Abstract
The immune system has two complementary arms: one is older and seemingly more primitive, called the innate immune system, found in both plants and animals. The second (already many millions of years old!) is the adaptive or antigen-specific immune system, limited to vertebrate animals. The human innate immune system has many cellular elements that include granulocytes, monocytes, macrophages, natural killer (NK) cells, mast cells, eosinophils, and basophils. Receptors for these cells are non-clonal, fixed in the genome, requiring no rearrangement, and recognize conserved molecular patterns that are specific to pathogens. The adaptive immune system (B cells and T cells) have receptors with great variation, able to recognize an almost an unlimited number of highly specific pathogens through rearrangement of receptor gene segments, and can also provide immunological memory so critical for vaccination. As the immune system has evolved to recognize non-self, malignant transformation of self can likely escape immune surveillance with relative ease. Contributors to this chapter are utilizing distinct components of either the innate or adaptive immune system that recognize non-self, in combination with what we know about differences between malignant and normal self, in an effort to develop novel and effective immunologic approaches against hematologic malignancies.
In Section I, Dr. Andrea Velardi reviews the benefits of NK cell alloreactivity in mismatched hematopoietic transplantation, provides updates on current clinical trials, and discusses further therapeutic perspectives emerging from murine bone marrow transplant models.
In Section II, Dr. David Scheinberg reviews novel leukemic antigens being targeted by humanized monoclonal antibodies as well as mechanisms by which antibody-mediated cytotoxicity occurs in vivo.
In Section III, Dr. Ivan Borrello reviews vaccine and adoptive T cell immunotherapy in the treatment of hematologic malignancies. Specifically, he discusses the various vaccine approaches used as well as strategies aimed at augmenting the tumor specificity of T cell therapies.
I. Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplantation
Andrea Velardi, MD,* Loredana Ruggeri, MD, PhD, Marusca Capanni, MSc, Antonella Mancusi, MSc, Emanuela Burchielli, MD, Katia Perruccio, MD, Franco Aversa, MD, and Massimo F. Martelli, MD
University of Perugia, Division of Hematology and Clinical Immunology, Ematologia, Policlinico Monteluce, Perugia 06122, Italy
Cure of leukemia by allogeneic hematopoietic transplantation relies on the action of donor T cells in the allograft, which are vital for promoting engraftment, eradicating malignant cells (graft vs leukemia [GVL] effect), and reconstituting immunity. Unfortunately, they mediate graft-versus-host disease (GVHD) which, in spite of progress in prophylaxis, is still responsible for significant morbidity and mortality.
In full haplotype-mismatched stem cell transplantation with donor and recipient pairs identical for one HLA haplotype and incompatible at the HLA class I and II loci of the unshared haplotype, all cases are at high risk of T cell–mediated alloreactions in the host-versus-graft (HvG) as well as in the graft-versus-host (GvH) direction. These T cell–mediated responses can be controlled to a large extent by (1) appropriate immunosuppressive intensity of the conditioning regimen followed by a graft containing a “megadose” of hematopoietic cells, both of which together prevent graft rejection, and (2) extensive T cell depletion of the graft which prevents GVHD.1 Unlike matched transplants, haploidentical transplants can rely on another type of alloreactivity, mediated by natural killer (NK) cells, which is triggered by major histocompatibility (MHC) class mismatches between killer cell immunoglobulin (Ig)-like receptors on donor NK cells and human leukocyte antigen (HLA) class I molecules on recipient cells.2
NK Cell Alloreactivity
Several years ago an inverse correlation was established between expression of surface MHC class I molecules on target cells and susceptibility to NK cell–mediated lysis, suggesting MHC molecules protect normal cells from NK cell–mediated lysis.3 The lack of expression of self MHC molecules on target cells resulted in susceptibility to NK cell-mediated lysis (“missing self” recognition).
NK cells are primed to kill by signals delivered through several different activating receptors (reviewed in 4 and 5). However, NK killing of autologous cells is prevented as each NK cell co-expresses at least one inhibitory receptor for self-MHC class I molecules. Human NK cells discriminate between allelic forms of MHC molecules via clonally distributed receptors, termed KIRs (from killer cell Ig-like receptor), that are specific for epitopes that are shared by MHC class I alleles, i.e., group 1 and group 2 HLA-C alleles, and HLA-Bw4 alleles (Table 1 ).
Although HLA and KIR genes are inherited independently, most individuals possess a full complement of KIR genes for inhibitory receptors for the three major class I ligands (group 1 and group 2 HLA-C alleles and HLA-Bw4 alleles)6 and can thus make an NK repertoire that is as diverse as the class I KIR ligands of the individual. In the 162 individuals we screened for KIR genotyping, 97% bore KIR2DL1, which is the receptor for HLA-C group 2 alleles; 100% expressed KIR2DL2/3 receptors, which are specific for HLA-C group 1 alleles; and 94% bore KIR3DL1, which is the receptor for HLA-Bw4 alleles (7 and Mancusi et al, unpublished observations).
The HLA class I genotype imposes selection during development of the NK-cell receptor repertoire by dictating which KIRs are to be used as inhibitory receptors for self HLA class I. In addition, the HLA class I genotype on the NK-cell KIR repertoire determines the frequencies of cells expressing a given KIR.8
The CD94-NKG2A inhibitory receptor complex is expressed primarily in NK cells that do not express an inhibitory KIR for self HLA class I, so it fills the gaps in the KIR repertoire. Alloreactive NK cells are not found among CD94-NKG2A-positive NK cells because HLA-E, the ligand for this receptor, is expressed on cells from all individuals (reviewed in 4 and 5).
Thus, in every individual, NK cell gene expression of different KIR and CD94/NKG2 receptors generates the NK cell repertoire of that person. As KIRs are clonally distributed, each cell in the repertoire bears a different receptor or, less frequently, two or more receptors. However, every mature NK cell expresses at least one receptor that is specific for self HLA class I molecules (because the individual’s HLA selects the self-tolerant repertoire). Consequently, when faced with mismatched allogeneic targets, NK cells in the repertoire will sense the missing expression of self HLA class I alleles and will mediate alloreactions.
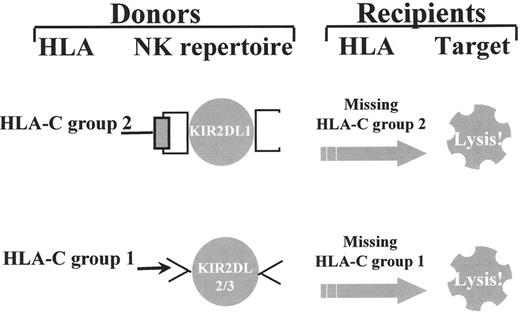
The great diversity of KIR expression ensures the generation of alloreactive NK cells between individuals who are mismatched for either one of the two subgroups of HLA-C alleles and/or the HLA-Bw4 allele group. For example, individuals who express Group 2 HLA-C alleles and possess NK cells that express KIR specific for Group 2 HLA-C alleles (KIR2DL1) are alloreactive against cells from individuals who do not express Group 2 HLA-C alleles (who are homozygous for Group 1 HLA-C alleles). Individuals who express Group 1 HLA-C alleles possess NK cells with KIR specific for Group 1 HLA-C alleles (KIR2DL2 and/or KIR2DL3) and are alloreactive against cells from individuals who do not express Group 1 HLA-C alleles (who are homozygous for Group 2 HLA-C alleles) (Figure 1 ). Likewise, HLA-Bw4 positive individuals expressing the Bw4-specific KIR3DL1 receptor may possess NK cells that are alloreactive against Bw4-negative cells.
Donor-Versus-Recipient NK Cell Alloreactivity in Haploidentical Transplants
In HLA haplotype-mismatched hematopoietic transplantation with a potential for graft-versus-host NK-mediated reactions, the engrafted stem cells give rise to an NK cell wave of donor origin that regenerates the same repertoire as the donor’s, and so includes high frequencies of donor-versus-recipient alloreactive NK cells.9 Donor-versus-recipient NK cell alloreactivity reduced the risk of leukemia relapse in 57 acute myeloid leukemia (AML) patients at high risk of relapse, while improving engraftment and protecting against GVHD.2
Experimental evidence confirmed NK cells were directly involved in controlling relapse of AML. In vitro studies showed that 100% of myeloid leukemias were killed by alloreactive NK cells, but only a minor fraction of common phenotype acute lymphoblastic leukemias (ALLs) were lysed. This was the only non-susceptible target in all the primary tumors of lympho-hematopoietic lineage we have tested to date (Table 2 ). Moreover, transfer of NK cells into NOD/SCID mice eradicated transplanted human AML provided that the NK cells were alloreactive toward the transplanted AML.2
An updated analysis of transplantation outcomes of 93 AML patients transplanted from haploidentical donors from 1993 through January 2004 demonstrates that transplantation from NK-alloreactive donors enhances engraftment (rejection rate 10% in the absence of NK alloreactivity vs 2% in its presence), does not increase the risk of GVHD but rather appears to protect from it (9% vs 3%), and exerts a remarkable control of leukemia relapse. Probability of relapse is 68% for the 53 patients transplanted from non-NK–alloreactive donors (26 transplanted in clinical remission [CR], 27 in relapse) versus 15% (P < 0.005) for the 40 patients transplanted from NK-alloreactive donors (25 transplanted in CR, 15 in relapse). Probability of event-free survival is 55% for patients with NK-alloreactive donors versus 12% for those without (P < 0.005). Thus, transplantation from an NK-alloreactive donor is a strong independent factor predicting survival (transplantation from NK-alloreactive vs non-NK-alloreactive donor: hazard ratio = 0.44, confidence interval [CI] = 0.25–0.77, P = 0.004), even when compared with disease status at transplant (remission vs relapse: hazard ratio = 0.47, CI = 0.28–0.73, P = 0.004).
Donor-Versus-Recipient NK Cell Alloreactivity in Unrelated-Donor Hematopoietic Transplantation
As approximately 50% of unrelated-donor transplants are performed in the presence of one or more HLA allele-level mismatches, NK cell alloreactivity may be expected to occur in this setting. Results from retrospective studies addressing this issue are now available,10–,16 but several show no advantage of transplantation from donors with potential to exert NK cell alloreactivity. Common to these reports is the lack of functional assessment of donor-versus-recipient NK cell alloreactivity and transplant protocols that differ from the haploidentical: they are heterogeneous in terms of patient populations, underlying diseases (including ALL, which is resistant to alloreactive NK killing, see above), conditioning regimens, graft composition, and post-transplant immunosuppressive regimens. Unlike the “megadose” of extensively T cell-depleted stem cells in haploidentical grafts, unrelated-donor transplants generally use unmanipulated bone marrow harvests (or, less frequently, peripheral blood progenitors) that contain ~4 log more T cells and up to 1 log fewer stem cells. Furthermore, they depend on post-transplant immune suppression to help prevent and/or control GVHD. The relatively few transplanted stem cells, immune-suppressive GVHD prophylaxis, and the further immune suppression given when GVHD develops all combine to adversely affect NK cell maturation from their bone marrow precursors. Indeed, under these conditions a recent study demonstrated a very delayed and poor reconstitution of potentially alloreactive KIR-bearing NK cells.17
Even under these adverse conditions, some of these studies documented an increased GvL effect in transplants from donors with potential to exert NK cell alloreactivity.11,13–,15 Interestingly, the one report that demonstrated a dramatic survival advantage for patients transplanted from donors with potential to exert donor-versus-recipient NK cell alloreactivity administered anti-thymocyte globulin (ATG) pre-transplant (which provided in vivo T cell depletion) and a graft containing 2- to 3-fold more nucleated cells than usual in unrelated-donor transplants.11
Prospective studies are needed to determine whether strategies from haploidentical transplantation that harness donor-vs-recipient NK cell alloreactivity—i.e., (1) transplantation of high doses of stem cells, (2) extensive T cell depletion, (3) no post-grafting immune suppression—improve outcomes in unrelated donor transplants for myeloid leukemias at high risk of relapse.
Work-Up for NK Alloreactive Donor Selection
One consequence of the haploidentical transplant studies is revision of current criteria for donor selection. Donor selection for AML now involves a search for the donor who is able to mount donor-versus-recipient NK cell alloreactivity.
The search for NK alloreactive donors may require extension from the immediate family (parents and siblings) to other family members such as aunts, uncles, and cousins. An extended search raises the chance of finding an NK-alloreactive donor from the random 30% to > 60% (which is close to the maximum, bearing in mind that HLA type of about 30% of the population makes them resistant to alloreactive NK killing).
HLA Typing
For NK alloreactive donor selection, first HLA type the recipient by high-resolution molecular techniques. Recipients who express class I alleles belonging to the 3 major class I groups (HLA-C group 1, HLA-C group 2, and HLA-Bw4 alleles) will block all NK cells from every donor. Recipients who express alleles belonging to 1 or 2 of these three class I groups have the chance of finding NK-alloreactive donors. Donor HLA typing will identify the relative who expresses the allele in the class I group that is not expressed by the patient. Most of these donors will possess NK cells that are alloreactive against the recipient’s.
KIR Genotyping
As we have observed, 3% of the population lack KIR2DL1 and 6% lack KIR3DL1, while 100% have KIR2DL2/3. KIR genotyping of the donors is necessary to make sure the donor possesses the relevant KIR gene and, thus, to improve accuracy of NK-alloreactive donor identification.
NK cloning
In some individuals, allelic variants in the HLA-Bw4 inhibitory NK receptor gene KIR3DL1 may not allow full receptor expression at the cell membrane.18 Others may express the corresponding NK clones in very low frequencies.7 Direct functional assessment of the donor-alloreactive NK repertoire through the generation of large numbers of donor NK clones and cytotoxicity assays against recipient target cells may be necessary to identify these donors.
Therapeutic Perspectives from Mouse Models
NK alloreactivity favors engraftment, as our murine transplant models show.2 Even after mild host immune suppression, infusion of donor-alloreactive NK cells ablated the host lympho-hematopoietic cells, thus preventing rejection of the MHC-mismatched BMT.
Experimental evidence suggests NK cells attack predominantly the hematopoietic cells of the host but not other tissues that are common targets for T cell–mediated GVHD. Murine-alloreactive NK cells, even when infused in large numbers, do not cause GVHD.2 Interestingly, alloreactive NK cells kill host-type dendritic cells, which prevents presentation of host antigens to donor T cells, i.e., the crucial step that initiates graft-versus-host reactions. Indeed, alloreactive NK cells in a pretransplant conditioning regimen protected mice from GVHD to such an extent as to allow a safe infusion of otherwise lethal doses of allogeneic T cells.2
Even in the overt absence of acute systemic GVHD, the NK conditioning itself, or the transfer of allogeneic T cells, might still cause acute thymic GVHD, i.e., damaged stroma, and impaired thymopoiesis and selection of the T cell repertoire.19 However, the use of an alloreactive NK-based conditioning regimen in conjunction with the adoptive transfer of large numbers of allogeneic T cells affected neither the architectural organization nor the cellular composition of the thymic stromal compartment nor did it impair regular thymopoiesis.20
Therefore, conditioning with alloreactive NK cells might protect from post-transplant infections because it allows T cell-replete mismatched transplants without GVHD and does not endanger long-term thymic T cell reconstitution. Indeed, the time-kinetics of donor-type T cells and dendritic cell recovery were much faster after NK-based than standard conditioning. Thus, mice responded immediately to infectious challenges and mounted a protective T cell-mediated immunity which allowed them to survive later high-virulence challenges and, concomitantly, were protected from GVHD.21
In conclusion, unlike T cell alloreactivity, NK alloreactivity combines all the features which make it uniquely suited for mismatched hematopoietic transplantation. In mice, alloreactive NK cells eradicate leukemia and favor engraftment by killing host lympho-hematopoietic cells. By eliminating host-type dendritic cells they reduce GVHD, to such an extent that T cell-replete mismatched bone marrow can be transplanted without GVHD (Figure 2 ). We envisage harnessing the potential of NK cell alloreactivity to enhance the graft-versus-leukemia effect and reduce transplant-related mortality and the incidence of infection-related deaths, thus offering survival to more leukemia patients.
II. Antibody Therapies of Acute Myeloid Leukemias
David A. Scheinberg, MD, PhD*
Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York NY 10021 Acknowledgments: Joseph Jurcic and Debra Mulford participated in the preparation of this chapter. David A. Scheinberg is a Doris Duke Distinguished Scientist.
AML remains a fatal disease for most patients. AML is well suited to treatment with the slowly diffusing, large monoclonal antibodies because of the accessibility of cells in the blood, bone marrow, spleen, and lymph nodes. In addition, immunophenotypes of the various types of AML allow the identification of antigenic targets that are relatively selective, though not truly tumor-specific. Many studies in AML have focused on CD33, a cell-surface glycoprotein found on most myeloid leukemias as well as myelomonocytic progenitor cells, but not on the ultimate stem cell nor on mature granulocytes or non-hematopoietic tissues (Table 3 ). CD45, expressed on most leukocytes and myeloid and lymphoid blasts, and CD66, a glycoprotein found on mature myeloid cells but not leukemic blasts, have also been targeted. Despite lack of true specificity, monoclonal antibodies offer the possibility of decreased toxicity compared with conventional chemotherapy, by increasing the therapeutic index and by having non-overlapping toxicities with conventional agents. We will discuss (1) the use of native unconjugated antibodies against overt AML and minimal leukemia; (2) the use of targeted radiotherapy for myeloablation before hematopoietic stem cell transplantation; (3) α-particle immunotherapy; (4) the use of antibody-directed chemotherapy with gemtuzumab ozogamicin (GO) (Mylotarg; Wyeth-Ayerst Pharmaceuticals, Radnor, PA); and (5) immunotoxins.
Native Antibody Therapy
Monoclonal antibodies can direct an inflammatory response against tumor cells that typically involves complement activation and resulting cellular infiltrates, or antibody dependent cellular cytotoxicity (ADCC,) in which the antibody Fc domain recruits natural killer (NK), polymorphonuclear (PMN), or macrophage cells to kill targets (Table 4 ). Additionally, antibodies may block growth factors or induce growth arrest and apoptosis. These latter mechanisms are not well described for the antibodies in current use against AML. Most antibodies in clinical use are chimeric mousehuman or fully humanized to reduce immunogenicity and also to increase immunologic activity compared to their murine counterparts. Resistance to complement-mediated cytotoxicity, but not ADCC, related to intracellular pH and expression of p-glycoprotein has been described.1
Targeting CD33 with Native Antibody
Murine M195 and genetically engineered humanized HuM195 are monoclonal antibodies that bind to the early myeloid surface-antigen CD33. A series of studies showed that these antibodies rapidly target CD33-positive leukemia cells in patients, with optimal targeting of leukemic sites seen at doses below 3–5 mg/m2. This is due to the low number of binding sites (approximately 10,000–20,000) on each leukemia cell. HuM195 mediates leukemia cell killing by ADCC, fixes human complement, and is not immunogenic in humans.2
In Phase I trials, HuM195 was well tolerated and produced occasional long-term objective responses. At higher doses (12 to 36 mg/m2 daily for 4 days), fever and rigors, generally seen with the first dose, were the most common toxicities.3 Among 50 patients with relapsed or refractory AML, 3 patients achieved a complete remission or CRp (a complete response except for full platelet recovery).4 These 3 patients had less than 30% blasts in the bone marrow before treatment, suggesting that HuM195 has limited activity against previously treated leukemia, especially in the treatment of lower leukemic burdens.
Native Antibody in Acute Promyelocytic Leukemia
Serial monitoring of bone marrow for PML-RARα fusion mRNA by reverse-transcriptase polymerase chain reaction (RT-PCR) allows sensitive monitoring of therapy in patients who are clinically in remission. Thirty-one patients with newly diagnosed acute promyelocytic leukemia (APL) in clinical CR immediately after retinoic acid were treated with HuM195 twice weekly for 3 weeks.5 Half of the 24 patients evaluable for conversion of positive RT-PCR assays became RT-PCR negative after HuM195; after additional therapy, 5-year disease-free survival was 93%, compared with 73% among historical patients receiving all-trans retinoic acid (ATRA) induction followed by consolidation chemotherapy without HuM195. Thus, the use of HuM195 and other newer agents, such as arsenic trioxide or other antibodies to CD33, may reduce the necessity of standard chemotherapy courses required for long-term remission in APL.
Based on the activity in APL, the use of HuM195 in conjunction with chemotherapy was studied in a randomized Phase 3 trial in 191 patients with relapsed or refractory AML using mitoxantrone, etoposide, and cytarabine with or without HuM195.6 A 36% overall response rate (CR + CRp) was seen in the HuM195 group versus 28% in the control group (P = 0.28).
Radiolabeled Antibodies
Key features of isotopes for conjugation to antibodies are the emission characteristics, physical half-life, and the metabolism of the immunoconjugate in vivo. These need to be matched to disease burden, antigen site number, internalization of targets, and clinical setting (Table 5 ). Alpha and beta particles are distinct, with markedly different properties that confer advantages and disadvantages to each radiation type. Alpha particles are heavy, slow-moving nuclei that have a short range in tissue (50–80 μm) and a high linear energy transfer (LET) (~100 keV/μm) compared to the far lighter beta particles, which have a 20- to 100-fold longer range (1–10 mm) and a much lower LET (~0.2 keV/μm).
Radioimmunotherapy with beta-emitting isotopes, such as iodine-131 (131I), yttrium-90 (90Y), and rhenium-188 (188Re), has the advantage of making a “crossfire effect,” destroying tumor cells to which the radioimmunoconjugate is not directly bound; but this also leads to significant dose-limiting toxicity to normal cells (usually in bone marrow [BM], liver, or lung). As a consequence, beta therapy is useful in the setting of bulky disease where marrow transplantation is possible if necessary. In contrast, alpha radioimmunotherapy, such as with conjugated bismuth-213 (213Bi), astatine-211 (211At), and actinium-225 (225Ac), results in more potent and more specific tumor cell kill and little damage to surrounding tissue. Thus, alpha particles are more likely applicable for the treatment of residual individual cells or small-volume tumors or leukemias (Tables 4 and 5 ).
Beta particle RIT: 131I-and 90Y-Anti-CD33, 131I-Anti-CD45, and 90Y- and 188Rh-Anti-CD66
A series of trials with antibodies targeting CD33 conjugated to beta emitters have been conducted.7,8 Gamma camera imaging demonstrated rapid uptake of the antibodies to leukemia targets in the bone marrow, liver, and spleen with retention for up to 3 days and internalization of the radiolabel. Myelosuppression was dose limiting at doses greater than 135 mCi/m2, necessitating bone marrow transplantation (BMT) in patients at these or higher levels. The studies demonstrated that selective beta targeting to the marrow yielded significant antileukemic effects with limited extramedullary toxicity. Thus, beta-emitting anti-CD33 antibodies were next used to safely intensify conditioning before busulfan/cytoxan-prepared BMT. Estimated absorbed radiation doses to the marrow ranged between 272 and 1470 cGy, and no toxicities were seen beyond those expected with busulfan and cyclophosphamide. Long-term (5+–8+ years) remissions were achieved in 20% of 15 patients.
BC8 is a murine immunoglobulin G1 (IgG1) antibody that targets the non-internalizing pan-leukocyte antigen CD45. In a Phase I trial, 44 patients with advanced acute leukemia or myelodysplastic syndrome (MDS) initially received BC8 labeled with trace doses of 131I. Thirty-four of these patients, with favorable biodistributions, received escalating therapeutic doses of 131I-BC8 (76–612 mCi) followed by cyclophosphamide, total-body irradiation (TBI), and allogeneic or autologous transplantation. Approximately one third of patients with acute leukemias achieved durable disease-free responses. Approximately 85% of patients in Phase II trials have favorable biodistributions. Therefore, Phase II trials were begun using the preparative regimen of 131I-BC8, busulfan, and cyclophosphamide in patients with AML in first remission (9 and Pagel, unpublished) in which 18 of 24 patients were alive and disease-free at a median follow-up of 42 months, and in older patients as part of a “nonmyeloablative” preparative regimen combined with fludarabine and 2 Gy TBI.
The limitations of 131I as a conjugated radiopharmaceutical (such as long isotope half-life and iodine metabolism and excretion) suggested a switch to the use of the radiometal 90Y or 188Rh (Tables 4 and 5 ). Nineteen patients with relapsed or refractory AML were treated with single escalating doses of 90Y-HuM195 (0.1–0.3 mCi/kg) (8 and Jurcic unpublished). Dose-dependent leukemia and myelosuppression were profound, with a maximum tolerated dose (MTD) of 90Y-HuM195 without stem cell rescue of 0.275 mCi/kg. One patient treated at the maximum tolerated dose achieved complete remission lasting 5 months. The advantages of these approaches allow 90Y-HuM195 to be studied as part of a reduced-intensity preparative regimen before allogeneic stem cell transplantation.
188Re (17-hour half-life) is a radiometal that emits both beta particles and gamma rays, which facilitate biodistribution and dosimetry studies. A monoclonal antibody to CD66 was used to deliver “crossfire” radiation from the long-ranged beta particles emitted by 188Re to the normal cells of the bone marrow of 54 patients with high-risk AML before related and unrelated transplants (10 and Bunjes unpublished). Possible late renal toxicity occurred in 11% of patients between 6 and 12 months after transplantation. Disease-free survival was 44% at 48 months. Additional patients are under study in several centers in Germany using both 90Y- and 188Rh-Anti-CD66 as pre-BMT conditioning.
Alpha Particle Emitters
Preclinical studies in several systems, including mice, dogs, and monkeys, support the hypothesis that alpha particle immunotherapy may be safer and possibly more effective in the treatment of small-volume disease than in the treatment of bulky tumors.11–,13 Because of the short half-life of 213Bi (45 minutes) and 217Bi (60 minutes), novel “pretargeting” strategies for radioimmunotherapy have been developed as well.14 A monoclonal antibody or fusion protein is first conjugated to the tetravalent streptavidin molecule and administered intravenously. Then, a biotinylated N-acetylgalactosamine-containing “clearing agent” is infused to remove excess circulating antibody. Finally, a rapidly targeting therapeutic radioisotope labeled with biotin is administered.
A generator of clinical grade 213Bi was constructed to allow 18 patients with advanced myeloid leukemias to be treated with 213Bi-HuM195 (0.28–1.0 mCi/kg) in a Phase I trial.15 Absorbed dose ratios between the marrow, liver, and spleen and the whole body were 1000-fold greater than those seen with β-emitting HuM195 constructs in similar patients. Significant toxicity was limited to myelosuppression and 78% of patients had reductions in the percentage of bone marrow blasts. MTD was not reached and no complete remissions occurred. A subsequent trial was undertaken in which high-risk patients were first treated with chemotherapy to achieve partial cytoreduction of the leukemic burden followed by 213Bi-HuM195.16 The maximum tolerated dose was 1 mCi/kg. At MTD or higher in this ongoing trial, approximately one third of patients had CR or CRp.
Another strategy to overcome the short half-life of 213Bi is to couple the 213Bi generator directly to the antibody (a so-called “atomic nanogenerator”).17
225Ac decays by alpha emission through three atoms, each of which also emits an α-particle, and can be conjugated to a variety of antibodies using derivatives of DOTA chelates. 225Ac-immunoconjugates are approximately 1000 times more potent than 213Bi-containing conjugates, but also potentially more toxic to the possibility of free daughter radioisotopes in circulation after decay of 225Ac. Anti-CD33 nanogenerators have completed murine and monkey studies and await clinical trials.
Toxin- and Drug-Antibody Conjugates
Drugs or toxins derived from either plants or bacteria, such as diphtheria toxin (DT), calicheamicin, Pseudomonas exotoxin A (PE) ricin, pokeweed antiviral protein (PAP), and gelonin, can be conjugated to antibodies to increase potency and reduce toxicity of the parent toxin. Often the toxins are enzymatically based inhibitors of extraordinary potency, which require small numbers of molecules to enter the cell for action. However, the toxins must enter the cell function, thus requiring internalizing antigens such as CD33.
The most widely studied (and now US Food and Drug Administration [FDA]-approved) agent is gemtuzumab ozogamicin (GO), which consists of a recombinant humanized anti-CD33 monoclonal antibody conjugated to calicheamicin, a potent antitumor antibiotic. Calicheamicin dissociates from the antibody in the lysosome and binds within the minor groove of DNA, causing double-stranded DNA breaks. GO’s efficacy has been demonstrated in large trials of 9 mg/m2 given 2 weeks apart to patients with AML in first relapse. Patients with secondary AML or prior MDS were excluded. Complete remission was achieved in 16%; CRp in 13%.18 Toxicities in these trials include significant hyperbilirubinemia (23%) and elevated levels of serum transaminases (17%). Hepatic veno-occlusive disease (VOD) has developed in some patients, especially after a subsequent stem cell transplant, where rates as high as 64% were seen.19 These toxicities were not seen in other anti-CD33 constructs.
GO is now under investigation for newly diagnosed AML in combinations, usually with dose reductions to avoid additive toxicities. For example, in two studies in patients with newly diagnosed AML, the maximum tolerated dose of GO was a single infusion of 3–6 mg/m2 in combination with standard inductions. However, high remission rates were seen (85%–86%).20 Early results suggest that GO in combination with ATRA in APL can produce high molecular remission rates as first-line therapy as well. GO appears less effective in patients with refractory or poor-risk AML.21
The anti-CD33 antibody HuM195 has been linked to gelonin, a ribosome-inactivating plant toxin.22 Gelonin irreversibly inactivates ribosomal subunits by enzymatically hydrolyzing the N-glycoside bond in a specific sequence of the rRNA critical for elongation binding factor. A Phase I clinical trial for the in vivo treatment of relapsed AML is underway.
Conclusion
While monoclonal antibodies and antibody conjugates have complicated pharmacology, they have become useful components of cancer therapy because of their unique mechanisms of action, their non-crossresistance and their non-addictive toxicities. In AML, native antibodies thus far have not shown sufficient antitumor activity to be approved alone. In contrast, native antibodies appear to have significant activity against minimal residual disease in APL. Radioimmunotherapy and antibody-drug conjugates provide far greater potency, but also toxicity. One drug conjugate (GO) is now FDA approved for sale. Other conjugates will require substantial additional study to demonstrate their efficacy and the best way to integrate their activity into standard regimens.
III. Vaccine and T Cell Therapy in Hematologic Malignancies
Ivan Borrello, MD*
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Dept. of Oncology, 1650 Orleans St., CRB 453, Baltimore MD 21231
Immunotherapy is increasingly playing a role in the treatment of hematologic malignancies. The recent successes obtained with monoclonal antibodies have firmly established these agents in the armamentarium of various therapeutic settings. The accessibility to large quantities of autologous tumor, the identification and potential purification of tumor-associated antigens, coupled with the initial responsiveness to chemotherapy for many of these diseases, highlight several advantages toward the integration of active immunotherapy in the treatment of these diseases. Furthermore, the clinical benefit conferred by allogeneic bone marrow transplantation underscores the role of the T cells in mediating a measurable and sustained antitumor response. However, significant toxicity and limited donor availability limit its broader use. Cancer vaccines aim to increase the applicability, increase the tumor specificity, and minimize the toxicity of active immunotherapy.
Unlike vaccines against infectious agents, which target foreign antigens in a prophylactic setting, cancer vaccines are administered in a therapeutic setting and must generate an immune response toward antigens to which the host has already been exposed. Recent advances in tumor immunology have increased our understanding of the critical requirements for overcoming the barriers of immune unresponsiveness in cancer-bearing hosts and identified strategies to augment the efficacy of immune-based therapeutic interventions.
Vaccine Therapy
Antigen-specific vaccines
Critical to effective vaccine design is the ability to overcome the intrinsic tolerogenic mechanisms and to target immunodominant tumor antigens capable of generating meaningful and sustained anti-tumor responses. Tumor antigens fall into at least five categories based on their expression patterns (Table 6 ). Their identification has enabled the development of antigen-specific vaccine formulations. Such an approach provides greater control in targeting the antitumor response as well as monitoring resultant in vivo T cell responses. For vaccines to be effective, tumor-associated antigens must be delivered, processed, and effectively presented by antigen-presenting cells (APCs) in the appropriate major histocompatibility (MHC) complex. The APCs must then traffic to the draining lymph nodes where the APC-T cell generates a T cell response and subsequently systemic immunity. Optimal design and delivery of antigens to maximize immunogenicity are areas of intensive ongoing research. As such, a variety of vaccine approaches are currently being tested in clinical trials (Table 7 ).
Protein vaccines
Idiotype (Id) vaccines against the monoclonal immunoglobulin probably represent the best-studied vaccine formulation in low-grade lymphomas and multiple myeloma.1 This approach is based on the concept that a given B cell clone produces a unique immunoglobulin through recombination in the variable regions of its heavy and light chains, thereby forming a tumor-specific antigen. Studies thus far in low-grade lymphomas have shown evidence of the induction of both humoral and cellular immune responses. Impressively, in patients with follicular lymphoma who achieved a CR with chemotherapy, Id-keyhole limpet hemocyanin (KLH) vaccines and adjuvant granulocyte-macrophage colony-stimulating factor (GM-CSF) achieved molecular remissions in 8 of 11 vaccinated patients examined.2 Based on these results, two currently ongoing randomized Phase III clinical trials are examining the clinical efficacy of this approach.
In multiple myeloma, the low surface expression of Id on plasma cells, the secretion of large amounts of Id into the serum—which can serve to both neutralize anti-Id humoral responses and possibly tolerize T cell responses—as well as the difficulty in achieving clinical complete remissions (a state of minimal tumor burden) with current therapy are all factors likely contributing to the absence of potent T cell responses of Id-KLH–vaccinated patients. However, encouraging immunologic and clinical activity has been reported with this approach.3
Peptide vaccines
Effective vaccination requires that the immunogen be efficiently recognized by T cells. CD8+ T cells recognize peptides 9 amino acids in length restricted by class I human leukocyte antigen (HLA) molecules, whereas CD4+ T cells recognize peptides 12–15 amino acids in length restricted by class II HLA molecules. While a synthetic peptide vaccine approach is limited to patients expressing the specific HLA molecules, advantages of such a vaccine strategy as compared to a whole-protein approach include the ease of production on a large scale, and more importantly, the ability to specifically monitor immune responses to these antigens through the use of peptide-HLA tetramers.
In chronic myeloid leukemia (CML), the bcr/abl fusion protein (p210bcr/abl) most frequently generated by the chimeric messenger RNA (mRNA) transcript (β3α2) represents a unique tumor antigen. A study using a multivalent vaccine (6 peptides) spanning the β3α2 region was administered with an adjuvant in patients with minimal residual disease. Of the 14 patients, 11 demonstrated a CD4+ response to vaccination, and 4 a peptide-specific CD8+ response. All 5 patients on α-interferon (IFN-α) ultimately achieved a complete cytogenetic response, and 3 patients in molecular relapse became transiently polymerase chain reaction (PCR) negative following vaccination.4 In this study, the clinical efficacy of the vaccine could not be determined, as patients were continued on their therapy (IFN-α, imatinib, hydroxyurea, or donor lymphocyte infusion [DLI]) throughout the vaccination period. Interestingly, Bocchia et al, utilizing similar peptides, began vaccination following the achievement of a therapeutic plateau with either IFN-α or imatinib and were able to demonstrate the induction of a molecular complete remission in a significant number of patients.5
Proteinase 3 (Pr3), a serine protease expressed in promyelocytes, plays a critical role in leukemogenesis. A correlation between cytogenetic remissions with IFN-α and the presence of Pr-1 CD8+ T cells was demonstrated utilizing tetramers to an HLA-A2 peptide (Pr1).6 These findings were followed with a clinical trial utilizing the Pr1 peptide administered in incomplete Freund’s adjuvant and GM-CSF in patients with a myeloid malignancy in which higher-avidity Pr-1 CD8+ T cells were noted in responders compared to nonresponders, and durable molecular remissions were also observed.7
Cell-based vaccines
A major drawback of the antigen-specific vaccines approach is that of limiting the therapeutic effect to priming immune responses to single antigens, with the subsequent risk of relapsing with tumors no longer expressing the antigens against which they were vaccinated, a phenomenon known as “antigen escape variants.” Furthermore, with few exceptions, it is still not clear whether the tumor-specific antigens identified to date also represent immunodominant proteins to which effective immune responses can be generated. In contrast, a vaccine formulation using the tumor cell itself as an antigen source offers the advantage of a broad spectrum of tumor antigens present on the tumor cell’s surface (polyvalent vaccination) that can potentially serve as targets for the immune system. This approach relies on the ability to induce stronger immunity against tumor-selective antigens than against normal tissue antigens present on the tumor cell’s surface. Critical to vaccine development is the ability to modify the tumor cell with genes encoding immunologically relevant molecules and producing a sustained, local release of its product, leading to a local inflammation at the vaccine site without systemic toxicity. A variety of tumor cells have been genetically modified to secrete cytokines locally. With this approach, the immunogenicity of malignant cells is increased by enhancing the presentation of tumor antigens to the T cell arm of the immune system. A systemic comparison of different cytokines or cell-surface molecule–based tumor vaccines showed that immunization with tumors transduced with a retroviral vector expressing GM-CSF produced the greatest degree of systemic immunity, which was enhanced relative to irradiated nontransduced tumors.22 Priming with GM-CSF–transduced tumor cells led to a potent, long-lived antitumor immunity that was both CD4+ and CD8+ dependent.1 Further dissection of the mechanisms mediating this strong antitumor effect showed that GM-CSF produced at the vaccine site promotes the recruitment and activation of the host’s antigen-presenting cells, which efficiently uptake, process, and present tumor antigens to antigen-specific T cells, leading to strong anti-tumor responses. Multiple reports have since confirmed the bioactivity of GM-CSF–transduced tumor cells in a number of different tumor model systems, including hematologic malignancies.
GM-CSF tumor cell–based vaccine strategies
To date, GM-CSF–secreting vaccines that have been tested clinically have fallen into two categories: autologous tumors virally transduced to secrete GM-CSF (renal cell carcinoma, melanoma, prostate carcinoma, and lung cancer) and GM-CSF–producing allogeneic tumor cell lines (pancreatic carcinoma and prostate carcinoma). In the former scenario, vaccine development was hampered by the ability to harvest adequate amounts of tumor, expense, labor intensity, and interpatient GM-CSF variability. With the latter, the strategy relies on the ability to prime immune responses to shared tumor antigens of similar histologies and is ideal in situations in which tumor tissue is limited. This strategy relies on the requirement of antigen processing and presentation by host APCs, and thus MHC compatibility between host and vaccinating tumor is not required. However, one limitation to this approach is the possible generation of allogeneic responses to the tumor vaccine itself that could ultimately reduce cell viability and, more importantly, antitumor efficacy with subsequent vaccinations.
Hematologic malignancies represent a unique situation in which, for many diseases, tumor is readily available. Furthermore, we have recently shown that although GM-CSF secretion is a critical parameter in generating systemic antitumor responses, autocrine secretion is not required and paracrine production is equally effective.8 We have therefore developed an allogeneic bystander cell line that secretes large and stable amounts of GM-CSF (K562/GM-CSF). This cell line was chosen because it can be easily grown in suspension and has no detectable levels of either HLA class I or class II expression, thereby minimizing the likelihood of anti-bystander allogeneic responses with multiple vaccinations. This universal bystander vaccine approach thus obviates the requirement of gene modification for each individual tumor source and guarantees uniform cytokine production.
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins currently has three Phase I/II clinical trials open examining the safety, feasibility, and efficacy of this approach. In the multiple myeloma trial, newly diagnosed patients undergo a bone marrow harvest to collect autologous tumor, whereas the AML trial utilizes the circulating tumor obtained via either peripheral phlebotomy or pheresis. The autologous tumor and the GM-CSF–producing bystander cell line will be admixed to compose the final vaccine formulation consisting of both an antigen and cytokine source, respectively, that will be administered pre- and post-autologous stem cell transplantation. Both these studies, sponsored by Cell Genesys, will determine whether vaccination early post-BMT during the period of immune reconstitution can augment the efficacy of vaccination. In contrast, in the CML trial, patients must have achieved a stable cytogenetic response to imatinib for at least 3 months with evidence of persistent molecular disease, following which they will be vaccinated with K562/GM-CSF—a CML-derived cell line serving as both the cytokine and antigen source. The bystander cell line (K562/GM-CSF) has been further modified to express the Epstein-Barr virus (EBV) antigens, EBNA-1 and LMP-2, that will be administered as a vaccine in EBV+ Hodgkin’s disease patients prior to and following lymphoablative chemotherapy. The primary endpoints of these studies will be the demonstration of tumor-antigen–specific immune responses.
A similar strategy using the universal bystander vaccine formulation is being evaluated in a Phase I trial at the H. Lee Moffitt Cancer Center & Research Institute in patients with mantle cell lymphoma. The vaccine in this study is slightly modified and consists of autologous tumor admixed with the GM-CSF–producing bystander cell line that has been also transfected to express the co-stimulatory molecule CD40 ligand (K562/GM-CSF/CD40L vaccine), in an attempt to provide further “activation signals” (via CD40L-CD40) to the APCs recruited to the vaccination site by the local secretion of GM-CSF.9
Dendritic cell vaccines
A critical requirement for the generation of effective anti-tumor immune responses is the ability to effectively process and present the tumor-specific antigens in the appropriate MHC context to the T cells. Dendritic cells (DCs) represent, by far, the most potent antigen-presenting cells capable of priming effective T cell responses. Many features are responsible for the unique antigen-presenting capabilities of DCs. They express 50-fold higher levels of MHC molecules than macrophages, thereby providing more peptide/MHC ligand for T cell receptor engagement. DCs also express extremely high levels of costimulatory and adhesion molecules critical for T cell activation. While effective generation of a vaccine-mediated antitumor response requires functional APCs, it is unclear whether the necessary antigen processing and presentation can occur with antigen or cell-based vaccine strategies that rely on the functionally defective, endogenous APCs of cancer-bearing patients. The enhanced ability to prime T cell responses, coupled with the recent development of techniques for obtaining large numbers of human DCs, has created the possibility of utilizing these cells for therapeutic vaccination following antigen loading. Newer approaches include tumor-DC fusions as a strategy to enable the presentation of the entire tumor antigenic repertoire on the surface of DCs.10 An alternative strategy exploits the myelomonocytic origin of DCs by utilizing autologous tumor cells from patients with either acute or chronic myeloid leukemia that are differentiated into DCs with the appropriate cytokines. This approach preserves the entire tumor antigenic repertoire and acquires the necessary costimulatory properties of DCs. These leukemic-DCs upregulated costimulatory molecules and were capable of stimulating autologous T cells to generate potent tumor-specific cytotoxic responses.11,12 Despite significant advances with the in vitro generation of DCs, a major limitation has been the inability of antigen-loaded APCs to efficiently traffic to secondary lymphoid organs and subsequently prime effective T cell responses. The transduction of hematopoietic stem cells with tumor-associated antigens is a technique recently described aimed at enhancing transduction efficiency, durability, and trafficking of these antigen-expressing DCs to secondary lymphoid organs.13
Few clinical trials have been reported to date utilizing DC-based vaccine strategies in hematologic malignancies. Hsu et al conducted a study with peripheral blood mononuclear cell (PBMC)-derived dendritic cells pulsed with either the idiotype protein or KLH and then infused intravenously in 4 patients with non-Hodgkin’s lymphoma.14 Three monthly infusions of Id-pulsed DCs were followed by Id-KLH vaccines administered subcutaneously and then an additional infusion of pulsed DCs. In this study, no toxicity was demonstrated, and evidence of cellular proliferative responses was shown in all patients. Clinical responses were also detected that included resolution of two pulmonary lesions in 1 patient, conversion from Id-specific PCR positivity to negativity in another, and stabilization of disease in the remaining 2 patients. Timmerman et al also reported on 35 patients with follicular lymphoma vaccinated with idiotype-pulsed DCs with either residual disease or in first remission following chemotherapy. They observed the ability of Id-pulsed DCs to generate both humoral and cellular anti-Id responses, with an overall clinical response rate of 70% in patients with residual disease at the time of vaccination. Interestingly, subsequent anti-tumor activity was observed in patients who relapsed after the Id-DC vaccine upon subsequent revaccination with an Id-KLH vaccine.1
Idiotype-pulsed DC vaccines were also evaluated in patients with multiple myeloma. In a study by Reichardt et al, 12 patients who had undergone autologous peripheral blood stem cell transplants (PBSCTs) went on to receive a series of monthly immunizations consisting of 2 intravenous infusions of Id-pulsed autologous DCs followed by boosters with Id-KLH subcutaneous vaccinations.15 This strategy was well tolerated, with patients experiencing only minor side effects. Furthermore, 2 of 12 patients developed an Id-specific, cellular proliferative immune response, and 1 of 3 patients studied developed an Id-specific cytotoxic T cell (CTL) response despite recent high-dose therapy. Therefore, DC-based Id vaccination is feasible after PBSCT and can elicit Id-specific T cell responses in multiple myeloma patients.15 More recently, Titzer et al demonstrated the feasibility of CD34-derived DC, Id-pulsed vaccines. These infusions were followed by Id–GM-CSF or Id-DC. Cellular or antibody immune responses were detectable in 4 of 10 patients upon completion of vaccination, and 1 patient demonstrated a reduction in the plasma cell infiltration of the bone marrow.16 Similar evidence of both cellular and humoral immune responses to Id-pulsed DCs were also observed in another study.17
Although these early results utilizing DC-based vaccine strategies are promising, the growing appreciation of different functional subtypes of DCs and the importance of the route of DC administration necessitates careful comparative studies to determine the best DC-based strategy that would result in maximal systemic anti-tumor immunity in vivo.
Adoptive T Cell Therapy
Critical to the success of cancer immunotherapy is the ability to generate immune responses that can effectively eradicate the tumor. While vaccines ideally prime tumor-specific immunity, the immune suppression accompanying tumor-bearing patients likely impairs the ability to generate sufficient effector T cells. Therefore, for adoptive immunotherapy to be most effective, it must overcome the intrinsic tolerogenic mechanisms present in tumor-bearing hosts, exhibit significant specificity toward a broad range of tumor antigens with minimal cross-reactivity toward normal tissue, infuse sufficient numbers of T cells to exert a measurable anti-tumor effect, and further expand as well as persist in vivo upon infusion.
CD3/CD28 stimulation
An artificial APC capable of delivering both signals I and II to T cells has been generated by coupling anti CD3 and CD28 antibodies to a magnetic bead.18 This technology has permitted the activation and logarithmic polyclonal expansion of T cells in patients with relapsed/refractory non-Hodgkin’s lymphoma, CML, chronic lymphocytic leukemia, and multiple myeloma. These studies, conducted in the context of an autologous peripheral blood stem cell transplant, demonstrated early and sustained lymphoid recovery indicative of their ability to expand also in vivo upon reinfusion. They also suggested the ability to reverse some of the intrinsic T cell defects as determined by normalization of the T cell repertoire upon in vitro stimulation and secretion of Th-1 cytokines. However, a strategy to augment the tumor specificity of the infused T cells would likely increase the therapeutic benefit of such an approach.
Marrow-infiltrating lymphocytes
Human studies performed in patients with breast cancer demonstrated enrichment of memory CD4 (CD45RO+) and CD8+ T cells in the bone marrow.19 The persistence of marrow-infiltrating T cells with a memory phenotype suggests that this T cell population may be more sensitive to reactivation and subsequent expansion as compared to peripheral blood lymphocytes. Marrow infiltrating lymphocytes (MILs) possess many of these unique features. These cells, when used in adoptive immunotherapy, could improve therapeutic outcome. In hematopoietic malignancies such as multiple myeloma, the bone marrow is the tumor microenvironment and thus represents a potentially unique site for the isolation and expansion of tumor specific T cells. Moreover, the easy accessibility of marrow makes this an attractive source for tumor-specific T cells.
Utilizing the anti-CD3/CD28 antibody-bound beads to activate MILs from myeloma patients, we have shown that MILs display an activated phenotype, display more of a Th1 cytokine profile, expand more rapidly, and possess enhanced myeloma specificity as compared to activated peripheral blood–derived lymphocytes from the same patients.20 However, effective cellular immunotherapy also requires the ability to traffic to the site of disease. Stromal cell–derived factor-1 (SDF-1) is a chemokine expressed by bone marrow stromal cells and plays a key role in trafficking and the retention of cells within the marrow through interactions with its cognate receptor CXCR4. Interestingly, upon activation, MILs express significantly greater levels of CXCR4 on their surface as compared to peripheral blood lymphocytes (PBLs), suggesting an increased tropism for the marrow. Furthermore, early work by Matsui et al demonstrated the presence of a clonogenic precursor of the terminally differentiated plasma cell in myeloma patients.21 Utilizing that assay, we have demonstrated the ability of stimulated MILs to inhibit myeloma clonogenic precursors to a far greater extent than activated peripheral lymphocytes, thereby suggesting that activated MILs target a broad range of antigen ranging from the terminally differentiated plasma cell to its clonogenic precursors.
Conclusions
Immunotherapy continues to play an increasing role in the treatment of many hematologic malignancies, yet significant and sustained remissions remain elusive. Advances over the next several years will likely come from several fronts. Vaccine formulations must be made more potent. Our understanding of the barriers that impair the development of vigorous immune responses in tumor-bearing hosts must increase to develop strategies aimed at overcoming them through in vivo manipulations of the host or in vitro modifications of certain cell populations so as to either augment the tumor efficacy of antigen presenting cells and/or effector T cells or eliminate suppressor populations. Also, we must integrate immunotherapy within the appropriate therapeutic context (lymphopenia, minimal residual disease, etc.) which, coupled with a better understanding of the genetic phenotype of individuals most likely to benefit from this approach, will contribute toward the increased success of immunotherapy in the treatment of hematologic malignancies.
HLA-class I allele specificity of the main inhibitory killer cell Ig-like receptor (KIR).
| KIR . | HLA-Class I Specificity . |
|---|---|
| KIR2DL1 | “Group 2” HLA-C alleles expressing Lys80 (such as, -Cw2, -Cw4, -Cw5, -Cw6) |
| KIR2DL2/3 | “Group 1” HLA-C alleles expressing Asn80 (such as -Cw1, -Cw3, -Cw7, -Cw8) |
| KIR3DL1 | HLA-Bw4 alleles (e.g., HLA-B27) |
| KIR . | HLA-Class I Specificity . |
|---|---|
| KIR2DL1 | “Group 2” HLA-C alleles expressing Lys80 (such as, -Cw2, -Cw4, -Cw5, -Cw6) |
| KIR2DL2/3 | “Group 1” HLA-C alleles expressing Asn80 (such as -Cw1, -Cw3, -Cw7, -Cw8) |
| KIR3DL1 | HLA-Bw4 alleles (e.g., HLA-B27) |
Malignancies of lympho-hematopoietic lineage and alloreactive natural killer (NK) cell killing.*
| Susceptible . | Resistant . |
|---|---|
| *Results of multiple 51Cr-release cytotoxicity experiments for each malignancy using alloreactive NK clones as effectors. | |
| T-cell phenotype acute lymphoblastic leukemia | Common phenotype acute lymphoblastic leukemia |
| Acute myeloblastic leukemias | |
| Chronic myelogenous leukemia | |
| Chronic lymphocytic leukemia | |
| Non-Hodgkin’s lymphomas | |
| Multiple myeloma | |
| Susceptible . | Resistant . |
|---|---|
| *Results of multiple 51Cr-release cytotoxicity experiments for each malignancy using alloreactive NK clones as effectors. | |
| T-cell phenotype acute lymphoblastic leukemia | Common phenotype acute lymphoblastic leukemia |
| Acute myeloblastic leukemias | |
| Chronic myelogenous leukemia | |
| Chronic lymphocytic leukemia | |
| Non-Hodgkin’s lymphomas | |
| Multiple myeloma | |
Antigenic targets in acute myeloid leukemia (AML) that have been used clinically.
| Antigen . | Distribution on Normal and Neoplastic Cells . |
|---|---|
| Abbreviations: ALL, acute lymphocytic leukemia. | |
| CD33 | Myeloid progenitors, monocytes, dendritic cells; most AMLs. Not earliest hematopoietic stem cells. |
| CD45 | Most myeloid and lymphoid cells of various stages of maturation. Most AMLs and ALLs. |
| CD66 | Normal and activated myeloid cells. Not AML blasts; some ALL. |
| CD15 | Le-X antigen. Normal and malignant myeloid and monocytic cells; epithelial cells and carcinomas. |
| CD13 | Broad myeloid and monocytic expression; epithelial and stromal cells and carcinomas. |
| Antigen . | Distribution on Normal and Neoplastic Cells . |
|---|---|
| Abbreviations: ALL, acute lymphocytic leukemia. | |
| CD33 | Myeloid progenitors, monocytes, dendritic cells; most AMLs. Not earliest hematopoietic stem cells. |
| CD45 | Most myeloid and lymphoid cells of various stages of maturation. Most AMLs and ALLs. |
| CD66 | Normal and activated myeloid cells. Not AML blasts; some ALL. |
| CD15 | Le-X antigen. Normal and malignant myeloid and monocytic cells; epithelial cells and carcinomas. |
| CD13 | Broad myeloid and monocytic expression; epithelial and stromal cells and carcinomas. |
Antibody effector mechanisms.
| Effectors Intrinsic to Antibodies . | Comment . | |
|---|---|---|
| * All require entry into cell to work; all capable of single cell kill | ||
| +All potentially immunogenic in humans | ||
| Complement | Affected greatly by antibody isotype and species, and antigen density. Resistance occurs. Reduced by antigen-antibody internalization (modulation). | |
| Antibody dependent cellular cytotoxicity (ADCC): NK, Macrophage, PMNs | Requires certain isotypes and adequate ratios of effector cells to target cells. Also reduced by modulation. | |
| Receptor-based signaling | Dependent on antigen target; may require cross-linking; effects may be additive with other drugs. | |
| Radiation Emitters From Conjugated Isotopes | ||
| Gamma rays | Poorly cytotoxic; long ranged; used for imaging. Many isotopes available; inexpensive. | |
| Beta particles | Weakly cytotoxic necessitating killing by a field effect; better for bulkier tumors or regions (e.g., bone marrow); significant bystander killing including normal or malignant cells; inexpensive, widely available isotopes produce betas. | |
| Alpha particles | High energy, short ranged (high linear energy transfer [LET]), heavy helium nuclei capable of killing single cells or small clusters of cells; minimal bystander killing; expensive and less widely available isotopes. | |
| Auger electrons | Low energy, short ranged, electrons (l μM range); capable of single cell kill, requires internalization and weakly potent. Inexpensive, widely available isotopes produce augers. | |
| Conjugated Cytotoxin* | Subclass (Example) | |
| Drug | Chemotherapies (Calicheamicin) | Minimal effects on pharmacology and immunogenicity of antibody; modest potency; subject to drug resistance mechanisms. |
| Toxins+ | Ribosomal inhibitory proteins (RIP). Heterodimer (ricin) | A-B chain heterodimers. Extremely toxic; blockade of toxin binding, B chain necessary. |
| RIP: hemitoxin (ricin A chain) | Binding site (B chain) removed, allowing less toxic use. | |
| RIP: Single chain toxin (gelonin) | Monomeric toxin; far less toxic when unconjugated from antibody. | |
| RIP: fusion toxin Ig (Pseudomonas exotoxin A fusion [FvPE]) | Truncated monomeric toxin (PE) genetically fused to truncated Ig (Fv) forming small targetable toxin. | |
| Effectors Intrinsic to Antibodies . | Comment . | |
|---|---|---|
| * All require entry into cell to work; all capable of single cell kill | ||
| +All potentially immunogenic in humans | ||
| Complement | Affected greatly by antibody isotype and species, and antigen density. Resistance occurs. Reduced by antigen-antibody internalization (modulation). | |
| Antibody dependent cellular cytotoxicity (ADCC): NK, Macrophage, PMNs | Requires certain isotypes and adequate ratios of effector cells to target cells. Also reduced by modulation. | |
| Receptor-based signaling | Dependent on antigen target; may require cross-linking; effects may be additive with other drugs. | |
| Radiation Emitters From Conjugated Isotopes | ||
| Gamma rays | Poorly cytotoxic; long ranged; used for imaging. Many isotopes available; inexpensive. | |
| Beta particles | Weakly cytotoxic necessitating killing by a field effect; better for bulkier tumors or regions (e.g., bone marrow); significant bystander killing including normal or malignant cells; inexpensive, widely available isotopes produce betas. | |
| Alpha particles | High energy, short ranged (high linear energy transfer [LET]), heavy helium nuclei capable of killing single cells or small clusters of cells; minimal bystander killing; expensive and less widely available isotopes. | |
| Auger electrons | Low energy, short ranged, electrons (l μM range); capable of single cell kill, requires internalization and weakly potent. Inexpensive, widely available isotopes produce augers. | |
| Conjugated Cytotoxin* | Subclass (Example) | |
| Drug | Chemotherapies (Calicheamicin) | Minimal effects on pharmacology and immunogenicity of antibody; modest potency; subject to drug resistance mechanisms. |
| Toxins+ | Ribosomal inhibitory proteins (RIP). Heterodimer (ricin) | A-B chain heterodimers. Extremely toxic; blockade of toxin binding, B chain necessary. |
| RIP: hemitoxin (ricin A chain) | Binding site (B chain) removed, allowing less toxic use. | |
| RIP: Single chain toxin (gelonin) | Monomeric toxin; far less toxic when unconjugated from antibody. | |
| RIP: fusion toxin Ig (Pseudomonas exotoxin A fusion [FvPE]) | Truncated monomeric toxin (PE) genetically fused to truncated Ig (Fv) forming small targetable toxin. | |
Characteristics of selected radioisotopes for therapy.
| Isotope . | Imaging Gamma Ray Available& . | Half-Life . | Mean Range of Emission (μM) . |
|---|---|---|---|
| & For imaging and dosimetry | |||
| * 4 different alphas emitted | |||
| $ An isotope generator | |||
| β-emitters | |||
| Iodine-131 | + | 8.1 days | 800 |
| Yttrium-90 | 2.5 days | 2700 | |
| Copper-67 | + | 2.6 days | 900 |
| Rhenium-188 | + | 17 hours | 2400 |
| α-emitters | |||
| Bismuth-212 | + | 1 hour | 70 |
| Bismuth-213 | + | 46 minutes | 70 |
| Astatine-211 | + | 7.2 hours | 70 |
| $Actinium-225 | 10 days | 40–80* | |
| $Lead-212 | + | 10.6 hours | 70 |
| Isotope . | Imaging Gamma Ray Available& . | Half-Life . | Mean Range of Emission (μM) . |
|---|---|---|---|
| & For imaging and dosimetry | |||
| * 4 different alphas emitted | |||
| $ An isotope generator | |||
| β-emitters | |||
| Iodine-131 | + | 8.1 days | 800 |
| Yttrium-90 | 2.5 days | 2700 | |
| Copper-67 | + | 2.6 days | 900 |
| Rhenium-188 | + | 17 hours | 2400 |
| α-emitters | |||
| Bismuth-212 | + | 1 hour | 70 |
| Bismuth-213 | + | 46 minutes | 70 |
| Astatine-211 | + | 7.2 hours | 70 |
| $Actinium-225 | 10 days | 40–80* | |
| $Lead-212 | + | 10.6 hours | 70 |
Tumor antigens in hematologic malignancies.
| Tumor Antigen Category . | Example . |
|---|---|
| Abbreviations: CML, chronic myeloid leukemia; Ig, immunoglobulin; APL, acute promyelocytic leukemia; NHL, non-Hodgkin’s lymphoma; AML, acute myeloid leukemia; EBV, Epstein-Barr virus. | |
| Unique antigens | bcr/abl protein (CML) |
| Immunoglobulin idiotype (myeloma, NHL) | |
| T cell receptor idiotype (T cell lymphomas) | |
| pml/rar-α protein (APL) | |
| Shared tumor-specific antigens | Mage |
| Bage | |
| NY-ESO-1 | |
| telomerase | |
| Tissue-specific differentiation antigens | Proteinase-3 (AML, CML) |
| WT-1 (AML) | |
| Oncogene/tumor suppressor antigens | p53 |
| mutated ras proteins | |
| Virus-associated antigens | EBV (Hodgkin’s, Burkitt’s lymphoma) |
| Tumor Antigen Category . | Example . |
|---|---|
| Abbreviations: CML, chronic myeloid leukemia; Ig, immunoglobulin; APL, acute promyelocytic leukemia; NHL, non-Hodgkin’s lymphoma; AML, acute myeloid leukemia; EBV, Epstein-Barr virus. | |
| Unique antigens | bcr/abl protein (CML) |
| Immunoglobulin idiotype (myeloma, NHL) | |
| T cell receptor idiotype (T cell lymphomas) | |
| pml/rar-α protein (APL) | |
| Shared tumor-specific antigens | Mage |
| Bage | |
| NY-ESO-1 | |
| telomerase | |
| Tissue-specific differentiation antigens | Proteinase-3 (AML, CML) |
| WT-1 (AML) | |
| Oncogene/tumor suppressor antigens | p53 |
| mutated ras proteins | |
| Virus-associated antigens | EBV (Hodgkin’s, Burkitt’s lymphoma) |
Partial summary of immunotherapy clinic trials in hematologic malignancies.
| Author . | Disease . | Patient Population . | Immune Intervention . | Response . | Ref . |
|---|---|---|---|---|---|
| Abbreviations: FL, follicular lymphoma; Id, idiotype protein; KLH, keyhole limpet hemocyanin; CR, complete response; MM, multiple myeloma; DTH, delayed type hypersensitivity; CML, chronic myeloid leukemia; PBSCT, peripheral blood stem cell transplant; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; Ad, adenovirus; NHL, non-Hodgkin’s lymphoma | |||||
| Bendandi | FL | CR | Id-KLH Vaccine | 8/11 molecular CRs | 2 |
| Rassmussen | MM | Stage I | Id-IL-12 ± GM-CSF | 4/6 reduced tumor burden (3/4 anti-Id Tcell response) | 3 |
| Massaia | MM | Auto-PBSCT | Id-KLH, GM-CSF v IL-2 | anti-Id DTH response 8/10 | 25 |
| Reichardt | MM | Auto-PBSCT | Id-DC | 2/12 anti-Id T cell proliferation 1/3 anti-Id CTL response | 15 |
| Cathcart | CML | Multi-valent bcr/abl fusion peptide | + peptide-specific response | 4 | |
| Bocchia | CML | Residual cytogenetic disease | Bcr/abl fusion peptide | 7/15 cytogenetic CR 3 molecular CR | 5 |
| Borrello | MM | autoPBSCT | Autologous tumor and GM-CSF–producing bystander cell line | 6/16 CRs 3/16 with M-spike declines post-BMT | 26 |
| Borrello | AML | CR prior to auto-PBSCT | Autologous tumor and GM-CSF–producing bystander cell line | 12/18 reduction in WT-1 transcript in marrow with pre-transplant vaccination | 27 |
| Hsu | FL | Id-DC | 1/4 molecular CR 1/4 complete tumor regression | 14 | |
| Timmerman | FL | Residual disease | Id-DC | 70% response rate in 35 pts | 1 |
| Titzer | MM | Heavily pretreated | Id-DC followed by Id-DC or Id-GM-CSF | 4/10 reduction in bone marrow plasma cell infiltrate | 16 |
| Wierda | CLL | Progressive intermediate/high risk CLL | Ad-CD154-transfected | Reduction in CLL burden autologous CLL cells | 23 |
| Laport | NHL | Relapsed/refractory | Anti-CD3/CD28 activated PBL | 5/16 CR 7/16 PR | 24 |
| Author . | Disease . | Patient Population . | Immune Intervention . | Response . | Ref . |
|---|---|---|---|---|---|
| Abbreviations: FL, follicular lymphoma; Id, idiotype protein; KLH, keyhole limpet hemocyanin; CR, complete response; MM, multiple myeloma; DTH, delayed type hypersensitivity; CML, chronic myeloid leukemia; PBSCT, peripheral blood stem cell transplant; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; Ad, adenovirus; NHL, non-Hodgkin’s lymphoma | |||||
| Bendandi | FL | CR | Id-KLH Vaccine | 8/11 molecular CRs | 2 |
| Rassmussen | MM | Stage I | Id-IL-12 ± GM-CSF | 4/6 reduced tumor burden (3/4 anti-Id Tcell response) | 3 |
| Massaia | MM | Auto-PBSCT | Id-KLH, GM-CSF v IL-2 | anti-Id DTH response 8/10 | 25 |
| Reichardt | MM | Auto-PBSCT | Id-DC | 2/12 anti-Id T cell proliferation 1/3 anti-Id CTL response | 15 |
| Cathcart | CML | Multi-valent bcr/abl fusion peptide | + peptide-specific response | 4 | |
| Bocchia | CML | Residual cytogenetic disease | Bcr/abl fusion peptide | 7/15 cytogenetic CR 3 molecular CR | 5 |
| Borrello | MM | autoPBSCT | Autologous tumor and GM-CSF–producing bystander cell line | 6/16 CRs 3/16 with M-spike declines post-BMT | 26 |
| Borrello | AML | CR prior to auto-PBSCT | Autologous tumor and GM-CSF–producing bystander cell line | 12/18 reduction in WT-1 transcript in marrow with pre-transplant vaccination | 27 |
| Hsu | FL | Id-DC | 1/4 molecular CR 1/4 complete tumor regression | 14 | |
| Timmerman | FL | Residual disease | Id-DC | 70% response rate in 35 pts | 1 |
| Titzer | MM | Heavily pretreated | Id-DC followed by Id-DC or Id-GM-CSF | 4/10 reduction in bone marrow plasma cell infiltrate | 16 |
| Wierda | CLL | Progressive intermediate/high risk CLL | Ad-CD154-transfected | Reduction in CLL burden autologous CLL cells | 23 |
| Laport | NHL | Relapsed/refractory | Anti-CD3/CD28 activated PBL | 5/16 CR 7/16 PR | 24 |
Donor-versus-recipient natural killer (NK) cell alloreactivity.
Individuals who express Group 2 HLA-C alleles and possess NK cells that express killer cell immunoglobulin-like receptors (KIRs) specific for Group 2 HLA-C alleles (KIR2DL1) are alloreactive against cells from individuals who do not express Group 2 HLA-C alleles (who are homozygous for Group 1 HLA-C alleles) (top). Individuals who express Group 1 HLA-C alleles possess NK cells with KIRs specific for Group 1 HLA-C alleles (KIR2DL2 and/or KIR2DL3) and are alloreactive against cells from individuals who do not express Group 1 HLA-C alleles (who are homozygous for Group 2 HLA-C alleles) (bottom).
Donor-versus-recipient natural killer (NK) cell alloreactivity.
Individuals who express Group 2 HLA-C alleles and possess NK cells that express killer cell immunoglobulin-like receptors (KIRs) specific for Group 2 HLA-C alleles (KIR2DL1) are alloreactive against cells from individuals who do not express Group 2 HLA-C alleles (who are homozygous for Group 1 HLA-C alleles) (top). Individuals who express Group 1 HLA-C alleles possess NK cells with KIRs specific for Group 1 HLA-C alleles (KIR2DL2 and/or KIR2DL3) and are alloreactive against cells from individuals who do not express Group 1 HLA-C alleles (who are homozygous for Group 2 HLA-C alleles) (bottom).
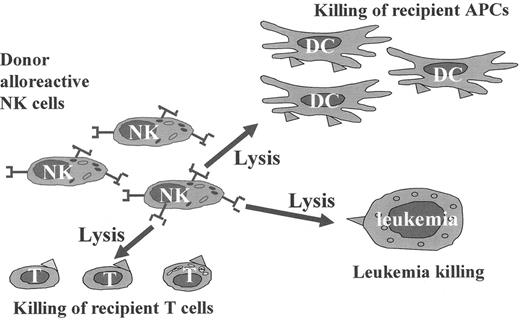
The figure summarizes data from preclinical murine models and clinical haploidentical transplantation.2
Donor-versus-recipient alloreactive natural killer (NK) cells eliminate residual leukemic cells. In addition, they ablate host-type dendritic cells, i.e., the cells that are responsible for triggering graft-versus-host disease (GVHD). NK cells also attack residual host lympho-hematopoietic cells, including T lymphocytes that are responsible for graft rejection.
The figure summarizes data from preclinical murine models and clinical haploidentical transplantation.2
Donor-versus-recipient alloreactive natural killer (NK) cells eliminate residual leukemic cells. In addition, they ablate host-type dendritic cells, i.e., the cells that are responsible for triggering graft-versus-host disease (GVHD). NK cells also attack residual host lympho-hematopoietic cells, including T lymphocytes that are responsible for graft rejection.