity could be abrogated by inhibitors of the respiratory burst.31
The two-event animal model of TRALI
The two-event model of TRALI has been verified in an animal model.19,32 Rats were treated with endotoxin (lipopolysaccharide [LPS]) for 2 hours to approximate active infection, one of the predisposing clinical conditions associated with TRALI.19,32 LPS activated the pulmonary vascular endothelium resulting in pulmonary sequestration of PMNs, which was confirmed by the pulmonary histology.19,32 The lungs were then isolated and perfused with buffer controls or 5% heat-treated plasma from fresh (day 0) or stored (day 5 or day 42) plasma from platelet concentrates (apheresis platelet concentrates [A-PLTs] or whole blood–derived platelet concentrates [WB-PLTs]) or PRBCs, respectively.19,32 The plasma fraction of PRBCs and platelet concentrates were taken from the same units so that the only variable among the different plasma fractions was storage time.19,32 The lungs isolated from buffer pretreated animals did not evidence ALI with any of the perfusates.19,32 In addition, the lungs from LPS-treated animals perfused with fresh (day 0) blood components also did not evidence acute lung injury. However, lungs from LPS pretreated animals, perfused with plasma from stored components (day 42 PRBCs or day 5 platelet concentrates) caused ALI as documented by pulmonary edema, lung histology, and increased leukotriene concentrations in the perfusate.19,32 In addition, both the lipid fraction and purified lipids from stored, but not fresh, PRBCs, WB-PLTs, and A-PLTs caused TRALI.19,32 Thus, both the plasma and lipids from stored blood products caused TRALI in this model.19,32
Apparently healthy patients who experience TRALI would seem to disprove the two-event model. However, by definition, patients who require transfusion are not healthy. Moreover, a study of the appearance and activity of PMNs from “healthy” donors indicated that the donors were in fact not well; all evidenced infections, 2 with sinusitis and 3 with viral syndromes, over the next 24 hours.33 Thus, it may be difficult to determine if transfused patients are indeed healthy. It is notable that TRALI has occurred, albeit rarely, in neutropenic patients. Hypotheses regarding these reactions have included the infusion of permeability factors including venule endothelial growth factor (VEGF) or class II HLA antibodies that may recognize antigens on pulmonary ECs and cause EC fenestration.7,34
Relevance of the two-event model to clinical TRALI
A retrospective clinical study of TRALI patients demonstrated that there was an effective PMN priming activity, which was a lipid, in the patients’ plasma at the time that TRALI was recognized, which was not in the patient’s pre-transfusion typing serum, and postulated that the clinical condition of the patient was important as a first event.3 In this study, patients with uncomplicated febrile and urticarial reactions comprised a control group that did not demonstrate PMN priming activity in their post-reaction blood samples.3 Importantly, two of the predisposing conditions postulated by this study to be involved with the two-event pathogenesis of TRALI, massive transfusion and recent major surgery, have since been implicated by other groups as predisposing conditions for TRALI.3,10,18
In a prospective analysis of TRALI, the role of cytotoxic HLA class I, class II and anti-granulocyte antibodies were examined.4 Of the donors tested, only 1/28 exhibited an antibody with specificity (HLA A26) similar to positive controls.4 The implicated blood products demonstrated significant plasma PMN priming activity as compared to similar products from the same facility and identical storage time that did not cause transfusion reactions.4 There was lipid priming activity in all TRALI patients at the time of recognition, which consisted of two classes of lipids: neutral lipids and lyso-PCs.4 In addition, the roles of IL-6 and IL-8 were examined and both increased during storage, but only IL-6 was significantly increased in the TRALI patients versus the pre-transfusion sample.4 Thus, in this series TRALI was due to two events: the first was the clinical condition of the patient, and the second was the infusion of bioactive lipids in the stored blood component.4
Prevention
Decreasing blood usage will diminish TRALI. In addition, all donors who have been implicated in TRALI reactions should be temporarily disqualified from donation until leukocyte antibody testing can be completed. If these donors have antibodies to high frequency leukocyte antigens, they should be disqualified from plasma or platelet donation; otherwise, if these studies are negative, they should be returned to the donor pool. Current data do not support the disqualification of multiparous, female donors at this time, and such disqualification may be disastrous to blood centers in which these individuals make up 20%–30% of the donor pool. For high-risk surgical procedures requiring transfusions, washing of cellular components removes both antibodies and lipids from the plasma fraction. Fresher products may be used to obviate the effects of lipids in high-risk patients who are not neutropenic such that the use of PRBCs < 21 days and platelet concentrates < 3 days will avoid much of the effects of these compounds that accumulate during storage.
Definition
Because of the increase in TRALI cases in the United States, a working group was convened in May of 2003 by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), to arrive at a consensus definition. TRALI was also the subject of a two-day Canadian Consensus Conference sponsored by Canadian Blood Services and Héma-Quebec, and a number of European groups, including the British and the Dutch, are currently developing a consensus definition of this life-threatening adverse event. TRALI is acute lung injury, as the name implies, and should be considered in all cases of respiratory distress with profound hypoxemia (PaO2/FiO2 < 300 mm Hg) temporally related to a transfusion (Table 3 ). TRALI may occur with the presence of risk factors for ALI or not as long as the observed ALI is temporally related to the transfusion (Table 4 ). Risk factors for ALI should not exclude TRALI, for it is a form of ALI that has a two-event etiology, with the second event related to factors present within the transfused component(s). Moreover, worsening pulmonary function following transfusion in a patient with compromised respiratory status should also be considered TRALI. Patients who are transfused are not healthy; even though patients who appear healthy may have the early stages of an acute illness whose presentation is sub-clinical. As stated previously, it is often difficult to assess patients who may have early phases of acute infection.33
III. Utilization of Recombinant Factor VIIa (rFVIIa) in Non-Approved Settings
Lawrence T. Goodnough, MD*
Transfusion Medicine Service, Washington University Medical School and Barnes-Jewish Hospital, St. Louis MO Current address: Stanford Medical Center, 300 Pasteur Drive, H-1402, Stanford CA 94304-5626
Recombinant FVIIa (rFVIIa) has been approved for treatment of bleeding in hemophilia patients with inhibitors. It has also been successfully used in nonhemophilia patients with acquired antibodies against FVIII (acquired hemophilia). Pharmacological doses of rFVIIa have been found to enhance the thrombin generation on already activated platelets and, therefore, may also likely be of benefit in providing hemostasis in other (non-approved) situations characterized by profuse bleeding and impaired thrombin generation,1 such as patients with thrombocytopenia and in those with functional platelet defects.2,3 Additionally, it has been used successfully in a variety of less well-characterized surgical bleeding situations4–,6 as well as in patients with impaired liver function.7
To date, case reports, anecdotal experience, and limited clinical trials describe these uses; data from randomized clinical trials are limited. Because of the recent trends in rFVIIa usage in non-approved settings among physicians from various disciplines, significant concerns about its safety, efficacy, and costs have arisen. Additionally, dosing of rFVIIa for these potentially broad clinical applications is not standardized. The decision to use rFVIIa for patients with uncontrolled bleeding continues to be one that is made by individual physicians, assisted by their hospital pharmacotherapeutics or transfusion committees.8
The transfusion medicine service of Barnes-Jewish Hospital in St. Louis, Missouri, therefore undertook a review of the currently available data and experience regarding rFVIIa therapy in non-approved settings and developed educational guidelines and policies for its potential use in these areas. These elements were approved by our hospital transfusion committee and published in our Laboratory Medicine Newsletter. These policies for rFVIIa therapy undergo periodic review and revision as relevant new information and data are generated, and are presented here.
Experience with rFVIIa in Non-Approved Settings
Complex surgery and traumas resulting in profuse bleeding
A hemostatic effect has been demonstrated following the administration of rFVIIa in a limited number of patients after trauma and bleeding.5,6 Seven trauma patients treated with rFVIIa after failure of conventional measures to achieve hemostasis6 reported cessation of diffuse bleeding and correction of abnormal coagulation assays; 3 of the 7 patients died of reasons other than bleeding or of thromboembolism.
Anecdotal case reports have been published that describe the successful use of rFVIIa in patients with substantial perisurgical bleeding.4 A prospective, randomized study of rFVIIa (20 μg/kg or 40 μg/kg) versus placebo perioperatively in 36 patients undergoing radical retropubic prostatectomy found that the cohorts receiving rFVIIa had substantially less median operative blood loss compared to placebo (1235 mL, 1089 mL, and 2688 mL, respectively).9
Nine patients with coagulopathy and urgent neurosurgical intervention were reviewed after receiving preoperative rFVIIa (40–90 μg/kg).10 Post-rFVIIa coagulation parameters normalized as early as 20 minutes after infusion, with no noted procedural or operative complications. No associated thromboembolic complications were observed.
The experience of rFVIIa use in trauma with excessive bleeding as well as in postoperative profuse bleeding, based largely on case reports, indicates a hemostatic effect of rFVIIa given in doses of 20–120 μg/kg. Initial dosage approval for administration is recommended to be a 4.8 mg vial, which for an adult patient is a dose range of 50–100 μg/kg for a body weight range of 100–50 kg (Table 5 ). One or two administrations seem to be enough to decrease the bleeding significantly. Controlled randomized studies are, however, required to prove any beneficial effect of rFVIIa in these patients.
Congenital FVII deficiency
In a randomized study, 17 FVII-deficient patients were treated with rFVIIa,11 ranging from 21 to 27 μg/kg based on the dose capable of normalizing the prothrombin time (PT) 15 minutes after injection. The treatment resulted in excellent resolution of all hemarthroses treated. An infant with severe FVII deficiency and massive intracranial hemorrhage was evaluated after administration of rFVIIa at three dose levels: 15 μg/kg, 22 μg/kg, and 30 μg/kg.12 FVII levels were > 100% between 30 and 180 minutes after each infusion, with mean trough levels above 25% at all three dose levels. The recommended dosage at the Transfusion Medicine Service at Washington University for rFVIIa replacement therapy in congenital FVII deficiency is therefore 20 μg/kg (Table 5 ).
Patients receiving oral anticoagulant therapy
One report describes the use of rFVIIa in 7 adult patients with prolonged INR, 3 of whom required surgery. The doses administered ranged from 20 μg/kg to 90 μg/kg, and all patients were reported to have a positive outcome.13 These observations indicate that rFVIIa may be used to reverse the effect of warfarin or other vitamin K-antagonist therapy in cases in which the administration of vitamin K alone has been found to be insufficient. Two published reports of 15 total patients treated with rFVIIa for reversal of excessive anticoagulation with coumadin support a dosage of 20 μg/kg, or 1.2 mg for an adult patient.14,15 A recent review of 19 patients with acute warfarin-associated intracranial hemorrhage over this same time period at our institution, who received rFVIIa (30–135 μg/kg) as well as vitamin K (10 mg/D × 3) and FFP (1307 ± 652 mL) for treatment, found that treatment was associated with rapid correction of INR and that single doses appeared safe in this high-risk population.16
Patients with impaired liver function
A hemostatic effect of rFVIIa has been proven in a limited number of liver disease patients.7 In one clinical trial, 10 cirrhotic patients whose PT did not correct to within 2 seconds above the control reference value were given three successive dosages of rFVIIa (5, 20, or 80 μg/kg) during a 3-week period in a randomized study.17 The PT transiently corrected to normal in all three dosage groups.
A multicenter trial studied 71 patients with advanced liver disease who were undergoing laparoscopic liver biopsy.18 The patients were randomized to receive one of four doses (5, 20, 80, or 120 μg/kg); 48 (74%) of 65 patients achieved hemostasis within 10 minutes. One thrombotic event and one case of disseminated intravascular coagulation (DIC) were reported, not felt by the authors to be related to rFVIIa therapy. Despite these complications, the authors concluded that laparoscopic liver biopsy can be performed safely and reliably by using rFVIIa in patients in whom the standard procedure might be contraindicated because of coagulopathy.
Safety
Of the more than 170,000 standard doses of rFVIIa given after its approval (almost all to patients with hemophilia and inhibitors), only rare (< 1:11,300) thrombotic events have been reported.1 Five patients with thromboembolic events have been reported. Six patients developed acute myocardial infarction in association with rFVIIa treatment. Five out of the 6 patients were over 70 years old, and 2 hemophilia patients had a history of cardiovascular disease, which also was the case in 2 patients with acquired hemophilia. Cerebrovascular disorders were reported in 4 patients, 3 of whom were more than 55 years old, 1 with hemophilia, and the others having acquired hemophilia.
Thrombotic complications have also been reported with rFVIIa therapy in patients without inhibitors to FVIII or FIX. An acute cerebral vascular accident and death occurred in a clinical trial of rFVIIa (90 μg/kg) before and after minor surgery or dental procedures in patients with FXII deficiency.18,19 The last of 10 patients enrolled in an open-label, dose-escalation trial to prevent rebleeding after subarachnoid hemorrhage developed middle cerebral artery thrombosis after receiving rFVIIa.20 In a high-risk trauma population, 3 of 40 (7.5%) patients who were deemed at high risk for thrombosis developed thrombotic complications after receiving rFVIIa.21
We reported a patient who had a fatal thrombosis after administration of activated prothrombin complex concentrate (APCC), who had also received two doses of rFVIIa more than 6 hours earlier, while supported by extracorporeal membrane oxygenation.22 Because of this experience, we recommend that patients should not receive combination therapy with both APCC and rFVIIa.
On the basis of these reports, use of activated factor concentrates should be used with caution in patients with known hypercoagulability (e.g., history of thrombotic complications, established thrombotic disorders like factor V Leiden, antiphospholipid syndrome, etc.) or who have excessive bleeding in the setting of DIC or other states of generalized activation of the hemostatic system (e.g., after cardiac surgery, patients on extracorporeal membrane oxygenation [ECMO] or ventricular assist devices) based on the potential for development of localized or systemic intravascular thrombosis.
Utilization
A review of our transfusion service for 2002 to 2004 indicated that 122 patients received rFVIIa therapy for non-approved indications (Table 6 ). The mean dose administered for all of these patients was 8.3 ± 7.7 mg (range 1.2–60 mg) at a mean cost of $7215 per patient. Over this same interval, 8 patients with hemophilia and inhibitors received a mean dose of 75.7 ± 80.8 mg at a mean cost of $60,800 per patient.
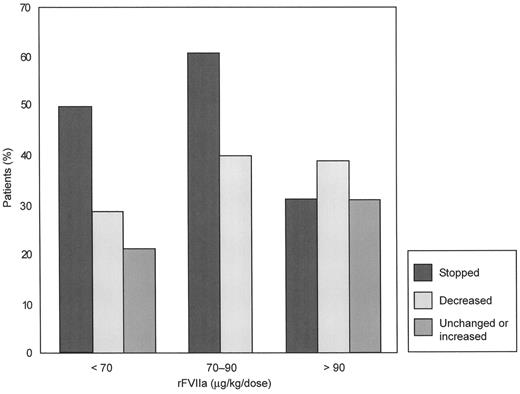
A retrospective review of 40 patients with coagulopathic bleeding in a variety of medical and surgical settings from 13 hospitals in a web-based database (excluding prior history of coagulopathy and trauma patients) who received rFVIIa (15180 μg/kg, with 38 patients receiving fewer than 5 doses) found that 32 (80%) achieved complete (n = 18) or partial (n = 14) cessation of bleeding.23 Responses occurred in all dose ranges, without any evidence of a dose-response effect; the percentages of complete, partial, or no response were not different at doses of < 70 μg/kg, 70 to 90 μg/kg or > 90 μg/kg (Figure 4 ). Significantly fewer blood products were administered after rFVIIa therapy. Twenty-three (58%) patients died, reflecting the unstable clinical status of the patients at the decision point for considering rFVIIa therapy.
Dose and timing of rFVIIa have yet to be defined in this diverse patient population, and formal prospective trials are needed; in the meantime, the decision on when and where to use rFVIIa for patients with uncontrolled bleeding continues to be one that must be made by individual physicians, assisted by their hospital pharmacotherapeutic or transfusion committees.
Features of the different forms of Creutzfeldt-Jakob disease (CJD).
| Form of CJD . | Distribution . | Age . | Duration of illness . | Clinical features . |
|---|---|---|---|---|
| Sporadic CJD | Worldwide | 60–80 | Median 4 months; rarely greater than 2 years | Rapidly progressive dementia Impaired movement Myoclonus |
| Variant CJD | Predominantly UK | Median age 27 (range 12–74) | Median 14 months (range 6–40) | Early psychiatric symptoms Unpleasant sensory symptoms Late dementia |
| Familial (genetic) CJD | Worldwide | 30–50 | Variable; may be up to 5 years | Varies with underlying mutation; may resemble sporadic CJD, or a progressive cerebellar ataxia |
| Iatrogenic CJD | Worldwide | Any | 2–18 months, occasionally some years | Depends upon route of infection. Intramuscular route: progressive cerebellar ataxia Neurological route: rapidly progressive dementia |
| Form of CJD . | Distribution . | Age . | Duration of illness . | Clinical features . |
|---|---|---|---|---|
| Sporadic CJD | Worldwide | 60–80 | Median 4 months; rarely greater than 2 years | Rapidly progressive dementia Impaired movement Myoclonus |
| Variant CJD | Predominantly UK | Median age 27 (range 12–74) | Median 14 months (range 6–40) | Early psychiatric symptoms Unpleasant sensory symptoms Late dementia |
| Familial (genetic) CJD | Worldwide | 30–50 | Variable; may be up to 5 years | Varies with underlying mutation; may resemble sporadic CJD, or a progressive cerebellar ataxia |
| Iatrogenic CJD | Worldwide | Any | 2–18 months, occasionally some years | Depends upon route of infection. Intramuscular route: progressive cerebellar ataxia Neurological route: rapidly progressive dementia |
Clinical criteria for the diagnosis of acute lung injury (ALI) and transfusion-related ALI (TRALI).35
| *For patients without an arterial blood gas, pulse oximetry less than 90% meets the criteria for hypoxemia. |
| †Irrespective of the pulmonary end expiratory pressure (PEEP). |
|
| *For patients without an arterial blood gas, pulse oximetry less than 90% meets the criteria for hypoxemia. |
| †Irrespective of the pulmonary end expiratory pressure (PEEP). |
|
Blood products implicated in transfusion-related acute lung injury (TRALI).*
| * The implicated blood products are listed in the order of numbers of published cases of TRALI. |
|
| * The implicated blood products are listed in the order of numbers of published cases of TRALI. |
|
Definition of transfusion-related acute lung injury (TRALI).
| *During or within 6 hours of the completion of the transfusion. |
| †Recognition by the clinical care team that ALI is most likely due to the transfusion. |
| Abbreviations: ALI, acute lung injury |
|
| *During or within 6 hours of the completion of the transfusion. |
| †Recognition by the clinical care team that ALI is most likely due to the transfusion. |
| Abbreviations: ALI, acute lung injury |
|
Recombinant factor VIIa (rFVIIa) dosing policies.*
| * Goodnough LT. Recombinant Factor 7a. Washington University Laboratory Medicine Newsletter 2003 ;9 :1 –4. |
| **Currently available in vials of 1.2 mg, 2.4 mg, and 4.8 mg. |
| Abbreviations: FFP, fresh frozen plasma; DDAVP, desmopressin acetate; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; VAD, vincristine, doxorubicin, and dexamethasone; INR, international normalized ratio; PT, prothrombin time; aPTT, activated partial thromboplastin time |
| Currently Approved Clinical Settings |
|
| Currently Nonapproved Clinical Settings |
|
| * Goodnough LT. Recombinant Factor 7a. Washington University Laboratory Medicine Newsletter 2003 ;9 :1 –4. |
| **Currently available in vials of 1.2 mg, 2.4 mg, and 4.8 mg. |
| Abbreviations: FFP, fresh frozen plasma; DDAVP, desmopressin acetate; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; VAD, vincristine, doxorubicin, and dexamethasone; INR, international normalized ratio; PT, prothrombin time; aPTT, activated partial thromboplastin time |
| Currently Approved Clinical Settings |
|
| Currently Nonapproved Clinical Settings |
|
Recombinant factor VIIa (rFVIIa) utilization.
| Series . | 2002 Jan–June . | 2002 July–Dec . | 2003 Jan–June . | 2003 July–Dec . | 2004 Jan–June . | Total . |
|---|---|---|---|---|---|---|
| * Values expressed as means ± SD with range in parenthesis. | ||||||
| All patients (n) | 5 | 14 | 26 | 47 | 38 | 130 |
| # of doses | 18 | 45 | 121 | 121 | 78 | 383 |
| Mean total dose* (mg) (range) | 12.2 ± 9.1 (2.4–24) | 12.0 ± 12.1 (2.4–48) | 21.2 ± 50.5 (2.4–246) | 10.4 ± 13.0 (1.2–60) | 8.6 ± 13.5 (1.2–84) | 12.5 ± 26.0 (1.2–246) |
| On-label – FVIII Inhibitors (n) | 2 | 0 | 2 | 2 | 2 | 8 |
| # of doses | 9 | 0 | 79 | 12 | 26 | 126 |
| Mean total dose* (mg) (range) | 14.4 ± 6.8 (9.6–19.2) | - | 125 ± 121 (4–246) | 57 ± 2.5 (55.2–58.8) | 43.2 ± 57.7 (2.4–84.0) | 75.7 ± 80.8 (2.4–246) |
| Off-label (n) | 3 | 14 | 24 | 45 | 36 | 122 |
| # of doses | 9 | 45 | 42 | 100 | 52 | 248 |
| Mean total dose* (mg) (range) | 10.8 ± 11.6 (2.4–24) | 12 ± 12.1 (2.4–48) | 8.2 ± 4.8 (2.4–24.5) | 8.3 ± 8.6 (1.2–60) | 6.7 ± 5.2 (1.2–27.6) | 8.3 ± 7.7 (1.2–60) |
| Series . | 2002 Jan–June . | 2002 July–Dec . | 2003 Jan–June . | 2003 July–Dec . | 2004 Jan–June . | Total . |
|---|---|---|---|---|---|---|
| * Values expressed as means ± SD with range in parenthesis. | ||||||
| All patients (n) | 5 | 14 | 26 | 47 | 38 | 130 |
| # of doses | 18 | 45 | 121 | 121 | 78 | 383 |
| Mean total dose* (mg) (range) | 12.2 ± 9.1 (2.4–24) | 12.0 ± 12.1 (2.4–48) | 21.2 ± 50.5 (2.4–246) | 10.4 ± 13.0 (1.2–60) | 8.6 ± 13.5 (1.2–84) | 12.5 ± 26.0 (1.2–246) |
| On-label – FVIII Inhibitors (n) | 2 | 0 | 2 | 2 | 2 | 8 |
| # of doses | 9 | 0 | 79 | 12 | 26 | 126 |
| Mean total dose* (mg) (range) | 14.4 ± 6.8 (9.6–19.2) | - | 125 ± 121 (4–246) | 57 ± 2.5 (55.2–58.8) | 43.2 ± 57.7 (2.4–84.0) | 75.7 ± 80.8 (2.4–246) |
| Off-label (n) | 3 | 14 | 24 | 45 | 36 | 122 |
| # of doses | 9 | 45 | 42 | 100 | 52 | 248 |
| Mean total dose* (mg) (range) | 10.8 ± 11.6 (2.4–24) | 12 ± 12.1 (2.4–48) | 8.2 ± 4.8 (2.4–24.5) | 8.3 ± 8.6 (1.2–60) | 6.7 ± 5.2 (1.2–27.6) | 8.3 ± 7.7 (1.2–60) |
Number of onsets per annum of variant Creutzfeldt-Jakob disease (vCJD) (UK).
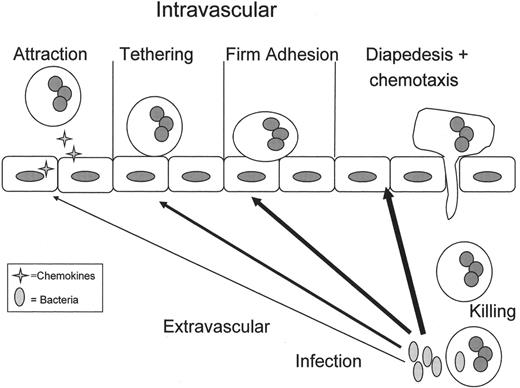
Normal polymorphonuclear leukocyte (PMN) emigration from the vasculature to the site of infection in the tissues.
In response to an infection in the tissues, inflammatory signals (arrows) diffuse to the vasculature and activate the vascular endothelium causing release of chemokines (4-pointed stars), which attract PMNs to the endothelial surface. Attraction is followed by selectin-mediated PMN rolling and β2-integrin:ICAM-1 mediated firm adhesion of PMNs to endothelial cells (ECs).10 These PMNs, which have undergone a change from a non-adhesive to an adhesive phenotype are now primed.10,31 Priming of PMNs enhances the microbicidal function of PMNs to a subsequent stimulus and changes the activity of PMNs such that stimuli that normally do not cause activation of quiescent neutrophils are able to activate primed PMNs.10,31 It is important to note that priming is part of the orderly process of PMN transmigration to the tissues, and although there are benefits to enhanced PMN function including efficient destruction of pathogens, it is clear priming may be detrimental to the host leading to PMN-mediated organ injury, especially acute respiratory distress syndrome (ARDS).10,31 The PMNs then diapedese through the endothelial layer, chemotax to the site of infection and phagocytize and destroy the bacterial invaders.10
Normal polymorphonuclear leukocyte (PMN) emigration from the vasculature to the site of infection in the tissues.
In response to an infection in the tissues, inflammatory signals (arrows) diffuse to the vasculature and activate the vascular endothelium causing release of chemokines (4-pointed stars), which attract PMNs to the endothelial surface. Attraction is followed by selectin-mediated PMN rolling and β2-integrin:ICAM-1 mediated firm adhesion of PMNs to endothelial cells (ECs).10 These PMNs, which have undergone a change from a non-adhesive to an adhesive phenotype are now primed.10,31 Priming of PMNs enhances the microbicidal function of PMNs to a subsequent stimulus and changes the activity of PMNs such that stimuli that normally do not cause activation of quiescent neutrophils are able to activate primed PMNs.10,31 It is important to note that priming is part of the orderly process of PMN transmigration to the tissues, and although there are benefits to enhanced PMN function including efficient destruction of pathogens, it is clear priming may be detrimental to the host leading to PMN-mediated organ injury, especially acute respiratory distress syndrome (ARDS).10,31 The PMNs then diapedese through the endothelial layer, chemotax to the site of infection and phagocytize and destroy the bacterial invaders.10
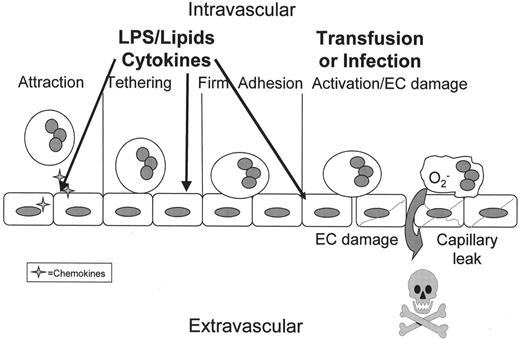
Polymorphonuclear leukocyte (PMN)–mediated tissue injury.
If the orderly process of PMN transmigration is altered by a stimulus coming from the intravascular space (arrows) rather than the tissues, these intravascular stimuli activate vascular endothelial cells (ECs) and cause attraction, firm adhesion and priming of PMNs. As shown the vascular endothelium is activated causing the release of chemokines (stars) that attract PMNs to the endothelial surface followed by selectin-mediated tethering and firm adhesion through the ICAM-1:β2-integrin interaction. However, since there are not signals to cause diapedesis and PMN chemotaxis into the tissues, the PMNs become sequestered, and these primed, hyper-reactive leukocytes may be activated by stimuli that normally have no effect including antibodies directed against specific leukocyte antigens or the lipids that accumulate during routine storage of cellular blood components. Activation of these adherent PMNs causes endothelial damage (ECs with diagonal lines), capillary leak (the large arrow), and organ injury.10
Polymorphonuclear leukocyte (PMN)–mediated tissue injury.
If the orderly process of PMN transmigration is altered by a stimulus coming from the intravascular space (arrows) rather than the tissues, these intravascular stimuli activate vascular endothelial cells (ECs) and cause attraction, firm adhesion and priming of PMNs. As shown the vascular endothelium is activated causing the release of chemokines (stars) that attract PMNs to the endothelial surface followed by selectin-mediated tethering and firm adhesion through the ICAM-1:β2-integrin interaction. However, since there are not signals to cause diapedesis and PMN chemotaxis into the tissues, the PMNs become sequestered, and these primed, hyper-reactive leukocytes may be activated by stimuli that normally have no effect including antibodies directed against specific leukocyte antigens or the lipids that accumulate during routine storage of cellular blood components. Activation of these adherent PMNs causes endothelial damage (ECs with diagonal lines), capillary leak (the large arrow), and organ injury.10
Response to recombinant factor VIIa (rFVIIa) in each of three dose groups as assessed by the treating clinician.
Number of patients per group: < 70 μg/kg/dose, n = 14; 70–90 μg/kg/dose, n = 10; > 90 μg/kg/dose, n = 16.
Reprinted with permission from O'Connell NM, et al. Transfusion. 2003;43:1711–1716.
Response to recombinant factor VIIa (rFVIIa) in each of three dose groups as assessed by the treating clinician.
Number of patients per group: < 70 μg/kg/dose, n = 14; 70–90 μg/kg/dose, n = 10; > 90 μg/kg/dose, n = 16.
Reprinted with permission from O'Connell NM, et al. Transfusion. 2003;43:1711–1716.