Abstract
The diagnosis and management of thrombocytopenia is a growing component in the practice of hematology. The frequency with which hematologists are called in consultation for thrombocytopenia continues to increase with the advent of routine automated platelet determinations and the introduction of new medications. For most patients, such as those with inherited and auto-immune thrombocytopenia, emphasis is focused on efforts to treat or forestall bleeding without excess drug-induced toxicity or burden to the patient. However, in disorders such as heparin-induced thrombocytopenia (HIT), avoidance of thrombotic complications is the key to management. In this chapter, we provide the pediatric and adult hematologist with new insights into the pathogenesis and recognition of congenital inherited thrombocytopenias (CTP), a hitherto difficult to comprehend constellation of clinical entities. We also highlight new approaches to the diagnosis and treatment of two of the more common thrombocytopenic conditions encountered in practice, autoimmune or idiopathic thrombocytopenic purpura (ITP) and HIT.
In Section I, Dr. James Bussel discusses CTPs and their distinction from childhood ITP. He emphasizes the clinical features that enable the pediatrician and hematologist to suspect the diagnosis of CTP and those that are of use to subcategorize the various entities, where possible. He also emphasizes newer molecular markers that afford definitive diagnosis in some cases and provide insight into platelet production. This section highlights the characteristic associated findings and differences in the natural history and approaches to management of the various entities.
In Section II, Dr. Robert McMillan discusses adult chronic ITP. He revisits the utility of platelet antibody determination in diagnosis and review new insights into pathogenesis. The role of Helicobacter pylori infection and the timing of splenectomy in the management of acute and emergent ITP are examined. New insights into the natural history of ITP post-splenectomy and management strategies for patients with severe, chronic, refractory ITP are discussed.
In Section III, Dr. James Zehnder updates us on HIT. He emphasizes new insights into the clinical presentation and pathogenesis of this condition. He critically reviews the utility of laboratory testing for heparin-dependent antibodies. Recent studies on the use of direct thrombin inhibitors are examined and the management of cardiopulmonary bypass surgery in patients with HIT is discussed.
I. Congenital Hereditary Familial Thrombocytopenias
James B. Bussel, MD*
New York Presbyterian Hospital, Weill Cornell Medical Center, 525 E 68th Street, Payson 695, New York NY 10021-4870
Congenital thrombocytopenias represent a very small percentage of the thrombocytopenias that are seen by hematologists and oncologists. Even when chemotherapy-induced thrombocytopenia and overt infections are excluded and only isolated thrombocytopenia is considered, at least 95% of these cases in both children and adults will be primarily autoimmune thrombocytopenia (ITP) or drug-induced thrombocytopenia. However, more and more routine platelet counts are obtained allowing the identification of asymptomatic or mildly symptomatic children and adults, which increases the number of isolated thrombocytopenias referred to specialists. Even in adults, a small percentage of cases represent a type of congenital thrombocytopenia (CTP), and a number of cases of CTP have been misdiagnosed as ITP and the patients subjected to splenectomy and/or cyclophosphamide, among other inappropriate therapies.1 Recent developments, especially in identification of molecular defects, have highlighted the manifestations of the congenital cases of nonimmune thrombocytopenia and improved the ability to identify and distinguish among them.2,3
Traditionally, the CTPs are divided into categories based on accessible information. Since blood smears are universally available, platelet size (as estimated on smear) is often considered to be the first point of triage. However, at least three additional triage points may be identified by history prior to review of the smear that would suggest CTP (Table 1 ). One is a family history of “ITP.” The second is the absence of an increase in the platelet count in response to ITP treatments. The third is the presence of certain “associated features” (see below) that are identified along with the thrombocytopenia. Some of these features, in the context of isolated thrombocytopenia, would suggest very specific diagnoses, e.g. thrombocytopenia with absent radii (TAR). Depending upon the specificity of the finding and the availability of specific testing, a suspected entity can then be confirmed or rejected by further testing, i.e., functional or molecular study, as needed.
Family History
The most obvious feature suggesting CTP, while nonspecific, is the presence of a family history of thrombocytopenia. This should immediately raise suspicion of CTP, rather than ITP, especially if more than two family members are involved and/or the family members are closely related, especially parent and child or maternal uncle and nephew. Many types of CTP have an autosomal dominant inheritance, but several are X-linked or autosomal recessive, which means that the affected child or adult may be the propositus/index case. A family history of “ITP” has been reported, for example, in familial autoimmune disease in which the family history may be more consistent with systemic lupus (SLE). Based on anecdotal experience, the infrequent cases of familial ITP often appear coincidental, e.g., two cousins. In the past, cases of familial CTP have been considered (and reported) to be ITP because of the presence of platelet-associated antibodies using tests now known to have low specificity. While current testing that is antigen-specific (involves a specific platelet glycoprotein such as GPIIb/IIIa) has greater predictive value than tests in which the whole platelet is the target, it remains to be demonstrated that this testing will distinguish ITP from CTP, especially in those subsets in which there may be an autoimmune component (see below).
Nonresponse to ITP Treatment
In cases where it is necessary to distinguish CTP from ITP, the most definitive finding available by history or clinically, prior to specific laboratory investigation for CTP including review of the smear where diagnostic, is the “failure” to respond to ITP-specific therapy. This includes not only IVIG and IV anti-D, but also splenectomy and steroids. It also potentially includes other immune-modulating therapies such as azathioprine, cyclophosphamide, and anti-CD20. While the lack of response in patients with CTP to these treatments is a universally accepted criterion based on anecdotal experience, an exact definition of lack of response has not been established or verified. For example, there are no well-defined response thresholds, i.e., how high does the platelet count have to go after treatment with IVIG to diagnose ITP and exclude CTP? Arbitrarily, a peak platelet response to treatment > 30,000/μl increase from baseline would suggest that the case in question is not CTP. Conversely, a < 10,000/μl peak increase is compatible with CTP, although it just as compatible with a diagnosis of “refractory” ITP. Numbers in between might favor ITP but are even more ambiguous. A confusing feature is that some children with Wiskott-Aldrich syndrome and velocardiofacial syndrome (also known as DiGeorge syndrome) respond to corticosteroids, IVIG, and/or splenectomy either because there is an immunological component to the thrombocytopenia or because impeding the clearance mechanism for aberrant platelets helps to offset impaired platelet production. Since “spontaneous” fluctuation in the platelet count may occur (for example as a result of a viral infection), the assessment of two treatment responses is probably more helpful as a diagnostic criterion.
Associated Features
Certain features, if identified in patients with persistent thrombocytopenia, suggest specific types of CTP, which can be classified in several different ways depending upon the other clinical features of the case and the laboratory techniques available to the physician (Table 2 ). In certain types of CTP, the uniformity of the presence and heterogeneity in the associated features are still uncertain because fewer than 10 cases have been described. These clinical features that might occur either in the patient or in a family member include (but are not limited to) presentation at birth, absence of radii, high-tone hearing loss, renal failure, bifid uvula, right-sided aortic arch, neonatal hematochezia, eczema, frequent (severe) infections, platelet dysfunction, and a family history of leukemia. Frequent infections may overlap with immune thrombocytopenias secondary to hypogammaglobulinemia. These associated features are listed and linked to molecular diagnosis in Table 3 .
Review of the Smear
The fourth clinically available parameter involves the review of the peripheral blood smear. Newer automatic blood cell analyzers are superior to the previous generation in their recognition of platelets, especially large platelets, so that size measurements appear to be more reliable than they had been in the past. However, particularly for very small or very large platelets, and for very low platelet counts, the accuracy of the MPV obtained in standard laboratories remains to be determined and may vary from case to case, so that visual inspection of the smear remains the “gold standard” for platelet size in clinical practice. If platelets appear very large (the size of red cells or even larger), this would be compatible with Bernard-Soulier syndrome or the MYH9 defects, e.g., May-Hegglin syndrome. Döhle-like bodies in neutrophils also suggest May-Hegglin syndrome. The presence of very small platelets is most consistent with Wiskott-Aldrich, whether the complete syndrome or the XLT form. Platelet clumping may suggest von Willebrand type IIb, although pseudothrombocytopenia would need to be excluded either by using citrate as the anticoagulant or by making smears directly from a drop of blood. Microcytosis suggests the XLT-T form involving a mutation in the DNA face of GATA-1.4
Specific Inherited Thrombocytopenias
Amegakaryocytic thrombocytopenias
Congenital amegakaryocytic thrombocytopenia (CAMT) typically presents as severe thrombocytopenia that is often recognized on day 1 of life or at least within the first month. CAMT is often initially confused with fetal and neonatal alloimmune thrombocytopenia, but the neonate fails to improve and responds only to platelet transfusion. Eventually a “diagnostic” bone marrow is performed; a marrow biopsy may be required which can be technically difficult in a neonate. Ten percent to 30% of cases have orthopedic or neurologic abnormalities. Intracranial hemorrhage is not rare (5 of 24 cases in one survey [PR Merola et al, manuscript in preparation]), and treatment other than platelet transfusions is largely ineffective.
Fifty percent of the 24 cases in the same survey progressed to aplastic pancytopenia within the first 5 years of life; 1 case of leukemia was also seen (PR Merola et al, manuscript in preparation). The underlying defect in the majority of cases is a mutation in the TPO-receptor, c-mpl (PR Merolta et al, manuscript in preparation). In the absence of a signal from thrombopoietin (TPO), megakaryocytes do not proliferate. The prevailing hypothesis to explain the development of aplastic pancytopenia is that c-mpl is also required for stem cell maturation. Therefore, in the absence of its anti-apoptotic effects of TPO, stem cell depletion may lead to aplasia.
While certain cytokines (interleukin [IL]-3, granulocyte-macrophage colony-stimulating factor [GM-CSF], IL-11) may have limited efficacy in individual patients, none are consistently effective and their use may result in substantial toxicity. TPO, or a thrombopoietic agent, seems unlikely to be of use because the underlying defect is a mutation in c-mpl. Platelet transfusions are administered for very low counts and as prophylaxis in patients who have had major bleeds. Matching strategies are generally pursued only in the context of developing refractoriness to leukocyte-reduced random donor units. The only effective treatment thus far has been allogeneic stem cell transplant (HSCT). An approach to gene therapy is being pursued in which insertion of a dimerized artificial TPO receptor is intended to convey a growth advantage to stem cells that take up and express the vector, allowing them to eventually replace the marrow.
Thrombocytopenia and absent radii
The diagnosis of TAR is suggested by the finding of isolated, severe neonatal thrombocytopenia (similar to CAMT) along with characteristic physical anomalies (associated features). These are not limited to absent radii but also include other orthopedic abnormalities, e.g. of the ulna, humerus, and tibia.5 For example, an isolated abnormality of the radii was only identified in 4 of 54 cases of TAR in a survey, whereas abnormalities of the ulna and knees occured in well over 50% of patients (PR Merola et al, manuscript in preparation). Patients with TAR have a high incidence of serious bleeding including intracranical hemorrhage (ICH) and gastrointestinal (GI) bleeding. However, unlike those with CAMT, patients with TAR tend to improve and their platelet counts increase with time. The dogma is that these patients will achieve normal platelet counts within 1 year of birth.5 However, milder thrombocytopenia often persists and the platelets may fall again during adulthood. Signaling via the TPO receptor is abnormal, but the defect in the signaling pathway has not been defined.
AMT with radial-ulnar synostosis
AMT with radial-ulnar synostosis (CTRUS) is a rare entity (3 cases reported) that behaves like TAR, including initially severe thrombocytopenia with subsequent improvement.6 Initially, the forearm appears normal or subtle abnormalities may be detected. The diagnosis of CTRUS is made later when pronation-supination of the forearm is discovered to be restricted. Hox 11a was reported to be abnormal in the initial cases but at least 1 other case did not have this abnormality. A study of cases of TAR did not identify mutations in this or other Hox genes.
Microcytosis and X-linked inheritance
There are two partially overlapping forms of familial thrombocytopenia: those with microcytic anemia, i.e., the XLT-T syndrome (X-linked thrombocytopenia– thalassemia), and those that include dyserythropoiesis. Patients with both types of familial thrombocytopenia have large platelets that, in combination with the microcytic erythrocytes, distinguish them from XLT/WASp. The second type of XLT and anemia could be considered to be a form of myelodysplasia (MDS). These cases reflect the importance of GATA-1 in thrombopoiesis and erythropoiesis. The recent evaluation of a second family with the XLT–β-thalassemia mutation suggests that anemia derived from mutations of GATA-1 that affect the binding site for FOG (Friend of GATA) is more severe than that derived from the one known mutation interfering with binding of GATA-1 to DNA.4 Fanconi anemia may also present as thrombocytopenia with mild anemia. Furthermore, the other anomalies characteristic of Fanconi anemia (abnormal thumbs, failure to thrive, renal anomalies, etc.) may not be present or immediately apparent at the time of presentation.
XLT-WASp
Patients with either Wiskott-Aldrich syndrome (WAS) or the XLT form of WAS classically have marked or severe thrombocytopenia and smaller than normal platelets. The only other entity in which such distinctly small platelets are found is in patients with TORCH (toxo-rubella-cytomegalovirus-herpes) infections, e.g. cytomegalovirus (CMV). In addition to severe thrombocytopenia, WAS is an important congenital immunodeficiency syndrome that includes an inability to make anti-polysaccharide antibodies, resulting in a predilection to pneumococcal sepsis. Eczema is common, although its relationship to the underlying defect is unclear. The platelets at birth may not appear small, possibly because of lack of splenic maturity, and newborns and very young infants may present with thrombocytopenia, milk allergy, and hematochezia. The XLT form seems to involve defects primarily in exon 2 of the WAS gene; these patients have minimal immunodeficiency. WAS/XLT is unusual in that the thrombocytopenia responds to splenectomy,7 apparently for the same reasons that hereditary spherocytic anemia does: the defective platelets are no longer destroyed efficiently. The possibility that they make anti-platelet antibodies as part of the immunodeficiency state is also possible although unlikely in other than exceptional cases.
Even when the thrombocytopenia is moderately severe, the risk of hemorrhage may be high because the platelet mass is low relative to the count and the platelets may also be dysfunctional. Treatment of the patient to increase the platelet count beyond what can be accomplished by platelet transfusion involves either splenectomy or stem cell transplantation (HSCT). In XLT or in patients with WAS for whom a HSCT cannot be performed, splenectomy is appropriate.7 However, in WAS and even in XLT, there may be an increased risk of overwhelming postsplenectomy sepsis. Determining an adequate response to pneumococcal vaccine, either Pneumovax or Prevnar, is important, and careful antibiotic prophylaxis as well as monitoring antibody levels is mandatory to minimize the risk of postsplenectomy sepsis and IVIG may need to be given monthly. There is approximately a 10% cumulative incidence of lymphoma in either form, WAS or XLT, in those who have not undergone transplantation, which is very close to the mortality from allogeneic HSCT.
Velocardiofacial syndrome (DiGeorge syndrome)
Another form of thrombocytopenia, with similarities to XLT, and the first of the large platelet syndromes to be discussed, is velocardiofacial syndrome (VCF; also known as DiGeorge syndrome).8 VCF, like WAS/XLT, involves a variable clinical immunodeficiency but the thrombocytopenia is generally mild. Rightsided heart disease, neonatal hypocalcemia, cleft palate, neuro-psychologic issues, and not only ITP but also Evans syndrome suggest the possibility of VCF. VCF is associated with mutations in chromosome 1q22 and 10p4 and molecular diagnosis is available. Although several gene defects have been identified, the mutation is yet to be identified in others with a similar clinical syndrome.
In VCF, as in WAS/XLT, two forms of thrombocytopenia are seen: one autoimmune and one hereditary. The autoimmune thrombocytopenia in VCF may respond to ITP therapies but is often severe and likely to be chronic. Evans syndrome may recur.
Milder forms of VCF without prominent heart disease or hypocalcemia are often not identified until adolescence or adulthood for the following reasons:
The immunodeficiency may be sufficiently subtle to be missed.
The thrombocytopenia may be asymptomatic and therefore avert detection.
The “heart disease” may be clinically silent, i.e., a right-sided aortic arch.
Either the oropharyngeal findings are subtle, such as a bifid uvula, or else a cleft palate was repaired in the first year of life and subsequently forgotten.
The nonimmune component of the thrombocytopenia may be linked to the Bernard-Soulier gene, resulting in marked macrothrombocytopenia; platelet function has not been well characterized but clinically has not seemed to be a major issue. The thrombocytopenia may have an autoimmune component. Underlying behavioral abnormalities that are associated with VCF may be initiated or worsened by corticosteroids administered to treat presumed autoimmune thrombocytopenia.
MYH9-related diseases
The most common forms of CTP are accompanied by macrothrombocytopenia, and among these the most common are a group now known collectively as the MYH9-related diseases.9 What had been separate but overlapping syndromes (May-Hegglin, Fechtner, Sebastian, and Epstein syndromes) have been shown in several laboratories to involve mutations of the gene that codes for myosin IIA. Myosin IIB is normally expressed in cell types other than platelets and neutrophils, which only express myosin IIA. While not yet precisely defined, there is consensus that the associated features of these overlapping syndromes involve the site of the mutation within the MYH9 gene. These associated features include leukocyte inclusions, renal failure, hearing loss, and cataracts. In the commonest form, May-Hegglin, Döhle-like bodies may be seen in neutrophils in addition to the very large (giant) platelets identified on peripheral smear. The platelet count varies and can be < 20,000/μL; however, the very large platelets often lead to the reporting of falsely low counts. Platelet function is generally preserved, and cases of these syndromes are frequently identified in asymptomatic patients.
Familial thrombocytopenia-leukemia (Tel-AML1)
The familial thrombocytopenia-leukemia (Tel-AML1) syndrome is important to identify because it is the thrombocytopenia most linked to malignancy. Fortunately, it has a somewhat unique presentation. The thrombocytopenia is usually mild, approximately 80–100,000/μL, but nonetheless signs and symptoms of bleeding are common. This is consistent with clinically significant platelet dysfunction, similar to the XLT form of WAS and Bernard-Soulier syndrome. Inheritance is autosomal dominant. Platelet function testing, where possible, reveals a storage pool disorder. Approximately half of the patients may go on to develop a malignancy, 2/3 of which are myeloid leukemia and 1/3 solid tumors. Recently, mutations in the transcription factor CBFA2 have been identified in two families with this syndrome.
Bernard-Soulier syndrome
The Bernard-Soulier syndrome should be considered when a patient presents with macrothrombocytopenia and bleeding out of proportion to the platelet count in the absence of other clinical or hematologic abnormalities. Bernard-Soulier syndrome is a result of the absence of the GPIb-V-IX complex on the platelet surface. This can occur either in the homozygous or heterozygous form, although clinically there may be some overlap in the clinical manifestations. In homozygotes, the platelets are comparable in size to those seen in the MYH9 syndromes. Automated platelet counts are often inaccurate because the platelets are so large. Epistaxis is relatively common. Diagnosis in the routine laboratory can be strongly suspected by lack of aggregation (agglutination) of platelets by “high dose” ristocetin in homozygotes. With this test result, the primary differential diagnosis is von Willebrand disease, which would be highly unlikely if the levels of von Willebrand factor (VWF) and the pattern of the VWF multimers were normal. Heterozygotes may be more difficult to identify. Flow cytometry to quantify platelet glycoproteins is confirmatory in homozygotes and diagnostic for heterozygotes if GPIb-V-IX is absent or greatly reduced in number. Milder variants, e.g. “Bolzano,” have been described in which greater than expected amounts of the platelet glycoproteins are expressed.
von Willebrand disease 2B
von Willebrand disease 2B (VW2B) is an autosomal dominant disorder caused by the production of an abnormal VWF molecule with a propensity to form ultra-large multimers that bind more avidly to platelets than normal vWF and may promote platelet clumping: a gain of function mutation. Pseudothrombocytopenia may occur as a result of in vitro clumping. Affected individuals have no associated findings. The platelet count tends to fluctuate especially with stress and hormonal changes such as those associated with pregnancy. Postpartum hemorrhage may occur as the levels of VWF fall. Bleeding at times of menses may be heavy and other mucous membrane bleeding is seen. Desmopressin acetate (DDAVP) may result in more marked thrombocytopenia by increasing the level of the overly avid VWF multimers exacerbating platelet agglutination. The platelets may be normal in size or large. Assessment of multimer size distinguishes Type IIb vWF from the less common platelet type von Willebrand disease in which mutations in GPIb are responsible for the enhanced interaction with von Willebrand factor molecule.
Mediterranean macrothrombocytopenia
Mediterranean macrothrombocytopenia is a disorder without associated features in which there is a defect in the demarcatory membrane system by which megakaryocytes divide up their cytoplasm into platelets. Fewer larger platelets are made and released, but it is thought that the overall platelet mass remains approximately normal.
A number of rarer syndromes are not covered here including Paris-Trousseau and Jacobsen’s syndrome, THC2, Gray platelet syndrome and Montreal platelet syndrome. These are reviewed by Balduini2,3 and are listed on-line at Mendelian Inheritance in Man (www.ncbi.nlm.nih.gov/omim/).
Management
Management of patients with CTP is not well defined. There is no uniform approach to the treatment of patients with CTP who are bleeding or are to undergo surgery. The available agents include DDAVP; antifibrinolytics such as Amicar (epsilon-aminocaproic acid) especially for mouth or nose bleeding; hormonal therapy to lessen menses; platelet transfusion; HSCT for severe cases, i.e., CAMT or homozygous Bernard-Soulier; and rFVIIa, which in the field of platelet disorders has been used most often with effect in Glanzmann thrombasthenia. The use of leukocyte-reduced platelets has lessened sensitization to platelets and development of refractoriness to platelet transfusion, but platelet transfusion should still be reserved for cases of true need. The dose of rFVIIa remains to be defined as only very anecdotal use has been reported. It may be in the range of 20–40 units/kg instead of the 90 units/kg used for hemophiliacs with inhibitors. Whether thrombopoietic agents will have a role in the future in at least some of these entities seems probable but entirely unexplored.
Conclusion
In summary, while a considerable amount has been learned about the hereditary thrombocytopenias in the past 10 years, much remains to be discovered. Several approaches can be taken to identify and classify these cases using either of two approaches. The first, as described in Table 1 , requires the suspicion that a case may represent CTP. The algorithm developed by the Italian group is one way to approach this complex and confusing area but necessitates testing that is not universally available for all patients. Table 2 lists the different variables that can be used to classify which type of CTP is involved and represents a second, similar approach that focuses first on suspicion of CTP and then on the distinctive features that often permit subclassification. This remains a confusing area for several reasons: a) almost all of the individual conditions are quite rare so that it is difficult to remember the details of each and not all conditions are necessarily well defined; b) some of the conditions appear similar, e.g., isolated macrothrombocytopenia; c) the highly specialized tools required to distinguish one from another are not universally available; and d) 40% of cases recently investigated by the Italian group according to their algorithm had to be classified as “unknown” despite extensive investigation. Nonetheless, the advent of molecular biology into this field has greatly clarified certain of these conditions and allowed unequivocal assignation of certain entities into specific groups. Furthermore, a number of these conditions have certain features that clearly point to specific entities. Table 3 describes the specifics of each disease including the molecular defect, if known, that can be used as a reference. Continued work in this area promises to reduce the number of unknown cases and may simultaneously teach us important lessons regarding the biology of thrombopoiesis.
II. Adult Chronic Immune Thrombocytopenic Purpura
Robert B. McMillan, MD*
The Scripps Research Institute, MEM 215, 10550 North Torrey Pines Road, La Jolla CA 92037
Adult chronic immune thrombocytopenic purpura (ITP) is an autoimmune disorder manifested by immunemediated thrombocytopenia. The diagnosis is one of exclusion, based on the American Society of Hematology (ASH) Practice Guidelines:1 Each patient’s history, physical examination, complete blood count and peripheral blood smear must be characteristic of ITP and other causes of thrombocytopenia must be excluded. This summary will concentrate on recent developments relevant to this disorder. More detail can be obtained from several recent reviews.2– 4
Pathogenesis
The fundamental disturbance leading to the autoimmune response in ITP is unknown. T lymphocytes and B lymphocytes that react with platelet autoantigens have been detected in the peripheral blood and spleen of ITP patients,5,6 and autoantibody production by cells from the spleen, blood and bone marrow has been demonstrated.6,7 In addition, skewing of cytokine production compatible with activation of Th0/Th1 cells (elevated IL-2 and interferon-γ [IFNγ], reduced IL-10), and a reduced Th2 response have been recognized.8
Antiplatelet autoantibody
Since the studies of Harrington9 and Shulman,10 it has been known that many ITP patients produce autoantibodies that cause platelet destruction. Using antigen-specific assays, autoantibodies that recognize one or more platelet surface glycoproteins (GP), including GPIIb-IIIa, GPIb-IX and GPIa-IIa, among others, can be demonstrated in about 50%–60% of ITP patients. In prospective studies, platelet-bound autoantibodies can be detected with a sensitivity of 49%–66% and a specificity of 78%–93% when ITP patients are compared to healthy individuals or patients with non-immune thrombocytopenia.11 A positive antigen-specific assay provides strong evidence for the diagnosis of immune thrombocytopenia while a negative test does not rule it out. The frequency of positive assays and the degree of positivity increases with disease severity.12 Antibody-coated platelets may be destroyed by either phagocytosis or complement-induced lysis.
Recent studies suggest that autoantibody may also affect platelet production. In the 1980s, it was shown that platelet turnover in the majority of ITP patients is either normal or reduced rather than increased as would be expected suggesting either inhibition of megakaryocytopoiesis or intramedullary destruction of platelets. Recent in vitro studies, showing reduced megakaryocyte production and impaired maturation in the presence of some ITP plasmas, provide evidence for autoantibody-induced suppression of megakaryocytopoiesis. In one study, plasma antibody from 12 of 18 adults with severe adult ITP suppressed in vitro megakaryocytopoiesis (Figure 1A ); antibody not only decreased the total number of megakaryocytes produced but also inhibited megakaryocyte maturation (Figure 1B ).13 Similar findings are noted in childhood ITP.14 The implications of this finding, in terms of responsiveness to various forms of therapy, merit study.
T lymphocyte-mediated platelet lysis
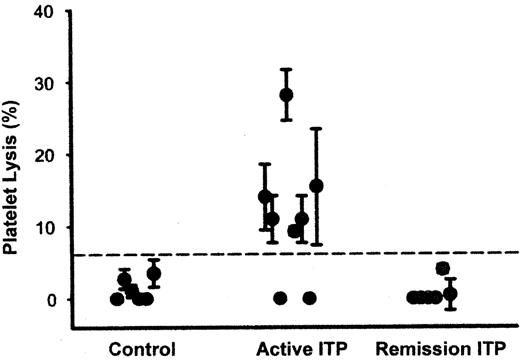
The absence of detectable autoantibody in about 40% of ITP patients suggests the presence of alternative platelet destruction mechanisms. A recent study suggests that T cytotoxic lymphocyte-induced lysis of platelets may be important.15 As shown in Figure 2 , incubation of platelets with anti-CD3-stimulated autologous CD14−/CD19− blood mononuclear cells from 6 of 8 patients with active ITP resulted in significant platelet lysis when compared with similar studies using cells from normal subjects or ITP patients in remission. The active cells were CD3+/CD8+ lymphocytes and the lysis was HLA-specific. The authors also noted increased expression of T cell cytotoxic genes (Apo-1/Fas, granzyme 1, granzyme 2 and perforin) and genes involved with the Th1 response (interferon γ and IL-2 receptor B) while genes from several members of the KIR receptor family, which downregulate cytotoxic T lymphocytes, were increased in remission patients when compared to those with active ITP. Since the number of cytotoxic T cells is small relative to the number of platelets produced, the in vivo importance of this mechanism in ITP remains to be shown.
Treatment
Hospitalization and emergency treatment
Hospitalization should be strongly considered for any patient with extremely low platelet counts (< 5,000/μL) or with significant bleeding. A rapid (although temporary) increase in the platelet count can usually be achieved with one of the following: intravenous gammaglobulin (IVIG, 1.0 g/kg/day IV for 2 days); methylprednisolone (1.0 g/day IV for 3 days) or a combination of both. In many cases, platelet transfusion should also be given. Anti-D (75 μg/kg) has been suggested as an alternative, although there is limited experience in the emergency setting. In unresponsive patients, we have had success with IVIG (1.0 μg/kg/day IV for 2 days) given by continuous infusion over the 48-hour period, combined with continuous platelet infusions (1 random unit/hour) until the platelet count increases. Recombinant factor VIIa may be useful in unresponsive patients with uncontrolled bleeding.16
Helicobacter pylori
Early studies from Italy and Japan reported an increased incidence of H pylori infection in ITP patients and persistent partial or complete remissions in many patients following its eradication. A recent prospective study in the US showed no increased incidence of H pylori in ITP patients and no platelet response following its eradication.17 A multi-institutional prospective randomized study is planned that should help define the role of H pylori in ITP. In the meantime, no evidence-based recommendations can be made about whether routine H pylori testing and/or treatment are indicated in chronic ITP.
Initial therapy
Patients with platelet counts < 25–30,000/μL should be treated with the aim of obtaining a stable, safe platelet count (> 30,000/μL) on no treatment. There is now good evidence that, if the platelet count can be supported, some adult ITP patients will achieve safe platelet counts without the need for splenectomy. Cooper et al18 reported on 28 Rh+, non-splenectomized ITP patients who received periodic anti-D therapy for platelet counts < 30,000/μL (68% responded consistently to anti-D). Patients were treated for 18 months unless they required splenectomy. Of the 28 patients, 12 (43%) are off all treatment (median 16, range 6–33 mos) with either platelet counts of > 100,000/μL (6 patients) or safe platelet counts > 30,000/μL (6 patients). Our group obtained follow-up on 21 patients in whom we advised splenectomy after they failed to attain a stable remission with prednisone. They decided to postpone splenectomy and continue treatment, in most cases with either prednisone or danazol, and their platelet counts eventually stabilized. Ten had normal platelet counts and 11 had stable counts > 50,000/μL off all treatment, with median follow-up of > 8 years.
Subsequently, IVIG and anti-D have each been compared to corticosteroids as the initial ITP treatment.19,20 In neither study was there a difference in terms of response rates, duration of response or the requirement for splenectomy.
From these observations, it is clear that some adult chronic ITP patients will remit without splenectomy. The following need to be determined: a) what percentage will remit and how will this subgroup be identified; b) whether remissions are permanent; c) how long should treatment should continue before advising splenectomy; and d) what is the best agent to maintain the platelet count.
Recommended initial treatment
Begin prednisone (1 mg/kg) and, if a response occurs, taper the dose slowly with the aim of maintaining safe platelet counts on doses causing tolerable side effects (< 10–15 mg/day). In Rh+ patients who either do not respond to prednisone or who cannot be tapered to safe doses, anti-D should be given whenever the platelet count falls below 25–30,000/μL. This approach should be continued for ~6 months, if possible, with the aim of eventually stopping all treatment. The duration of this approach, prior to advising splenectomy, must be decided by the patient and treating physician since there are no data which establish a definite stopping point.
Splenectomy.
Splenectomy is recommended if: a) safe platelet counts cannot be maintained; b) remission is considered unlikely; c) drug toxicity is severe; or d) the approach becomes too burdensome (frequent blood tests, office visits, lost work time, etc). Immunization with pneumococcal, H influenzae and meningococcal vaccines is advised at least 2 weeks prior to surgery. IVIG, anti-D or steroids are used to boost the platelet count prior to surgery. Prophylactic platelet transfusions are unnecessary, although platelets should be available. Approximately 75%–85% of patients have an initial response to splenectomy; of these, 25%–40% relapse within 5–10 years. The outcomes of laparoscopic and conventional transabdominal approaches are comparable, although the former hastens recovery. Life-long use of phenoxymethylpenicillin (250–500 mg BID) or erythromycin (500 mg BID) is recommended by the United Kingdom guidelines, although antibiotics are not used routinely in the US. All postsplenectomy febrile illnesses demand careful evaluation and prophylactic antibiotics should be given for any illness with fever ≥ 101°F until sepsis can be ruled out.
Treatment of refractory patients.
About 40%–50% of ITP patients fail splenectomy and require additional treatment. Refractory patients, defined in this manner, often respond slowly to subsequent treatment, have significant morbidity from the disease and its therapy, and have a mortality rate of 10%–16%.21,22 As reported recently,23 there are few randomized, controlled trials to support the use of any therapy for refractory ITP patients, and treatment is based on small uncontrolled studies and physician experience. A recent report,22 evaluating the long-term outcome of 105 refractory adult ITP patients (median follow-up, 110 months), showed that 75 patients eventually attained a stable platelet count > 30,000/μL either off all therapy (51 patients) or on maintenance therapy with danazol or low-dose prednisone (24 patients). Remissions occurred slowly with a mean time to remission of about 4 years.
All refractory patients should be evaluated for an accessory spleen with either abdominal CT scan, magnetic resonance imaging or, preferably, with a sensitive scanning method (e.g., radiolabeled heat-damaged red cells); if present, strong consideration should be given for its removal. Therapy of refractory patients must be individualized, depending upon the patient’s level of activity and other clinical situations (e.g., female patients wishing to have children should avoid alkylating agents while other agents may adversely affect comorbid conditions such as bone, cardiac or prostate disease).
The presence of significant bleeding will require periodic emergency therapy (see above). Table 4 lists the doses, expected response times and side effects of each treatment modality. References on individual treatment reports can be obtained from comprehensive reviews.1,21,23,24 The following treatment progression is recommended although modifications may be required depending on the individual clinical situation. Most treatments require the concomitant use of corticosteroids to stabilize the platelet count during the early phases of therapy.
First-line therapy
Corticosteroids.
Prednisone (1.0 mg/kg/day) is the drug of choice in refractory patients if “safe” platelet counts can be maintained on doses acceptable for long-term use (≤ 10 mg/day). Some patients who require small doses of prednisone, to maintain safe platelet counts, can be successfully switched to either colchicine (0.6 mg BID or TID PO) or dapsone (75 mg PO daily) to avoid steroid side effects. These drugs are given initially with prednisone and the latter is then tapered and discontinued.
Danazol.
A dose of 200 mg QID PO is given initially with full dose prednisone. Responses occur slowly and therapy should be continued for 3–6 months before abandoning it. In responding patients, prednisone is tapered and, if possible, stopped while danazol is continued at full doses for at least 1 year and then tapered slowly over several months. Some patients require danazol maintenance therapy, with or without low-dose prednisone.
Rituximab.
Patients who fail corticosteroids and danazol should receive rituximab (375 mg/M2 IV q week × 4). Responses are usually noted within 3–4 weeks after the first infusion. A stable complete or partial remission occurs in about one-third of treated patients. Some patients who relapse will respond to subsequent courses.
Second-line therapy
Oral cyclophosphamide or azathioprine should be used next. Responses to cyclophosphamide occur more rapidly but potential serious side effects are greater. Therefore, drug selection depends on the urgency of the clinical situation since the overall response rates are similar (20%–40%). In the event of relapse following a response to either agent, the long-term risks (e.g., secondary malignancy, myelodysplasia) must be weighed against the potential benefits of resuming therapy.
Cyclophosphamide.
The starting dose (150 mg/day PO) is adjusted to maintain mild neutropenia. Responses occur within 8–12 weeks and, if the count normalizes, full doses are given for 3 additional months and then treatment is stopped. Patients should drink at least 2 quarts of liquid daily, to prevent hemorrhagic cystitis, and the blood count should be monitored each week.
Azathioprine.
The initial dose of 150 mg/day PO is adjusted to maintain mild neutropenia. Responses occur slowly, over 3–6 months, and this agent is often stopped prematurely. In responding patients, therapy should be continued at full doses for 12–18 months and then gradually tapered and discontinued.
Cyclosporine.
The dose of 1.25–2.5 mg/kg BID is adjusted based on cyclosporine and creatinine levels. Of 18 post-splenectomy patients in one study, 5 complete and 5 partial remissions were noted; 30% stopped therapy due to side effects.25
Mycophenolate mofetil.
Few patients have been studied; responses were noted in 15 of 23 patients with durations ranging from 1–39 months. Start with 500 mg BID PO and increase to 1,000 mg BID after 2 weeks. Few side effects were noted.26
Third-line therapy
These treatments should be reserved for patients with life-threatening symptoms or extremely low platelet counts (< 10,000/μl). If severe neutropenia occurs, prophylactic antibiotics should be given until counts reach safe levels. Stop therapy if there is no response after 2 courses. Responding patients should receive 3–6 courses even if the platelet count normalizes.
High-dose cyclophosphamide.
A dose of 1.0–1.5 g/M2 IV is given at 4-week intervals. A high fluid intake is mandatory and frequent blood counts are required.
Combination chemotherapy.
Various combinations have been used and few patients have been treated.27 The long-term outcome of 12 patients (follow-up 35–150 months) showed 5 complete remissions and 1 partial remission. Monitoring depends on the drugs used.
Experimental therapy
Stem cell transplantation.
One group transplanted 14 patients with refractory ITP.28 Of these, 6 attained stable platelet counts > 100,000/μL and 2 patients had partial responses (follow-up 9–42 months). There were no deaths associated with the procedure and no major complications.
Platelet growth factors.
A new approach to the treatment of ITP is the use of thrombopoietic factors. A recent dose-finding study using AMG 531, a molecule that activates the thrombopoietin receptor, showed a temporary increase in the platelet count in 8 of 12 ITP patients (5 splenectomy failures) at a dose of ≥ 3.0 μg/kg.29
III. Heparin-Induced Thrombocytopenia: ASH Update
James L. Zehnder, MD*
Stanford University Medical Center, Stanford CA 94305
This update highlights specific aspects of heparin-induced thrombocytopenia (HIT) from a review of the literature of the past two years. A comprehensive review of HIT was provided in Hematology 2003.1
Incidence/Epidemiology/Pathogenesis
HIT occurs in 1%–4% of individuals treated with unfractionated heparin for a minimum of 7 days. Surgical patients are at highest risk. In a recent analysis of consecutively studied medical patients treated with subcutaneous unfractionated heparin Girolami et al2 diagnosed HIT in 5 of 598 (0.8%). All 5 cases of HIT occurred in patients who had been receiving prophylactic heparin. The prevalence of thrombotic complications was 60% in the HIT cases and 3.5% in the remaining 593 individuals without HIT, for a relative risk of 40. Thus, although the rate in medical patients was lower than that in surgical patients, the thrombotic event rate was similar, which highlights the need for vigilance in this large population. HIT is seen less frequently in pediatric populations (but has been reported, particularly in the critical care setting) and is rare in pregnant women. The risk of HIT is higher in individuals treated with unfractionated heparin of bovine origin compared with porcine heparin and far lower with low molecular weight heparin (LMWH).3
Almost all individuals with HIT have antibodies to heparin-platelet factor 4 (PF4) complexes in their plasma at the time the disease develops. PF4 is a platelet-specific C-X-C chemokine that is released in high concentrations at sites of platelet activation. Recent evidence in a transgenic mouse model suggests a role for PF4 in thrombus formation.4 Heparin-PF4 complexes form though an interaction between the highly sulfated anionic heparin and a circumferential band of lysine and arginine residues on PF4. Binding of PF4 to polyanions appears to expose new antigenic epitopes that lead to antibody formation. This phenomenon is not heparin-specific; other negatively charged polyanions can also induce antigenic sites. Although all major classes of antibodies have been detected, it appears that patients with high titer IgG responses are at greatest risk to develop clinical disease.5,6 Anti-heparin-PF4 antibodies also appear to be more prevalent among individuals undergoing cardiac surgery with bypass, where high doses of heparin are used in the setting of intense platelet activation. Up to 50% of individuals who undergo cardiac surgery with bypass will become seropositive. However, at present, there are no reliable means to predict which seropositive individuals will develop thrombocytopenia or the even smaller fraction who will manifest thrombotic complications.
IgG/heparin-PF4 complexes activate platelets through the platelet Fc receptor, resulting in further PF4 release and amplification of this process. In addition, this process results in thrombin activation, thereby eventuating in a systemic hypercoagulable state. Morbidity and mortality in HIT are related to thrombotic events. Venous thrombosis occurs more commonly, but occlusion of peripheral or central arteries is not infrequent. If an indwelling catheter is present, the risk of thrombosis is increased in that extremity.7 Skin necrosis has been described, particularly in individuals treated with warfarin in the absence of a direct thrombin inhibitor, presumably due to acute depletion of the vitamin K–dependent anticoagulant protein C occurring in the presence of high levels of procoagulant proteins and an active hypercoagulable state.
Diagnosis
HIT remains a clinical diagnosis, supported by confirmatory laboratory testing. The traditional clinical definition of HIT is an acquired platelet count of < 150K or a 50% fall from baseline occurring 5–10 days after initiation of heparin therapy in the absence of another etiology. This means that some patients with HIT have platelet counts that are still within the normal range, but always lower than the pre-heparin exposure baseline. “Isolated HIT” refers to heparin-induced thrombocytopenia in the absence of thrombosis. Individuals with isolated HIT are at high risk for thrombotic events, as discussed below. A thrombotic event occurring in an individual on heparin therapy within this temporal window should always trigger consideration of HIT. Variant presentations of HIT have been described recently, including delayed HIT (e.g., a patient presenting to the emergency room with pulmonary embolism a week after being discharged from a hospitalization with heparin exposure) and acute HIT in patients with recent (< 3 months) heparin exposure.
One of the challenges in HIT diagnosis is that these patients are often critically ill or post-surgery, and often have alternative explanations for thrombocytopenia (e.g., infection, other drugs). To assist in the evaluation of such patients, useful scoring systems based on clinical criteria have been developed to aid in assessing the likelihood of HIT.1
Laboratory Testing: Heparin-PF4 ELISA
Platelet-activation assays are available only at specialized centers and require considerable skill in specimen handling, control selection and interpretation. Optimal use of these tests requires knowledge of a particular laboratory’s test performance characteristics. Washed platelet activation assays performed by experienced laboratories have been reported to have a sensitivity of > 90% and specificity approaching 80%–100%.8
The most commonly used (and only US Food and Drug Adminstration [FDA]–approved) tests for HIT are ELISA kits measuring the presence of anti-heparin-PF4 antibodies of all antibody classes. These tests are reported as positive if the ELISA result exceeds a threshold value. The actual optical density (OD) result is usually not reported and differs between the two approved kits. The sensitivity of this test is high (> 90%, depending on the cutoff used). The most common clinical problem with this test is the high incidence of “false positive results” (i.e., positive tests in the absence of HIT or even thrombocytopenia), which varies from 1%–3% in dialysis patients, to 10%–15% of medical patients and > 20% of patients receiving heparin for peripheral vascular surgery. This is most troublesome in intensive care units, where there is ubiquitous heparin exposure and often multiple potential etiologies for thrombocytopenia. In certain populations, such as that undergoing cardiopulmonary bypass, the seroconversion rate approaches 50%. There may be utility in performing both ELISA and an activation assay in borderline cases; if both are positive the likelihood of HIT is high; conversely, if both are negative, the likelihood is low.9 Knowledge of the ELISA titer may have utility. Refaai and colleagues recently studied the clinical significance of borderline results using the Stago H-PF4 ELISA in patients referred for HIT testing and reported that 43% of patients with a borderline positive result (defined as 66.7%–99.9% of the test threshold) became positive upon repeated testing.10 Based on this finding, laboratories should consider reporting titers and borderline results, and these tests should be repeated to detect rising titers when clinically appropriate.
Using a particle gel immunoassay system, Chilver-Stainer and colleagues reported that patients with clinically suspected HIT and high titers of antibody had higher levels of F1+2, TAT and D-dimers compared to those without detectable antibodies.11 This relationship remained statistically significant when controlled for concomitant thromboembolic complications. Thus, the H-PF4 titer identified a subset of patients with biochemical evidence of increased thrombin generation, and may have utility in identifying patients at high risk for thrombosis. Such methods may eventually be useful to limit the number of patients with isolated HIT who require prophylactic treatment with direct thrombin inhibitors.
Hematologists are often asked to evaluate a patient with a positive H-PF4 ELISA result in the absence of a diagnostic decrease in platelet count or other clinical suspicion of HIT. When the pre-test probability of HIT is low, the positive predictive value of the ELISA is low and “false-positive” test results are common. However, this situation can generate considerable anxiety given the potentially catastrophic consequences of not treating a bona fide case of isolated HIT. If there is a strong clinical suspicion of HIT, or if both the platelet activation assay and ELISA are positive, prophylactic therapy should be given serious consideration in the absence of a major contraindication.
Treatment
Direct thrombin inhibitors
Two direct thrombin inhibitors (DTIs) are approved for treatment of HIT in the US: argatroban and lepirudin. Argatroban is also approved for prevention of thrombosis in HIT patients and for coronary interventions. Danaparoid has been used extensively for HIT therapy but is not currently available in the US.
Hirsh and colleagues recently published a critical review on HIT treatment.12 They examined four issues: risk of thrombosis in patients with HIT where heparin was discontinued in the absence of an alternative anticoagulant; the efficacy of DTIs in a patients with HIT (with or without thrombosis); the use of DTIs in patients with a history of HIT requiring a coronary intervention; and bleeding risk associated with this therapy. The criteria for inclusion were a study design of randomized controlled trial, prospective cohort study or retrospective observational study enrolling patients consecutively; treatment with lepirudin, argatroban or danaparoid; and a minimum of 30 patients. Of 999 potentially relevant citations identified, only 9 met these criteria; no studies using danaparoid met the criteria. There were no randomized controlled trials comparing treatments. The incidence of new thromboembolic events in patients with HIT treated by discontinuing heparin alone or by heparin withdrawal and initiation of warfarin in the absence of a DTI ranged from 19% to 52%.
The use of historical controls in the lepirudin and argatroban trials, together with differences in trial design, made it difficult to compare the efficacy of the two drugs directly. For example, patients in the lepirudin trials were treated twice as long on average as those in the argatoban trial. All patients in the lepirudin trial had a positive test for HIT antibodies, compared with 65% of those in the argatoban trial. Even with treatment, mortality ranged from 9% to 22%; 6%–18% of patients required amputation or experienced a new thrombotic event. Neither outcome was statistically significantly different from historical controls. However, as Warkentin and others have pointed out,13 using a single endpoint of new thrombosis (rather than the composite endpoint of all cause mortality, new thrombosis and limb amputation) may be a better measure of DTI efficacy. Amputation of a limb with severe ischemic injury, for example, might well be required despite the use of an effective anticoagulant following the injury. Both drugs significantly reduced the incidence of new thromboembolic events. Bleeding rates of 6%–18% were reported for both drugs. The bleeding rate in coronary inventions was approximately 1%.
In the absence of properly designed drug comparison trials, the choice of drug is generally based on differences in their mode of elimination; lepirudin is renally cleared whereas argatroban is cleared by the liver. There is no antidote for either drug. Therefore, in patients with renal insufficiency, argatroban would be preferred; in those with liver disease lepirudin would be a better initial choice. Lepirudin is a recombinant form of the leech anticoagulant hirudin. Up to 40% of individuals receiving the drug will develop antihirudin antibodies, which sometimes prolongs the drug half-life. There have been a few cases of fatal anaphylactic reactions in patients re-exposed to lepirudin.14 Either drug should be used cautiously in critically ill and elderly patients. For example, because the serum creatinine does not correlate with the creatinine clearance as well in the elderly population as it does in younger individuals, lepirudin may be cleared more slowly than expected. Argatroban clearance may be prolonged in ICU patients with normal liver function tests (LFTs) but compromised hepatic blood flow due to congestive heart failure or hemodynamic instability.
Other drugs
Obviously, continued treatment with heparin is contraindicated, as is use of LMWH due to the high incidence of crossreactivity. Warfarin should never be used alone in HIT because of the risk of precipitating skin necrosis. Fondaparinux appears not to crossreact with HIT antibodies and may provide an alternative to direct thrombin inhibitors, but this remains to be tested. Bivalirudin has been used in HIT patients during angioplasty (see below) and clinical trials in other settings are underway.
Conversion from DTIs to warfarin
Almost all patients require oral anticoagulation either because of the underlying indication for heparin or the risk of new thromboembolic events due to HIT, which persists for about a month. Conversion to warfarin may be accomplished while on the DTI, after there is evidence of clinical stability and platelet recovery. Prolongation of the PT is rarely an issue with lepirudin. This conversion is more complicated in patients treated with argatroban, which generally prolongs the INR. Thus, the observed INR in a patient on argatroban is a reflection of the effect of both warfarin and argatroban. A nomogram has been developed by the manufacturer to assist in this conversion; however, the effect on the INR depends on both the argatroban infusion rate and thromboplastin used. A validation of this nomogram in HIT patients on concurrent argatroban and warfarin is in progress.
Managing HIT in Cardiac Surgery
Whenever possible, such surgery should be postponed in patients with clinical or serologic evidence of HIT. HIT antibodies are relatively short-lived, and not all patients develop HIT upon re-exposure to heparin.15 Thus, the generally preferred strategy is to wait for several weeks and perform the surgery using unfractionated heparin (UFH) when serologic tests are negative. Postoperatively, it is prudent to monitor patients for the development of HIT antibodies and to have a low threshold for prophylactic use of a DTI; a DTI or other non-heparin anticoagulant should be used for acute coronary syndromes or interventions requiring anticoagulation. Argatroban is approved for use in HIT patients requiring percutaneous coronary interventions (PCI). The hirudin analog bivalirudin has also been studied in HIT patients requiring PCI, and while not FDA-approved, appears to be a safe and effective alternative.16
If surgery cannot be delayed, then the approach will depend on the experience of the institution. Surgery should be performed “off-pump,” if possible, to decrease the anticoagulant requirement. Alternative approaches are not FDA-approved, involve off-label use of available anticoagulants, are based primarily on anecdotal evidence and involve a significant risk of bleeding. Lepirudin and bivalirudin have been used successfully in this setting. However, there is no neutralizing agent and monitoring drug levels on the pump optimally requires use of the ecarin clotting time or chromogenic substrate thrombin assay.17 The use of these drugs for cardiac bypass has recently been reviewed in detail.15 Another alternative is to use UFH along with prostacyclin to inhibit platelet activation.18 Norepinephrine or similar agents must be used concurrently to counteract the vasodilatory effects of prostacyclins.
Prevention
UFH remains the drug of choice for bypass surgery, and continues to be widely used for vascular procedures and DVT prophylaxis. Thus, inpatients will likely continue to be exposed to the most antigenic form of heparin for the foreseeable future. To the extent that LMWH and other anticoagulants replace UFH, the risk of HIT will decrease. Until that time, HIT will remain a common clinical problem. Unfortunately, when HIT develops, catastrophic outcomes are all too common and frequently result in litigation as well. A typical scenario is a patient who undergoes a surgical procedure with heparin exposure, is left on UFH post-operatively for DVT prophylaxis without monitoring of platelet counts. The patient then suddenly presents with a serious thrombotic event and is subsequently found to be thrombocytopenic and the diagnosis is confirmed serologically. Another typical scenario is where there is a clinical suspicion of HIT but the medical personnel fail to eliminate unintended heparin exposure from catheters, arterial flushes, hyperalimentation solutions, etc. It should be considered standard of care to monitor platelet counts in all patients exposed to heparin. If a patient has a clinical picture consistent with HIT (falling platelet count in proper temporal sequence with exposure), then use of an alternative anticoagulant should be strongly considered even before confirmatory serology is available and treatment should be continued until the platelet count has recovered to least 100,000/μL. As outlined above, simply stopping the heparin may not suffice to prevent a thrombotic event; these patients should be considered to have an active and protracted hypercoagulable state. A recent report from the surgical literature suggests that some physicians are still treating HIT with LMWH or even by continuing UFH.19 Thus, hematologists have an important role in prevention and treatment of HIT by educating physicians, nurses and other health professionals caring for patients exposed to heparin.
Reasons to suspect hereditary thrombocytopenia.
|
|
Classification schemes for hereditary thrombocytopenias.
|
|
Classification of congenital thrombocytopenias including gene defect, inheritance, and associated features.
| Inherited Thrombocytopenias . | Abbre- viation . | Gene (Localization) . | Inherited Pattern . | Clinical and Laboratory Features . |
|---|---|---|---|---|
| Wiskott-Aldrich syndrome | WAS | WAS (Xp11) | X-L | Severe immunodeficiency. Defective WAS protein. Small platelets. Eczema. |
| X-linked thrombocytopenia | XLT | WAS (Xp11) Exon2 | X-L | Mild immunodeficiency. Defective WAS protein. Small platelets. |
| Familial platelet disorder with predisposition to acute myelogenous leukemia | FPD/AML | CBFA2 (21q22) | AD | Propensity to develop myelodysplastic syndrome or acute myelogenous leukemia. Normal platelet size. Dysfunctional platelets. |
| Amegakaryocytic thrombocytopenia | CAMT | c-Mpl (1p34) | AR | Hypomegakaryocytic thrombocytopenia evolving into bone marrow aplasia. Normal platelet size. |
| Amegakaryocytic thrombocytopenia with radio-ulnar synostosis | CTRUS | HOXA11 (7p15-14) | AD | Reduced-absent megakaryocytes. Radio-ulnar synostosis ± other malformations. Possible sensorineural hearing loss. Normal platelet size. |
| Thrombocytopenia with absent radii | TAR | n.d. | AR | Thrombocytopenia usually severe in the first year of life. Reduced megakaryocytes. Bilateral radial aplasia ± other malformations. Normal platelet size. |
| Bernard-Soulier syndrome | BSS | GPIbα (17p13) GPIbβ (22q11) GPIX (3q21) | AD | Defective GPIb/IX/V. Homozygous subjects: defective ristocetin- induced platelet agglutination. Giant platelets. Heterozygous subjects: mild thrombocyto- penia, normal ristocetin-induced platelet agglutination. Large platelets. |
| Velocardiofacial syndrome/DiGeorge | VCFS | 1q22, 10 p4 | AD | Right heart defect. Palate defect. T cell immune deficiency. Evans syndrome. Large platelets. |
| Von Willebrand disease type 2B Platelet type von Willebrand disease | VW2B PltVWF | GP1bα (17p13) | AD AD | Platelet clumping. Abnormal (hyperreactive) ristocetin-induced platelet agglutination. |
| Benign Mediterranean macrothrombocytopenia | n.d. | n.d. | AD | Dysmegakaryocytopoiesis. Large platelets. |
| X-linked thrombocytopenia and dyserythropoiesis with or without anemia X-linked thrombocytopenia- thalassemia | XLTT | GATA-1 (Xp11) | X-L | Anemia (mild to nil), unbalanced globin chain synthesis resembling β-thalassemia, peripheral red cell hemolysis, dysmegakaryocytopoiesis, splenomegaly. Large platelets. |
| MYH9-related disease | ||||
| May-Hegglin anomaly | MHA | MYH9 (22q12-13) | AD | Neutrophil inclusions ± hearing loss, cataract and/or renal defect. Giant platelets. |
| Sebastian syndrome | SBS | MYH9 (22q12-13) | AD | Neutrophil inclusions ± hearing loss ± cataract ± renal defect. Giant platelets. |
| Fechtner syndrome | FTNS | MYH9 (22q12-13) | AD | Neutrophil inclusions ± hearing loss ± cataract ± renal defect. Giant platelets. |
| Epstein syndrome | EPTS | MYH9 (22q12-13) | AD | Neutrophil inclusions ± hearing loss ± cataract ± renal defect. Giant platelets. |
| Gray platelet syndrome | GPS | n.d. | AD | Pale platelets on blood films due to reduced- absent α-granules. Large platelets. |
| Inherited Thrombocytopenias . | Abbre- viation . | Gene (Localization) . | Inherited Pattern . | Clinical and Laboratory Features . |
|---|---|---|---|---|
| Wiskott-Aldrich syndrome | WAS | WAS (Xp11) | X-L | Severe immunodeficiency. Defective WAS protein. Small platelets. Eczema. |
| X-linked thrombocytopenia | XLT | WAS (Xp11) Exon2 | X-L | Mild immunodeficiency. Defective WAS protein. Small platelets. |
| Familial platelet disorder with predisposition to acute myelogenous leukemia | FPD/AML | CBFA2 (21q22) | AD | Propensity to develop myelodysplastic syndrome or acute myelogenous leukemia. Normal platelet size. Dysfunctional platelets. |
| Amegakaryocytic thrombocytopenia | CAMT | c-Mpl (1p34) | AR | Hypomegakaryocytic thrombocytopenia evolving into bone marrow aplasia. Normal platelet size. |
| Amegakaryocytic thrombocytopenia with radio-ulnar synostosis | CTRUS | HOXA11 (7p15-14) | AD | Reduced-absent megakaryocytes. Radio-ulnar synostosis ± other malformations. Possible sensorineural hearing loss. Normal platelet size. |
| Thrombocytopenia with absent radii | TAR | n.d. | AR | Thrombocytopenia usually severe in the first year of life. Reduced megakaryocytes. Bilateral radial aplasia ± other malformations. Normal platelet size. |
| Bernard-Soulier syndrome | BSS | GPIbα (17p13) GPIbβ (22q11) GPIX (3q21) | AD | Defective GPIb/IX/V. Homozygous subjects: defective ristocetin- induced platelet agglutination. Giant platelets. Heterozygous subjects: mild thrombocyto- penia, normal ristocetin-induced platelet agglutination. Large platelets. |
| Velocardiofacial syndrome/DiGeorge | VCFS | 1q22, 10 p4 | AD | Right heart defect. Palate defect. T cell immune deficiency. Evans syndrome. Large platelets. |
| Von Willebrand disease type 2B Platelet type von Willebrand disease | VW2B PltVWF | GP1bα (17p13) | AD AD | Platelet clumping. Abnormal (hyperreactive) ristocetin-induced platelet agglutination. |
| Benign Mediterranean macrothrombocytopenia | n.d. | n.d. | AD | Dysmegakaryocytopoiesis. Large platelets. |
| X-linked thrombocytopenia and dyserythropoiesis with or without anemia X-linked thrombocytopenia- thalassemia | XLTT | GATA-1 (Xp11) | X-L | Anemia (mild to nil), unbalanced globin chain synthesis resembling β-thalassemia, peripheral red cell hemolysis, dysmegakaryocytopoiesis, splenomegaly. Large platelets. |
| MYH9-related disease | ||||
| May-Hegglin anomaly | MHA | MYH9 (22q12-13) | AD | Neutrophil inclusions ± hearing loss, cataract and/or renal defect. Giant platelets. |
| Sebastian syndrome | SBS | MYH9 (22q12-13) | AD | Neutrophil inclusions ± hearing loss ± cataract ± renal defect. Giant platelets. |
| Fechtner syndrome | FTNS | MYH9 (22q12-13) | AD | Neutrophil inclusions ± hearing loss ± cataract ± renal defect. Giant platelets. |
| Epstein syndrome | EPTS | MYH9 (22q12-13) | AD | Neutrophil inclusions ± hearing loss ± cataract ± renal defect. Giant platelets. |
| Gray platelet syndrome | GPS | n.d. | AD | Pale platelets on blood films due to reduced- absent α-granules. Large platelets. |
Characteristics of the treatments used in adult chronic immune thrombocytopenic purpura (ITP).
| Therapy . | Dose . | Response Time . | Common Side Effects . |
|---|---|---|---|
| * Acute leukemia or myelodysplasia have resulted from therapy with alkylating agents. | |||
| † Lymphoproliferative disorders or acute leukemia have occurred in patients with other disorders receiving these drugs. | |||
| # Several combinations have been used.27 | |||
| Abbreviations: PO, by mouth; IV, intravenous; SC, subcutaneously; qd, daily; bid, 2 times daily; tid, 3 times daily; qid, 4 times daily; GI, gastrointestinal | |||
| Prednisone | 1.0 mg/kg PO qd | 1–4 weeks | Hypokalemia, gastric upset, sodium and fluid retention, hyperglycemia, hypertension, myopathy, osteoporosis, infection risk, psychosis. |
| Colchicine | 0.6 mg PO tid | 4–8 weeks | Diarrhea (may limit therapy), nausea, vomiting. |
| Dapsone | 75–100 mg PO qd | 4–8 weeks | Hemolysis, agranulocytosis, aplastic anemia, exfoliative dermatitis, toxic hepatitis, choleostatic jaundice, peripheral neuropathy. |
| Danazol | 200 mg PO qid | 3–6 months | Weight gain, fluid retention, seborrhea, hirsutism, vocal changes, amenorrhea, acne, headache, liver toxicity. |
| Rituximab | 375 mg/M2 IV q week × 4 | 3–4 weeks | Infusional symptoms: (fever, chills, headache, broncho- spasm), severe B cell reduction and potential for infection. |
| Cyclophosphamide | 150 mg PO qd | 6–8 weeks | Cytopenias, hemorrhagic cystitis, GI symptoms, sterility, secondary malignancies.* |
| Azathioprine | 150 mg PO qd | 2–10 months | Cytopenias, GI symptoms, secondary malignancies.† |
| Cyclosporine | 1.25–2.5 mg/kg PO bid | Variable | Renal insufficiency, hepatotoxicity, hypertension, tremor, hirsutism, gum hyperplasia, hypomagnesemia, secondary malignancies.† |
| Mycophenolate mofetil | 0.5–1.0 g BID PO | 3–4 weeks | Diarrhea, leukopenia, headache, secondary malignancies.† |
| High-dose cyclophosphamide | 1.0–1.5 g/M2 IV q 4 weeks | 1–4 weeks | Cytopenias, hemorrhagic cystitis, GI symptoms, alopecia, sterility, myocardiopathy, secondary malignancies.* |
| Combination chemotherapy | See below# | 1–4 weeks | Cytopenias, hemorrhagic cystitis, alopecia, dermatitis, anaphylaxis, GI symptoms, sterility, myocardiopathy, mucositis, secondary malignancies.* |
| Therapy . | Dose . | Response Time . | Common Side Effects . |
|---|---|---|---|
| * Acute leukemia or myelodysplasia have resulted from therapy with alkylating agents. | |||
| † Lymphoproliferative disorders or acute leukemia have occurred in patients with other disorders receiving these drugs. | |||
| # Several combinations have been used.27 | |||
| Abbreviations: PO, by mouth; IV, intravenous; SC, subcutaneously; qd, daily; bid, 2 times daily; tid, 3 times daily; qid, 4 times daily; GI, gastrointestinal | |||
| Prednisone | 1.0 mg/kg PO qd | 1–4 weeks | Hypokalemia, gastric upset, sodium and fluid retention, hyperglycemia, hypertension, myopathy, osteoporosis, infection risk, psychosis. |
| Colchicine | 0.6 mg PO tid | 4–8 weeks | Diarrhea (may limit therapy), nausea, vomiting. |
| Dapsone | 75–100 mg PO qd | 4–8 weeks | Hemolysis, agranulocytosis, aplastic anemia, exfoliative dermatitis, toxic hepatitis, choleostatic jaundice, peripheral neuropathy. |
| Danazol | 200 mg PO qid | 3–6 months | Weight gain, fluid retention, seborrhea, hirsutism, vocal changes, amenorrhea, acne, headache, liver toxicity. |
| Rituximab | 375 mg/M2 IV q week × 4 | 3–4 weeks | Infusional symptoms: (fever, chills, headache, broncho- spasm), severe B cell reduction and potential for infection. |
| Cyclophosphamide | 150 mg PO qd | 6–8 weeks | Cytopenias, hemorrhagic cystitis, GI symptoms, sterility, secondary malignancies.* |
| Azathioprine | 150 mg PO qd | 2–10 months | Cytopenias, GI symptoms, secondary malignancies.† |
| Cyclosporine | 1.25–2.5 mg/kg PO bid | Variable | Renal insufficiency, hepatotoxicity, hypertension, tremor, hirsutism, gum hyperplasia, hypomagnesemia, secondary malignancies.† |
| Mycophenolate mofetil | 0.5–1.0 g BID PO | 3–4 weeks | Diarrhea, leukopenia, headache, secondary malignancies.† |
| High-dose cyclophosphamide | 1.0–1.5 g/M2 IV q 4 weeks | 1–4 weeks | Cytopenias, hemorrhagic cystitis, GI symptoms, alopecia, sterility, myocardiopathy, secondary malignancies.* |
| Combination chemotherapy | See below# | 1–4 weeks | Cytopenias, hemorrhagic cystitis, alopecia, dermatitis, anaphylaxis, GI symptoms, sterility, myocardiopathy, mucositis, secondary malignancies.* |
Effect of immune thrombocytopenic purpura (ITP) plasma on megakaryocyte production and maturation.
1A. CD34+-rich cells were cultured in media containing marrow growth and development factor and either ITP or control plasma. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (CD41+ cells by FACS)]. % Suppression = 100 – [mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100]. The results of the 12 of 18 ITP plasmas tested, which showed significant suppression of megakaryocytopoiesis, are shown.
1B. The histograms show the megakaryocyte ploidy distribution of CD34+ cells cultured for 10 days in the following plasmas (left to right): control, ITP-1 and ITP-2. Cells cultured in control or ITP-2 plasma (no evident suppression of in vitro megakaryocyte production) show 4 distinct peaks (2N, 4N, 8N, 16N) while cells cultured in ITP-1 plasma (suppressed in vitro megakaryocyte production) show primarily 2N cells.
Reprinted by permission from McMillan et al. Blood. 2004;103:1364–1369.
Effect of immune thrombocytopenic purpura (ITP) plasma on megakaryocyte production and maturation.
1A. CD34+-rich cells were cultured in media containing marrow growth and development factor and either ITP or control plasma. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (CD41+ cells by FACS)]. % Suppression = 100 – [mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100]. The results of the 12 of 18 ITP plasmas tested, which showed significant suppression of megakaryocytopoiesis, are shown.
1B. The histograms show the megakaryocyte ploidy distribution of CD34+ cells cultured for 10 days in the following plasmas (left to right): control, ITP-1 and ITP-2. Cells cultured in control or ITP-2 plasma (no evident suppression of in vitro megakaryocyte production) show 4 distinct peaks (2N, 4N, 8N, 16N) while cells cultured in ITP-1 plasma (suppressed in vitro megakaryocyte production) show primarily 2N cells.
Reprinted by permission from McMillan et al. Blood. 2004;103:1364–1369.
Cell-mediated cytotoxicity against platelets in patients with chronic immune thrombocytopenic purpura (ITP).
CD14−CD19− blood mononuclear cells (75 ± 4% CD3+ and 14 ± 3% NK cells) were incubated with 111In-labeled platelets at an effector/target ratio of 0.625:1 in the presence of anti-CD3 antibody (to stimulate cytolytic effector T cells) for 4 hours followed by centrifugation. Platelet lysis was determined as follows: (observed lysis-spontaneous lysis)/(maximal lysis-spontaneous lysis). The dashed line is the mean ± 2 s.d. recorded for controls. Data points represent mean ± s.d. of five measurements for each individual, using different CD3 antibody concentrations (15.6 ng/mL to 4 μg/mL). Patients with active ITP had significantly increased platelet lysis compared with patients in remission and control patients.
Reprinted with permission from Olsson et al. Nature Medicine. 2003;9:1123–1124.
Cell-mediated cytotoxicity against platelets in patients with chronic immune thrombocytopenic purpura (ITP).
CD14−CD19− blood mononuclear cells (75 ± 4% CD3+ and 14 ± 3% NK cells) were incubated with 111In-labeled platelets at an effector/target ratio of 0.625:1 in the presence of anti-CD3 antibody (to stimulate cytolytic effector T cells) for 4 hours followed by centrifugation. Platelet lysis was determined as follows: (observed lysis-spontaneous lysis)/(maximal lysis-spontaneous lysis). The dashed line is the mean ± 2 s.d. recorded for controls. Data points represent mean ± s.d. of five measurements for each individual, using different CD3 antibody concentrations (15.6 ng/mL to 4 μg/mL). Patients with active ITP had significantly increased platelet lysis compared with patients in remission and control patients.
Reprinted with permission from Olsson et al. Nature Medicine. 2003;9:1123–1124.

![Figure 1. Effect of immune thrombocytopenic purpura (ITP) plasma on megakaryocyte production and maturation. / 1A. CD34+-rich cells were cultured in media containing marrow growth and development factor and either ITP or control plasma. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (CD41+ cells by FACS)]. % Suppression = 100 – [mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100]. The results of the 12 of 18 ITP plasmas tested, which showed significant suppression of megakaryocytopoiesis, are shown. / 1B. The histograms show the megakaryocyte ploidy distribution of CD34+ cells cultured for 10 days in the following plasmas (left to right): control, ITP-1 and ITP-2. Cells cultured in control or ITP-2 plasma (no evident suppression of in vitro megakaryocyte production) show 4 distinct peaks (2N, 4N, 8N, 16N) while cells cultured in ITP-1 plasma (suppressed in vitro megakaryocyte production) show primarily 2N cells. / Reprinted by permission from McMillan et al. Blood. 2004;103:1364–1369.](https://ash.silverchair-cdn.com/ash/content_public/journal/hematology/2004/1/10.1182_asheducation-2004.1.390/4/m_cines_fig1.jpeg?Expires=1764962365&Signature=qsV4p-GuU1MJ-fYSEWrGeR48wEWkcvNan~htsa7A8LDszzkuNUrlMKVls9u-9oH~haLSWLJah~L9Iu9YODuKB260LIZ1bW6Ue3K-HvGy-EcOf8HoEBV0PaFkr6XsHIQ3om~y8lAwBJUWL0B9GQcf8hFqW5X98od4~tHE3nl6VdZxROc4r~~0VnEBHl2zsQwXcDl-ZC4xvhZjVPgiY~ca6ClPn-QwZhBLO5QyMlWpS-xfu83jjmNScfdmA9ENhiFVo8cBqVYEJw-sv6Wt0JKpo8cWvWNlJm1bF0ufa~zeLAuoLn0xLIF-AL2Qs0PJ658qC75kLAx~4-sma9JW6EL4KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)