Abstract
New developments in the epidemiology, treatment and prognosis of thalassemia have dramatically altered the approach to the care of affected patients, and these developments are likely to have an even greater impact in the next few years. Demographic changes have required an awareness and understanding of the unique features of thalassemia disorders that were previously uncommon in North America but are now seen more frequently in children and recognized more consistently in adults. New methods for measuring tissue iron accumulation and new drugs to remove excessive iron are advancing two of the most challenging areas in the management of thalassemia as well as other transfusion-dependent disorders. Improved survival of patients with thalassemia has given new importance to adult complications such as endocrinopathies and hepatitis that have a major impact on the quality of life. This chapter describes how these changes are redefining the clinical management of thalassemia.
In Section I, Dr. Renzo Galanello describes recent advances in iron chelation therapy. Several new chelators are either licensed in some countries, are in clinical trials or are in the late stages of preclinical development. Some of these iron chelators, such as deferiprone (DFP) and ICL670, are orally active. Others, such as hydroxybenzyl-ethylenediamine-diacetic acid (HBED) and starch deferoxamine, require parenteral administration but may be effective with less frequent administration than is currently required for deferoxamine. Chelation therapy employing two chelators offers the possibility of more effective removal of iron without compromising safety or compliance. Other strategies for chelation therapy may take advantage of the ability of particular chelators to remove iron from specific target organs such as the heart and the liver.
In Section II, Dr. Dudley Pennell addresses cardiac iron overload, the most frequent cause of death from chronic transfusion therapy. The cardiac complications related to excessive iron may result from long-term iron deposition in vulnerable areas or may be due to the more immediate effects of nontransferrin-bound iron. Cardiac disease is reversible in some patients with intensive iron chelation therapy, but identification of cardiac problems prior to the onset of serious arrhythmias or congestive heart failure has proven difficult. New methods using magnetic resonance imaging (MRI) have recently been developed to assess cardiac iron loading, and studies suggest a clinically useful relationship between the results using these techniques and critical measures of cardiac function. Measurements such as T2* may help guide chelation therapy in individual patients and may also enhance the assessment of new chelators in clinical trials. The use of MRI-based technology also holds promise for wider application of non-invasive assessment of cardiac iron in the management of patients with thalassemia.
In Section III, Dr. Melody Cunningham describes some of the important complications of thalassemia that are emerging as patients survive into adulthood. Hepatitis C infection is present in the majority of patients older than 25 years. However, antiviral therapy in patients with thalassemia has been held back by the absence of large clinical trials and concern about ribavirin-induced hemolysis. More aggressive approaches to the treatment of hepatitis C may be particularly valuable because of the additive risks for cirrhosis and hepatocellular carcinoma that are posed by infection and iron overload. Thrombosis is recognized with increasing frequency as a significant complication of thalassemia major and thalassemia intermedia, and pulmonary hypertension is now the focus of intense study. Risk factors for thrombosis such as splenectomy are being identified and new approaches to anticoagulation are being initiated. Pregnancies in women with thalassemia are increasingly common with and without hormonal therapy, and require a better understanding of the risks of iron overload and cardiac disease in the mother and exposure of the fetus to iron chelators.
In Section IV, Dr. Elliott Vichinsky describes the dramatic changes in the epidemiology of thalassemia in North America. Hemoglobin E-β thalassemia is seen with increasing frequency and poses a particular challenge because of the wide variability in clinical severity. Some affected patients may require little or no intervention, while others need chronic transfusion therapy and may be appropriate candidates for hematopoietic stem cell transplantation. Enhancers of fetal hemoglobin production may have a unique role in Hb E-β thalassemia since a modest increase in hemoglobin level may confer substantial clinical benefits. Alpha thalassemia is also being recognized with increasing frequency in North America, and newborn screening for Hemoglobin Barts in some states is leading to early detection of Hb H disease and Hb H Constant Spring. New data clarify the importance of distinguishing these two disorders because of the increased severity associated with Hb H Constant Spring. The use of intrauterine transfusions to sustain the viability of fetuses with homozygous alpha thalassemia has created a new population of patients with severe thalassemia and has raised new and complex issues in genetic counseling for parents with alpha thalassemia trait.
I. New Iron Chelators
Renzo Galanello, MD*
Universita di Cagliari, Ospedale Microcitemico, Dip. di Scienze Biomediche E Biotec., Via Jenner s.n., Cagliari 09121, Italy
In the last 30 years, conventional treatment of β-thalassemia major, based primarily on regular blood transfusions and iron chelation therapy with desferrioxamine (DFO), has markedly improved the prognosis of the disease. Adequate administration of parenteral DFO reduces or prevents iron accumulation and iron-mediated organ damage, resulting in a consistent decrease of morbidity and mortality.1 However, for several reasons chelation with DFO may be inadequate. In emerging Middle Eastern and Far Eastern countries, where thalassemia is a substantial public health problem, the high costs of the drug and of the supplies for its administration make it unavailable for most patients. Worldwide, it has been estimated that DFO is prescribed for only 25,000 of 72,000 patients with thalassemia major who are regularly transfused.2 Despite wide availability of DFO in Western countries, some patients are unable to comply sufficiently with the prescribed treatment because the effective chelation regimen is unpleasant and cumbersome, requiring prolonged daily subcutaneous or intravenous administration. Moreover, some patients may develop side effects of variable severity that affect compliance or sometimes result in cessation of treatment. In a recent report, 105 of 328 patients from North America who had ever received chelation therapy with DFO reported complications requiring modifications of the dose or route of administration of the chelator and 20 reported stopping DFO.3 The inability to cope with the rigorous and demanding long-term use of DFO may result in death from iron overload.
The unavailability of DFO for most patients with thalassemia major and the failure of prescribed therapy to prevent complications in other patients have led to a search for alternative iron chelators. Over the last 25 years more than 1000 molecules of synthetic, microbiological or plant origin have been tested in vitro and in vivo and several interesting compounds have been identified. One of them, deferiprone (DFP), has been commercially available for 9 years in India and 5 years in Europe, while others are under intensive clinical investigation.
Iron accumulates at different rates in various organs, and these organs show a different susceptibility to the damage induced by reactive iron species such as nontransferrin bound iron (NTBI) and the intracellular labile iron pool (LIP). More work is needed to understand the relative accessibility of iron chelators to those different pools. Methods for measurement of these iron species are becoming available and more sensitive, and they could improve the understanding of the mechanism of action of the various chelators and facilitate the design of specific chelation strategies.4 Based on these findings, targeted chelators that remove iron from specific organs could prove useful. Therefore, besides the overall reduction of iron, effective chelation should aim for a decrease of iron in specific organs, especially the heart, since cardiac disease is the most important life-limiting consequence of iron overload in thalassemia.1
General Characteristics of Iron Chelators
Iron has six electrochemical coordination sites that should be tightly bound to block the ability of the iron ions to catalyze redox reactions and to allow efficient transport and excretion without iron redistribution. Iron chelators should reduce tissue iron levels, prevent excessive organ iron accumulation and neutralize toxic labile iron pools. Based on the number of the coordination sites, iron ligands are termed hexadentate, tridentate and bidentate. Denticity is directly related to the molecular weight: hexadentate chelators have a higher molecular weight as compared to tri- and bidentate molecules. However, the diffusion through biological membranes and hence the absorption from the gastrointestinal tract and the cellular penetration are governed not only by molecular size, but also by lipophilicity and net molecular charge.5 For example, DFO, like many hexadentate chelators, is not absorbed in the gastrointestinal tract, but derivatives of hydroxybenzyl-ethylenediamine-diacetic acid (HBED), another hexadentate iron chelator, possess good oral availability. In recent years extensive research has been directed toward bidentate and tridentate ligands that are more likely than hexadentate chelators to be orally active and to penetrate cells.
Selectivity and affinity for the ferric (Fe3+) oxidation state are important characteristics of an iron chelator (Table 1 ). These properties reduce the chelation of other biologically important bivalent metals, such as copper and zinc, while the effect on non-essential trivalent cations, such as aluminum and gallium, remains negligible. Under biological conditions the affinity of chelators for iron and the stability of ligand-metal complexes is expressed as pF3+ value, that is, the negative logarithm of the concentration of the free Fe3+, measured in a solution of 10 μM ligand and 1 μM Fe3+ at pH 7.4.5 The larger the pF3+, the higher is the stability of the ligand-metal complex.
Rapid conversion to glucuronidate metabolites limits the efficacy of many iron chelators. Most of the administered DFO and DFP is rapidly metabolized to the inactive glucuronide, resulting in a net chelating efficiency lower than 10%. Therefore, one strategy to increase chelating efficiency is to design compounds with a reduced rate of glucuronidation.
Toxic Effects of Iron Chelators
Designing an ideal iron chelator is a difficult challenge because of the iron paradox: iron is an essential element for many important metabolic functions (oxygen transportation and utilization, DNA synthesis, electron transport and many other biological processes), but it becomes toxic when accumulated. A chelator should remove only excess iron. Thus, in addition to possible direct toxic effects, iron chelators may alter iron homeostasis (absorption, distribution and utilization), interfere with iron-dependent enzymes (ribonucleotide reductase, lipooxygenase) or remove other metals such as zinc or calcium from essential metabolic pools. Characteristics of the compound such as molecular weight, lipophilicity/hydrophilicity balance, affinity and selectivity for Fe3+, pharmacokinetics, distribution and metabolism play a role in determining the boundary between safety and toxicity.
Development of Iron Chelators
Development of an iron chelator requires several distinct and rigorous procedures, the first being the definition of the chemical properties of the ligand and of the ligand-iron complex. The second step consists of cellular studies using red blood cells, reticulocytes, cell lines and primary cell cultures (hepatocytes, myocardial and reticuloendothelial cells). Subsequently, animal models, both non-iron overloaded and iron-overloaded, are critical to evaluate the safety and efficacy of iron chelators. Unfortunately, the predictive power of the animal models is low because of the frequent differences between iron-overloaded animals and humans, and because of differences between species in iron metabolism. Efficacy in animals may not translate into comparable efficacy at tolerable doses in man. Both acute and long-term toxicity studies in different species of animals are essential for planning clinical trials.
To obtain the registration of a new compound, Phase I, II and III clinical trials, performed according to stringent requirements established by regulatory agencies, are necessary to define the safety and pharmacokinetic and pharmacodynamic characteristics of the chelator in humans, to find the therapeutic dose range and to evaluate the tolerability and the efficacy in comparison with the reference drug. Although the process of product development is very rigorous, the intrinsic limitations of clinical trials (e.g., efficacy-oriented, highly selected population, short duration, limited sample size, risk factors and exclusion criteria) make it difficult to detect all safety concerns during this phase. Post-marketing pharmacovigilance is defined as the ongoing process of evaluating and improving the safety of drugs. Once a product is marketed, there is generally a large increase in the number of patients exposed, including those with comorbid conditions and those being treated with concomitant medical products. Therefore, pharmacovigilance is critical to establish and regularly review the risk/benefit ratio of iron chelators.
New Iron Chelators
Deferiprone
Deferiprone or L1 (DFP) is currently the only orally active iron chelator available for clinical use. It was licensed first in India in 1995 and subsequently in Europe in 1999. At present, DFP is licensed in Europe for patients for whom treatment with DFO is inadequate. Overall DFP is currently available in approximately 50 countries. Studies have demonstrated a stable or declining mean serum ferritin and liver iron concentration during long-term therapy in most transfusiondependent patients, although iron accumulation continues in others.6 Agranulocytosis is the most serious side effect, with a reported incidence of 0.6 per 100 patient-years.7 More common but less serious side effects are gastrointestinal symptoms (e.g., nausea, vomiting, gastric discomfort), arthralgia, zinc deficiency, and fluctuating ALT levels, particularly in anti-hepatitis C virus (HCV)–positive patients. Retrospective studies in patients treated with DFP have shown significant improvement in cardiac magnetic resonance imaging (MRI), consistent with a reduction in cardiac iron overload and improved cardiac function, in comparison with patients treated with DFO.8,9 These observations support a potential cardioprotective role of DFP that needs to be confirmed in prospective randomized trials.
Desferrithiocin
Desferrithiocin (DFT) is a siderophore originally isolated from Streptomyces antibioticus in 1980 and then produced by chemical synthesis (Figure 1 ). DFT is an orally available, tridentate, potent iron chelator with a high affinity for Fe3+. Soon after its discovery, DFT was actively tested in iron overloaded rats, where effective reduction of liver ferritin iron was demonstrated. Initial single dose safety data showed no relevant acute toxic effects, but longer exposure in rats showed moderate to severe degenerative changes in the proximal tubules of the kidney. The damage was believed to be due to toxic effects of the Fe3+ complex, ferrithiocin.10 Several DFT analogs have subsequently been synthesized and tested in animals. One of these analogs, 4-OH-desaza-desmethyldesferrithiocin, appears to be less toxic while remaining biologically active as an orally administered iron chelator in Cebus apella primates.11 This analog may enter Phase I human trials shortly.
Hydroxybenzyl-ethylenediamine-diacetic acid
HBED is a synthetic hexadentate phenolic aminocarboxylate chelator, first synthesized more than 30 years ago, which forms a 1:1 complex with Fe3+ with high affinity and selectivity (Figure 1 ). Initial studies in rodents showed that it was able to clear radiolabeled iron when administered parenterally and that it remained active after oral administration. However, further evaluation in both iron-loaded primates and humans revealed that the oral activity was too small to be of value in the treatment of iron overload.12 Recently Bergeron et al13 continued the preclinical evaluation of the efficacy and safety of HBED monosodium salt for the treatment of both transfusional iron overload and of acute iron poisoning in animals. Na-HBED was compared with DFO at equimolar doses in iron-loaded Cebus apella monkeys, either as a subcutaneous (SC) bolus or a 20-minute intravenous infusion (IV). In both conditions Na-HBED was consistently about twice as efficient as DFO in promoting iron excretion. Safety evaluation showed no systemic toxicity after either IV administration once daily or SC administration every other day for 14 days in dogs without iron overload. No local irritation at injection sites was found when the Na-HBED concentration was reduced to 15% or less in a volume that would be clinically tolerable. Interestingly, the rapid IV infusion of Na-HBED, as may be required for treatment of acute iron poisoning, had little detrimental effect on blood pressure and heart rate. If these results are confirmed in human studies, Na-HBED may prove to be an alternative to DFO for the treatment of acute iron poisoning or chronic iron overload, although it would still require parenteral administration.13 This alternative may be particularly important for patients allergic to DFO since HBED is a member of a different family of chelators.
Pyridoxal isonicotinoyl hydrazone
Pyridoxal isonicotinoyl hydrazone (PIH) is one of a family of aromatic hydrazones produced by chemical synthesis and identified as an effective iron chelator in 1979. PIH is a tridentate chelator (Figure 1 ) with a selectivity for iron comparable to that of DFO. Initially it was found to be effective when given parenterally to mice and orally to rats. Interestingly, PIH and some of its analogs were shown to be potent inhibitors of the production of toxic oxygen-free radicals.14 Over the years, various PIH analogs have been synthesized and some of them, such as pyridoxal-benzoyl hydrazones, were as much as 280% more effective than PIH. Studies in iron-overloaded patients treated with 30 mg/kg/day of PIH have shown a modest net iron excretion of 0.12 ± 0.07 mg/kg/day, which is much less than the 0.5 mg/kg/day that is required, on average, to achieve negative iron balance in regularly transfused patients.15 Despite these unsatisfactory results several arguments have been raised in favor of PIH.16 First, the dose used was lower than the effective dose of 125–500 mg/kg used in experimental animals. Second, the preparation was given to patients after calcium carbonate to prevent acid hydrolysis of PIH, but this approach might have limited the chelator’s absorption because of the low solubility of PIH at a neutral pH. Progress in the development of PIH and its derivatives has been very slow, not only because of the discouraging results in humans, but also because its patent rights are limited. However, recently investigators have examined the chelating potential of two additional PIH analogs, alone or in combination with DFO, in cultured iron-loaded heart cells and in hypertransfused rats.17 The latter studies have shown that the orally administered analogs are about 2–6 times more effective than intraperitoneally administered DFO in mobilizing liver iron in rats.
GT56-252
GT56-252 is a novel orally available iron chelator derived from DFT that forms a 2:1 complex with Fe3+.18 Pharmacokinetics studies conducted in rats, dogs and Cebus apella monkeys showed that the compound was orally bioavailable in all three species, and the elimination half-life ranged from 3 hours in the rats to 7–8 hours in the dogs. Pharmacokinetic (PK) profiles were similar in iron-loaded and non-iron-loaded animals, plasma levels increased in a dose-related manner and maximum plasma levels were reached about 1 hour after dosing. In Cebus apella monkeys, the iron-clearing efficiency was 13%–18%, about three times that of DFO in the same model. Approximately 80% of iron was excreted in the feces. Pharmacodynamics of repeat doses of GT56-252, from 5 to 45 mg/kg once a day over a 3-day period, in 8 iron-loaded Cebus monkeys showed a dose-related clearing efficiency in a range from 0.06 mg/kg/dose to 0.53 mg/kg/dose.19 These studies provided sufficient background data to undertake Phase I clinical trials. Eighteen adult patients with β-thalassemia received 3–8 mg/kg in 2 doses with food or fasting. The compound was well tolerated, with no related serious adverse clinical events, laboratory abnormalities or changes in the electrocardigram (ECG). GT56-252 was very well absorbed and area under the curve (AUC) values with or without food were similar.20 Further studies are in progress to define the effect of GT56-252 on iron balance.
40SD02 (CHF1540)
40SD02 is a new entity synthesized by chemically attaching DFO to a modified starch polymer.21 The resulting high molecular weight chelator has a prolonged half-life and preserves the affinity and specificity of DFO for Fe3+, but does not produce the acute toxic effects of DFO such as hypotension. A Phase I study in 10 patients with thalassemia and chronic iron overload showed that single doses of up to 600 mg/kg of the compound were safe and well tolerated, and stimulated a clinically significant amount of iron excretion. Average total iron excretion over 7 days was 0.46 mg/kg and 0.72 mg/kg in the 150 and 300 mg/kg dose groups, respectively. In a more recent Phase I clinical trial 12 patients were treated, each subject receiving a single dose, with 4 patients at 150 mg/Kg, 4 at 300mg/Kg, and 4 at 600 mg/Kg.22 No drug-related adverse effects on vital signs, ECG, or laboratory tests have been described. A skin reaction, judged to be related to the drug, was reported in 3 different patients. At the highest dose level of 900 mg/kg, a single infusion induced cumulative urinary iron excretion of 0.84–1.93 mg/kg for 7 days following drug administration.
ICL670
ICL670 is an orally active chelating agent developed for the treatment of chronic iron overload. The compound is an N-substituted bis-hydroxyphenyl-triazole that was selected from more than 700 compounds that were screened as part of a drug development program. ICL670 represents a new class of tridentate iron chelators with a high specificity for iron that was developed on the basis of computer modeling.10 Efficient and selective mobilization of tissue iron has been demonstrated in several animal models, with efficiency being greater than DFO and considerably greater than DFP at comparable levels of iron binding potential.
The Phase I clinical evaluation of ICL670 showed this novel agent to be well tolerated, with no major safety concerns at doses up to 80 mg/kg/day.23 Iron excretion is dose-dependent and is almost entirely in the feces. Excretion averaged 0.127, 0.344 and 0.564 mg/kg/day at the 10, 20 and 40 mg/kg doses, respectively.24 The plasma half-life (11–19 hours) supports the once daily oral dosing regimen used in subsequent clinical studies.
A 12-month Phase II study assessed the tolerability and efficacy of ICL670 in comparison with DFO in 71 patients with transfusional hemosiderosis. Patients were randomized to receive ICL670 (10 or 20 mg/kg/day) or DFO (40 mg/kg SC 5 days per week). The overall incidence of adverse events was similar in all groups. Transient mild to moderate nausea and vomiting were more common in patients receiving ICL670 at 20 mg/kg/day but did not require discontinuation of the drug. One patient receiving ICL670 at a dose of 10 mg/kg/day developed a mild skin rash with a suspected relationship to the study drug; the rash subsided spontaneously despite continued therapy. Elevations in urinary β-2 microglobulin, sometimes accompanied by a mild increase in urinary 24-hour total protein excretion, have been observed in all dose groups but predominantly in patients treated with ICL670 at 20 mg/kg/day. The clinical significance of these renal findings remains uncertain. There were no significant changes in serum creatinine levels. Decreases in liver iron concentration (LIC), determined by biomagnetic susceptometry, were similar in the groups treated with ICL670 at 20 mg/kg/day and DFO. At baseline, LIC values in the two groups were 8.5 and 7.9 mg/g dry weight respectively, falling to 6.6 and 5.9 mg/g dry weight at 12 months. A Phase III international study including about 500 patients is expected to be completed by the end of 2004.
Combined Chelation Therapy
New chelators, particularly those that are orally available, are expected to have a major impact on the management of patients with thalassemia. A choice of more than one chelator would permit a flexible approach to chelation therapy and would probably improve compliance with treatment and overall quality of life. As an example, two chelators might be administered simultaneously or sequentially. Combined chelation offers several potential advantages (Table 2 ). Chelators with distinct chemical properties may have different ironcarrying capacities and accessibility to different iron pools. Formal balance studies and clinical trials with DFO and DFP have shown that two chelators may have additive or synergistic effects, resulting in an increased efficacy.25–,28 To explain these effects, it has been proposed that a bidentate or tridentate ligand, with access to a variety of tissues, acts as a “shuttle” to mobilize iron from tissue compartments to the bloodstream, where the chelator may exchange iron with a larger hexandentate “sink.”25 The “shuttle effect” has been directly demonstrated by following the fate of chelated iron in patients with thalassemia treated with DFO and DFP.29 While monotherapy with DFP resulted in temporary accumulation of chelated iron in the plasma, the addition of DFO produced a transfer of DFP-chelated iron to DFO and an increase in total chelated iron. Mourad et al reported that giving DFP every day and DFO 2 days a week produced iron excretion comparable to that achieved with DFO administered 5 days a week.27 This regimen of chelation is more tolerable and may be attractive for patients who are unable to comply with regular daily use of DFO. Since some of the toxic effects of the chelators are dose-dependent, combination therapy might make it possible to lower the dose of one or both drugs, reducing toxicity but maintaining effective chelation. Giving two drugs sequentially, e.g., DFP during the day and DFO at night, might also reduce the level of NTBI. To date, no unexpected side effects have been consistently observed in patients receiving combination therapy with DFO and DFP. However, prospective, randomized studies are needed to establish firmly the risks and benefits of this approach.
Other combinations of chelators have been utilized in studies in vitro and in vivo. Co-administration of DFP and HBED to patients with thalassemia greatly enhanced the effectiveness of HBED.30 Total iron excretion was 148% and 227% of that with each drug alone.
Using the iron-loaded gerbil model, combined DFP and DFO treatment was particularly efficient in reducing liver iron concentration and normalizing the mitochondrial respiratory enzyme activity.31 PIH analogs and DFO have shown an additive effect on hepatocellular iron excretion.17 Studies in heart cell cultures have shown a favorable interaction between DFO and ICL670, manifested as improved chelating efficiency of ICL670.32
Conclusions
During the last 25 years, investigators have made great strides in developing new iron chelators for the treatment of iron overload in thalassemia. Many candidate drugs have been screened, but only a few have had the physicochemical and biological properties suitable for potential clinical application. Some of these are now under intensive investigation (Table 3 ). Patients may ultimately benefit from having a choice between several chelators, including orally active drugs. New strategies of chelation, such as combination therapy and organ-targeted chelation, may soon have a considerable impact on the therapeutic outcome and quality of life of patients with thalassemia.
II. Iron Overload and the Heart
Dudley J. Pennell, MD*
Royal Brompton Hospital, Director, Cardiovascular MR Unit, Sydney Street, London SW3 6NP, UK
Clinical Presentation
The cardiac complications of thalassemia major were first described prior to the introduction of chelation therapy. Engle described an increase in heart size after 10 years of age with first-degree heart block in a third of the patients. Episodes of pericarditis occurred in half of the patients after 11 years of age. Congestive cardiac failure developed at a mean age of 16 years. As heart failure progressed, atrioventricular (AV) block worsened and either complete heart block or right or left bundle branch block occurred in a third of patients. Atrial arrhythmias were noted in half and repetitive ventricular tachycardia was present in a minority. The duration of life after the onset of heart failure was less than 3 months in over half of the patients.
Currently, cardiac complications are reported to cause 71% of deaths in patients with thalassemia major. Therefore the heart is the target lethal organ in thalassemia. Once heart failure develops, the outlook is usually poor. The cardiomyopathy may be reversible if iron chelation treatment is intensified in time, but the diagnosis is often delayed by the unpredictability of cardiac iron deposition and the late development of symptoms and echocardiographic abnormalities. The early diagnosis of iron-induced cardiomyopathy with established clinical techniques such as echocardiography and stress radionuclide angiography has had limited success. In particular, overt dysfunction on echocardiography presents late in the disease process and the progression from mildly abnormal echocardiographic parameters to fulminant cardiac failure is often rapid and relentless.
Pathophysiology
Iron deposition is reported to be greatest in the ventricular walls with less in the atria and conduction system. There is also greater iron in the epicardial layer. The degree of cardiac dysfunction is considered to depend on the quantity of iron deposited in individual myocardial fibers and the number of fibers affected. In patients with only mild cardiac dysfunction, iron deposits are usually limited to the perinuclear areas, with only a few fibers involved, whereas in patients with significant cardiac dysfunction, iron deposits occupy large areas of the myocardial fibers. The relatively mild degree of fibrosis in most autopsy studies suggests that even when advanced, the cardiomyopathy is potentially reversible. The iron is stored in intracellular lysosomes in the relatively non-toxic forms of ferritin or hemosiderin but above a certain concentration, NTBI is released and the cell begins to fail. The toxicity of iron depends on intracellular iron concentration and its oxidative state. Reduction of ferric to ferrous iron promotes hydroxyl radical formation via the Haber-Weiss reaction.
Iron Chelation Therapy and the Reversibility of Iron-Induced Cardiomyopathy
The iron chelator DFO was first introduced more than 30 years ago. DFO reduces hepatic iron and the progression of hepatic fibrosis. Subsequent studies showed improved survival in thalassemia patients on long-term chelation therapy. However, many patients still die of iron overload, and this continued early mortality may be due to several factors: late presentation of cardiac disease, difficulties in assessing myocardial iron, and difficulties in compliance to long-term treatment with daily parenteral iron chelation regimens.
The reversibility of iron-induced cardiomyopathy has been demonstrated in patients with hereditary hemochromatosis treated with recurrent venesection and in patients with thalassemia major who are treated with chelation therapy. In vitro, DFO removes iron from cardiac myocytes, reverses lipid peroxidation, and reverses the iron-induced abnormalities of cellular contractility and rhythmicity. However, the clinical reversibility of iron-induced cardiac failure has not been universally accepted, partly due to the high mortality of patients presenting with advanced cardiac failure despite chelation treatment, and more recently by alternative explanations for the heart failure such as myocarditis.
Assessment of Tissue Iron Stores
There is variability in iron deposition between and within different organs. Serum ferritin is the most commonly used indirect estimate of body iron stores, but this reflects only 1% of the total iron storage pool. In addition, interpretation of ferritin values may be complicated by a variety of conditions. Reliance on serum ferritin alone can lead to an inaccurate assessment of body iron stores in individual patients. The hepatic iron concentration by liver biopsy is considered the most accurate and sensitive method for determining the body iron burden. However, this is an invasive technique with a low but recognized complication rate; the result is affected by hepatic fibrosis (especially in small biopsies), which is common in thalassemia as a result of increased liver iron and HCV infection; and iron has been shown to be unevenly distributed in the thalassemic liver even in the noncirrhotic stages. Endomyocardial biopsy can evaluate iron deposition in the heart in thalassemia, but is not a routine part of patient management due to the invasiveness of the technique and the heterogeneous deposition of iron in the heart. In addition, iron has been reported to be absent from the right ventricular subendocardium in some patients with cardiac iron overload. The relation between myocardial iron and other measures such as serum ferritin and liver iron has been unknown until recently (see below), and therefore assessing cardiac risk from these other measures has at best been uncertain.
There are noninvasive techniques that can assess tissue iron. Both magnetic resonance (MR) and magnetic susceptometry are significantly affected by iron, and both show changes in iron overload. However, to date only MR can be applied to a moving organ such as the heart. Fortunately there has been substantial progress in the speed and image quality of cardiovascular MR in recent years, and therefore we investigated its use in assessing myocardial iron in thalassemia.
Choice of MR Technique for Measuring Myocardial Iron
Signal intensity ratios
There are reports of the use of signal intensity ratios for the assessment of tissue iron levels, particularly the liver, but rather less so in the heart.1 These measurements are semi-quantitative because while they rely on alterations in relaxation time caused by iron, they effectively relate the relaxation of one tissue of interest to another such as skeletal muscle, which it is assumed has a low propensity for iron uptake and therefore acts as a reference tissue. Attempts to derive absolute myocardial iron concentration using this approach have relied on tissue calibration curves from liver and the use of an empiric correction factor related to hearts with normal iron levels.2 Reproducing the results between scanners is very difficult when imaging parameters vary even slightly, and this makes it difficult to compare results between centers. The multiple assumptions and problems associated with signal intensity ratio measurements limit their value and suggest that direct measurement of myocardial relaxation would have significant potential advantages.
T2
On first exploring the optimal way to determine myocardial relaxation, we attempted to measure myocardial T2, a measure which in animals has been shown to reflect iron levels.3 This requires the use of spin echo cardiac magnetic resonance (CMR) techniques, but in our experience with the techniques routinely available in the late 1990s for human in vivo use, these were not sufficiently reliable or reproducible. For example, there was marked motion artifact in noncompliant patients. Some groups have pursued myocardial T2 measurements.4 Although there are now more advanced T2 imaging techniques, we have focused on assessing myocardial T2* measurement.5 T2* is measured from gradient echo images, which are fast and easy to acquire, are robust to motion for CMR, and in principle are more sensitive to iron levels than T2. In addition, adjustment of the echo time within the sequence to build up a signal decay curve at multiple echo times that were short enough for moderate or severe iron overload was significantly easier using gradient echo techniques.
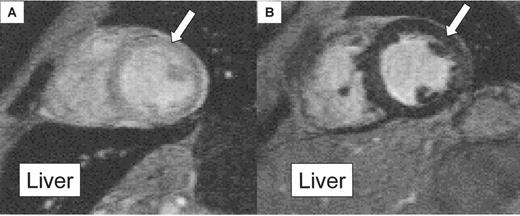
T2*
The principle of using T2* relaxation measurements to determine tissue iron is simple. As the echo time of the images is increased, the signal intensity of all tissues decreases and this rate of decay is strongly enhanced in the presence of iron deposition. Thus, increased myocardial iron reduces myocardial T2*. The key issue in evaluating myocardial T2* was to use echo times that allowed reproducible measurements. Initial experiments showed that echo times from approximately 4 to 20 ms separated by 2 ms increments allowed the best assessment of T2* in mild to severe iron overload. Patients with higher T2* values would have decreased measurement reproducibility, but we had already established that such patients had normal myocardial iron and therefore would not require intensive follow-up to monitor response to a change in treatment.
In our first studies, where we were unable to control the sequence repeat time, we chose a low flip angle to minimize T1 effects that can cause errors. The scan resolution was fixed to allow typical breath-hold times per acquisition of 8–10 seconds, which is suitable for children as well as adults. In our early studies, one breath-hold acquisition was required per single image, which had a single echo time. We then acquired up to 9 images, each at a lengthening echo time. A fixed gating delay of 0 ms after the R-wave was chosen in order to obtain myocardial images in a consistent position in the cardiac cycle irrespective of the heart rate. The resulting image is therefore acquired in early systole. An end-systolic image would have offered the benefits of decreased wall motion and thicker ventricular myocardium in which to draw the region of interest, but the delay time would require alteration to end-systole for each patient and also between breath-holds if there was any variation in the patient’s heart rate. A short axis mid-ventricular slice was chosen to minimize artifacts from blood flow. The region of interest was drawn in the ventricular septum, encompassing all layers of the myocardium from the right ventricle through to the left ventricle (LV) endocardium. The region was drawn away from the cardiac veins and the lungs, which cause susceptibility artifacts. More recently, we have developed a single breath hold technique (15 seconds) that acquires all the images at lengthening echo times.6 This has advantages of speed of acquisition and excellent registration, such that the heart position is constant between images of different echo times, which improves the ease of quantitative analysis. To calculate the myocardial T2*, the signal intensity of the myocardial region of interest was measured in each of the nine images using in-house software (CMRtools, Cardiovascular Imaging Solutions, London, UK). The value was plotted against the echo time for each image. A trendline was fitted to the resulting exponential decay curve, with an equation of the form y = Ke−TE/T2* where K represents a constant, TE represents the echo time and y represents the image signal intensity.
Normal Ranges for T2* Relaxation Times
To determine normal ranges for T2* values in the heart we scanned 15 healthy volunteers. The normal value for the myocardium was 52 ± 16 ms. There is limited literature with which to compare these results. Previous reports of myocardial T2* values using a variety of echo times include values of 33 ± 6.5 ms, 48 ± 9 ms and 38 ± 6 ms. Importantly, however, the lower limit of normal (defined as the mean minus 2 standard deviations) with our technique was 20 ms, which is very similar to that of the other reports. Therefore, the lower boundary limit we have adopted for myocardial T2* is 20 ms, and values below this represent iron overload. No other clinical scenarios other than iron overload have been shown to cause myocardial T2* below this value.
Calibration of the T2* Technique
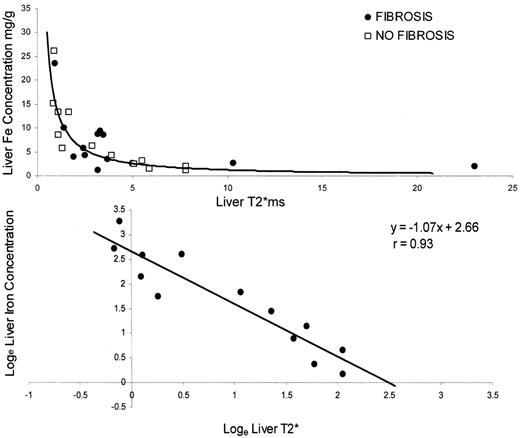
To investigate the relationship between tissue iron and T2* relaxation, we prospectively studied 30 patients undergoing liver biopsy. The biopsy iron concentrations were compared with the liver T2* measurements. We found a significant, curvilinear, inverse correlation between liver T2* and the liver iron content for all samples (r = 0.81, Figure 2 ). As predicted from the known variability of biopsy iron determination in the presence of hepatic fibrosis, there was a better correlation with the non-fibrotic liver samples (r = 0.93, P < 0.0001) than the fibrotic samples (r = 0.68, P < 0.0001). Therefore, we subsequently employed nonfibrotic samples to generate predictions of liver iron content from the measured T2* values. Log transformation for the nonparametric data showed a linear relation between liver iron and liver T2*, confirming that tissue T2* can be used as a quantitative measure of tissue iron. These data confirm that tissue T2* is sensitive to tissue iron levels, but they do not provide calibration for myocardium.
Reproducibility of T2* Measurements
In order for the T2* sequence to be suitable for the clinical assessment of tissue iron, good reproducibility of the technique is required. Therefore, patients with thalassemia major were scanned on two occasions to assess the interstudy reproducibility of the T2* technique. The coefficient of variation (SD of differences divided by the mean) was found to be 3.3% for the liver and 5.0% for the heart.5 Reproducibility between scanners was also shown to be very good at 9.4%.7 To assess intercenter reproducibility, patient scans were performed in 2 different countries and the patients rescanned in our center in London.8 The myocardial reproducibility in each country was 2.4% and 3.5%. Finally, myocardial reproducibility of the single breath-hold technique was 2.3% for abnormal T2* values.6 All these reproducibility values were considered appropriate for clinical application. The improved reproducibility of these gradient-echo T2* techniques over the fast-spin-echo and spin-echo techniques results principally from improved quality of the original images from which T2* is measured.
Myocardial T2*, Cardiac Function and Normality
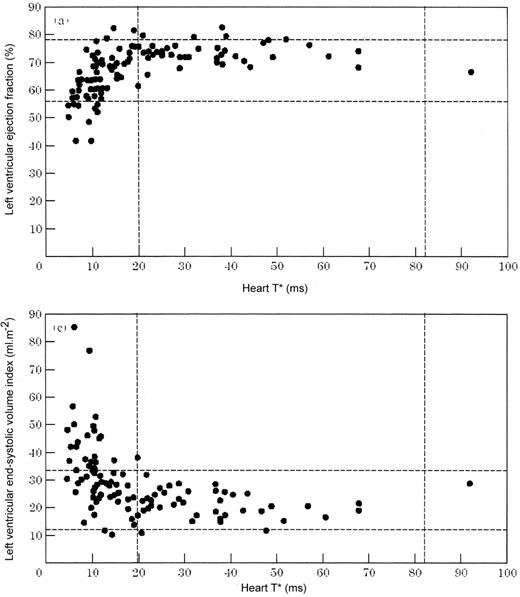
LV function was shown to fail in association with myocardial T2* values below the normal range (< 20 ms). As myocardial iron increased, the LV dilated, ejection fraction fell and mass increased (Figure 3 ).5 Importantly, we have not observed any patient to have heart failure who did not have a low myocardial T2* unless there was an obvious alternative cause, such as congenital heart disease. Another notable finding in this cohort study was that in patients with normal myocardial T2* the ejection fraction was usually in the high range for normal subjects. Because of the anemia in these patients, it is possible that to compare their cardiac volumes and other parameters against normal ranges for non-anemic subjects could lead to a significant underestimation of the number of thalassemia patients recognized as having the early stages of LV impairment. We therefore studied a further cohort of thalassemia patients and sought to determine the normal ranges for LV function in those patients in whom myocardial iron loading could be confidently excluded by the presence of a myocardial T2* which was greater than 20 ms.8 This study showed a significant difference for normal LV parameters between the published normal ranges and “normal function” in thalassemia. The latter group had increased LV end-diastolic volume, ejection fraction and mass with reduced end-systolic volume in comparison with normal subjects. These findings confirm that great care must be taken to interpret LV function parameters in thalassemia if significant misdiagnosis of the presence of early cardiomyopathy is not to occur.
Further analysis of other parameters of cardiac function has also been related to myocardial T2*. Diastolic function is significantly affected by T2* in the abnormal range of < 20 ms, and there is a progressive increase in the ratio of early to late peak filling rate.8 However, the early detection of cardiomyopathy using diastolic function is still relatively poor when compared with the more direct measure of myocardial T2*. There is also a strong inverse relation between right ventricular function and myocardial T2*, indicating that myocardial iron is responsible for bi-ventricular dysfunction.9
Validation of the T2* Technique
Some have questioned whether the myocardial T2* technique has been adequately validated. In brief, a technique can be validated in relation to established clinical parameters known to reflect a disease process.10 As described above, there are important relationships between T2* in the range below normal and the remodeling of heart failure, which is the classic response of the heart in cardiomyopathy. There is no doubt, therefore, that since nothing is known to reduce T2* to abnormal levels other than iron loading, iron affects T2*, and T2* is related, in turn, to cardiomyopathy. Therefore, myocardial T2* is validated clinically against cardiac function. What has not yet been achieved is calibration of myocardial T2* against absolute myocardial iron levels. In discussion of whether absolute calibration is vital, the question is whether it is more important to know the relationship between myocardial T2* and cardiac function or between myocardial T2* and directly measured myocardial iron levels. The former is vital, while the latter has additional scientific merit. To date, the myocardial T2* technique can be considered validated but not yet calibrated.
Relation of Cardiac Iron to Liver Iron and Serum Ferritin
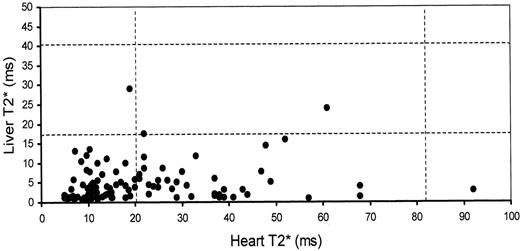
No significant correlation could be found between liver and heart T2* in a large cohort (r = 0.15, P = 0.11, Figure 4 ).5 This lack of correlation has been confirmed by Wood et al.11 Similarly, no significant correlation was found between heart T2* and serum ferritin level at the time of the scan (r = 0.10, P = 0.32, Figure 5 ). To confirm that this finding was not due to spurious individual ferritin readings, the mean ferritin for 12 months prior to the scan was also compared to heart T2*, and once again there was no significant correlation (r = 0.09, P = 0.35). Wood found a weak correlation between myocardial T2* and ferritin.11 This strongly suggests that assessment of myocardial iron from the liver iron is unreliable, and that from ferritin is not ideal.
Relation of Cardiac Function to Liver Iron and Serum Ferritin
In a cohort study of 205 patients, there was no significant difference in left ventricular ejection fraction in patients with liver iron above 15 mg/g (left ventricular ejection fraction [LVEF] 66.1 ± 6.3%) or below 15 mg/g (LVEF 66.2 ± 9.0%, P = 0.96) or with serum ferritin levels above 2500 mg/L (LVEF 65.1 ± 10.1) or below 2500 mg/L (LVEF 66.6 ± 8.2, P = 0.22).9 No significant correlation could be demonstrated between liver iron and LVEF (r = 0.13, P = 0.07) or serum ferritin and LVEF (r = 0.08, P = 0.27) in the cohort of 205 patients. These findings strongly suggest that assessment of the risk of heart failure from either the liver iron or serum ferritin alone is unreliable.
Reversibility of Cardiac Iron in Heart Failure
A study was performed of 7 thalassemia patients presenting with heart failure who were converted to intravenous DFO treatment, with follow-up over 12 months.11 One patient died. The survivors showed improved LV function and improved myocardial T2*. This rare prospective study in this condition clearly demonstrates that aggressive iron chelation with DFO is effective in patients with heart failure, and that myocardial T2* improves in concert with parameters of LV function. Of great interest, however, was the clearance rate of iron from the heart, which was substantially slower than from the liver (5.0 ± 3.3% vs 39 ± 23% per month, P = 0.02). Excess myocardial iron persisted in all patients at 1 year, despite liver iron normalization in 4 patients. This suggests that monitoring the duration of treatment by liver iron alone would be misleading in guiding the risk from myocardial iron loading, as the liver would be clear of iron loading very much earlier (by tens of months) than the heart.
Cross-Sectional Study of Cardiac Iron Between Deferiprone and Desferrioxamine
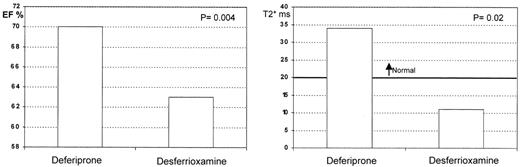
A cross-sectional study was performed comparing myocardial T2* and LV function between 2 cohorts of thalassemia patients: one treated with standard DFO therapy, and one treated with DFP.12 The median myocardial T2* was significantly higher in the DFP group, and their ejection fraction was also significantly better (Figure 6 ), suggesting less cardiac iron and better ventricular function than in patients treated with DFO. The liver T2* was higher in the DFO group, suggesting more rapid removal of iron from this organ than with DFP. These data require prospective confirmation in randomized controlled trials (these are now underway and will be reported in late 2004 or 2005), but the current data suggest that oral DFP may have preferential access to the heart, in which case it has a potentially unique and valuable role in the treatment of iron overload.
Conclusions
Myocardial T2* measurements have started to unravel some of the complexities of why patients with thalassemia have such a high mortality rate from cardiomyopathy despite clinical management guided by surrogate measures. Myocardial CMR demonstrates the relationship between T2* and LV function. Calibration with myocardial iron is awaited for scientific completeness. Because myocardial T2* is transferable to other scanners and is reproducible, there is considerable potential for widespread application of this technique among the approximately 22,000 MR scanners worldwide.
III. Hepatitis C, Thrombosis and Fertility in Adult Patients with Thalassemia
Melody J. Cunningham, MD*
Children’s Hospital Boston, 300 Longwood Avenue, Fegan 703, Boston MA 02115
As clinical care of patients with thalassemia has improved and the patient population is aging due to improved survival, new issues are evolving. These include long-term complications of infection with hepatitis C (HCV), thrombosis, and fertility. HCV and its consequences of fibrosis, cirrhosis and hepatocellular carcinoma (HCC) are prevalent. Increasing evidence of thrombotic risk in patients with thalassemia intermedia and thalassemia major is being reported in the literature. Further, the improved lifespan and clinical status of the thalassemia population has allowed preservation of fertility and successful term pregnancies in some patients. Without exception, large-scale definitive trials addressing these problems in patients with thalassemia are still needed.
Hepatitis C Virus
Worldwide, no patients get more red cell products than those with thalassemia major. The life-long need for transfusion renders patients vulnerable to transfusion-transmitted viral infections. HCV has emerged as the paramount risk. HCV was identified in 1989 and serologic screening of the blood supply was initiated in 1990. Data from the Registry of the Thalassemia Clinical Research Network (TCRN) demonstrate that this screening has been successful in preventing new HCV infection via blood transfusion in younger patients with thalassemia major in North America. Only 5% of patients under age 15 (transfused since screening was available) are positive for HCV antibody or RNA, whereas the prevalence of HCV exposure in those over age 25 years is 70%.1 These results are encouraging. However, there are still hundreds of adults in North America and thousands worldwide with chronic HCV who are at risk for significant complications of liver fibrosis, cirrhosis and HCC. Additionally, in developing countries, the prevalence of HCV is as high as 63.8% in patients with thalassemia, including young patients, as shown in a recent report from the Pasteur Institute of Iran.2
The major issues influencing the rate of HCV exposure and chronic infection in patients with thalassemia (and other patients requiring blood transfusions) are appropriate choice of donors and viral testing of donated units. Voluntary donation has been demonstrated to be a critical component in maintaining a safe blood supply. At the time of a 2000 Pan American Health Organization press release, only 5 countries in the Americas exclusively accepted volunteer donors and 16 nations tested 100% of donated units for human immunodeficiency virus (HIV), hepatitis B virus (HBV) and HCV. According to the American Association of Blood Banks, the chance of becoming infected with HCV from a blood transfusion in the United States is currently 1 per 1 million units transfused. However, the risk is much higher in other countries, so many patients with thalassemia worldwide continue to become infected with HCV. Until universal testing is a reality, treatment must be a focus for these patients.
HCV infection and iron overload are risk factors for cirrhosis and HCC. Patients with thalassemia major often suffer from both of these complications. The only large survey, from 52 Italian centers, regarding HCC was reported in 2004 by Borgna-Pignatti et al.3 Twenty-two patients (estimated denominator of 5000) were identified with HCC. The mean age was 43 years. Only one patient had no evidence of exposure to either HCV or HBV, and 17 of 19 HCV exposed patients were HCV-RNA positive. The most common presenting symptoms were ascites, fatigue, edema, and jaundice. The survival of patients with HCC is negatively associated with size of tumor, and the authors recommended screening of HCV-RNA–positive patients with ultrasound and serum alpha-fetoprotein (AFP) every 6 months.3 Although there are no prospective, randomized studies of screening for HCC in patients with chronic, active HCV infection, many hepatologists perform biannual screening with serum AFP and liver ultrasound.4 Other centers, including ours, perform annual screening.
The apparent rate of spontaneous HCV clearance among North American patients with thalassemia major in the TCRN Registry was 33%,1 which exceeds the rate of 10%–20% reported in the literature but still leaves many patients chronically infected. Combination therapy with interferon and ribavirin is now considered standard treatment for patients with chronic HCV without hemolytic anemia based on a randomized, controlled clinical trial of 1121 previously untreated adults.5 However, the product information for ribavirin still states, “patients with hemoglobinopathies should not be treated with [Rebetol] capsules or oral solution.” Because hemolysis is a common side effect of ribavirin therapy, the concern in patients with transfusion-dependent thalassemia and other hemolytic anemias is that the increased iron burden and damage to the liver will outweigh the benefits of clearing the virus. This potential harm would be magnified, theoretically, in patients who fail to respond to treatment including ribavirin since they would have both increased hepatic iron and continuing infection with HCV. Interferon alone in patients with thalassemia has been reported to have a successful clearance rate of only 15%–40%.6,7 Several reports have demonstrated the safe and successful treatment of chronic HCV infection with combination therapy in patients with thalassemia major.6–,8 These studies and case series demonstrate variable but substantial, sustained biochemical and virological response rates of 45.5%7 to 72.2%6 at 6 months following completion of therapy. The increase in transfusion requirements is highly variable but may be as great as 152% over baseline.6
The paucity of large prospective trials and the cautionary notes about use of ribavirin in the face of hemolytic anemia pose challenges for clinicians who must decide on therapy for adults with thalassemia who have chronic, active HCV infection. Until adequate data become available, we have taken an individualized approach.
Whenever possible, we enroll such patients in clinical trials designed to answer the outstanding questions.
We optimize chelation regimen and assess cardiac status prior to therapy.
We consider treatment with PEG-interferon/ribavirin with careful monitoring of viral load, ferritin, liver enzymes, transfusion requirements, liver biopsy prior to and after 1 year of therapy and alteration of chelation regimen according to increases in transfusion requirements.
Should the viral load not substantially decrease after 6 months of therapy, it is unlikely that further treatment will be effective and we would discontinue therapy.
Thrombosis
In a recent review, Eldor and Rachmilewitz summarize data from many series and present compelling clinical evidence for increased risk of thrombosis in patients with not only β-thalassemia intermedia, but also β-thalassemia major, α-thalassemia syndromes and hemoglobin E/β-thalassemia (E/β-thal).9 These data are compiled in Table 4 . The difficulty in ascertaining clinical risk and determining preventive and treatment care is that many of these patients are not fully characterized in that their transfusion regimens are not known. Additionally, although in one series the incidence of deep venous thrombosis and pulmonary embolism was inversely correlated with hemoglobin levels, the details are lacking.9 A retrospective review by Cappellini et al of 83 patients with thalassemia intermedia and 65 patients with thalassemia major demonstrated a prevalence of venous thromboembolic events (VTE) of 29% and 2%, respectively.10 Twenty-three of the 24 patients with thalassemia intermedia and VTE had been splenectomized. The author recommends short-term antithrombotic therapy both perioperatively and when patients are exposed to thrombotic risk factors.10 We recommend this approach and additionally recommend low-dose daily aspirin for all of our splenectomized patients with thalassemia and also for our splenectomized patients with thalassemia major who have platelet counts in excess of 600,000/μL.
Biologic risk factors for thrombosis include splenectomy, red cell phosphatidylserine exposure, and plasma coagulation factor abnormalities.9,11,12 Comparison of E/β-thal patients with splenectomy, without splenectomy and age-matched controls demonstrated statistically increased levels of thrombin-antithrombin III (“TAT”) complex in the splenectomized patients compared to the other two groups.11 Levels of prothrombin fragment 1.2 and RBC phosphatidylserine were statistically increased in the splenectomized patients when compared to the controls.11 These findings suggest ongoing thrombin generation related to anionic phospholipid exposure. Plasma β2-thromboglobulin and platelet aggregation studies demonstrated statistically significant hyperaggregation in the splenectomized group when compared to the nonsplenectomized and normal controls.12 The precise clinical significance of such a finding is unknown.
Studies suggest that a significant, often subclinical, manifestation of increased risk of clotting is development of pulmonary hypertension from small pulmonary emboli9 or thrombi11 in the 100–800 μ-sized arterioles.11 This is attributed to high cardiac output from chronic tissue hypoxia and increased peripheral vascular resistance,13 silent small pulmonary emboli9 and increased pulmonary vascular resistance indices secondary to thrombotic pulmonary arteriopathy.14 A mechanism related to nitric oxide scavenging by free hemoglobin has been implicated in the pulmonary arterial disease of sickle cell anemia and, by extension, could be present in thalassemia as well.15 It will be critical to study the biologic risk factors, thrombosis risk and prophylaxis in prospective clinical trials with precise data ascertainment regarding transfusion regimens, phenotype, genotype, and other thrombosis risk factors such as oral contraceptive pills and genetic hypercoagulable states.
Fertility
Hypogonadotrophic hypogonadism is common in young adults with thalassemia major and is thought to contribute to low fertility in this population. In addition, endocrinologic and cardiac complications of iron overload may cause pregnancy complications in women with thalassemia. Nevertheless, case reports and small published series demonstrate that successful pregnancy and delivery of healthy babies is possible in women with thalassemia major.16–,19 Spontaneous pregnancy without hormonal assistance has been reported.17–,19 Recent literature on hypogonadal hypogonadism suggests that early intervention with hormonal therapy and aggressive iron chelation therapy to prevent permanent damage may help to preserve innate fertility.20
The TCRN Registry recently reported data on successful delivery of healthy babies in 8 women with thalassemia major.19 The ages of the birthing mothers, pre- and post-delivery ferritin and liver iron content, and need for hormonal replacement therapy were reviewed. Age-matched women with thalassemia major who were not birth-mothers were the controls. The Registry includes 109 women over age 18 years. The review reported 9 term deliveries in 8 women 23–32 years of age. All of the women discontinued DFO during the pregnancy but the date of discontinuation relative to conception was not known. Four of 9 pregnancies (44%) were assisted by ovulation induction therapy. Significant preconception to postdelivery increases were found for ferritin (n = 7 women, mean increase 590 ng/mL, 95% confidence interaval [CI] 311–1113 ng/mL, P < .03) but not for liver iron (n = 5 women, mean increase 2.2 mg/g, 95% CI −3.8–8.1 mg/g, P = .59). Ferritin values of non-birthing women were 60% greater than the pre-conception ferritins of the pregnant women (2251 vs 1392 ng/mL after controlling for age); however, the difference was not significant (P = .35).19 As more women with thalassemia have successful pregnancies and deliveries, careful review of iron accumulation, cardiac stress and potential deterioration, appropriate increase in transfusion support and use of DFO or other chelators during pregnancy is imperative.
Successful pre-implantation genetic diagnosis has recently been reported for carriers of thalassemia.21 Using in vitro fertilization technology, single cells from an early embryo can be assayed by polymerase chain reaction (PCR) for known mutations, including those of globin genes. This method of biopsy of blastomeres and subsequent transfer in utero of blastomeres unaffected by β-thalassemia major may be used instead of prenatal testing in utero. This technology has also recently been utilized to determine β-thalassemia and HLA genotype for planned HLA-matched cord blood transplantation for an affected sibling.22 Selection of nonthalassemia-affected, HLA-matched siblings as a source of cells for subsequent hematopoietic stem cell transplantation for affected older siblings is gaining favor, but this expensive, “cutting edge” technology should not be considered standard therapy at present.
Future Considerations
The novel issues now coming to the fore in older patients with thalassemia deserve careful attention. Optimal management requires new standards of care for management of hepatitis C, for prevention of silent and clinically overt thrombotic events and for determination of necessary support for successful pregnancy and for identification of patients who may suffer undue harm from the physiologic stress of pregnancy and delivery.
IV. Emerging Thalassemia Syndromes
Elliott Vichinsky, MD*
Oakland Children’s Hospital, Director, Hematology-Oncology Children’s Hospital Research Center, Oakland CA 94610
Today’s epidemiology of thalassemia is strikingly different from that of the past. Thalassemia is now a heterogeneous group of diseases with varied ethnicities, phenotypes and treatments. In the past, Hb E-β-thalassemia and Hb H disease were rarely seen in North America and Europe. Now, as a result of changing demographics, these disorders have become more common than classical β-thalassemia major in many regions1–,5 Hb H, Hb H-Constant Spring and homozygous α-thalassemia affect at least a million people worldwide.3 The natural history of these disorders is highly variable and poorly studied. The phenotype, for patients with similar mutations, can range from asymptomatic to transfusion dependency.
Ethnic groups originating from regions with malaria have a high frequency of thalassemia mutations. There are almost 300 million carriers of hemoglobin disorders in the world, with the majority living in South East Asia.1,3,5,6 Worldwide, the Asian, Indian and Middle Eastern regions account for 95% of thalassemia births. The frequency of α-thalassemia reaches 25% in Thailand, and Hb E approaches 60% in many regions of Thailand, Laos and Cambodia. Hb Constant Spring is found in 1%–10% of the population in these areas. The World Health Organization estimates that Thailand will have over 250,000 symptomatic thalassemia patients diagnosed over the next few decades.3–,7 At least 100,000 new cases of Hb E-β-thalassemia are expected. Similar estimates are predicted for India, Sri Lanka and Malaysia.8 In southern China, with a population of over 350 million, approximately 5% are carriers for α-thalassemia and 4% for β-thalassemia or Hb E.6
In the last decade, three-quarters of immigrants to North America came from areas where thalassemia mutations were prevalent. These demographic changes have resulted in thalassemia becoming a more significant health problem in North America. Hb E-β-thalassemia and α-thalassemia disorders are now the most common thalassemias in California. In response to the high prevalence of α-thalassemia and its associated morbidity, California has initiated universal newborn screening for Hb H and Hb H-Constant Spring.2
Hemoglobin E
Worldwide, Hb E-β-thalassemia may be the most important hemoglobinopathy because of the high gene frequencies for both Hb E and β-thalassemia.8 Since the classic description of Hb E-β-thalassemia by Chernoff, it has been noted to be an important hemoglobin disorder in the Indian subcontinent and Southeast Asia. It has recently replaced β-thalassemia as the most common disorder in many regions, including coastal North America.1,2,5
Hb E is synthesized at a mildly reduced rate and in its homozygous state is similar to β-thalassemia trait. Heterozygotes and homozygotes for Hb E are microcytic, slightly anemic and asymptomatic. The homozygous form is very common, with thousands of cases already detected in North America through newborn screening programs. Without further testing, benign homozygous E disease can be misidentified as hemoglobin E-β-thalassemia. The compound heterozygote state of Hb E-β-thalassemia results in a variable, often severe anemia, with the phenotype ranging from a complete lack of symptoms to transfusion-dependence.4,8– 10 Approximately one-half of the patients are phenotypically similar to patients with thalassemia major who require regular transfusion therapy. The clinical course for the other half resembles thalassemia intermedia. The classification of thalassemia intermedia includes a diverse group of patients. As some of the patients with thalassemia grow older, they become transfusion-dependent. Others remain asymptomatic throughout late adulthood.
The marked variability and clinical course in Hb E-β-thalassemia is largely unexplained. The variability is probably due, in part, to whether the patient is heterozygous for β+-thalassemia or β0-thalassemia mutations. Co-inheritance of α-thalassemia mutations occurs in 15% of patients and may also modulate severity. Hb F level is the strongest predictor of morbidity.4,9–,11 Although the basis of increased Hb F is usually unknown, inheritance of a β-thalassemia chromosome with the XmnI +/+ genotype of the G-gamma globin gene is an example of an associated mutation that is responsible for increased Hb F and a milder clinical course.4,10,11
The pathophysiology of Hb E-β-thalassemia is complex. Ineffective erythropoiesis, apoptosis, and oxidative damage are central components of the disease and its shortened red cell survival.9,12,13 The instability of Hb E and the expression of alpha hemoglobin stabilizing protein do not appear to be major factors in the pathophysiology.14 Non-transfusional iron overload can result in widespread organ injury in the absence of markedly elevated ferritin levels.13,15 A chronic hypercoagulable state may result in pulmonary thrombosis and other thromboembolic events. Splenectomy and increased exposure of erythrocyte phosphatidylserine appear to increase the prothrombotic risk.12,16 Oxidative damage with deficiency of antioxidants may also be important in the cellular pathophysiology of this disease.17
The availability of prenatal diagnosis, chronic transfusion, bone marrow transplants and chemotherapy adds to the importance of characterizing the clinical course of Hb E-β-thalassemia. In earlier reports by Chernoff and Wasi, patients were classified as having severe thalassemia if they manifested with massive abdominal enlargement, growth failure and marked skeletal deformities. There was a uniformly increased risk of life-threatening infections. Patients suffered from recurrent anemic events due to hypersplenism, aplastic crises and megaloblastic anemia.18 As most patients could not be transfused, they developed large tumor-like masses secondary to massive erythroid hyperplasia. When these masses impinged on the spinal cord, they caused paraplegia and other complications. As noted above, severe iron overload may develop despite the absence of regular transfusion therapy. Early reports on the hematologic findings of E-β-thalassemia stress the severity of the illness and report a mean hemoglobin of 6.3 g/dL. However, the thalassemia-related severity of Hb E-β-thalassemia in these reported patients may be overestimated because of the additional effects of acquired causes of anemia, such as infection and poor nutrition, which were endemic during that period in the studied region.
In recent studies of Hb E-β0-thalassemia, the hemoglobin concentrations ranged from 3 to 13 g/dL with an average of 7.7 g/dL. Even with screening for genetic modifiers, early predictors of severity have yet to be defined. The time of onset and the severity of anemia may be useful in predicting the long-term clinical phenotype.8– 10 Severe anemia that begins at 6–12 months suggests a thalassemia major phenotype. A stable clinical condition at 5 years of age is generally sustained into at least early adulthood.
Splenomegaly often develops in severely affected patients. In the past, splenectomy was routinely performed in an attempt to increase hemoglobin levels. Splenectomy is often less successful than expected. Increasing evidence of the risk of thromboembolism in splenectomized patients has led to reconsideration of the role of splenectomy.
The bone marrow expansion and increased metabolic rate that are found in Hb E-β0-thalassemia result in growth failure, delay in secondary sex development and osteoporosis. In older patients, severe bone pain may lead to the initiation of transfusion therapy.
Iron overload in nontransfused patients is common, secondary to increased gastrointestinal absorption of iron.18 End organ failure secondary to iron overload may not be suspected because the serum ferritin level is disproportionately low. The cause of the lack of correlation between serum ferritin levels and liver iron stores is unknown. Vitamin C deficiency may play a role. Quantitative liver iron measurements are necessary to identify iron overload even in nontransfused patients with Hb E-β0-thalassemia.
Cardiac disease and pulmonary disease are the most common causes of death in Hb E-β-thalassemia. Increased cardiac output, severe anemia and, in some cases, thromboembolism result in cardiac failure and pulmonary hypertension. Transfusion therapy appears to reverse this process in some patients.16
Patients with Hb E-β-thalassemia may be excellent candidates for agents directed at elevating Hb F production. A small increase in the steady state hemoglobin concentration might be of major clinical benefit. Pilot data suggest that some patients sustain long-term benefit from hydroxyurea, short chain fatty acids such as butyrate, or erythropoietin.19 However, individual responses are inconsistent.
Alpha Thalassemia Disorders
The gene frequencies of α-thalassemia exceed those of β-thalassemia. Hb H disease is a serious health problem in Southeast Asia and southern China.7,18,20,21 Thousands of affected patients live in the Middle East, Mediterranean region and North America.2,7 Hb H disease may be a mild disorder, but recent studies suggest its clinical course is often more severe than previously recognized. Hb H is unstable and forms intracellular precipitates that result in early cell death. Hemolysis rather than ineffective erythropoiesis is the primary cause of anemia in Hb H disease. Many patients require intermittent transfusions. The clinical severity is strongly influenced by the type of mutation. Deletions on chromosome 16 are responsible for 75% of Hb H mutations, and these deletions cause a milder form of the disorder. The remaining 25% of patients with Hb H disease have two deletions plus a point mutation or insertion in the alpha globin gene. Non-deletional Hb H is often severe and likely to require transfusions.21– 24 In both groups, however, there is marked phenotypic variability.
The diagnosis of Hb H may be difficult. Hb H and Hb Barts are fast moving hemoglobins that may appear on electrophoresis. However, they are unstable and often go undetected. Patients with Hb H disease have > 20% Hb Barts at birth. In the California newborn screening for Hb H disease, 90% of cases were diagnosed by the finding of elevated levels of Hb Barts on high performance liquid chromatography (HPLC). DNA testing was required to distinguish Hb H Constant Spring (CS) from Hb H disease.2
After the newborn period, Hb Barts is no longer seen on electrophoresis, but Hb H may be detected. Red cells from patients with Hb H form inclusions when stained with brilliant cresyl blue or methylene blue, but these tests are nonspecific. DNA testing is often helpful in identifying both deletional and nondeletional mutations.
The diagnosis of deletional Hb H disease is often made only after the detection of complications such as cholelithiasis, exacerbations of the anemia induced by infection, or the findings of splenomegaly and growth failure.22,23 The mean hemoglobin in deletional Hb H is quite variable, but averages 9.5 g/dL. Twenty-nine to 50% of patients with deletional Hb H require intermittent transfusion therapy, but the need for chronic transfusion therapy is uncommon. Pregnancy is often associated with an increased severity of anemia as well as pre-eclampsia. Iron overload and iron-induced heart failure have occasionally occurred in patients not receiving transfusions.
Hb CS12,20–,22,25 is the most common nondeletional α-thalassemia mutation associated with Hb H disease. The gene frequency of Hb CS approaches 8% in Southeast Asia. Its messenger RNA is highly unstable, producing less than 1% of the total protein output of a normal gene. Cells containing Hb CS become overhydrated, modifying the expected microcytosis. In comparison with cells from patients with deletional Hb H disease, cells containing Hb CS have increased hemoglobin bound to the membrane, increasing the rate of hemolysis.22,23 Hb H/CS disease has significantly more ineffective erythropoiesis and erythroid apoptosis than Hb H disease.7 The laboratory and clinical course of Hb H/CS disease is more severe than Hb H disease.1,20–,22 The average hemoglobin is 2 g/dL less than in deletional Hb H disease. The mean MCV is a near normal 72 fL compared to 59 fL for deletional Hb H disease, since cells containing Hb CS become overhydrated, modifying the expected microcytosis. Most patients have moderately severe splenomegaly and over 50% require splenectomy. Splenectomy often results in improved hemoglobin levels but is associated with a high rate of portal vein thrombosis. Ninety percent of patients with Hb H/CS disease have been intermittently transfused, and up to 40% have required repeated transfusions, particularly in early infancy and in later adulthood. Iron overload occurs in 75% of patients by adulthood. Rarely, Hb H/CS disease and other nondeletional Hb H disorders have caused fatal hydrops foetalis syndrome.7
Homozygous α-thalassemia, caused by a deletion of all four α-globin genes, leads to the formation of high levels of Hb Barts in utero. Hb Barts has an extremely high oxygen affinity and therefore delivers little oxygen to fetal tissues. The severe hypoxia results in cardiac failure, massive ascites and intrauterine death.4 Congenital malformations associated with homozygous α-thalassemia include hypospadias, other genitourinary defects, and limb malformations. Infants surviving to delivery without prenatal intervention are usually hydropic and commonly have neurologic impairment. Intrauterine transfusions following early detection of homozygous α-thalassemia have resulted in the birth of several nonhydropic infants, some but not all of whom have no significant neurologic abnormalities or congenital anomalies. Affected infants who survive gestation and the neonatal period subsequently require chronic transfusion therapy or may be appropriate candidates for hematopoietic stem cell transplantation.26,27 Occasionally infants with homozygous α-thalassemia are born without hydrops even in the absence of intrauterine transfusions.26,27 Increased levels of Hb Portland, a normally functioning embryonic hemoglobin, may be responsible for this improved clinical course.
In pregnancies known to be at risk, chorionic villous sampling with molecular analysis identifies homozygous α-thalassemia within the first months of pregnancy. The ethical issues of managing the fetus known to have homozygous α-thalassemia are complex. Obstetric complications and the necessity for long-term transfusion therapy are serious considerations. Increased risk of both maternal and fetal morbidity should be included in counseling families at risk for an affected fetus.
The prognosis for patients with the hemoglobinopathies that are newly emerging in North America and elsewhere is strongly dependent on the health care system’s approach to these disorders. Prenatal diagnosis, neonatal screening, comprehensive care and access to new therapies including transfusion therapy, stem cell transplantation and Hb F–enhancing agents are important components of care. Simple approaches such as the use of tea to inhibit iron absorption may have profound benefits. Addressing the economic and cultural barriers and increasing the knowledge of physicians about these disorders should improve the overall care of affected patients.
Important properties for an ideal iron chelator.
|
|
Potential advantages of combined chelation therapy.
|
|
Current status of iron chelators.
| Chelator . | Current Status . |
|---|---|
| Abbreviations: HBED, hydroxybenzyl-ethylenediamine-diacetic acid; PIH, pyridoxal isonicotinoyl hydrazone | |
| Deferiprone | Approved in over 50 countries |
| ICL670 | Phase III studies in progress |
| GT56-252 | Phase I study completed |
| 40SD02 | Phase I study completed |
| HBED | Phase I study planned |
| PIH | Preclinical studies |
| Desferrithiocin | Preclinical studies |
| Chelator . | Current Status . |
|---|---|
| Abbreviations: HBED, hydroxybenzyl-ethylenediamine-diacetic acid; PIH, pyridoxal isonicotinoyl hydrazone | |
| Deferiprone | Approved in over 50 countries |
| ICL670 | Phase III studies in progress |
| GT56-252 | Phase I study completed |
| 40SD02 | Phase I study completed |
| HBED | Phase I study planned |
| PIH | Preclinical studies |
| Desferrithiocin | Preclinical studies |
Evidence of hypercoagulable pathology in patients with thalassemia syndromes.*
| Diagnosis . | No. of Patients . | Thromboembolic Diagnosis . | % . | Comments . |
|---|---|---|---|---|
| * This table summarized data from the Eldor and Rachmilewitz review article in Blood 2002.9 Data included are from multicenter studies, retrospective autopsy reviews and observational studies. Not all studies looked for evidence of all findings, nor did all report on the presence or absence of the spleen in patients. | ||||
| Abbreviations: TM, thalassemia; TI, thalassemia intermedia; E, hemoglobin E; TIA, transient ischemic attack; MRI, magnetic resonance imaging; DVT, deep venous thromboembolism; PE, pulmonary embolism; y, year; Pulm, pulmonary; HTN, hypertension | ||||
| β-TM | 138 | Stroke | 0.02 | Not regularly transfused |
| TIA | 20 | Not regularly transfused | ||
| β-TM | 735 | Stroke | 0.02 | Not regularly transfused |
| β-TI | 41 | Asymptomatic cerebral ischemia | 37 | By MRI, Incidence correlated positively w/age and negatively w/hgb |
| β-TM | 685 | DVT or PE | 3.95 | |
| β-TI | 52 | DVT or PE | 9.61 | |
| β-TM | 1146 | DVT or PE | 1.1 | |
| β-TM | 421 | DVT or PE | 3.3 | Median age 28 y, 15% congenital or acquired risk factors |
| β-TI | 74 | DVT or PE | 16.2 | Median age 28 y, 15% congenital or acquired risk factors |
| β-TI | 83 | DVT, PE or portal vein thrombosis | 29 | 11% of total with recurrent VTE, 99% splenectomized |
| β-TM | 33 | Pulm HTN | 85 | Age range 2–24 y, suspected microthrombi |
| β-TM+/-E | 16 | Pulm HTN | 94 | Age range 5–14 y, suspected microthrombi |
| β-TM | 35 | Pulm HTN | 75 | |
| β-TM/E | 43 | Pulm arteriolar organized thrombi | 19 | Autopsy findings, 17 splenectomized |
| β-TM | 2 | Pulm arteriolar platelet microthrombi | 2 | Autopsy findings |
| Diagnosis . | No. of Patients . | Thromboembolic Diagnosis . | % . | Comments . |
|---|---|---|---|---|
| * This table summarized data from the Eldor and Rachmilewitz review article in Blood 2002.9 Data included are from multicenter studies, retrospective autopsy reviews and observational studies. Not all studies looked for evidence of all findings, nor did all report on the presence or absence of the spleen in patients. | ||||
| Abbreviations: TM, thalassemia; TI, thalassemia intermedia; E, hemoglobin E; TIA, transient ischemic attack; MRI, magnetic resonance imaging; DVT, deep venous thromboembolism; PE, pulmonary embolism; y, year; Pulm, pulmonary; HTN, hypertension | ||||
| β-TM | 138 | Stroke | 0.02 | Not regularly transfused |
| TIA | 20 | Not regularly transfused | ||
| β-TM | 735 | Stroke | 0.02 | Not regularly transfused |
| β-TI | 41 | Asymptomatic cerebral ischemia | 37 | By MRI, Incidence correlated positively w/age and negatively w/hgb |
| β-TM | 685 | DVT or PE | 3.95 | |
| β-TI | 52 | DVT or PE | 9.61 | |
| β-TM | 1146 | DVT or PE | 1.1 | |
| β-TM | 421 | DVT or PE | 3.3 | Median age 28 y, 15% congenital or acquired risk factors |
| β-TI | 74 | DVT or PE | 16.2 | Median age 28 y, 15% congenital or acquired risk factors |
| β-TI | 83 | DVT, PE or portal vein thrombosis | 29 | 11% of total with recurrent VTE, 99% splenectomized |
| β-TM | 33 | Pulm HTN | 85 | Age range 2–24 y, suspected microthrombi |
| β-TM+/-E | 16 | Pulm HTN | 94 | Age range 5–14 y, suspected microthrombi |
| β-TM | 35 | Pulm HTN | 75 | |
| β-TM/E | 43 | Pulm arteriolar organized thrombi | 19 | Autopsy findings, 17 splenectomized |
| β-TM | 2 | Pulm arteriolar platelet microthrombi | 2 | Autopsy findings |
Validation of T2* as a measure of tissue iron.
The upper graph shows the relation between liver iron from liver biopsy, and liver T2* measured by MR. The curvilinear relation becomes linear on the lower graph by using a log-log plot (non-fibrotic samples). This confirms that tissue iron affects tissue T2* and provides a calibration curve for liver T2*. Calibration of myocardial T2* is not yet available, but there is no doubt that myocardial T2* can be used to quantify relative myocardial iron levels.
Reproduced with permission from Anderson LJ, Holden S, Davies B, et al. Cardiovascular T2* (T2 star) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179.
Validation of T2* as a measure of tissue iron.
The upper graph shows the relation between liver iron from liver biopsy, and liver T2* measured by MR. The curvilinear relation becomes linear on the lower graph by using a log-log plot (non-fibrotic samples). This confirms that tissue iron affects tissue T2* and provides a calibration curve for liver T2*. Calibration of myocardial T2* is not yet available, but there is no doubt that myocardial T2* can be used to quantify relative myocardial iron levels.
Reproduced with permission from Anderson LJ, Holden S, Davies B, et al. Cardiovascular T2* (T2 star) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179.
The relation of myocardial T2* to left ventricular size and function.
T2* in the abnormal range (< 20 ms) indicates increased myocardial iron. When T2* is > 20 ms, the left ventricular ejection fraction and end-diastolic volume are normal. There is a clear relation between left ventricle dilation and failure with T2* values in the abnormal range, and this is particularly apparent once T2* is < 10 ms.
Reproduced with permission from Anderson LJ, Holden S, Davies B, et al. Cardiovascular T2* (T2 star) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179.
The relation of myocardial T2* to left ventricular size and function.
T2* in the abnormal range (< 20 ms) indicates increased myocardial iron. When T2* is > 20 ms, the left ventricular ejection fraction and end-diastolic volume are normal. There is a clear relation between left ventricle dilation and failure with T2* values in the abnormal range, and this is particularly apparent once T2* is < 10 ms.
Reproduced with permission from Anderson LJ, Holden S, Davies B, et al. Cardiovascular T2* (T2 star) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179.
The relation of myocardial T2* and liver iron.
In this cohort, there was no significant relation between myocardial T2* and liver iron. There was also no significant relation of myocardial iron to serum ferritin. These findings strongly indicate that management of myocardial iron needs to be guided by direct myocardial iron measurements and not via surrogates.
The relation of myocardial T2* and liver iron.
In this cohort, there was no significant relation between myocardial T2* and liver iron. There was also no significant relation of myocardial iron to serum ferritin. These findings strongly indicate that management of myocardial iron needs to be guided by direct myocardial iron measurements and not via surrogates.
Discordance of liver and myocardial iron.
In A the patient has heavy liver iron loading (black liver), but the heart retains signal indicating light loading (arrow). If management or myocardial iron were based on a liver biopsy in this patient, chelation treatment might be increased, but the risk of cardiac complications from iron overload is low. In B the patient has little liver iron loading (grey liver), but the heart has low signal indicating heavy loading (arrow). If management or myocardial iron were based on a liver biopsy in this patient, chelation treatment might be maintained at current levels or even decreased, but the risk of cardiac complications from iron overload is high.
Discordance of liver and myocardial iron.
In A the patient has heavy liver iron loading (black liver), but the heart retains signal indicating light loading (arrow). If management or myocardial iron were based on a liver biopsy in this patient, chelation treatment might be increased, but the risk of cardiac complications from iron overload is low. In B the patient has little liver iron loading (grey liver), but the heart has low signal indicating heavy loading (arrow). If management or myocardial iron were based on a liver biopsy in this patient, chelation treatment might be maintained at current levels or even decreased, but the risk of cardiac complications from iron overload is high.
T2* and ejection fraction between deferiprone (DFP) and desferrioxamine (DFO).
The ejection fraction and myocardial T2* was measured in a cross-sectional study of matched thalassemia patients on monotherapy with DFP (due to intolerance to DFO) or DFO. The ejection fraction and the myocardial T2* was significantly higher in the DFP group. Randomized controlled studies are now underway to determine if these preliminary findings represent improved access of DFP to myocardial iron, which, if true, would represent a significant clinical advance in the management of the cardiac complications of thalassemia.
T2* and ejection fraction between deferiprone (DFP) and desferrioxamine (DFO).
The ejection fraction and myocardial T2* was measured in a cross-sectional study of matched thalassemia patients on monotherapy with DFP (due to intolerance to DFO) or DFO. The ejection fraction and the myocardial T2* was significantly higher in the DFP group. Randomized controlled studies are now underway to determine if these preliminary findings represent improved access of DFP to myocardial iron, which, if true, would represent a significant clinical advance in the management of the cardiac complications of thalassemia.