Abstract
The therapeutic approach to the patient with acute myeloid leukemia (AML) currently evolves toward new frontiers. This is particularly apparent from the entree of high-throughput diagnostic technologies and the identification of prognostic and therapeutic targets, the introduction of therapies in genetically defined subgroups of AML, as well as the influx of investigational approaches and novel drugs into the pipeline of clinical trials that target pathogenetic mechanisms of the disease.
In Section I, Dr. Bob Löwenberg reviews current issues in the clinical practice of the management of adults with AML, including those of older age. Dr. Löwenberg describes upcoming possibilities for predicting prognosis in defined subsets by molecular markers and reviews experimental strategies to improve remission induction and postinduction treatment.
In Section II, Dr. James Griffin reviews the mechanisms that lead to activation of tyrosine kinases by mutations in AML, the consequences of that activation for the cell, and the opportunities for targeted therapy and discusses some examples of developing novel drugs (tyrosine kinase inhibitors) and their effectiveness in AML (FLT3).
In Section III, Dr. Martin Tallman describes the evaluation and management of patients with acute promyelocytic leukemia, a notable example of therapeutic progress in a molecularly defined entity of leukemia. Dr. Tallman focuses on the molecular genetics of APL, current curative treatment strategies and approaches for patients with relapsed and refractory disease. In addition, areas of controversy regarding treatment are addressed.
I. Current Issues in Treating AML
Bob Löwenberg, MD, PhD*
University Hospital, Erasmus University Medical Center, Department of Hematology, PO Box 2040, Rotterdam 3000 CA, The Netherlands
The term acute myeloid leukemia (AML) collectively refers to a mixture of distinct diseases that differ with regard to their pathogenetic evolution, genetic abnormalities, clinical features, response to therapy, and prognosis. Cytogenetic and molecular analyses have been instrumental in identifying disease entities among the mixed bag of AML types. They are also guiding the way to targeted treatment interventions. Treatment of AML begins with establishing a precise diagnosis. The treatment usually involves a remission induction phase aimed at establishing a complete remission and a postinduction phase aimed at eradicating “occult” residual disease.
Remission Induction Therapy
Since the introduction of the anthracyclines (daunorubicin, idarubicin) and cytarabine, these therapeutic agents have been the cornerstones of remission induction therapy for adult AML.1 With some variations, most centers apply treatment schedules based on these drugs, sometimes supplemented with etoposide. Instead of anthracyclins, remission induction programs may incorporate mitoxantrone and amsacrine. These combinations induce complete remissions (CR) in an average of 70% to 80% of adults aged less than 60 years. Continuous efforts are being made to improve the efficacy of remission induction treatment. Improved induction therapy could yield more CRs or CRs of longer duration. The overexpression of a membrane protein designated P-glycoprotein (P-gp) is a typical phenotypic marker of pleiotropic drug resistance. P-gp belongs to a group of phosphorylated glycoproteins that function in the cell membrane as efflux pumps. In patients, primary or acquired resistance to chemotherapy is associated with the expression of P-gp. Efforts to overcome chemotherapy resistance by including multidrug resistance modifiers (e.g., cyclosporin or its analogue PSC 833) in the induction schedule have as yet not met with reproducible success in prospective comparative studies.2– 4 Due to the impact of the modulators on chemotherapy pharmacokinetics and the increased toxicity associated with their use, the dosages of chemotherapeutic drugs in the experimental groups had to be reduced. The dose reductions and the enhanced early toxicity may have jeopardized any potential benefit. P-gp modulators without pharmacokinetic side effects are currently in clinical development.
Induction with Hematopoietic Growth Factor Priming
AML is a prototype malignancy expressing functional hematopoietic growth factor receptors on their cellular surface.5 Growth factor receptors offer potential targets for therapeutic intervention. Coculture of AML cells with the cell cycle dependent chemotherapeutic agent cytarabine and granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) increases intracellular levels of the active metabolite cytosine arabinoside-triphosphate, elevates incorporation of cytarabine into cellular DNA and enhances cytarabine cytotoxicity against leukemic blasts and leukemic progenitor cells. Until recently, the therapeutic concept of sensitizing AML to chemotherapy with G-CSF or GM-CSF, a phenomenon frequently referred to as growth factor priming, has been examined mainly in uncontrolled and few small size randomized studies only. In two larger randomized studies in which GM-CSF was applied for priming during the days of chemotherapy, it was also administered concomitantly with the chemotherapy.6,7 The latter two studies were performed in patients of older age, thus representing mainly patients with AML of unfavorable prognosis. In one of these studies, in 240 patients of 55+ years of age, GM-CSF conferred a better disease-free survival,7 but a benefit from GM-CSF was not apparent in the first study.6 A recent, large randomized study (enrolling 640 patients) selectively focused on the G-CSF priming question with no G-CSF being administered postchemotherapy.8 The study was conducted in young and middle aged adults with previously untreated AML, representative of a broader prognostic diversity. G-CSF was only applied from day −1 of chemotherapy through the last day of chemotherapy of both induction cycles I and II. In addition, in this study the anthracyclin was scheduled at the end of the cycle to avoid interference with G-CSF induced cell cycle dependent cytarabine cytotoxicity. G-CSF was temporarily withheld to avoid problems of leukostasis, in case the white blood cells would exceed a value of 30 × 106/mm3. Among patients in the study attaining CR, the probability of relapse was reduced when they had been assigned to treatment with G-CSF along with induction chemotherapy. This difference translated into a 9% disease-free survival (DFS) benefit at 4 years for G-CSF primed patients. The benefit of chemotherapy-sensitization by G-CSF was particularly evident among the intermediate-risk (for definition see below) subset of patients (72% of cases) as evidenced by improvements of overall survival, disease-free as well as event-free survival.8 These observations have revitalized the interest in CSF priming as a strategy of enhancing killing of subpopulations of leukemic cells relatively insensitive to chemotherapy and reducing risk of relapse.
Response Evaluation After Induction Therapy
Attaining a CR is a prerequisite for long-term disease-free and overall survival. Therefore the assessment of CR is an important step in the management of patients with AML. CR has traditionally been defined as a cellular marrow with less than 5% of blasts, no circulating blasts, no evidence of extramedullary leukemia, and recovery of granulocyte (PMN 1.5 × 109/L) and platelet (100 × 109/L) counts. Since the NCI criteria for CR were published in 1990, treatment, however, has changed quite considerably, and this has challenged the current validity of the definition of CR. Chemotherapy has become more dose intensive. This directly impacts on the cellularity of the marrow following chemotherapy and the ability for prompt hematological recovery. Furthermore, the next cycle of treatment often follows before full hematological recovery. In a recent analysis (still unpublished) in 1250 patients treated with contemporary strategies in 3 successive AML study protocols from HOVON (Dutch-Belgian Cooperative Hemato-Oncology) and SAKK (Swiss Cancer) Cooperative Groups, the prognostic impact of each of the definition elements of hematological CR following cycle I was assessed. The analysis confirms that % marrow blasts and extramedullary leukemia after induction therapy are powerful predictive hematological determinants of outcome (relapse, disease-free survival). It also reveals that the cutoff value of 5% is still valid. Patients with 6%, 7%, or 6%–10% blasts after cycle I have a significantly greater risk of relapse. When the two conditions of less than 5% marrow blasts and absent extramedullary leukemia are fulfilled, the marrow cellularity does not add impact on prognosis. Patients in the HOVON/SAKK database with no or slow platelet recovery, however, have inferior survival and increased relapse rates, and those with no granulocyte recovery show a similar trend. Thus, of the traditional CR parameters, % marrow blasts, absence of extramedullary leukemia and hematological recovery continue to stand out as predictors of outcome. In clinical management it has become quite common to conduct an “early” bone marrow assessment (at approximately days 7–10 after the first cycle) to identify those with refractory disease at an early point posttherapy. Recently, a group of experts have revisited the definition of CR and updated the scoring methodology for CR.38 For reasons of international intercomparability between studies and standardization, they recommend to define CR in operational terms and distinguish morphological CR, CR with incomplete blood count recovery (CRi), cytogenetic CR (CRc), and molecular CR (CRm). Immunological approaches based on multiple parameter flow cytometry and quantitative (real-time) reverse transcriptase polymerase chain reactions for fusion genes in t(8;21) and inv(16) show promise as regards further refinement of the assessment of CR.
Postremission Therapy
During the past 20 years there has been a shift from low-dose maintenance chemotherapy administered for prolonged times (1–2 years) toward intensified cycles of chemotherapy delivered within a concentrated time (4–6 months). These dose-escalated and time-condensed cycles are given once a CR is induced and serve the objective of eradicating minimal residual leukemia. Most commonly, these regimens have been based on intensive additional cycles of chemotherapy (e.g., high-dose cytarabine)1 or on high-dose cytotoxic therapy followed by autologous or allogeneic hematopoietic stem cell transplantation. Survival rates in large Phase III studies in patients 60 years of age or younger range between 30%–40% at 4 years.
Postremission Therapy: Autologous Stem Cell Transplantation
Whether autologous stem cell transplantation (autoSCT) following high-dose cytotoxic therapy is better than intensive chemotherapy has been an issue of ongoing investigation during the past decade. In 2 comparative trials9,10 but not in 2 others,11,12 disease-free survival was improved after autoSCT due to a reduction in the probability of relapse. In none of these studies a significant advantage in overall survival has been apparent. The lack of a survival advantage over chemotherapy was in part caused by the fact that a proportion of patients relapsing after chemotherapy could still be salvaged by an autograft in second remission (CR2). Also, the somewhat greater procedure-related mortality following autoSCT has offset part of the advantage of the reduced relapse rate after autoSCT. Importantly, only a minority of complete responders (approximately 30%–40% effectively) proceed to autoSCT, which dilutes any possible therapeutic advantage in an intent-to-treat analysis of autoSCT. Premature withdrawal from autografting has mainly been caused by early relapse of leukemia, or the harvest of an insufficient hematopoietic cell graft. The earlier scheduling of autoSCT (e.g., after 2 cycles of induction therapy) might reduce this problem. The introduction of peripheral blood stem cell grafts permits faster hematopoietic recovery. The question of whether autoSCT might benefit particular prognostic subgroups, has not definitely been settled. Prospective studies do not provide direct support for a beneficial effect of autoSCT on disease-free or overall survival in any of the distinct AML risk categories.10,12 However, these prognostic subgroup analyses are based on relatively small numbers and have limited statistical power.
Postremission Therapy: Allogeneic Stem Cell Transplantation
Prospective studies involving allogeneic stem cell transplantation (alloSCT) are not based on true randomizations since the entry is determined by the availability of a matched donor and transplantation-specific eligibility criteria (age). Comparative analyses in AML-CR1 consistently show markedly reduced relapse frequencies following alloSCT.9,11–,17 Thus, alloSCT following myeloablative cytotoxic therapy currently is considered the most powerful antileukemic treatment modality for adults with AML in remission. However, alloSCT is associated with enhanced transplant related mortality,15,16 which significantly offsets the relapse advantages of patients assigned to alloSCT. Therefore, the gain from reduced risk of relapse after alloSCT is largely sacrificed to excess procedure-related mortality. The latter effect is dependent on age.15,17 Improved disease-free survival after alloSCT has been apparent in certain studies9,15,16 but not in others.11,12 This implies the challenge to develop alloSCT toward a less toxic treatment strategy.
What strategy should be pursued in clinical practice? In patients with good-risk AML (e.g., based on cytogenetics) with an a priori risk of relapse of 25% or less, it makes no sense to apply alloSCT in first CR considering the procedure-related death rate of alloSCT which, on average, is in the order of 10% to 25%. Also, patients with good-risk AML have a greater chance of rescue with alloSCT in case of relapse. Indeed, patients with favorable risk AML show no better disease-free survival nor overall survival after alloSCT in donor versus no donor comparisons. In intermediate-risk AML (relapse probabilities of 40%–50%), and poor-risk AML with comparatively high relapse rates (70%–80%), the value of the greater antileukemic efficacy of allografting may outweigh the risk of greater transplant-related toxicity and mortality,14–,16 although data on this are still controversial.12,17 The benefit of alloSCT appears more prominent in the adult younger age category due to decreased treatment-related deaths.15,16 Since patients with high-risk AML are frequently withdrawn from actual transplantation because of early relapse, most centers now deliberately plan alloSCT at an earlier phase in the treatment plan.
HLA-matched unrelated donor (MUD) transplants are increasingly employed when a genotypically HLA-matched donor is not available. With improved molecular donor matching the results have been promising. Although such transplants are mainly applied to restricted categories of high-risk cases (poor-risk AML in CR1, or AML in CR2 or CR3 or in early relapse) their value remains to be critically assessed in prospective series of patients. Reduced-intensity alloSCT protocols are now actively being investigated as efforts of reducing treatment-related mortality. While donor chimerism can be established and graft-versus-leukemia effects can be exploited, the overall value of these strategies as regards treatment outcome is not yet clear.18– 20
Treatment of Older Patients
The majority of patients with AML are 60 years of age or older. While results of treatment have improved steadily in younger adults over the past 20 years, there have been limited changes in outcome among individuals of 60+ years of age. When treated with chemotherapy alone, this age group has an estimated 2-year survival of approximately 20% and 10% at 4 to 5 years.1 The reasons for the unsatisfactory outcome in the elderly likely relate to the increased frequency of unfavorable cytogenetics among older patients with AML, a greater frequency of antecedent myelodysplasia, as well as their limited abilities to tolerate intensive chemotherapy. High-dose chemotherapy is not beneficial to the elderly with AML.
There has been an intense interest in the introduction of new modalities. Examples of these strategies are the use of antibody directed treatment (e.g., the use of the antiCD33-calicheamycin toxin conjugate, Mylotarg), and the development of molecular targeting (e.g., farnesyl transferase inhibitors, kinase inhibitors). Particularly interesting is the development of alloSCT following chemotherapy with nonmyeloablative preparative regimens in older individuals not able of to tolerate high-dose cytotoxic treatment. The goal of these approaches is to establish allogeneic chimerism following immunosuppressive therapy and then exploit the graft-versus-leukemia effects of the allografts, so that donor chimerism can also be used as a platform for subsequent infusions of donor lymphocytes. Early clinical trials afford proof of principle of this approach. In older patients, donor chimerism can be established. For the time being these studies are based on small patient numbers and have limited follow-up.18– 20
What Has (Cyto)Genetics to Offer to the Management of AML?
Cytogenetic classifications of AML employed with some variation by different groups, roughly distinguish 3 risk groups: first, a group with favorable outcome (probability of relapse of 25% or less and a 4-year survival of 70% or more); second, an intermediate prognostic group with a risk probability of relapse of 50% and an overall survival at 4 years of 40% to 50%; and third, an adverse prognostic category characterized by a high relapse rate (more than 70%) and an overall survival rate at 4 years of 20% or less. Age expresses independent prognostic value. The above-mentioned values of outcome may thus vary for different age categories. In order to refine the prognostic predictive value of these classifications, additional parameters have been introduced into these models. One common prognostic parameter has been the rapidity of attaining a CR. Patients achieving a CR following the first induction cycle of chemotherapy (early CR) have a significantly better outcome than those with a CR attained after induction cycle II (late CR).21 High white blood cell counts, when considered in combination with favorable cytogenetics, recognize an unfavorable subset among good-risk AML.22,23 More recently, various new molecular markers have been identified that allow for dissecting these heterogeneous risk categories. For instance, FLT3 internal tandem duplications (FLT3-ITDs) have been recognized as the single most common genetic abnormality in AML. FLT3-ITDs represent activating mutations of the FMS-like tyrosine kinase 3 (FLT3), a hematopoietic receptor. AML with FLT3-ITDs is seen in 15%–30% of pediatric and adult patients. FLT3-ITDs are associated with a significantly greater risk of relapse and reduced survival,24–,27 although some studies with large numbers of patients could not (yet) unquestionably reproduce the prognostic value of FLT3-ITDs for survival.28,29 It has been suggested that a high mutant/wild type FLT3 ratio enhances the predictive power of FLT3 mutations for survival as well. Interestingly, FLT3 mutations are mainly seen in the largest AML category of intermediate cytogenetic risk. Hence, detection of FLT3-ITDs offers an important addition to recognize a new subset of poor-risk AML. Another recurrent Asp835 point mutation of the FLT3 receptor, seen in approximately 5% to 10% of de novo AML, has not (or not yet) been correlated with prognosis. Mutations of the tumor suppressor gene p53 predict for negative outcome30 (Table 1 ). In addition, high BCL2 and WT1 expression have been suggested to define AML with poor risk.31 EVI-1 (ecotropic virus integration site 1) is an oncogene overexpressed in AML with translocations of 3q26 and characterizes a notoriously poor risk AML. Recently it was shown that EVI-1 mRNA overexpression in AML in the absence of 3q26 cytogenetic abnormalities also predicts for notably bad prognosis.32 Thus EVI-1 generally defines an intracellular pathway of poor therapy response in approximately 10% of cases. Similarly, partial tandem duplications of a portion of the MLL (mixed leukemia) gene define an unfavorable subset among AML with intermediate risk cytogenetics.33 Each of these molecularly defined groups is of relatively small size, consistent with the considerable genetic heterogeneity of AML. High expression of a gene designated BAALC (Brain and Acute Leukemia, Cytoplasmic), which is normally expressed on neuro-ectoderm-derived tissues and hematopoietic progenitors, has recently been suggested in a study of limited size (86 cases) to predict for poor survival among patients with AML with normal cytogenetics.37
C/EBP-α (CCAAT enhancer-binding protein alpha) is a transcription factor that has a key role in myelopoiesis. C/EBP-α mutations have been found in patients with AML in a few percent of cases. The latter mutations define AML with relatively good-risk leukemia.34,35 Point mutations of the hematopoietic receptor c-kit are seen in 30% of patients with abn(16) AML and t(8;21) AML. AMLs with abn(16) and t(8;21) represent leukemias of favorable prognosis. The presence of c-kit mutations among this subgroup defines those with an enhanced risk of recurrence.36 With the introduction of high-throughput analysis for molecular abnormalities and gene expression profiling, it will become possible to uncover new classes of AML (e.g., reference39). These distinctions are foreseen to provide useful guides in the management of patients with AML. The recognition of AML subsets with distinct pathogenetic origin may also furnish insights into the intracellular pathways causing unresponsiveness to traditional chemotherapy, and offer keys toward novel therapeutic drug development.
II. Tyrosine Kinases as Therapeutic Targets in AML
James D. Griffin, MD*
Dana-Farber Cancer Institute, 44 Binney Street, Boston MA 02115
More than 200 different chromosome translocations and other mutational events have been described in AML cells, and one of the long-term goals of leukemia investigators is to develop therapies that target these oncogenes. The best example of oncogene-targeted therapy in AML currently is all-trans retinoic acid (ATRA), which can specifically inhibit the transforming activities of the PML-RARα oncogene in acute promyelocytic leukemia. Whereas other AML oncogenes that involve transcription factor mutations have been difficult to target with small molecule drugs, many are currently being considered as attractive targets for immunotherapeutic approaches.
Kinases in general, and tyrosine kinases in particular, are attractive as drug targets for a number of reasons. Most significantly, the impressive clinical effects of imatinib in chronic myeloid leukemia provide a proof of principle that inhibition of a kinase target is possible in humans with acceptable toxicity, and can be associated with rapid and dramatic tumor responses.1,2 There are a growing number of tyrosine kinases now known to be activated by mutation in blast cells from patients with AML, and here we will review the current state of knowledge in this field, and provide an update on clinical development of kinase inhibitors in AML.
A “Two-Hit” Model of Oncogenes in AML
In contrast to chronic myelogeous leukemia (CML), there is abundant evidence that mutations in 2 or more genes are necessary to cause AML. First, many of the major fusion oncogenes resulting from balanced translocations, such as PML-RARα or AML1-ETO, are not sufficient by themselves to induce acute leukemia in murine models.3 Also, small numbers of cells bearing leukemia translocations can sometimes be detected at birth in children that do not develop overt leukemia for many years.
Many of the common fusion oncogenes involve genes encoding transcription factors and appear to function by inhibiting differentiation of myeloid cells, often by interfering with the function of transcription factors such as the core binding factor complex that are necessary for normal myeloid differentiation. In contrast, there is another class of AML oncogene that has less ability to inhibit differentiation, but rather induces cell cycle deregulation, proliferation, and/or inhibition of apoptosis. Mutated tyrosine kinases such as FLT3 or KIT, and activated alleles of N-RAS or K-RAS are typical oncogenes of this type. These two classes of oncogenes, sometimes referred to as Class II (that block differentiation) and Class I (that induce proliferation), respectively, cooperate to cause acute leukemia in numerous animal models.3 While this model is likely an oversimplification, it is testable and has a number of therapeutic implications. The model predicts that AML requires 1 oncogene of each class, and suggests the possibility that targeted inhibition of either type of oncogene would have potential therapeutic activity, although simultaneous inhibition of multiple oncogenes in the same cell would likely be of significant further advantage.
Tyrosine Kinase Oncogenes in AML
Tyrosine kinases are enzymes that phosphorylate proteins on tyrosine residues and typically function in signal transduction cascades. The enzyme class is divided into 2 large subfamilies, receptor and nonreceptor tyrosine kinases.4 Receptor tyrosine kinases cross the cell membrane, with an external ligand-binding domain and an internal kinase domain. Binding of ligand induces dimerization and conformational changes that activate the kinase, resulting in receptor autophosphorylation, attraction of signaling intermediates, and initiation of signal transduction. These signals mediate a variety of cellular activities including differentiation, growth, and cell death. Nonreceptor tyrosine kinases are found in both the cytoplasm and the nucleus and play many roles in the cell, including regulation of growth, adhesion, response to stress, and many other functions. Activating or gain-of-function mutations in tyrosine kinases have been identified in many acute and chronic leukemias.5 These mutations may result in continuous and ligand-independent proliferation and viability signals. Selective inhibition of these mutated tyrosine kinases by small molecule inhibitors represents a strategy to disrupt signaling pathways that promote neoplastic growth and survival. This review will focus on those tyrosine kinases that are known to have gain of function mutations in AML, and are therefore candidates for the use of small molecule tyrosine kinase inhibitors. Previous excellent reviews give more detail about the signaling pathways of these enzymes and their roles in normal hematopoiesis and malignancy.5,6
Activation of Tyrosine Kinases by Balanced Chromosome Translocations
Although relatively uncommon, there are a number of examples of tyrosine kinase activation due to fusion with another gene, typically TEL on chromosome 12, by a chromosome translocation. The first example was TEL-PDGFRβ (platelet-derived growth factor receptor beta), identified in 1994 by Golub and Gilliland as the product of a t(5;12) in CMML.7 Since then, TEL-JAK2, TEL-ABL, TEL-ARG, and others have been described.8 In each case, it seems likely that the fusion partner acts to oligomerize and/or relocate the kinase, resulting in constitutive activity. However, much like BCR/ABL, it is quite possible that the fusion partner also has signaling activities of its own. Since PDGFRβ ABL and ARG are sensitive to inhibition by imatinib, it is important to identify these fusion oncogenes when present. These kinases have been the subject of recent reviews.9
Class III Receptor Tyrosine Kinases
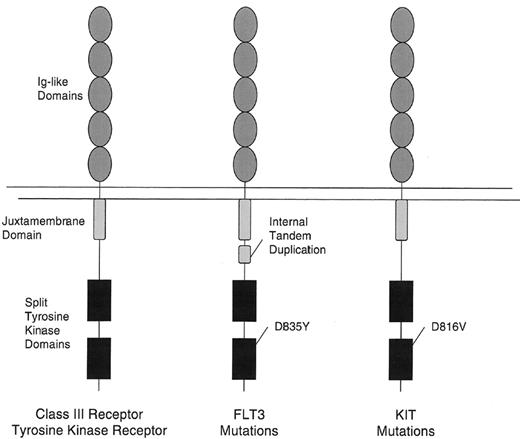
The majority of known mutations in tyrosine kinases in AML are members of the Class III receptor tyrosine kinase family, which includes KIT, PDGFR, FLT3, and FMS.10 These receptors are characterized by an extracellular domain comprised of 5 immunoglobulin-like (Ig-like) domains and by a cytoplasmic domain with a split tyrosine kinase motif (Figure 1 ). Here we will focus on the 2 most commonly mutated receptors, KIT and FLT3.
C-KIT Tyrosine Kinase
C-KIT is expressed on hematopoietic progenitor cells, mast cells, germ cells, and the pacemaker cells of the gut.11 Kit is essential for the normal development of hematopoiesis, particularly the erythroid system. c-KIT has been found to be mutated and constitutively activated in some cases of gastrointestinal stromal cell tumor (GIST), mastocytosis/mast cell leukemia and acute myelogenous leukemia.11 Activating mutations can occur in many different exons of the c-KIT gene. Ultimately, these mutations result in ligand independent autophosphorylation and activation of downstream signaling pathways.
c-KIT Mutation in Mastocytosis, Mast Cell Leukemia, and AML
Valine substituted for aspartic acid at codon 816 (D816V mutation) in the activation loop of the kinase catalytic domain is the most common activating mutation in c-KIT, and is commonly detected in systemic mastocytosis or mast cell leukemia, and less commonly in myeloproliferative disorders and AML.5,11,12 This mutation results in a 10-fold increase in the kinase activity. Although imatinib inhibits wild type KIT, the D816V mutation is resistant to imatinib, presumably because this mutation alters the configuration of the kinase pocket, causing it to no longer bind the drug.13
The small molecules SU5416 and SU6668 inhibit c-kit, as well as VEGFR-2 (KDR), FGFR, FLT-3, and PDGFR.14,15 SU5416 was tested in the treatment of patients with refractory, c-KIT positive AML.16 Only 1 patient of 38 had a complete response, 7 patients had a partial response (PR) (defined as reduction of blasts in blood and/or bone marrow by at least 50%) which lasted 1–5 months. It is unclear how many had activating mutations of c-kit.
FLT3
FLT3 (Fms-like tyrosine kinase-3), also known as FLK-2 (fetal liver kinase-2) and STK-1 (human stem cell kinase-1) was cloned independently by 2 groups in 1991.17,18FLT3 has strong sequence similarities with other members of the class III receptor tyrosine kinase (RTKIII) receptor family, and is expressed in immature hematopoietic cells, placenta, gonads, and brain.19 In normal bone marrow, expression appears to be restricted to early progenitors. FLT3 is also expressed at high levels in a spectrum of hematologic malignancies including 70%–100% of AML of all FAB subtypes, B-precursor cell acute lymphoblastic leukemias, a fraction of T cell ALL, and CML in lymphoid blast crisis. Targeted disruption of FLT3 results in healthy adult mice with normal mature hematopoietic populations.20 However, there are deficiencies in primitive B lymphoid progenitors, and bone marrow transplantation experiments show a reduced ability of stem cells lacking FLT3 to reconstitute both T cells and myeloid cells. FLT3 ligand stimulates the proliferation of FLT3 expressing primary AML cells.
Mutations of FLT3 in Human Leukemias
Nakao and colleagues first reported the presence of internal tandem duplications in the juxtamembrane domain of FLT3 in AML in 1996.21 They noted that in 17% (5/30) of patients with AML, there were length polymorphisms in the juxtamembrane (JM) domain. Sequence analysis of genomic DNA demonstrated that each of the 5 patients harbored in-frame internal tandem duplication mutations in the JM domain. These observations have been subsequently confirmed by many groups.22,23 FLT3-ITDs have also been detected at lower frequency in MDS, and are rarely detected in acute lymphoblastic leukemia except in infants with mixed lineage leukemia, MLL. FLT3-ITDs have been detected in all FAB subtypes of AML, with the highest reported frequency in the M3 subtype, and less frequently in the M2 subtype. In addition to length mutations in one allele of FLT3, several studies have demonstrated bialleleic mutations in FLT3 (both FLT3 genes having undergone mutations), as well as patients in whom the residual wild-type allele is lost.24
Mutations at or near asparagines 835 in the so-called activation loop of FLT3 have also been reported with a frequency of about 7%.25 The activation loop is a general component of tyrosine kinases and when the kinase is in the “inactive” state, it functions to block access of adenosine triphosphate (ATP) and substrate to the kinase domain. D835 in FLT3 is analogous to D816 in KIT. D825Y has been the most common substitution, but other substitutions included D835Vm D835H, D835E and D835N. Taken together these data indicate that approximately 30%–35% of AML patients have acquired mutations in FLT3.
Biological Activity of FLT3-ITD and Activating Loop Mutations
The available evidence indicates that either length mutations in the juxtamembrane domain or activating loop mutations result in constitutive activation of the FLT3 kinase, and activation of growth-related signaling pathways. Retroviral transduction of FLT3-ITD or activating loop mutations into primary murine bone marrow cells results in a myeloproliferative phenotype in a bone marrow transplant (BMT) assay.26 FLT3-ITDs cooperate with oncogenes such as PML-RARα to induce acute leukemia in mice.27
Prognostic Significance of FLT3 Mutations in Leukemia
The majority of retrospective data indicate that FLT3 mutations are an independent variable that confers a poor prognosis in AML, in particular due to an enhanced rate of relapse (Section I and Gilliland and Griffin28).
FLT3 Inhibitors
There has been great interest in developing FLT3 inhibitors because of the high frequency and poor prognosis of AML patients with mutant FLT3. At least 4 compounds are currently under development. CEP-701 (Cephalon, Inc, West Chester, PA), is a novel indolocarbazole derivative that inhibits the autophosphorylation of wild-type and constitutively activated FLT3 in vitro with an half-maximal inhibitory concentration (IC50) of 2–3 nM.29 A Phase II clinical trial using single agent CEP-701 in the treatment of patients with refractory or relapsed AML expressing FLT3 activating mutations is ongoing.30 Preliminary results of 5 patients treated initially at a dose of 60 mg by mouth twice a day have been reported, indicating that 1 patient has had a complete remission. Side effects include nausea, emesis, and fatigue (grade 1 and 2).30
CT53518 (MLN518) is a piperazinyl quanzoline with activity against PDGFR, c-kit, and FLT3.31 In BA/F3 cell lines expressing FLT3-ITD mutants, CT53518 inhibited interleukin (IL)-3-independent cell growth and FLT3-ITD autophosphorylation with an IC50 of 10–100 nM. In a murine bone marrow transplant model of FLT3-ITD-induced myeloproliferative disease, CT53518 treatment resulted in prolonged survival compared to controls. This drug is currently in a Phase I clinical trial in patients with relapsed or refractory AML or high-risk myelodysplasia, who may or may not demonstrate a FLT3-ITD.
PKC412 (Novartis Pharmaceuticals, Basel, Switzerland), a benzolystaurosporine originally developed as a vascular endothelial growth factor receptor (VEGFR) and protein kinase C (PKC) inhibitor, has been shown to be a potent inhibitor of FLT3-ITD in cell lines and prolongs survival in a murine bone marrow transplant model of FLT3-ITD-induced myeloproliferative disease.32 A Phase I trial of PKC412 in patients with advanced solid malignancies showed it to be a well-tolerated oral therapy.33 The most frequent treatment-related toxicities were nausea, vomiting, fatigue, and diarrhea.34 A Phase II trial of PKC412 at 75 mg by mouth 3 times a day was undertaken in patients with AML that expressed either a FLT3-ITD or an activation loop mutation. In the first 14 patients treated, all but 1 patient had at least a transient reduction in the number of peripheral or bone marrow blasts.34 SU5416, SU5614, and SU11248 (Sugen, San Francisco, CA) also reportedly have FLT3 inhibitor activity.35,36 A Phase I study of SU11248 has been completed in patients with AML.37 Five patients had FLT3 gene mutations (3 ITD and 2 activating loop mutations), demonstrating a decrease in peripheral blast counts in some patients following a single dose of SU11248.
Promise and Challenges for Tyrosine Kinase Inhibition in AML
FLT3 and KIT are promising molecular targets for therapy of AML. However, enthusiasm should be tempered by several considerations. First, as noted above, other mutations will always be present in AML, and it will likely be necessary to combine kinase inhibitors with other drugs to get substantial clinical benefit.19 None of the inhibitors currently under investigation is truly specific for FLT3. Although these agents are selective, they also target other kinases including PDGFRβ, c-FMS, SYK, c-KIT, VEGFR, and PKC to name a few. The toxicities of these drugs will need to be carefully evaluated. However, as has been suggested for STI571, the additional targets may in some cases prove beneficial. Thus, while a number of different inhibitors may be found to effectively inhibit FLT3 in vivo, therapeutic efficacy may vary considerably depending on the other targets. Unfortunately, we can almost certainly expect resistance to develop to FLT3 inhibitors, as has been observed with STI571 therapy of CML blast crisis.
In summary, FLT3 and KIT are important new molecular targets for therapy of AML. There is promise that these new therapies will improve outcome without increasing toxicity in treatment of AML.
III. Curative Strategies in Acute Promyelocytic Leukemia
Martin S. Tallman, MD*
Northwestern University Feinberg School of Medicine, Division of Hematology-Oncology, 676 N St. Clair St, Suite 850, Chicago IL 60611
Acute promyelocytic leukemia (APL) deserves special attention among the subtypes of AML for several important reasons. First, the disease has become the most curable of all of the subtypes of AML. With current therapy, including ATRA and anthracycline-based induction, anthracycline-based consolidation and maintenance, 70%–80% of patients are alive and free of disease at 5 years. Second, the disease is associated with unique genetic features including the t(15;17) translocation and the formation of the PML-RARα fusion transcript. The fusion transcript permits precise diagnosis and provides the marker for the identification of minimal residual or recurrent disease. Third, insights into the mechanism of leukemogenesis and resistance in APL serve as a paradigm for other AMLs. Fourth, treatment with ATRA-based regimens demonstrates that the novel strategy of differentiation therapy can be highly effective. Finally, the curability of APL reflects what can be accomplished from the union of progress in both laboratory science and well-designed clinical trials.
Disease Description
Epidemiology
Acute promyelocytic leukemia represents approximately 10%–15% of AMLs in adults. The median age is approximately 40 years, which is considerably younger than the other subtypes of AML (70 years). There appears to be an increased incidence among Hispanic patients (20%–30%).1 Finally, there is no apparent increase in incidence with age, unlike other subtypes of AML.2
Molecular genetics and pathogenesis
The leukemic cells from virtually every patient with APL have a balanced reciprocal translocation, t(15;17). This translocation leads to a fusion of two otherwise disparate genes, the promyelocytic (PML) gene on chromosome 15 and the retinoic acid receptor-α (RARα) on chromosome 17. The presence of the PML-RARα fusion protein inhibits myeloid differentiation. Rare patients have variant translocations. Although by morphology these cases may be difficult to distinguish from classic APL, they represent alternative fusion partners with RARα including promyelocytic leukemia zinc finger gene (PLZF), nucleophosmin (NMP), nuclear mitotic apparatus (NUMA), and STAT5b. In general, other than some cases involving the PLZF gene, the leukemic promyelocytes from patients with these variants are not sensitive to the differentiating effects of ATRA.
Recent laboratory investigations have provided important insights into the molecular basis of leukemogenesis in APL. As a result of the fusion of the RARα to the PML gene, there is increased affinity for the nuclear repressor protein complex. The formation of this protein complex attracts histone deacetylase which alters chromatin confirmation and therefore inhibits transcription.3 The presence of retinoic acid functions in part, at least, by inducing release of the nuclear corepressor complex with histone deacetylase. This leads to normal chromatin confirmation and normal transcription (Figure 2 ). In patients with the variant t(11;17) involving PLZF, resistance is conferred by the inability of retinoic acid to release histone deacetylase. Histone deacetylase inhibitors may be able to release histone deacetylase and restore sensitivity to retinoic acid.4
Induction Therapy
The choice of anthracycline
Until the late 1980s, induction therapy for patients with APL was similar to that of patients with other subtypes of AML and included an anthracycline and cytarabine. However, the leukemic cells from patients with APL are particularly sensitive to anthracyclines, perhaps because of significantly lower P-gp expression and other resistance markers in APL cells compared to other subtypes of AML.5 Both daunorubicin and idarubicin as single agents induce CR in 60%–80% of patients.6,7
The role of cytarabine
Two retrospective comparisons show no difference in the CR rate between patients treated with daunorubicin alone or daunorubicin with cytarabine.8,9 In addition, in a prospective randomized trial comparing idarubicin plus cytarabine to idarubicin alone in the pre-ATRA era, no difference in the CR rate was observed.10 The CR rate was 76.3% with the single agent alone compared with 66.6% in the combination arm. The event-free survival (EFS) was 35% in the idarubicin arm compared to 35% in the combination arm (P = .0429). However, the dose of idarubicin in the single agent arm was 72 mg/m2 compared to 40 mg/m2 in the combination arm. In a retrospective analysis, the Southwest Oncology Group showed excellent survival in patients with APL when a daunorubicin dose of 70 mg/m2/day was used compared to the dose used in most studies of 45–50 mg/m2/day, but without a change in cytarabine dose.11 In the PETHEMA Trial, patients receive ATRA with idarubicin alone and have an excellent CR rate.12
Combining ATRA with Chemotherapy for Induction: The Current Standard Approach
The introduction of ATRA prompted several study groups to first, compare ATRA to chemotherapy for induction and then, to study a concomitant versus sequential approach. The European APL group compared ATRA to daunorubicin plus cytarabine, with a provision for the ATRA-treated patients to introduce chemotherapy early for a rapidly rising white blood cell count (WBC)13 (Table 2 ). The North American Intergroup Trial also compared ATRA to daunorubicin plus cytarabine, but with no provision for early chemotherapy except hydroxyurea. In both studies, the CR rates and early death rates were not statistically different, but the early (2- to 3-year) disease-free survival/event-free survival (DFS/EFS) was better for the ATRA-treated patients.14 The second European APL Group trial compared concomitant ATRA plus chemotherapy versus a sequential approach.15 The CR rates and early death rates did not differ, but patients receiving concomitant therapy had an improved EFS. The Medical Research Council (MRC) in the United Kingdom explored a sequential approach with a limited exposure to ATRA (5 days) versus concomitant ATRA and chemotherapy until CR.16 The DFS was superior for the patients receiving the long duration of ATRA. Combining ATRA and chemotherapy for induction has the additional benefit of possibly reducing the incidence of the retinoic acid syndrome (RAS) from 25%,17,18 with ATRA to 10% with concurrent chemotherapy and ATRA.12,19 Hemorrhage remains a major cause of induction mortality. It appears reasonable to begin treatment first with ATRA for 2–4 days to ameliorate the coagulopathy prior to initiating chemotherapy, provided the WBC is not high (< 10,000/μL). If the WBC is high, initial therapy with both ATRA and chemotherapy is appropriate.
Postremission Therapy
Consolidation chemotherapy
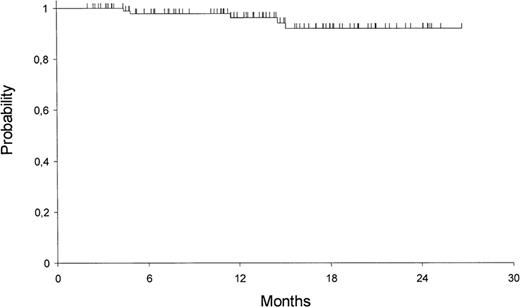
It is mandatory to administer consolidation chemotherapy after CR because initial studies showed that most patients relapsed after ATRA alone. In most studies, consolidation chemotherapy has been anthracycline-based. Three trials have included high-dose (1–3 g/m2) cytarabine in consolidation.14,16,20 The North American Intergroup study administered 1 cycle of daunorubicin 45 mg/m2 per day for 3 days and standard-dose cytarabine 100 mg/m2 per day for 7 days as a first consolidation course followed by high-dose cytarabine 2 g/m2 twice daily for 4 days with 2 days of daunorubicin 45 mg/m2 d for 2 days.14 Patients in the MRC trial younger than 60 years received consolidation with cytarabine 1 g/m2 twice daily on days 1–3.16 The German AML Cooperative Group administered intensified double induction therapy including high-dose cytarabine with ATRA to newly diagnosed patients.20 Patients in CR received standard-dose cytarabine, daunorubicin, and 6-thioguanine consolidation and 3 years of monthly maintenance. The European APL group and JALSG studies included standard-dose cytarabine as consolidation.15,21 However, just as there appears to be little role for cytarabine during induction, emerging data suggest that there is no role for high-dose cytarabine in consolidation. A recent prospective nonrandomized study published by the Spanish Cooperative Group PETHEMA, suggests that patients do as well without cytarabine in either induction or consolidation12 (Figure 3 ). It has become routine to administer at least 2 courses of postremission therapy, usually daunorubicin or idarubicin, following induction with ATRA and an anthracycline, with or without cytarabine, although the best number of cycles is not known. The most important goal of postremission therapy is complete eradication of the leukemic clone as determined by the conversion to a polymerase chain reaction (PCR)-negative status, since persistence of such minimal residual disease (MRD) predicts relapse.16,22
The role of maintenance therapy
Prior to the introduction of ATRA, several studies suggested a role for maintenance chemotherapy in patients with APL.8,23,24 Two large prospective randomized trials now suggest that maintenance therapy with ATRA is useful14,15 (Table 3 ). In the North American Intergroup study, patients in CR after 2 courses of consolidation chemotherapy were randomized to either a year of daily maintenance ATRA at standard doses or observation.14 This study showed a significant benefit when a year of daily maintenance ATRA is administered to patients whether they were induced into remission with chemotherapy alone, or with ATRA. The best outcome was observed in patients who received ATRA during both induction and as maintenance with a 5-year DFS of 74%. The European APL 93 trial randomized patients in remission after anthracycline-based consolidation to 1 of 3 maintenance regimens or observation: ATRA in standard doses for 15 days every 3 months; or 6-mercaptopurine (6-MP) 90 mg/m2 per day plus methotrexate 25 mg/m2 per week; or the combination of ATRA and 6-MP/methotrexate as above.15 Patients receiving both ATRA and chemotherapy had the lowest relapse rate. In addition, overall survival (OS) was improved in patients receiving maintenance chemotherapy (P = .01) and there was a trend toward better survival in patients who receive maintenance ATRA (P = .22).
Furthermore, the combination of intermittent maintenance ATRA and continuous maintenance chemotherapy appears to be particularly useful for patients presenting with a high WBC count. Therefore, at the present time, it appears that patients benefit from maintenance ATRA with or without continuous low-dose chemotherapy, particularly those at high risk of recurrence such as those presenting with a high WBC count and older adults (age > 60). The combination appears to be associated with the lowest relapse rate. The PETHEMA trial of ATRA plus idarubicin for induction, anthracycline/anthracenedione consolidation and maintenance with ATRA, and low-dose chemotherapy is associated with a relapse rate of 5%. The GIMEMA Cooperative Group is currently randomizing patients to the identical 3 maintenance regimens or observation and the North American Intergroup is currently randomizing patients in CR to either ATRA every other week with 6-mercaptopurine (6-MP) and methotrexate or ATRA alone. The results of these studies will aid in determining the optimal maintenance regimen, and the patient population most likely to benefit. It is possible that higher doses of anthracyclines in induction or consolidation may obviate the need for maintenance therapy for some patients.11
Prevention and Management of the Retinoic Acid Syndrome
The major toxicity of ATRA is the RAS, a cardiorespiratory distress syndrome manifested by fever, weight gain, respiratory distress, interstitial pulmonary infiltrates, pleural and pericardial effusion, episodic hypotension, and acute renal failure.25 Among patients induced into remission with ATRA alone, the incidence is approximately 25%17,18,25 (Table 4 ). The mortality rate of patients with the syndrome has declined over time (29% in the New York study versus 5% in the North American Intergroup study) possibly reflecting earlier recognition and institution of dexamethasone. There are no factors clearly predictive of the syndrome including WBC count,14,15,18,25 although the presence of M3v morphology was protective in one study.18 The diagnosis may be elusive and difficult to establish since patients may develop toxicities and complications of therapy such as pneumonia, congestive heart failure, and sepsis, with manifestations that mimic the RAS. This emphasizes the importance of a uniform definition.
The concurrent administration of chemotherapy with ATRA may decrease the incidence of the syndrome. However, this has not been clearly established. In the first report of the GIMEMA (ATRA plus idarubicin) trial in which all patients received concurrent therapy, the incidence of the syndrome was 10%.19 As experience with concurrent ATRA plus idarubicin accumulated, the incidence of the syndrome decreased to approximately 4%.26 In the Japanese Adult Leukemia Study Group (JALSG) trial in which chemotherapy was introduced early for the prevention of hyperleukocytosis, the incidence of the syndrome was 6%.21 An overall incidence of 15% was reported by the European APL group with a mortality rate of 8%.15 A review of the first North American Intergroup trial showed that 26% of patients treated with ATRA developed the syndrome at a median of 11 days; however, none of the patients who received ATRA as maintenance therapy developed the syndrome.18 The Australian Leukemia Study Group (ALSG) has explored the benefits of prophylactic corticosteroids in patients who develop leukocytosis (WBC > 10,000/μL). In a small nonrandomized study, 87 patients received prophylactic corticosteroids at a dose of 75 mg of prednisone per day and 16% of patients developed the syndrome which was fatal in 3%.27 This approach cannot be routinely recommended for all patients since no prospective randomized trial has confirmed the benefits given the potential toxicities of several weeks of corticosteroids in this setting.
It has been suggested that development of the syndrome may be associated with an increased incidence of extramedullary relapse.28 A number of reports have emerged suggesting that the incidence of extramedullary relapse in APL particularly in the central nervous system (CNS) is higher among patients previously exposed to ATRA than historically observed in patients treated with chemotherapy alone.29 This may be related to modulation of adhesion molecules by ATRA.30 This suggests that in patients who have relapsed in the marrow, prophylactic treatment of the CNS may need to be considered.
The Prognostic Significance of a Positive Molecular Test for the PML/RARα Fusion Transcript after Chemotherapy
Reverse transcriptase polymerase chain reaction (RT-PCR) has been shown to be an effective method to detect MRD in patients with APL in apparent CR.22 Approximately 95% of patients are rendered molecularly negative after intensive consolidation chemotherapy.16,26 However, a negative PCR test does not guarantee the absence of relapse.16
A positive PML/RARα test after consolidation reliably predicts subsequent hematologic relapse, whereas repeatedly negative results are associated with long-term survival in the majority of patients. Diverio and colleagues reported a prospective study in which 163 patients were induced into remission by ATRA combined with chemotherapy, and were tested at regular preestablished time intervals after the end of treatment.22 Twenty of 21 patients who converted to a positive PCR relapsed within a median of 3 months, whereas the 3-year estimate of relapse risk for patients who tested negative at least twice after consolidation was less than 10%. The molecular tests were carried out at similar time points from CR (at the end of consolidation, every 3 months during the first and second year, and then every 6 months during the third and fourth years) and the duration of follow-up was similar. Since patients who convert to a positive PCR can be salvaged early with chemotherapy prior to overt disease,31 this approach resulted in a significantly improved outcome compared to delaying treatment until morphologic evidence of relapse. It is anticipated that therapy at the time of molecular relapse will be associated with a lower mortality rate than that observed with reinduction of overt disease. Furthermore, aggressive chemotherapy such as high-dose cytarabine may be effective for patients who fail to achieve molecular remission with ATRA and daunorubicin.32 It is currently accepted that the persistence of a negative PCR is associated with long-term survival.33 A reasonable schedule of testing is to obtain at least 2 successive marrow samples at the end of treatment done every 3 months for the first 2 years of CR then every 6 months for the next 2–3 years. Results from a large Intergroup trial suggest quantitative PCR may be useful in identifying high-risk thresholds for relapse in the postconsolidation period.34
Long-Term Outcome with ATRA-Based Regimens
The long-term outcomes for several randomized and nonrandomized trials have been reported. Review of these studies suggests an apparent improvement in outcome as therapeutic strategies have evolved (Table 5 ). Among the patients treated on the APL 91 trial with ATRA plus daunorubicin and cytarabine for induction, daunorubicin and cytarabine for consolidation, but no maintenance, the 5-year DFS is 63%.35 In the North American Intergroup study, patients treated with ATRA plus daunorubicin and cytarabine for induction, high-dose cytarabine plus daunorubicin for consideration, and day maintenance ATRA, have a 5-year DFS of 74%.36 In the nonrandomized PETHEMA trial, patients treated with ATRA plus idarubicin for induction, anthracycline, or anthracenedione for consolidation and maintenance with ATRA plus 6MP and methotrexate, but without any cytarabine, had a 4-year DFS of 90%. Several principles have emerged which represent new concepts in the treatment of AML. First, differentiation therapy with ATRA is responsible for the high cure rate. Second, cytarabine may not be required in induction or consolidation. Third, maintenance with ATRA or low-dose chemotherapy or both improves outcome.
The Current Role of Arsenic Trioxide in APL
Arsenic trioxide to induce second remission in relapsed or refractory patients
Investigators from China reported that arsenic trioxide induces CR in patients with relapsed and refractory APL38,39 (Table 6 ). Soignet and colleagues conducted a pilot study of twelve relapsed APL patients treated with arsenic trioxide at doses ranging from 0.06 to 0.2 mg/kg/day until leukemic cells were eliminated from the bone marrow as determined by light microscopy. Eleven patients obtained CR, with 8 of the 11 patients who initially tested positive for the PML/RARα fusion transcript later becoming negative.40 A multicenter trial of 40 patients confirmed the high CR rate (85%).41 Furthermore, approximately 78% of patients had no evidence of the leukemic clone by PCR after 2 courses of arsenic trioxide. The most important toxicities include prolongation of the QTc interval and the APL differentiation syndrome, a cardiorespiratory distress syndrome with pulmonary infiltrates, reminiscent of the RAS and responsive to dexamethasone.42 Preliminary studies in a small cohort of patients testing lower doses of arsenic trioxide suggest efficacy similar to that of the standard dose with less toxicity.43 Currently, arsenic trioxide is considered the treatment of choice for patients with relapsed or refractory disease.
Approach for patients in second CR
Once patients achieve a second CR, the best postremission strategy is not known. There are few data addressing the duration of remission and PCR negativity with arsenic alone in patients with relapsed APL. One study of a small number of patients suggested that the disease-free survival may be better when patients in a CR after arsenic are treated with arsenic plus chemotherapy compared to arsenic alone (2/11 relapses versus 12/18 relapses; P < .01, respectively).38 Although some patients do well with maintenance arsenic trioxide with or without chemotherapy, others relapse and may be considered for either allogeneic (alloSCT) or autologous stem cell transplantation (ASCT) in second CR. Some form of consolidation therapy after an arsenic-induced second CR has become routine practice.
Stem Cell Transplantation in APL
Stem cell transplantation in patients in second CR previously exposed to ATRA
Meloni and colleagues reported a very small study of 15 consecutive patients with relapsed APL who underwent ASCT with unpurged marrow.44 Thirteen patients received anthracycline-based chemotherapy as initial treatment, and 2 were treated by combined ATRA and idarubicin. All patients received 3 cycles of consolidation therapy. The first CR duration ranged from 6 to 40 months. Second CR was achieved in all patients with oral ATRA. All but 3 patients received consolidation therapy with intravenous cytarabine at 1 g/m2 days 1 through 4 and intravenous mitoxantrone at 6 mg/m2 days 1 through 4. In this study, 6 (45%) of the 15 patients remain alive and well and in molecular remission. All 7 patients who underwent ABMT with persistent PCR-detectable MRD in the transfused cells relapsed within 9 months after transplant, which confirms the value of PCR positivity during remission as a predictor of relapse in APL. Only 1 of 8 patients with negative PCR relapsed, and 1 developed secondary leukemia (Table 7 ). This study demonstrates that patients with negative PCR after their second CR will fare well with ASCT and patients with positive PCR should not routinely be offered an ASCT. In addition, these patients were treated prior to the routine use of ATRA as a primary therapy, or as a therapy for relapsed disease. Sanz and colleagues on behalf of the European Blood and Marrow Transplantation Group (EBMT) have reported an overall survival (OS), leukemia-free survival (LFS), relapse rate and treatment-related mortality (TRM) for patients in first CR undergoing AlloSCT of 77%, 70%, 15%, and 20%, respectively, and for ASCT, 73%, 70%, 24%, and 12%, respectively.45 For patients in second CR, the results for AlloSCT were 58%, 57%, 15%, and 33%, respectively compared to 40%, 45%, 44%, and 25%, for ASCT.
Currently, there is little role for alloSCT in first CR since the outcome with current ATRA-based strategies is excellent. Most patients in first relapse achieve a second CR with arsenic trioxide. However, many patients relapse after arsenic-induced second CR and there may be a benefit for postarsenic chemotherapy.37 Once a patient has achieved a second CR, it is appropriate to consider transplantation. The outcome of ASCT with molecularly negative cells appears excellent and the TRM associated with alloSCT may be obviated. The ability to detect MRD by molecular studies provides the unique opportunity to collect minimally contaminated stem cells. The role of ASCT in first CR for patients at high risk of relapse based on a variety of prognostic factors has not been studied.
Prognostic Factors in APL
The presenting WBC has been the most important prognostic factor in patients treated with ATRA plus chemotherapy. Various levels of WBC have been reported to predict for outcome, with thresholds including 10,000/μL, 5,000/μL16 and 2,000/μL. The PETHEMA and GIMEMA groups have identified risk based on WBC and platelet count, for patients treated with ATRA plus idarubicin for induction and ATRA plus 6-MP and methotrexate for maintenance following anthracycline-based consolidation.45 Patients at low risk of relapse were those with a presenting WBC < 10,000/μL and a platelet count ≥ 40,000/μL, high-risk if the WBC was > 10,000/μL, and intermediate risk if the WBC was < 10,000/μL and platelet count < 40,000/μL. Female gender has been shown in several trials to confer a favorable outcome compared to male gender. The long and short PML-RARα fusion transcripts have been examined for their prognostic importance. Although a less favorable outcome for the short form has been reported by several groups,17 others have not shown a difference. Expression of CD56, which reflects the neural crest adhesion molecule believed to be involved in trafficking of leukemia cells, has also been shown to be an unfavorable prognostic factor.46 The importance of cytogenetic abnormalities in addition to the t(15;17) in ATRA-treated patients has not been completely established. Slack and colleagues reported that secondary cytogenetic abnormalities do not confer a poor prognosis among patients treated with chemotherapy only without ATRA.47 However, a recent study of newly diagnosed patients treated with ATRA-containing induction shows an apparent adverse effect for those patients with additional cytogenetic abnormalities or complex karyotypes, yet a report from the European APL Group shows that secondary chromosomal abnormalities do not confer a poor prognosis.48,49 A recent study suggested that HLA-B13 was significantly associated with relapse.
Conclusions
Acute promyelocytic leukemia was once characterized by a high early death rate, but now has become the most curable subtype of AML. Approximately 70% to 80% of patients can now be expected to be cured with contemporary strategies, which include ATRA plus anthracycline-based induction, anthracycline-based consolidation, and ATRA-based maintenance (Table 8 ). Patients who relapse after initial ATRA-based therapy can be reliably induced into a second hematologic, cytogenetic, and molecular remission with arsenic trioxide. Autologous stem cell transplantation for patients in second molecular CR and allogeneic stem cell transplantation for those persistently molecularly positive are appropriate strategies.
Several questions remain to be addressed in future studies. First, can the induction mortality rate, which remains approximately 10% in most studies, be reduced? Second, is there a benefit to adding arsenic trioxide in induction or consolidation? The latter is being explored in the current North American Intergroup Trial. Third, are there additional prognostic factors other than age, white blood cell, and platelet count, which can guide therapy to determine who will require other novel approaches such as gemtuzumab ozogamicin51 and who may or may not benefit from maintenance?
Molecular markers additional to cytogenetics with independent prognostic significance for remission duration or survival in acute myeloid leukemia (AML) of adults.
| Marker . | Frequency . | (%) . | Predictive for Relapse . | Survival . | Reference . |
|---|---|---|---|---|---|
| *normal cytogenetics only. †AML with t(8;21) and inv(16) only. | |||||
| P53 mutation | 9/200 | 4.5 | -- | Unfavorable | Nakano et al30 |
| High BCL2 and WT1 mRNA expression | 35/98 | 36 | Unfavorable | Unfavorable | Karakas et al31 |
| MLL partial tandem duplication | 18/221* | 8 | Unfavorable | Not significant | Döhner et al33 |
| High EVI1 mRNA expression | 32/319 | 10 | Unfavorable | Unfavorable | Van Waalwijk et al32 |
| C/EBP alpha mutation | 15/135 | 11 | Favorable | Favorable | Preudhomme et al34 |
| 12/277 | 4.3 | Favorable | Favorable | Van Waalwijk et al35 | |
| c-KIT mutation | 34/110† | 31 | Unfavorable | Not significant | Care et al36 |
| Marker . | Frequency . | (%) . | Predictive for Relapse . | Survival . | Reference . |
|---|---|---|---|---|---|
| *normal cytogenetics only. †AML with t(8;21) and inv(16) only. | |||||
| P53 mutation | 9/200 | 4.5 | -- | Unfavorable | Nakano et al30 |
| High BCL2 and WT1 mRNA expression | 35/98 | 36 | Unfavorable | Unfavorable | Karakas et al31 |
| MLL partial tandem duplication | 18/221* | 8 | Unfavorable | Not significant | Döhner et al33 |
| High EVI1 mRNA expression | 32/319 | 10 | Unfavorable | Unfavorable | Van Waalwijk et al32 |
| C/EBP alpha mutation | 15/135 | 11 | Favorable | Favorable | Preudhomme et al34 |
| 12/277 | 4.3 | Favorable | Favorable | Van Waalwijk et al35 | |
| c-KIT mutation | 34/110† | 31 | Unfavorable | Not significant | Care et al36 |
Prospective randomized trials of all-trans retinoic acid (ATRA) in acute promyelocytic leukemia (APL).
| Trial . | n . | Induction . | CR (%) . | ED (%) . | DFS/EFS, 2–3 yrs, (%) . |
|---|---|---|---|---|---|
| Abbreviations: CR, complete response; ED, early death; DFS, disease-free survival; EFS, event-free survival; ATRA, all-trans retinoic acid; chemo, chemotherapy; MRC, Medical Research Council. | |||||
| APL9113 | 54 | ATRA (+Chemo) | 97 | 9 | 79 |
| 47 | Chemo | 81 | 8 | 50 | |
| APL9315 | 109 | ATRA → Chemo | 95 | 8 | 75 |
| 99 | ATRA + Chemo | 94 | 7 | 86 | |
| No. Am. Intergroup14 | 172 | ATRA | 72 | 11 | 69 |
| 174 | Chemo | 69 | 14 | 29 | |
| MRC16 | 119 | ATRA(5d) → Chemo | 70 | 23 | 59 |
| 120 | ATRA + Chemo | 87 | 12 | 78 | |
| Trial . | n . | Induction . | CR (%) . | ED (%) . | DFS/EFS, 2–3 yrs, (%) . |
|---|---|---|---|---|---|
| Abbreviations: CR, complete response; ED, early death; DFS, disease-free survival; EFS, event-free survival; ATRA, all-trans retinoic acid; chemo, chemotherapy; MRC, Medical Research Council. | |||||
| APL9113 | 54 | ATRA (+Chemo) | 97 | 9 | 79 |
| 47 | Chemo | 81 | 8 | 50 | |
| APL9315 | 109 | ATRA → Chemo | 95 | 8 | 75 |
| 99 | ATRA + Chemo | 94 | 7 | 86 | |
| No. Am. Intergroup14 | 172 | ATRA | 72 | 11 | 69 |
| 174 | Chemo | 69 | 14 | 29 | |
| MRC16 | 119 | ATRA(5d) → Chemo | 70 | 23 | 59 |
| 120 | ATRA + Chemo | 87 | 12 | 78 | |
Maintenance therapy in acute promyelocytic leukemia (APL).
| Study . | n . | Maintenance . | Relapse Rate (%) . |
|---|---|---|---|
| Abbreviations: ATRA, all-trans retinoic acid; CT, chemotherapy (6-mercaptopurine plus methotrexate) | |||
| No. Amer. Intergroup14 | 94 | ATRA | 32 |
| 105 | Observation | 57 | |
| APL15 | 63 | ATRA | 20 |
| 64 | ATRA + CT | 9 | |
| 63 | CT | 22 | |
| 67 | Observation | 32 | |
| PETHEMA12 | 123 | ATRA + CT | 5 |
Comparison of the incidence and outcome of the retinoic acid syndrome (RAS).
| Study . | n . | Induction . | Incidence (%) . | Mortality (%) of Pts with RAS . | Mortality (%) of all treated Pts due to RAS . |
|---|---|---|---|---|---|
| ALSG27 | 87 | ATRA + steroids | 16 | 21 | 3 |
| No. Amer. Intergroup14 | 172 | ATRA | 26 | 5 | 1 |
| JALSG21 | 196 | ATRA ± chemo | 6 | 9 | 0.5 |
| APL 9315 | 413 | ATRA ± chemo | 15 | 8 | 1 |
| PETHEMA12 | 123 | ATRA + chemo | 6 | 17 | 0.8 |
Long-term outcome with all-trans retinoic acid (ATRA)-based regimens.
| Study . | n . | Regimen . | DFS/EFS/RFS, 3–5 yrs, (%) . |
|---|---|---|---|
| Abbreviations: DFS, disease-free survival; EFS, event-free survival; RFS, relapse-free survival; ATRA, all-trans retinoic acid; DNR, daunorubicin; Ara-C, cytosine arabinoside; IDA, idarubicin | |||
| Randomized | |||
| APL9135 | 54 | ATRA+DNR+Ara-C | 63 |
| North American Intergroup14,36 | 49 | ATRA+DNR+Ara-C+maint. | 74 |
| Nonrandomized | |||
| GIMEMA37 | 108 | ATRA+IDA+maint. | 90 |
| PETHEMA37 | 109 | ATRA+IDA+maint. (no Ara-C) | 90 |
| Study . | n . | Regimen . | DFS/EFS/RFS, 3–5 yrs, (%) . |
|---|---|---|---|
| Abbreviations: DFS, disease-free survival; EFS, event-free survival; RFS, relapse-free survival; ATRA, all-trans retinoic acid; DNR, daunorubicin; Ara-C, cytosine arabinoside; IDA, idarubicin | |||
| Randomized | |||
| APL9135 | 54 | ATRA+DNR+Ara-C | 63 |
| North American Intergroup14,36 | 49 | ATRA+DNR+Ara-C+maint. | 74 |
| Nonrandomized | |||
| GIMEMA37 | 108 | ATRA+IDA+maint. | 90 |
| PETHEMA37 | 109 | ATRA+IDA+maint. (no Ara-C) | 90 |
Patients with relapsed and refractory acute promyelocytic leukemia (APL) achieving complete response (CR) after 1 course of arsenic trioxide therapy.
| Study . | N . | No. CR . | % CR . |
|---|---|---|---|
| Zhang37 | 42 | 22 | 52 |
| Niu38 | 47 | 40 | 85 |
| 25 | 24 | 96 | |
| Soignet39 | 12 | 11 | 92 |
| Soignet40 | 40 | 35 | 85 |
Correlation between pretransplant PCR for PML-RARα and occurrence of relapse following ASCT for patients in second CR.44
| . | Relapsed < 14 months . | CCR > 14 months . |
|---|---|---|
| Abbreviations: PCR, polymerase chain reaction; PML-RARα, promyelocytic-retinoic acid receptor-alpha; ASCT, autologous stem cell transplantation; CR, clinical remission; CCR, second clinical remission | ||
| Pre-ASCT PCR+ | 7 | 0 |
| Pre-ASCT PCR- | 1 | 7 |
| . | Relapsed < 14 months . | CCR > 14 months . |
|---|---|---|
| Abbreviations: PCR, polymerase chain reaction; PML-RARα, promyelocytic-retinoic acid receptor-alpha; ASCT, autologous stem cell transplantation; CR, clinical remission; CCR, second clinical remission | ||
| Pre-ASCT PCR+ | 7 | 0 |
| Pre-ASCT PCR- | 1 | 7 |
Current recommendations for treatment of APL for patients not participating in a clinical trial.
| Abbreviations: ATRA, all-trans retinoic acid; 6-MP, 6-mercaptopurine; MTX, methotrexate; PCR, polymerase chain reaction; PML-RARα, promyelocytic-retinoic acid receptor-alpha; PBSCs, peripheral blood stem cells; CR, clinical remission; ASCT, autologous stem cell transplantation; PBSC, peripheral blood stem cells |
| Newly Diagnosed Patients |
| Induction† |
| ATRA 45 mg/m2/day until CR + an anthracycline, either daunorubicin 50–60 mg/m2/day for 3 days or idarubicin 12 mg/m2/day every other day for 4 days, although other schedules may be as effective. |
| Consolidation |
| 2–3 cycles of anthracycline-based chemotherapy, or high-dose cytarabine can be considered for patients who remain PCR positive after such consolidation, or allogeneic stem cell transplantation or ASCT with previously harvested molecularly negative cells. |
| Maintenance |
| ATRA 45 mg/m2 daily for 15 days every 3 months + 6-MP 100 mg/m2/day + MTX 10 mg/m2/week all for 2 years for all patients. |
| Follow-up and Molecular Monitoring |
| PCR for PML-RARα every 3–6 months for 2 years then every 6 months for 2 years |
| Relapsed Disease |
| Arsenic trioxide 0.15 mg/kg/day or Monday–Friday, to second CR followed by ASCT with reinfusion molecularly-negative PBSCs or allogeneic transplant considered in younger patients if a suitable donor is available. Allogeneic transplant should be considered in patients who remain molecularly positive. |
| † For pediatric patients, although by an infusional schedule, a dose of daunorubicin of 405 mg/m2 may be exceeded, the total dose should not exceed 500 mg/m2. |
| Abbreviations: ATRA, all-trans retinoic acid; 6-MP, 6-mercaptopurine; MTX, methotrexate; PCR, polymerase chain reaction; PML-RARα, promyelocytic-retinoic acid receptor-alpha; PBSCs, peripheral blood stem cells; CR, clinical remission; ASCT, autologous stem cell transplantation; PBSC, peripheral blood stem cells |
| Newly Diagnosed Patients |
| Induction† |
| ATRA 45 mg/m2/day until CR + an anthracycline, either daunorubicin 50–60 mg/m2/day for 3 days or idarubicin 12 mg/m2/day every other day for 4 days, although other schedules may be as effective. |
| Consolidation |
| 2–3 cycles of anthracycline-based chemotherapy, or high-dose cytarabine can be considered for patients who remain PCR positive after such consolidation, or allogeneic stem cell transplantation or ASCT with previously harvested molecularly negative cells. |
| Maintenance |
| ATRA 45 mg/m2 daily for 15 days every 3 months + 6-MP 100 mg/m2/day + MTX 10 mg/m2/week all for 2 years for all patients. |
| Follow-up and Molecular Monitoring |
| PCR for PML-RARα every 3–6 months for 2 years then every 6 months for 2 years |
| Relapsed Disease |
| Arsenic trioxide 0.15 mg/kg/day or Monday–Friday, to second CR followed by ASCT with reinfusion molecularly-negative PBSCs or allogeneic transplant considered in younger patients if a suitable donor is available. Allogeneic transplant should be considered in patients who remain molecularly positive. |
| † For pediatric patients, although by an infusional schedule, a dose of daunorubicin of 405 mg/m2 may be exceeded, the total dose should not exceed 500 mg/m2. |
Mutations of two class III receptor tyrosine kinases in acute myelogenous leukemia (AML).FLT3 is mutated in approximately 30% of patients with AML and KIT in 5%. The most common type of mutation consists of internal tandem duplications of amino acids in the juxtamembrane domain. These are variable in length from patient to patient, but are always in frame. These repeat sequences may serve to disrupt auto-inhibitory activity of the juxtamembrane domain resulting in constitutive tyrosine kinase activation. The second type of mutation are point mutations in the so-called “activation loop” of the second tyrosine kinase domain. Mutations at a specific aspartic acid residue, D835, which is highly conserved among tyrosine kinases, results in constitutive FLT3 activation. KIT mutations in AML are typically found in the analogous asparagine, D816. Activating loops are thought also to exert auto-inhibitory function by limited access of adenosine triphosphate (ATP) and substrate to the catalytic domain. Mutations at this asparagine are thought to alter the configuration of the activation loop in a manner similar to that of ligand induced conformational changes. KIT mutations in gastrointestinal stromal cell tumors are found in the juxtamembrane (JM) domain or the extracellular domain.
Mutations of two class III receptor tyrosine kinases in acute myelogenous leukemia (AML).FLT3 is mutated in approximately 30% of patients with AML and KIT in 5%. The most common type of mutation consists of internal tandem duplications of amino acids in the juxtamembrane domain. These are variable in length from patient to patient, but are always in frame. These repeat sequences may serve to disrupt auto-inhibitory activity of the juxtamembrane domain resulting in constitutive tyrosine kinase activation. The second type of mutation are point mutations in the so-called “activation loop” of the second tyrosine kinase domain. Mutations at a specific aspartic acid residue, D835, which is highly conserved among tyrosine kinases, results in constitutive FLT3 activation. KIT mutations in AML are typically found in the analogous asparagine, D816. Activating loops are thought also to exert auto-inhibitory function by limited access of adenosine triphosphate (ATP) and substrate to the catalytic domain. Mutations at this asparagine are thought to alter the configuration of the activation loop in a manner similar to that of ligand induced conformational changes. KIT mutations in gastrointestinal stromal cell tumors are found in the juxtamembrane (JM) domain or the extracellular domain.
A model for the interactions of APL fusion proteins with the N-Co-R-mSin3-histone deacetylase complex.Reprinted with permission from
A model for the interactions of APL fusion proteins with the N-Co-R-mSin3-histone deacetylase complex.Reprinted with permission from
Kaplan-Meier product limit estimate of disease-free survival (DFS) from the time of complete response (CR).Reprinted with permission from
Kaplan-Meier product limit estimate of disease-free survival (DFS) from the time of complete response (CR).Reprinted with permission from