Abstract
Even during this past year, further advances have been made in understanding the molecular genetics of the disease, the mechanisms involved in the generation of myeloma-associated bone disease and elucidation of critical signaling pathways as therapeutic targets. New agents (thalidomide, Revimid, Velcade) providing effective salvage therapy for end-stage myeloma, have broadened the therapeutic armamentarium markedly.
As evidenced in Section I by Drs. Kuehl and Bergsagel, five recurrent primary translocations resulting from errors in IgH switch recombination during B-cell development in germinal centers involve 11q13 (cyclin D1), 4p16.3 (FGFR3 and MMSET), 6p21 (cyclin D3), 16q23 (c-maf), and 20q11 (mafB), which account for about 40% of all myeloma tumors.
Based on gene expression profiling data from two laboratories, the authors propose 5 multiple myeloma (MM) subtypes defined by the expression of translocation oncogenes and cyclins (TC molecular classification of MM) with different prognostic implications. In Section II, Drs. Barillé-Nion and Bataille review new insights into osteoclast activation through the RANK Ligand/OPG and MIP-1 chemokine axes and osteoblast inactivation in the context of recent data on DKK1. The observation that myeloma cells enhance the formation of osteoclasts whose activity or products, in turn, are essential for the survival and growth of myeloma cells forms the basis for a new treatment paradigm aimed at reducing the RANKL/OPG ratio by treatment with RANKL inhibitors and/or MIP inhibitors.
In Section III, Dr. Fenton reviews apoptotic pathways as they relate to MM therapy. Defects in the mitochrondrial intrinsic pathway result from imbalances in expression levels of Bcl-2, Bcl-XL and Mcl-1. Mcl-1 is a candidate target gene for rapid induction of apoptosis by flavoperidol. Antisense oglionucleotides (ASO) lead to the rapid induction of caspace activity and apoptosis, which was potentiated by dexamethasone. Similar clinical trials with Bcl-2 ASO molecules alone and in combination with doxorubicin and dexamethasone or thalidomide showed promising results.
The extrinsic pathway can be activated upon binding of the ligand TRAIL. OPG, released by osteoblasts and other stromal cells, can act as a decoy receptor for TRAIL, thereby blocking its apoptosis-inducing activity. MM cells inhibit OPG release by stromal cells, thereby promoting osteoclast activation and lytic bone disease (by enhancing RANKL availability) while at the same time exposing themselves to higher levels of ambient TRAIL. Thus, as a recurring theme, the relative levels of pro- versus anti-apoptotic molecules that act in a cell autonomous manner or in the milieu of the bone marrow microenvironment determine the outcome of potentially lethal signals.
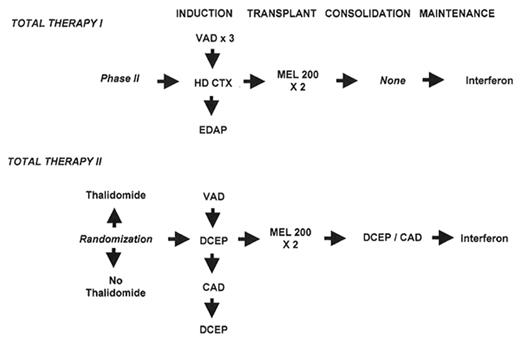
In Section IV, Dr. Barlogie and colleagues review data on single and tandem autotransplants for newly diagnosed myeloma. CR rates of 60%–70% can be reached with tandem transplants extending median survival to ~7 years. Dose adjustments of melphalan in the setting of renal failure and age > 70 may be required to reduce mucositis and other toxicities in such patients, especially in the context of amyloidosis with cardiac involvement.
In Total Therapy II the Arkansas group is evaluating the role of added thalidomide in a randomized trial design. While data are still blinded as to the contribution of thalidomide, the overriding adverse importance of cytogenetic abnormalities, previously reported for Total Therapy I, also pertain to this successor trial. In these two-thirds of patients without cytogenetic abnormalities, Total Therapy II effected a doubling of the 4-year EFS estimate from 37% to 75% (P < .0001) and increased the 4-year OS estimate from 63% to 84% (P = .0009).
The well-documented graft-vs-MM effect of allotransplants can be more safely examined in the context of non-myeloablative regimens, applied as consolidation after a single autologous transplant with melphalan 200 mg/m2, have been found to be much better tolerated than standard myeloablative conditioning regimens and yielding promising results even in the high-risk entity of MM with cytogenetic abnormalities.
For previously treated patients, the thalidomide congener Revimid and the proteasome inhibitor Velcade both are active in advanced and refractory MM (~30% PR).
Gene expression profiling (GEP) has unraveled distinct MM subtypes with different response and survival expectations, can distinguish the presence of or future development of bone disease, and, through serial investigations, can elucidate mechanisms of actions of new agents also in the context of the bone marrow microenvironment. By providing prognostically relevant distinction of MM subgroups, GEP should aid in the development of individualized treatment for MM.
I. Molecular Genetics for Disease Classification and Identification of Novel Drug Targets
W. Michael Kuehl, MD,*and P. Leif Bergsagel, MD
National Naval Medical Center, Bldg. 8, Room 5101, 8901 Wisconsin Avenue, Bethesda MD 20889
Multiple myeloma (MM), currently an incurable malignancy that often is preceded by premalignant monoclonal gammopathy of undetermined significance (MGUS), has a yearly incidence of nearly 14,000 in the US.1 For both MGUS and MM, the incidence is markedly age dependent, about 2-fold higher in American blacks than Caucasians, and significantly higher in males.2 The roles of genetic background and environment are poorly defined, although there may be clustering within families.3
MM Is a Plasmablast/Plasma Cell Tumor of Postgerminal Center B Cells
Most B cell tumors, including MM, involve germinal center (GC) or post-GC B cells4. Germinal center B cells uniquely modify their DNA through sequential rounds of somatic hypermutation and antigen selection, and also by immunoglobulin heavy chain (IgH) switch recombination. Post-GC B cells can generate plasmablasts (PB) that have successfully completed somatic hypermutation and IgH switching before migrating to the bone marrow (BM), where stromal cells enable terminal differentiation into long-lived plasma cells (PC). Although PC can be generated from either pre-GC or post-GC B cells, premalignant nonIgM MGUS and malignant MM are post-GC clonal tumors with phenotypic features of PB/PC, and are distributed at multiple sites in the bone marrow. A critical feature shared by MGUS and MM is the presence of a substantial tumor mass despite an extremely low rate of proliferation, usually with less than 1% of tumor cells synthesizing DNA until late stages of MM.5
Stages of Multiple Myeloma
There are a number of clinically defined stages for MM tumors.6 A clonal PC neoplasm must expand to about 109 cells before it produces enough immunoglobulin to be recognized as a monoclonal Ig “spike” (M-Ig) by serum electrophoresis. For premalignant MGUS, which typically is asymptomatic and stable, the M-Ig is ≤ 3 g/dl and the tumor cells comprise no more than 10% of the mononuclear cells in the BM. However, depending on the level of M-Ig (a surrogate measure of the number of tumor cells), 0.6%–3% per year of patients with non-IgM MGUS progress to MM expressing the same M-Ig.7 At present, there are no unequivocal genetic or phenotypic markers that distinguish MGUS from MM tumor cells, so it is not possible to predict if and when a particular MGUS tumor will progress to MM.4 Also, it remains unclear to what extent intrinsic genetic or epigenetic changes in the MGUS tumor cell versus extrinsic changes in nontumor cells (e.g., immune cells) are responsible for this progression. Primary amyloidosis, which accounts for about 4000 deaths per year in the United States, appears to represent premalignant MGUS that is symptomatic because of pathological deposits of the M protein (generally the intact or fragmented Ig light chain) in various tissues.8 MM is distinguished from MGUS by having a BM tumor cell content of > 10%. Smoldering MM (SMM), which has a stable intramedullary tumor cell content of > 10% but no osteolytic lesions or other complications of malignant MM, has a high probability of progressing to frankly malignant MM, which is distinguished by having osteolytic bone lesions and/or an increasing tumor mass. Further progression of MM is associated with increasingly severe secondary features (lytic bone lesions, anemia, immunodeficiency, renal impairment), and in a fraction of patients, the occurrence of tumor in extramedullary locations. Extramedullary MM is a more aggressive tumor that often is called secondary or primary plasma cell leukemia, depending on whether preceding intramedullary myeloma has been recognized. Human MM cell lines (HMCL) can sometimes be generated, but usually only from extramedullary tumors.
Oncogene Dysregulation by Ig Translocation: A Hallmark of B Cell Tumors
A seminal event in most kinds of B cell tumors (chronic lymphocytic leukemia being a major exception) is dysregulation of an oncogene that is juxtaposed near a strong Ig enhancer as a result of translocation to the IgH locus (14q32), or (less often) to an IgL locus (κ, 2p11, or λ, 22q11).9 These translocations appear to be mediated mainly by errors in 1 of 3 B cell specific DNA modification mechanisms: V(D)J recombination that occurs during early B cell development, IgH switch recombination, and somatic hypermutation, the latter 2 processes occurring mainly in germinal center B cells. The mechanism(s) by which double-stranded DNA breaks are generated in the partner chromosome are poorly understood. However, for many kinds of B cell tumors, there is a consistent Ig translocation that involves only 1 major partner (e.g., cyclin D1 at 11q13 in mantle cell lymphoma or bcl-2 at 18q21 in follicular lymphoma).
Translocations involving either an IgH or IgL locus are frequent in myeloma
The combination of karyotypic complexity, an inability to efficiently perform conventional cytogenetics on low proliferative tumors, and the telomeric location of some translocation partners delayed the identification of Ig translocations in MGUS and MM. An important initial step in solving this problem was the identification and cloning of IgH translocation breakpoints in HMCL.10 The subsequent application of interphase fluorescence in situ hybridization (FISH) using specific genetic probes to identify karyotypic abnormalities even in nondividing cells that have a PC phenotype enabled analysis of primary MGUS and MM tumors.11,12 Many recent studies have shown that many MM tumors have an IgH translocation that nonrandomly involves one of many potential chromosomal partners (for example, Bergsagel and Kuehl,10 Avet-Loiseau et al,13 and Fonseca et al14,15). The prevalence of IgH translocations varies with the stage of disease: 46%–48% in MGUS or SMM, 55%–73% in intramedullary MM, 85% in primary plasma cell leukemia, and > 90% in HMCL. Limited studies indicate a much lower prevalence of Igλ translocations: 11% in MGUS, 17% in advanced MM tumors, and 23% in HMCL.4,15 The prevalence of Igκ translocations is even lower, and appears to be no more than 2%–3% based on studies of advanced MM tumors and HMCL.16,17 Importantly, although all 34 HMCL fully analyzed have either an IgH or Igλ translocation, nearly 50% of MGUS tumors and at least 26% of advanced MM tumors have neither an IgH nor an IgL translocation.4,15
Recurrent Chromosomal Partners for Ig Translocations
Apart from c-myc at 8q24 (see below), there are 5 well-defined recurrent chromosomal partners (oncogenes) that are involved in IgH translocations in MGUS and MM: 11q13 (cyclin D1), 4p16.3 (FGFR3 & MMSET), 6p21 (cyclin D3), 16q23 (c-maf), and 20q11 (mafB), with at least the latter 3 also involved in Igλ translocations.10 Together the combined prevalence of these 5 IgH translocation partners is about 40%, with approximately 15% 11q13, 15% 4p16, 3% 6p21, 5% 16q23, and 2% 20q11.4,13,16–,19 The t(4;14) translocation is unusual in that it appears to dysregulate 2 potential oncogenes, FGFR3 on der (14) and MMSET on der (4), but FGFR3 on der (14) is lost or not expressed in about 20% of MM tumors that have a t(4;14) translocation.20–,22 There is conflicting data regarding the issue of whether the prevalence of t(4;14) and t(14;16) translocations is the same or much lower in MGUS/SMM compared to MM. However, the apparently lower incidence of 4p16 and/or 16q23 in MGUS/SMM compared to MM may be due to these translocations resulting in de novo MM without preceding MGUS, or a more rapid progression of MGUS to MM, an hypothesis supported by the fact that patients with translocations involving 4p16 or 16q23 have an extremely poor prognosis.13,14,21,23
Primary Versus Secondary Translocations in MM
Primary translocations occur as early and perhaps initiating events during tumor pathogenesis, whereas secondary translocations occur as progression events.10 For B cell tumors, most primary translocations appear to be simple reciprocal translocations that juxtapose a partner chromosomal locus (and oncogene) and one of the Ig enhancers, and usually are mediated by 1 of the 3 B-cell–specific DNA modification mechanisms described above. For MM, most translocations involving the 5 recurrent translocation partners described above appear to be primary translocations that occurred from errors in IgH switch recombination (possibly less often errors in somatic hypermutation) during B cell development in germinal centers. Occasionally, however, it appears that secondary translocations might involve 1 or more of these 5 recurrent partners4,15–,17 (C Cultraro and A Gabrea, unpublished). In addition, there are rare tumors that have independent translocations involving 2 of these 5 recurrent partners; all combinations have been documented except translocations that involve both 6p21 and 11q134,15 (C Cultraro and A Gabrea, unpublished). For normal PC and PC tumors, it appears that B-cell–specific DNA modification mechanisms are inactive. Therefore, unless one of these mechanisms could be reactivated, secondary translocations in PC tumors would be mediated by other kinds of recombination mechanisms that do not specifically target the Ig loci but could involve an Ig locus. In contrast to primary translocations, secondary translocations usually are complex, unbalanced translocations or insertions, often involving 3 different chromosomes and sometimes with associated inversion, deletion, duplication, or amplification. Primary translocations should be present in all tumor cells in both MGUS and MM, whereas secondary translocations are expected to be less frequent in MGUS than in MM, and might be present in only a subset of MGUS or MM tumor cells. Obviously, however, the only definitive way to distinguish primary from secondary translocations would be to document the time(s) at which translocations occur during the progression of individual tumors. In the absence of this definitive test, the criteria described above provide some help in distinguishing primary from secondary translocations.
Dysregulation of Myc: A Paradigm for Late Secondary Translocations in MM
Similar to other kinds of B cell tumors, translocations that dysregulate c-myc represent an important pathogenic event in MM.4 Chromosomal translocations that dysregulate c-myc by juxtaposing it with one of the three Ig loci represent an essentially invariant and apparently primary event in human Burkitt’s lymphoma and murine plasmacytoma tumors. The nontranslocated c-myc allele is not expressed, corresponding to the absence of c-myc expression, in resting germinal center B cells and terminally differentiated plasma cells. Strikingly, L-myc (1 HMCL) or 1 c-myc allele is expressed selectively in all informative HMCL, consistent with cis-dysregulation of L-myc or 1 c-myc allele in all HMCL. In addition, by our analysis of published24,25 gene expression profiling, N-myc (which is not expressed in normal PB or PC) is expressed in 2 of 82 primary MM tumors. Three-color FISH analyses of metaphase chromosomes show that nearly 90% of HMCL and 50% of advanced MM tumors have similar karyotypic abnormalities involving c-myc, L-myc (1 HMCL), or N-myc (1 tumor). Simple, reciprocal t(8;14) and t(8;22) translocations are infrequent. Most karyotypic abnormalities are complex translocations and insertions that often are nonreciprocal, and frequently involve 3 different chromosomes. Karyotypic abnormalities involving c-, L-, or N-myc often do not include association with an Ig enhancer, which suggests that secondary translocations can dysregulate c-myc by juxtaposition to non-Ig enhancers. By interphase FISH analyses, it is reported that the c-myc locus is rearranged in 3% of MGUS/SMM tumors, 10% of MM tumors with a low tumor mass, and 19% of MM tumors with a high tumor mass (β2 microglobulin > 3), and frequently is heterogeneous within a tumor.26 Cloned t(8;14) translocation/insertion breakpoints usually do not occur at the IgH sites targeted by the 3 B-cell–specific DNA modifications. All of these results support a model for MM in which dysregulation of c-, L-, or N-myc occurs as a late progression event that is mediated by secondary translocations not involving the 3 B-cell–specific DNA modification mechanisms.
Promiscuous Partners for Ig Translocations
Interphase FISH studies suggest that approximately 20% of MM tumors have IgH translocations not involving 1 of the recurrent loci cited above.13,27 Similarly, spectral karyotypic (SKY) analyses of metaphase chromosomes from 150 advanced MM tumors show that 15% of tumors have IgH translocations that do not involve a myc gene or one of the five recurrent partners described above.16,17 Most of these novel chromosomal loci are involved in only one MM tumor, and none are involved in more than three of the 150 MM tumors analyzed. It is unclear to what extent these promiscuous partners are generated by primary translocations versus secondary translocations. However, it is notable that IgH translocations involving the recurrent 5 chromosomal loci are found mainly in nonhyperdiploid tumors, whereas translocations involving other partners are represented to a similar extent in hyperdiploid and nonhyperdiploid tumors.16,17,27 More importantly, the identity and significance of the promiscuous partners (with the exception of IRF-4 at 6p25) remain enigmatic.
Dysregulation of Cyclin D1, 2, or 3: A Possible Unifying Oncogenic Event in MM
In terms of proliferation, MGUS and MM seem closer to normal, nonproliferating PC than to normal, but highly proliferating PB, for which 30% or more of the cells can be in S phase. Surprisingly, however, our analysis46 of combined gene expression profiling data from two laboratories24,25 shows that the expression level of cyclin D1, cyclin D2, or cyclin D3 mRNA in MM (and MGUS based on analysis of a limited number of samples) is relatively high, comparable to the levels of cyclin D2 mRNA expressed in normal proliferating PB, and distinctly different from normal PC. Normal hematopoietic cells, including normal B lymphocytes and PB, predominantly express cyclin D2, usually together with lower levels of cyclin D3, but do not express cyclin D1 (reviewed in Shaughnessy et al28). Given the lack of cyclin D1 expression in normal lymphocytes and the occurrence of Ig translocations that dysregulate cyclin D1 or cyclin D3 in a subset of MM tumors it seems apparent that virtually all MM tumors dysregulate at least 1 of the cyclin D genes. From cyclin D1 transgenic mice and cyclin D transfection experiments, it is known that overexpression of cyclin D is insufficient by itself to cause cell cycle progression. Instead, it has been suggested that the overexpression of cyclin D renders a cell more sensitive to growth activating signals and/or less sensitive to growth inhibitory signals.29 The apparent universal enhanced expression/dysregulation of one of the cyclin D genes in low proliferative MM tumors seems consistent with what is known about the effect of dysregulated cyclin D expression in these model systems.
Five MM Subtypes Defined by Expression of Translocation Oncogenes and Cyclin D
In addition to determining the expression level of cyclin D1, 2, and 3, gene expression profiling can effectively identify MM tumors that overexpress the oncogenes dysregulated by the 5 recurrent IgH translocations: 11q13 (cyclin D1); 6p21 (cyclin D3); 4p16 (MMSET and usually FGFR3); 16q23 (c-maf); and 20q11 (mafB)25 (and unpublished Affymetrix Hu95Av2 and Hu133A+B data from J. Shaughnessy lab). Unsupervised hierarchical cluster analysis of microarray gene expression profiles (Affymetrix Hu95Av2) confirms that there are a minimum of 5 distinct groups, with only limited overlap of the different groups. These groups (Table 1 ) can be distinguished based on the Ig translocation present, and cyclin D expression (TC classification): Group TC1 tumors (18%) express high levels of either cyclin D1 or cyclin D3 as a result of an Ig translocation; Group TC2 tumors (43%) ectopically express low to moderate levels of cyclin D1 despite the absence of a t(11;14) translocation; Group TC3 tumors (17%) are a mixture, with most expressing cyclin D2, but a few expressing only very low levels of cyclin D2 and/or cyclin D3; Group TC4 tumors (15%) express high levels of cyclin D2, and also MMSET (and in most cases FGFR3) as a result of a t(4;14) translocation; Group TC5 tumors (7%) express the highest levels of cyclin D2, and also high levels of either c-maf or mafB, consistent with the possibility that both maf transcription factors up-regulate the expression of cyclin D2.
Other Thoughts about the Roles of Ig Translocations and Cyclin D Dysregulation in MM
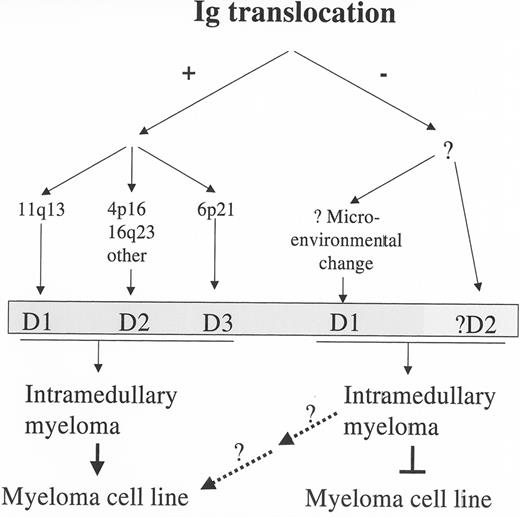
As indicated above, the incidence of IgH translocations is correlated with the stage of the disease. The occurrence of 2 (or sometimes 3) independent IgH translocations is even more prominently correlated with the stage of disease (Table 2 ).4 In addition, the prevalence of the 5 recurrent IgH translocations (particularly 16q23, and 20q11, but also 4p16 and 11q13) and c-myc translocations is lower in intramedullary tumors than in HMCL that are derived from extramedullary tumors representing both primary PC leukemia and terminal progression of intramedullary MM (Table 2 ).4,13 The increased incidence of secondary c-myc translocations in HMCL is consistent with accumulation of these translocations during disease progression. However, the increased incidence of primary translocations in HMCL compared to intramedullary tumors most likely occurs as a result of selective progression of intramedullary tumors with Ig translocations to an extramedullary phase from which virtually all HMCL are generated. Consistent with this latter hypothesis, Group TC2 tumors that ectopically express cyclin D1 without a t(11;14) translocation are not represented among the 34 HMCL that we have analyzed. On the basis of these results, we suggest the hypothesis that progression from stromal dependent, intramedullary MM to stromal independent, extramedullary MM requires a minimum of 2 genetic events, 1 of which can be an Ig translocation that dysregulates expression of one of the cyclin D genes (Figure 1 ). As a corollary to this hypothesis, we suggest that the ectopic expression of cyclin D1 in the group TC2 MM tumors requires the interaction of the MM tumor cells with bone marrow stromal cells.
A Current Model for the Molecular Pathogenesis of Multiple Myeloma
Considering the results summarized above, we propose the following model for the molecular pathogenesis of MM (Figures 1 and 2; see Appendix, page 606).4 In at least 40%—but possibly up to 60%—of tumors, a primary chromosome translocation (mediated mostly by aberrant switch recombination, and less frequently by aberrant somatic mutation), or possibly an early secondary translocation, results in the ectopic expression of an oncogene. This may lead directly (11q13–cyclin D1 and 6p21–cyclin D3) or indirectly (4p16, 16q23, other–cyclin D2) to cyclin D dysregulation. Alternatively, in at least 40%—but possibly up to 60%—of tumors there is no primary translocation, and cyclin D1 (possibly sometimes cyclin D2) is dysregulated by an as yet undefined mechanism that may involve aberrant interaction with bone marrow stromal cells. The dysregulation of 1 of 3 cyclin D genes, which provides a unifying model for the pathogenesis of MM, may render these clonal cells more susceptible to proliferative stimuli, resulting in selective expansion of this clone as a result of interaction with bone marrow stromal cells that produce interleukin (IL)-6 and other cytokines. Numeric (and possibly structural) karyotypic abnormalities, most notably including trisomies of chromosomes 3, 5, 7, 9, 11, 15, 19, and 21, and monosomy of chromosome 13 or deletion of 13q14, often are present in premalignant MGUS, the earliest identified stage of tumorigenesis.13,15 It remains to be determined if karyotypic abnormalities occur before or after primary IgH translocations. However, it is noteworthy that monosomy of chromosome 13 (or 13q14 deletion), which is present in approximately 50% of MM tumors,14,30 is present in most MM tumors that have a t(4;14) or t(14;16) translocation, perhaps consistent with the occurrence of this karyotypic abnormality preceding these translocations.13,14,17 It also is of interest that hyperdiploid tumors, which have heterogeneous combinations of trisomies involving the odd number chromosomes cited above, have a very low incidence of the 5 recurrent translocations compared to nonhyperdiploid tumors.27,31 It would appear that the hyperdiploid tumors are enriched for the group TC2 tumors that ectopically express cyclin D1 but probably have only infrequent IgH translocations. It is not clear if there is a high rate of ongoing karyotypic instability in MM, but tumor progression is associated with secondary chromosome translocations, of which c-myc provides a paradigm. The secondary translocations of c-, N-, or L-myc, which are associated with mono-allelic expression of the corresponding myc gene, are present at a low frequency in intramedullary MM tumors, but occur in nearly 50% of advanced tumors that generate metaphase chromosomes, and are almost universally present in HMCL. By timing, this dysregulation of a myc gene would appear to be associated with progression to a more aggressive, proliferative phenotype, perhaps decreasing the requirement for stromal cell cytokines that stimulate c-myc expression and proliferation at earlier disease stages. Mutually exclusive activating mutations of K- or N-Ras (or FGFR3 when there is a t(4;14) translocation) are rare or absent in MGUS, whereas RAS mutations are present in 30%–40% of early MM but perhaps a somewhat higher fraction of advanced MM, and the FGFR3 mutations are more frequent in the advanced stages of MM.20,32,33 Mutations and/or mono-allelic deletion of p53 are seen late in the course of the disease.34 Interestingly, although dysregulation of a cyclin D gene appears to be a nearly universal event in the pathogenesis of MM, inactivation of Rb or the INK4 cyclin dependent kinase inhibitors still occurs, but perhaps late in the more aggressive phase of the disease: p16INK4a by methylation, and Rb or p18INK4a by bi-allelic deletions.35– 37
Molecular Phenotypes Predict Prognosis and Response to Existing Therapies
In addition to tumor mass and secondary features that represent a host response to malignant MM (anemia, bone disease, immunodeficiency, etc.), intrinsic properties of the tumor cell are also informative in predicting prognosis and response to existing therapies. For example, it has been well documented that an unfavorable outcome is associated with each of the following: increased plasma cell labeling index, the generation of tumor cells with an abnormal karyotype (perhaps a surrogate for increased proliferation), hypodiploidy compared to hyperdiploidy, monosomy of chromosome 13/13q, and monosomy of chromosome 17/deletion of p53.5,14,27,38 It also has been reported but not confirmed that activating mutations of K-Ras (but not N-Ras) represent an adverse prognostic factor.33 More recently, it has become clear that specific IgH translocations, which represent early if not initiating events in tumorigenesis, also have a profound prognostic significance14,23 (Table 1 ). In particular, patients with tumors that have a t(4;14) translocation (TC4) have a substantially shortened survival with either standard or intense therapy, and patients with a t(14;16) (TC5) have a similarly poor if not worse prognosis. By contrast, patients with tumors that have a t(11;14) translocation (TC1) appear to have a marginally better survival following conventional chemotherapy but apparently a remarkably better response to intense therapy. These results suggest that the TC classification, based on translocation and cyclin D expression, and supported by hierarchical cluster analysis, may be a clinically useful way to classify patients into groups that have distinct subtypes of MM (and MGUS) tumors. In practice it would be very hard to get sufficient patients with either 6p21 or 20q11 translocations for a meaningful analysis. Therefore, given the similar pathogenesis and shared pattern of gene expression for tumors with 11q13 and 6p21 (TC1) and tumors with 16q23 and 20q11 (TC5) we suggest that in each case the patients be considered as part of the same group.
Identification of Novel Therapeutic Strategies
The critical role of cyclin D dysregulation in the pathogenesis of MM highlights the importance of the cyclin D/RB pathway, and suggests that there may be a therapeutic window in targeting this pathway39 for all molecular subtypes of MM. For example, epigenetic silencing of CDK inhibitor (INK4A) mRNA expression might be reversed by histone deacetylase inhibitors (SAHA, depsipeptide), or inhibitors of DNA methyl transferase (5 aza-2′deoxy-cytidine).40 To target cyclin D per se, there are a number of possible strategies including modulation of mRNA translation, posttranslational modifications, enzyme function, and perhaps even expression of cyclin D mRNA. First, the cyclin D mRNA is under strict translational control, and agents that inhibit the translation have been identified (e.g., desferroxamine, eicosapentaenoic acid).41,42 Second, cyclin D and many other cell cycle regulated proteins are posttranslationally regulated by ubiquitination and proteasomal degradation, which might provide another therapeutic target.43,44 Third, important steps downstream of cyclin D are dependent on kinases (CDKs) that may be targeted by selective kinase inhibitors.29,39 Finally, as suggested above, it is particularly intriguing that the TC2 group, which includes nearly half of MM tumors, may be absolutely dependent on an interaction with BM stromal cells for the ectopic expression of cyclin D1 that appears to be a critical property for these tumor cells. Clearly we need to elucidate the mechanism that is responsible for the ectopic expression of cyclin D1 in the TC2 group, but there may already be data with new emerging therapies that will help to address this issue. Recently, there have been promising therapeutic results with both thalidomide and its derivatives, and also bortezomib (PS-341), a proteasome inhibitor.45 Both kinds of treatment appear to target the MM tumor cells but also the interaction of tumor cells with the bone marrow microenvironment, and in each case an as yet undefined subset of patients may be more responsive. It is possible, for example, that the TC2 subgroup is selectively targeted. In any case, for this and for other treatments, it obviously is critically important to determine the response among the different TC groups, and to study the changes in protein levels of cell cycle proteins induced by treatment.
Additional specificity may be achieved by targeting the genes directly dysregulated. This seems to be especially true in the case of the t(4;14) where two enzymes are overexpressed: FGFR3, a tyrosine kinase receptor, and MMSET, which has homology to histone methyltransferases. As a surface receptor, FGFR3 may be targeted by monoclonal antibodies, and as a tyrosine kinase, by selective tyrosine kinase inhibitors. Preclinical studies have validated FGFR3 as a therapeutic target in t(4;14) MM, and plans for a clinical trial are under way (S Trudel and L Bergsagel, unpublished). Histone methyltransferases are being developed, and studies are under way to validate MMSET as a target in MM.
Concluding Thoughts
The past year has seen the synthesis of a number of different observations into a clearer picture of the molecular pathogenesis of MM. There appear to be two pathways of pathogenesis that are associated with specific cytogenetic features,27,31 one that is hyperdiploid, and usually lacks primary Ig translocations (TC2 and perhaps some TC3), and one that is nonhyperdiploid and has primary Ig translocations (TC1, TC4, TC5, and perhaps some TC3). Subtypes are identified on the basis of the Ig translocation and cyclin D expression (TC classification). This dichotomy between hyperdiploidy and translocation superficially appears very similar to what is seen in ALL,47 and the analogy deserves further scrutiny. The TC classification identifies clinically important molecular subtypes of MM with different prognoses and with unique responses to different treatments (e.g., high-dose therapy (HDT) and 11q13-TC1, FGFR3 inhibitor and 4p16-TC4). Prospective studies will be required to validate this classification for use in clinical trials. Almost all of the preclinical studies of new agents in MM are based on the induction of apoptosis at 24 or 48 hours in 1 or 2 cell lines (primarily 8226 and MM.1, both with 16q23 translocations— TC5). It now is clear that there are a minimum of 5 different molecular subtypes of MM, and an effort should be made to include the more common ones in preclinical studies (e.g., at a minimum, U266 for 11q13-TC1, H929 for 4p16-TC4, and 8226 or MM.1 for maf-TC5). It also is obvious that we desperately need a model for the large group of patients (TC2) for which we do not appear to have representative cell lines. For the reasons stated above, this may depend on developing models that are able to reproduce or replace critical features of the host BM microenvironment. Importantly, we need to gain a more comprehensive understanding of the molecular phenotype of each subtype of MM tumor, including identification of changes that remain essential for survival and growth of the tumor, thereby representing potential therapeutic targets. Although much remains to be learned, it seems essential that we rapidly incorporate what we have already learned about the molecular biology of myeloma into the clinical arena.
II. New Insights in the Biology and Treatment of Myeloma-Induced Bone Disease
Sophie Barillé-Nion, PhD, and Régis Bataille, MD, PhD*
INSERM U463, Institute of Biology, 9 Quai Moncousu, Cedex 01, Nantes 44035, France
Supports: Ligue Nationale contre le Cancer (équipe labelisée 2001) and Chugaï Company (Japan).
MM is a disorder in which malignant plasma cells accumulate in the BM and usually produce a monoclonal immunoglobulin (Ig) of IgG or IgA type. MM is responsible for about 1% of all cancer-related deaths in Western countries and epidemiological studies have shown that at least one third of MM emerge from a preexisting benign monoclonal plasma cell disorder, i.e., MGUS.1 One prominent feature in MM is the occurrence of skeletal events including bone pain, pathological fractures secondary to lytic bone lesions, and hypercalcemia. Up to 80% of patients with MM present with bone pain, and over 70% of the patients will develop pathologic fractures during the course of their disease. Bone lesions and hypercalcemia correlate directly with the presence of the total mass of myeloma cells and have prognostic value. The excessive bone resorption is an early symptom in MM and a hallmark of malignancy in individuals with MGUS.2
Bone Disease in MM
Myeloma cells grow in the BM, where the microenvironment supports their growth and protects them from apoptosis. The accumulation of myeloma cells within the BM is associated with increased rates of bone turnover. Histomorphometric analyses of bone biopsies from patients with MM have shown that an unbalanced bone remodeling formation was the characteristic feature of patients with osteolytic bone lesions, which on one hand increased osteoclastic resorption and on the other hand lowered bone formation.3– 5
Increased osteoclastic resorption
A significant increase both in the recruitment of new osteoclasts and in single osteoclast activity occurs in the close vicinity of myeloma cells, suggesting that bone disease results from local production of an osteoclast activating factor (OAF) secreted by either myeloma cells or BM stromal cells. Recently, two kinds of factors have been identified as such OAF: RANKL/OPG system and the chemokine macrophage inflammatory protein-1 (MIP-1).
RANKL/OPG:Evidence from gene-deleted and transgenic mice indicates that generation of activated osteoclasts from monocytic precursors is controlled by coordinate expression of the RANKL (receptor activation of NF-κB ligand, also known as TRANCE, OPGL, TNFSF11) and its decoy receptor osteoprotegerin (OPG, TNFRSF11B). RANKL is expressed by osteoblastic cells and binds to its receptor (RANK) present on osteoclastic cells triggering differentiation and activation signals in osteoclast precursors, thereby promoting bone resorption.6 Osteoprotegerin (OPG) is a naturally occurring factor that antagonizes the effects of RANKL, thereby preserving bone integrity.7 Therefore, the ratio between RANKL and OPG (RANKL/OPG) is determining to regulate osteoclast activity and bone resorption. Moreover, it has been suggested that the interactive network of bone-resorbing and antiresorptive cytokines and hormones converges at the RANKL/OPG system. RANKL/OPG then serves as the final common effector system to regulate osteoclast formation from precursors in the BM and its subsequent activation. Because the RANKL/OPG system is likely to play a pivotal role in the control of bone resorption, this axis was evaluated in MM-induced osteolysis. Recent studies have shown that myeloma cells are able to induce increased RANKL expression and decreased OPG production in the BM environment.8–,10 First, an overexpression of RANKL has been observed in BM biopsies from patients with MM. RANKL is overexpressed in stromal cells at the interface of MM with normal BM elements, rather than in myeloma cells. RANKL also may be produced by myeloma cells in some patients as described by Heider et al.11 Of note, in contrast to human myeloma cell lines, RANKL was detected in the murine myeloma cell line 5T2MM.12 In vitro coculture experiments have indicated that myeloma cells were able to induce RANKL expression in stromal/osteoblastic cells in part through cell-to-cell contact involving the integrin VLA-4 and in part through a soluble factor. Several OAF, including IL-1β, IL-6, and tumor necrosis factor (TNF)-α, have been reported as overproduced by stroma in response to MM. However, the RANKL overexpression seems unrelated to these cytokines since the addition of blocking antibodies against IL-1β, IL-6, and TNF-α in cocultures did not prevent RANKL upregulation.8 The soluble RANKL-inducing factors involved in MM are still unidentified but may implicate IL-7, which is produced by myeloma cells.13 Such abnormalities of RANKL overexpression in bone environment participate in the pathogenesis of various osteolytic diseases especially osteolytic metastasis in breast cancer, in which the parathyroid hormone (PTH)-related peptide plays a major role.14 In addition to increased expression of RANKL, MM-infiltrated BM exhibit decreased production of the natural RANKL inhibitor OPG. Two mechanisms have been involved in that process. First, a decrease of OPG production by stromal cells has been described as induced by MM cells.8,9 Second, myeloma cells sequestrate OPG, internalize, and degrade this factor within the lysosomal compartment. This process is dependent on physical interactions between OPG and heparane sulfates present on syndecan-1 highly expressed on myeloma cells.15 Both mechanisms may contribute to low local and systemic OPG levels observed in patients with MM.8,16 In summary, inhibition of OPG production at both transcriptional and posttranscriptional levels by myeloma cells associated with increased expression of RANKL in BM deeply disrupt RANKL/OPG ratio in favor of the osteoclastogenic factor RANKL. Finally, the main role of RANKL/OPG axis deregulation in MM-induced osteolysis is highlighted by the high potency of RANKL inhibitors such as OPG or RANK-Fc to prevent both excessive osteoclast development and lytic bone lesion occurrence in different murine myeloma models.9,12,17 Furthermore, as discussed below, disruption of RANKL/OPG axis may promote tumor progression, since treatment of mice with RANKL antagonists decreased tumor burden.
Chemokines MIP-1: Two different groups have recently shown that the chemokines MIP-1α and -β significantly participate in myeloma-induced bone disease. The first group found that MIP-1α was overproduced in myeloma BM18 and the second that both MIP-1α and MIP-1β were secreted by myeloma cells.19 The chemokines MIP-1 belong to the RANTES family and act as chemoattractants and activators of monocytes. Both osteoclast precursors and stromal cells express the chemokine receptor for MIP-1α and MIP-1β (CCR5). Data demonstrated that MIP-1α as well as MIP-1β induce expression of RANKL in stromal cells and consequently enhance osteoclast formation and resorbing activity. In line with these results, administration of neutralizing anti-MIP-1α antibodies to 5TGM1 myeloma–bearing mice limited development of osteolytic lesions and intact RANK/RANKL signaling is necessary since MIP-1α had no effect in RANK null mutant mice.20 Choi et al21 recently cloned the human MIP-1α promotor and characterized the transcription factor (TF) that controls MIP-1α expression in MM cells. They reported that the ratio of both alternatively spliced variants of the TF acute myeloid leukemia-1 (AML-1), AML-1A and AML-1B, regulates MIP-1α, and that abnormal expression of these TF in MM correlates with increased MIP-1α expression. The clinical correlation between severity of bone lesions and MIP-1 production by MM cells corroborates these results. In addition, the gene expression profile study of 92 primary MM indicated that the MIP-1α gene is overexpressed in osteolytic MM.22 Furthermore, because the chemokine receptor, CCR5, is also expressed by MM cells, MIP-1α and MIP-1β may act on MM cells in an autocrine paracrine fashion. In fact, it has been recently shown that MIP-1α triggers migration and signaling cascades mediating survival and proliferation in MM cells.23 In addition to their osteoclast-inductive capacity, MIP-1α and MIP-1β have other biologic activities that may be relevant to clinical features of patients with MM. In fact, these chemokines have been suggested to be potent modulators of hematopoiesis: MIP-1α inhibited early erythropoiesis24 and MIP-1β increased apoptosis in pre-B cells.25 Therefore, MIP-1α and -β are pluripotent chemokines that may play important roles in the pathogenesis of several clinical features of MM including not only destructive bone lesions, but also suppression of erythropoiesis, of B lymphopoiesis and of immunoglobulin production.
Direct interaction with osteoclasts: Several studies have demonstrated that myeloma cells enhanced osteoclast formation and activity through osteoblastic cells (i.e. RANKL and MIP-1α). Moreover, studies on mice demonstrate the dependence of myeloma cells on osteoclast activity and highlight the importance of the myeloma-osteoclast loop for sustaining the disease process. But direct interactions between myeloma cells and osteoclasts remain unclear. Like this, the chemokine MIP-1α, in addition to acting through osteoblastic cells to enhance osteoclast activity, may also be a potent osteoclastogenic factor that acts directly on osteoclast precursors that express CCR5 to induce late stage differentiation.18 Furthermore, the gene coding for Gas6, the ligand for the receptor tyrosine kinase Tyro-3, is overexpressed in plasma cells.26 As Tyro-3 is expressed on mature osteoclasts and involved in stimulation of osteoclastic bone resorption,27 overproduction of its ligand by plasma cells may be at the origin of strong direct interactions between myeloma cells and osteoclasts. These observations suggest that an interdependence could truly exist between myeloma cells and osteoclasts but further data are needed to sustain this hypothesis.
Decreased bone formation
Histomorphometric studies and biochemical indicators of bone turnover in MM have shown that although osteoclast number and function are increased in MM, the key difference in vivo between the presence and absence of lytic lesions is that osteoblasts are fewer and less active in patients with lytic lesions. In the early stages of MM, bone formation is increased reflecting the coupling of resorption to formation. However, as the disease progresses, bone formation is decreased and this leads to an uncoupling resorption and formation and rapid bone loss.5 This suggests that myeloma cells could first stimulate osteoblastic function during the early stages of the disease then inhibit it or even be toxic for these cells during overt expansion of the tumor. Few inhibiting interactions between osteoblasts and MM have been described so far. Recently, Shaughnessy et al28 reported the production of the potential osteoblast inhibitor DKK1 by myeloma cells. Actually, DKK1 can block Wnt signaling, an important pathway involved in osteoblast differentiation and function, and its overexpression in MM is associated with lytic bone disease. However, further experiments need to be done to confirm these data. Other potential means for the interplay between osteoblasts and myeloma cells could be through homophilic binding by the neural cell adhesion molecule NCAM/CD56. On the one hand, NCAM is known to be overexpressed by MM cells mainly of kappa subtype,29 in correlation with the presence of lytic bone lesions.30 Conversely, the lack of or weak expression of NCAM by MM cells delineates a subset of MM at diagnosis mainly characterized by a lambda light chain subtype, a lower osteolytic potential and a trend for malignant cells to circulate in the peripheral blood.31 Of note, NCAM is also strongly expressed by human osteoblasts.30,32 Thereby, NCAM-NCAM homophilic binding between CD56+/NCAM-positive MM cells and osteoblasts may induce a decrease in osteoblast function as we previously described for osteocalcin production.32 On the other hand, such negative interactions lacked in CD56−/NCAM negative MM.
Biochemical Markers of Bone Turnover
Classic biochemical markers of bone turnover remain poor predictive parameters in MM. Therefore, as RANKL/OPG axis and MIP-1α are particularly involved in the biology of bone resorption, their study as bone markers could be useful. First, median OPG serum levels were lower in patients with MM at the time of diagnosis than in healthy age- and sex-matched controls. Moreover, OPG levels were correlated with serum levels of carboxy-terminal propeptide of type I procollagen (PICP bone formation marker) but not with clinical stage or survival.16,33 Second, serum levels of sRANKL were elevated in patients with MM and correlated with bone disease. The ratio sRANKL/OPG was also increased and correlated with markers of bone resorption (TRACP-5b, NTX), osteolytic lesions, and markers of disease activity (β2-microglobulin but not CRP).34 Furthermore, Abe et al found that MIP-1 production by MM cells in BM correlated with the severity of bone lesions.19 In conclusion, these markers may have a clinical utility.
New Therapeutical Approaches in Myeloma-Induced Osteolysis
The development of lytic bone lesions is a major cause of morbidity in patients with MM. However, the therapeutic arsenal available to control excessive bone resorption remains insufficient, despite the emergence of new bisphosphonates. The recent description of the RANKL/OPG system and its main role in bone remodeling regulation has opened new avenues in the therapeutic approach of excessive bone resorption. In the close future, MIP-1 inhibitors could also represent a new effective therapeutical target to treat MM-induced bone disease. The 5T mouse model using the murine MM lines (5T2, 5T33, and its derived subclone 5TGM1) and the SCID-hu model have provided important tools for validation of in vitro observations and for preclinical studies. MM growth in the BM microenvironment was observed together with the appearance of osteolytic destruction of the human bone. The effect of potential antiresorptive drugs on bone disease and tumor growth has been evaluated in these models. These studies demonstrate that using bisphosphonates or blocking RANKL and the MIP-1α axis strongly affect not only bone resorption but also tumor development.
Bisphosphonates
Bisphosphonates, which are potent inhibitors of bone resorption, are widely used in MM-associated hypercalcemia. Placebo-controlled studies, generally including patients with stage III MM, have shown that bisphosphonates, mainly clodronate, pamidronate, and zoledronate, contribute to the long-term control of bone disease.35–,39 They reduced the incidence of skeletal events, prevented hypercalcemia, alleviated bone pain, and improved the patient’s quality of life. But they neither induced bone lesion healing nor improved the survival of patients, certainly because of their use at a too advanced stage of the disease (Table 3 ). Even though in vitro studies demonstrated that nitrogen-containing bisphosphonates induce apoptosis using human MM cell lines40 and in vivo use of pamidronate or zoledronate in the SCID-hu model and zoledronate in 5T217,41 halted MM bone resorption and decreased tumor burden, there is no proof so far that bisphosphonates really improve survival in vivo in patients. Interestingly, it has been recently shown that both pamidronate and zoledronate stimulate OPG production by primary human osteoblasts.42 These observations strongly argue for the early use of bisphosphonates in MM to prevent bone disease and slow down tumor development.
RANKL Inhibitors
The treatment of mice with MM-associated bone disease (5T2 and SCID-hu) with recombinant OPG or RANK-Fc, a fusion protein of the extracellular domain of the murine RANK with the constant region of human IgG1, resulted in a significant reduction in the number of osteolytic lesions.12,17 Histologic analysis demonstrated that OPG or RANK-Fc treatment was also associated with a partial preservation of cancellous bone volume and a significant increase of total bone marrow density. In addition to inhibiting the development of MM bone disease, OPG or RANK-Fc treatment resulted in a decrease in serum paraprotein, raising the possibility that they may have an antitumor effect. This effect was not direct since neither OPG nor RANK-Fc could induce myeloma cell death by themselves but rather depended on decreased bone remodeling. Similar results obtained in SCID-hu hosts treated with bisphosphonates17 also support the notion that antimyelomatous effect of RANKL inhibitors or bisphosphonates is closely related to inhibition of osteoclast activity. RANKL inhibitors have been successfully used to treat osteolytic metastases, tumor-induced bone pain, and humoral hypercalcemia in various animal models of nonmyeloma malignancies.9,43,44 Furthermore, 2 studies have evaluated the skeletal effect of a single dose of first OPG in a series of postmenopausal women with increased bone turnover45 and second OPG construct (AMGN-0007) in MM patients with lytic bone lesions.46 In both cases, OPG caused a rapid, sustained dose-dependent decrease of bone resorption as indicated by a decrease of biological bone turnover markers. The treatment was well tolerated and without adverse effects. However, bone mass, number of osteolytic lesions, and patient survival have not been assessed. Importantly, OPG inhibits TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis47 and can function in vitro as a paracrine factor for human MM cells.48 Altogether, these observations strongly suggest that RANKL inhibitors may represent a novel interesting approach to the treatment of MM-induced bone disease and various osteolytic diseases, but further studies should be done to evaluate adverse effects of OPG tumor protection against TRAIL-induced apoptosis.
MIP-1 inhibitors
Because the chemokines MIP-1α and MIP-1β on the one hand are among the leading candidates for MM-derived factors that enhance osteoclast differentiation and function and on the other hand may also be involved in the development of major clinical features of MM such as anemia and hypogammaglobulinemia, inhibition of their production or activities could be a novel and powerful therapeutic target in MM. Such inhibitors could come from antibodies blocking MIP-1 chemokines or their receptors, i.e., small-molecule nonpeptide receptor (CCR5) antagonists or modified chemokines.49
Conclusion
RANKL and OPG play an essential role in osteoclast formation and activation, and various bone tumors act through that system to trigger bone resorption. As described in this review and summarized in Figure 3 , the interaction of MM with stroma results in deregulation of the RANKL/OPG axis, both in increasing RANKL and decreasing OPG. This disruption of the RANKL/OPG ratio in the bone environment increases osteoclast activity, triggers bone destruction, and promotes tumor growth. Moreover, the chemokines MIP-1α and MIP-1β produced by myeloma tumor also enhance osteoclast activity both through RANKL expression in bone environment and direct effect on osteoclast precursors leading to increased bone resorption. Finally, in vivo use of osteoclast inhibitors (bisphosphonates or specific inhibitors of RANKL) halted MM-induced bone resorption and resulted in inhibition of myeloma growth and survival. These observations demonstrate a strong interdependence between myeloma cells and osteoclasts: myeloma cells enhance the formation of osteoclasts, whose activity or products, in turn, are essential for the survival and growth of myeloma cells. In line with this concept, a recent study has shown that IL-6 and osteopontin highly produced by osteoclasts played a central role in survival and growth of myeloma cells.50– 52 Indeed, the use of effective osteoclast inhibitors in vivo could break down this vicious circle and both suppress bone resorption and decrease tumor growth. It is tempting to speculate that interfering with BM cultivation by myeloma cells may inhibit the development of myeloma especially in early or premalignant stages (MGUS). Altogether, these observations strongly suggest that reducing the RANKL/OPG ratio by treatment with RANKL inhibitors and/or MIP inhibitors should provide a high therapeutic interest to decrease both bone resorption and tumor burden in MM.
III. Therapeutic Targets
Robert G. Fenton, MD, PhD*
University of Maryland Greenebaum Cancer Center, 655 W Baltimore St, Bressler Research Bldg, Room 7-023, Baltimore MD 21201
Supported by grants from the Multiple Myeloma Research Foundation and the Leukemia & Lymphoma Society.
Cancer cells arise through the progressive acquisition of mutations that deregulate cell cycle checkpoints, inactivate DNA repair mechanisms, disrupt apoptosis pathways, and alter host-tumor interactions allowing invasion and metastasis.1 Because of the acquisition of genetic lesions that perturb normal cell physiology, the hypothesis has been put forth that tumor cells differ from normal cells in their apoptotic burden.2 In normal cells, genetic abnormalities are “sensed,” and signaling pathways are activated that lead to cell cycle arrest or the induction of apoptosis. The “rheostat”’ that regulates activity of the core cell death machinery (i.e., the threshold of stimuli required to induce apoptosis) is set at a low level to ensure that renegade cells with genetic lesions conferring a growth advantage are destroyed. As tumor cells evolve, they acquire (through the process of mutation and selection) a myriad of mechanisms enabling them to survive even in the face of death signals that should lead to their demise.3,4
Because of this increased apoptotic burden, it can be hypothesized that tumor cells live on the precipice of apoptosis: even partial inhibition of antiapoptotic mechanisms operative in tumor cells would be expected to render them vulnerable to cell death.5 Therefore, therapies designed to downregulate antiapoptotic pathways would be expected to enhance the demise of cancer cells without affecting normal cells. This hypothesis will be testable when therapeutic agents are developed that specifically target the key regulatory elements of the apoptotic machinery. The Bcl-2 antisense oligonucleotide Genasense may represent the first of this class of agents, as discussed below. Note also that since most cytotoxic drugs exert their antitumor effects by activation of apoptosis pathways,4 interventions that inhibit antiapoptosis mechanisms in tumor cells should overcome many forms of drug resistance.
We believe that MM fits this paradigm. From the earliest stages of proliferation and differentiation in the lymph node germinal center, incipient MM cells accumulate genetic lesions including translocations involving the Ig-H switch region, and gross chromosomal abnormalities leading to aneuploidy.6 Late in the disease, MM cells lose the requirement for the BM microenvironment as they acquire additional mutations involving oncogenes such as myc, ras, and p53.7 One would hypothesize that even during the earliest stages of disease (MGUS), genetic lesions result in an increased apoptotic burden, and the surviving cells must have developed antiapoptotic mechanisms to counterbalance the death signals.8,9 This concept is consistent with the notion that while MM is a disease of deregulated proliferation, early in the disease the labeling index is low (< 1%), and an increased survival of malignant plasma cells may be a more important factor for the initial expansion of malignant plasma cells in the BM.7
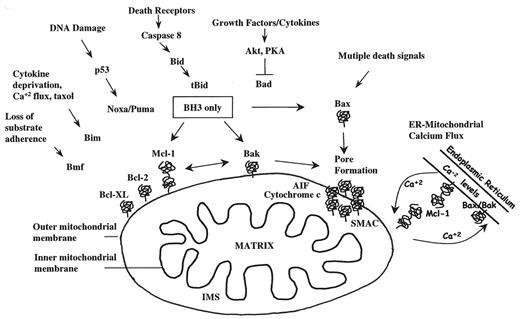
Apoptosis is induced through two distinct yet intertwined pathways: (1) the extrinsic or death receptor pathway composed of tumor necrosis factor (TNF)-family receptors and ligands and (2) the intrinsic pathway in which the release of mitochondrial constituents regulates caspase activation. Although the mitochondrial pathway of apoptosis is the main topic of this review, it should be noted that death receptors 4 and 5 (DR4, DR5) are expressed on MM cell lines and primary MM isolates, and can efficiently activate the extrinsic pathway after binding of the ligand TRAIL.10,11 This leads to the rapid activation of caspase 8 that can either directly activate effector caspases 3, 6, or 7 or cleave Bid, leading to amplification of the apoptotic signal by recruiting the mitochondrial pathway.12 Regulation of TRAIL-induced death can also occur at the level of the BM microenvironment, as OPG released by osteoblasts and other stromal cells can act as a decoy receptor for TRAIL, thereby blocking its apoptosis-inducing activity.13 MM cells inhibit OPG release by stromal cells, thereby promoting osteoclast activation and lytic bone disease (by enhancing RANKL availability), while at the same time exposing themselves to higher levels of ambient TRAIL. As a recurring theme, the relative levels of proapoptotic versus antiapoptotic molecules that act in a cell autonomous manner or in the milieu of the BM microenvironment determine the outcome of potentially lethal signals.
Regulation of the Mitochondrial Pathway of Apoptosis
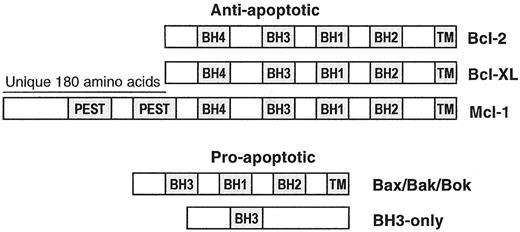
Myeloma cells are exposed to multiple noxious stimuli with the potential to induce apoptosis, such as chromosomal instability or hypoxia, and those induced by different forms of therapy, which work through mechanisms that are as varied as the therapeutic agents themselves (e.g., dexamethasone, melphalan, thalidomide, Velcade). Resistance to apoptosis in these cases ultimately rests on the ability of the MM cells to prevent activation of the mitochondrial pathway of apoptosis. Whether noxious signals activate this pathway is determined by members of the Bcl-2 family, and understanding the molecular functions of these proteins is required for the design of novel therapeutics to overcome the resistance to apoptosis exhibited by MM cells. Bcl-2 family members are divided into 3 functional groups; these encode 1 or more Bcl-2 homology domains (BH1-BH4) and act as inhibitors or inducers of the mitochondrial apoptosis pathway (Figure 4 ). Antiapoptotic family members (Bcl-2, Bcl-XL, Mcl-1) are localized to the outer mitochondrial membrane (OMM) via a hydrophobic carboxy-terminal tail, and regulate the release of apoptotic molecules from the intermembrane space.14 The apoptosis inducers (e.g., Bax and Bak) encode BH1, BH2, and BH3 domains, and can be induced by apoptotic signals to homo-oligomerize and form pores in the outer membrane, thus permitting efflux of apoptosis-inducing molecules including cytochrome c, dATP, SMAC/Diablo, and AIF.14,15 The mechanism by which mitochondrial outer membrane permeabilization (MOMP) allows efflux of apoptogenic proteins is controversial; however, it has been shown that Bax oligomers can form pores in liposomes that permit the passage of cytochrome c.16 Under normal growth conditions, Bak is tethered to the mitochondrial outer membrane, while Bax translocates to the mitochondria in response to apoptosis-induced conformational changes that unmask its carboxy-terminal hydrophobic domain.17 Cells isolated from Bax/Bak double knock-out mice exhibit a dramatic resistance to the induction of apoptosis by many different noxious insults, including overexpression of BH3-only proteins.18
BH3-only proteins promote apoptosis by monitoring the status of cell “health” from different locations within the cell (Figure 5 ).19 Thus, Bid is activated by caspase cleavage in response to signals from TNF-family death receptors,20 BAD senses the activity of growth factor regulated survival pathways (i.e., acting through Akt, PKA, and other kinases),21 Bmf and Bim appear to monitor microfilaments and microtubules, respectively,22,23 while Noxa and Puma are p53-regulated genes that respond to DNA damage.24,25 The BH3 domains contain an amphipathic α-helix that functions as a “death domain” in these proteins, and is essential for both proapoptotic activity and, not coincidentally, the ability to bind to multidomain Bcl-2 family members.26 Whether a cell undergoes programmed cell death (PCD) in response to a potential apoptosis-inducing signal depends on the interactions of the BH3-only proteins with mitochondrial-localized multidomain Bcl-2 family members.27 If the BH3-only proteins are sequestered by Bcl-2, Bcl-XL, and Mcl-1, apoptosis is prevented.28 If specific BH3-only family members are able to interact with Bax or Bak, then oligomerization is induced and cytochrome c is released (Figure 5 ).16,29
Much has been learned about how Bcl-2 and Bcl-XL interact with Bax, Bak, and BH3-only proteins.15 The core 3-dimensional (3D) protein structure is conserved between Bcl-XL and Bcl-2, and is composed of a globular bundle of 5 amphipathic α-helices surrounding 2 central hydrophobic α-helices.30 Of great functional importance is the formation of a hydrophobic binding groove by the BH1, BH2, and BH3 domains that is required for binding to BH3 domains and for survival functions.30,31 In addition, the 3D structures of Bcl-2, Bcl-XL, and Bax resemble the pore-forming subunits of bacterial toxins and have weak channel-forming activity for small ions through lipid membranes.31 As described above, pore formation by Bax and Bak may be the critical event in MOMP.
Recent elegant experiments have led to a revised model for the regulation of the mitochondrial pathway of apoptosis by BH3-only proteins (Figure 6 ).28,32 A critical aspect of this model is based on the relative affinities of distinct BH3-only proteins for antiapoptotic versus proapoptotic Bcl-2 family members, and the relative abundance of each class of protein within the cell. Letai et al have determined the binding affinity of Bcl-2 for a variety of peptides encoding the functional alpha-helical regions of killer protein BH3 domains, and have determined which of these peptides can directly induce cytochrome c release from purified mitochondria.32 BH3-domain peptides from Bid and Bim directly induced MOMP and cytochrome c release through a process that required Bax or Bak. A BH3-domain peptide from BAD could not induce cytochrome release directly. However the BAD peptide did bind to Bcl-2 with high affinity and could displace the lower affinity Bid peptide. A model was proposed that some BH3-only proteins (e.g., Bid and Bim) directly target Bax and Bak and induce pore formation; apoptosis can be averted if BH3-only proteins are bound by antiapoptotic Bcl-2 family members. A second class of BH3 proteins, represented by BAD, cannot directly induce pore formation, but can occupy the hydrophobic binding pocket of Bcl-2 (and presumably Bcl-XL or Mcl-1) thus enabling subthreshold levels of Bid to target Bax or Bak.
The regulation of the mitochondrial pathway is even more complex, as the Ca+2 content of the endoplasmic reticulum (ER) has recently been shown to determine a cell’s sensitivity to apoptosis.33 Apoptosis induced by some stimuli is associated with emptying of ER Ca+2 stores with a concomitant increase in mitochondrial Ca+2 pools.34 The steady-state level of Ca+2 in the ER and its release by apoptotic stimuli are regulated in part by Bcl-2 family members (e.g., Bcl-2, Bax, Bak) localized to ER membranes.34 The “mitochondrial pathway of apoptosis” may therefore be regulated by 2 hits: the Ca2+ flux from ER stores to the mitochondria, and the induction of mitochondrial outer membrane permeability by BH3-only proteins, both of which are regulated by multidomain Bcl-2 family members.
The Mitochondrial Pathway in MM
In MM, defects in programmed cell death pathways are frequently caused by imbalances in expression levels of the Bcl-2 family of proteins. It has become clear that every nucleated cell requires protection by at least one prosurvival Bcl-2 homologue, and that the abundance of these guardians regulates tissue homeostasis.15 MM cells conform to this model; they express Bcl-2, Bcl-XL, and Mcl-1, and both clinical and in vitro data suggest important roles for these proteins in maintaining MM cell survival and in clinical resistance to therapy.35,36 Although aberrant switch recombination leading to the activation of multiple translocation partners plays an important role in the pathogenesis of MM, these translocations do not include Bcl-2 family members.6 Nevertheless, Bcl-2 is expressed in many (but not all) MM cell lines and primary clinical isolates.36,37 Bcl-XL is expressed in most cell lines and clinical isolates, and was detected more often at the time of patient relapse and correlated with resistance to chemotherapy.35 Mcl-1 appears to be expressed in virtually all MM cell lines and in all clinical isolates thus far examined by numerous groups.37– 40 In a comparison of the expression of Mcl-1 in MM cells from 150 patients to that of plasma cells from 31 normal volunteers, a statistically significant increase in the myeloma cells was demonstrated (J. Shaughnessy et al, personal communication). Thus it appears that most MM cells express some level of Bcl-2, Bcl-XL, and Mcl-1, and it remains to be determined if these are purely overlapping in function, or also have distinct activities to promote tumor cell survival in the face of a myriad of apoptotic stimuli.
Experimental approaches to ablate expression of Mcl-1, Bcl-2, and Bcl-XL have begun to address this issue. A number of laboratories have correlated the induction of MM cell apoptosis with decreased expression of Mcl-1.37,38,40 Mcl-1 mRNA and protein have a short half-life, and inhibition of Mcl-1 synthesis led to the rapid induction of apoptosis of MM cells.41 The main apoptosis-inducing activity of the CDK-inhibitor flavopiridol can be linked to inhibition of CDK9/cyclin T1 with subsequent inhibition of transcription elongation; Mcl-1 was shown to be a candidate target gene for the rapid induction of apoptosis by flavopiridol.41,42 The critical role for Mcl-1 as a survival factor in MM has been demonstrated in vitro using antisense oligonucleotides (ASO) to specifically inhibit Mcl-1 expression. Mcl-1 ASO led to the rapid induction of caspase activity and apoptosis (within 3 hours in some cases) when used as a single agent, and killing was potentiated by the addition of dexamethasone.38,40 In the latter study, ASO-mediated inhibition of Bcl-XL or Bcl-2 did not induce apoptosis as single agents, even though expression of the molecular targets was shown to be significantly reduced. However, addition of dexamethasone to Bcl-2 ASO-treated cells did promote apoptosis in some cell lines.40 Others have demonstrated that in MM cell lines with a low level of Bcl-2 expression, apoptosis can be induced using the specific Bcl-2 ASO G3139, and that killing was potentiated by dexamethasone or taxol.43 A second study cultured purified primary MM cells with high concentrations of G3139 (10 μM, corresponding to 56 μg/mL; in clinical studies serum concentrations during the 7-day infusion were 3–7 μg/mL) and demonstrated a significant reduction of Bcl-2 RNA and protein in most patients.44 G3139 alone was not toxic to these cells, but it enhanced killing by doxorubicin and dexamethasone. It remains to be clarified if inhibition of Bcl-XL can promote MM cell death as only 1 study has critically examined this question.40 No studies have systematically evaluated the role of Bfl-1/A1 in primary MM cell isolates.
A number of signal transduction pathways have been shown to regulate the expression of antiapoptotic Bcl-2 family members in MM cells, and the role of IL-6 has been closely scrutinized. STAT3 was recently shown to be constitutively activated in primary MM cells, and was shown to induce the upregulation of Bcl-XL in the U266 cell line.45 A number of groups have determined that Mcl-1 is upregulated by IL-6, perhaps through the activity of the STAT3 pathway.37,46 However the role of STAT3 was based on experiments using the tyrosine kinase inhibitor AG490, whose specificity and mechanism of action are not at all clear. Our group demonstrated that approximately one third of MM cell lines and primary MM cells responded to IL-6 with the upregulation of Mcl-1, while in the other two thirds of cases, Mcl-1 was expressed at high levels in the absence of IL-6, without further increase upon IL-6 addition.39 An analysis of archival BM specimens indicated that while Mcl-1 expression was detected by in situ staining in all samples, phosphorylated STAT3 was only observed in 48%.47 This supports the notion that in some primary MM cells, Mcl-1 is expressed despite the absence of STAT3 activation. Although IGF-1 promotes MM cell proliferation and survival, it does not appear alter expression levels of Bcl-2 family members. Overall, the data are consistent with our current understanding of MM as a genetically heterogeneous disease, and therefore it is highly likely that the molecular mechanisms regulating Mcl-1 expression will also be heterogeneous.
In summary, data indicate that Mcl-1 is expressed in all MM, and that this is required for the viability of myeloma cells. This may related to the important role of Mcl-1 during B cell terminal differentiation in the germinal center.48 Its expression at the time of B-cell proliferation, class switch, and somatic hypermutation in the germinal center may be required for survival of developing plasma cells, and its expression may then be maintained during the evolution of MM cells. This is consistent with studies of the IL-6-mediated differentiation of Epstein-Barr virus (EBV)-transformed B cells to IgG expressing cells where Mcl-1 expression peaked as cells expressed high levels of IgG, and then declined in association with the induction of apoptosis.49 Bcl-2 and Bcl-XL are also expressed in most primary MM isolates, and it is likely that all 3 antiapoptotic Bcl-2 family proteins play important roles in preventing activation of the mitochondrial pathway. Future studies will be focused on determining how these molecules differ in their antiapoptosis functions, and whether individual cases of clinical resistance to treatment can be overcome by inhibition of one or more of these prosurvival factors.
Antiapoptosis Therapy in MM: Targeting the Mitochondrial Pathway
Understanding the intricacies of BH3 domain interactions will enhance efforts to develop novel antimyeloma agents that engage the apoptosis pathways in tumor cells. Cytotoxic insults that induce apoptosis through activation of BID-like BH3-only proteins act in a mechanistically different way from stimuli that promote apoptosis through the activation of BAD-like BH3-only proteins.32 From a therapeutic perspective, drugs that mimic the action of the Bad-like BH3 domain might be expected to sensitize tumor cells to a variety of apoptotic stimuli. In the case of MM, it remains to be determined which Bcl-2 family interactions are most important in regulating MM cell survival or inducing apoptosis in response to different environmental signals. For instance, it will be important to understand which BH3-only proteins are activated by individual chemotherapeutic agents. Does melphalan induce 1 or a few different BH3-only proteins? Does p53 status make a difference (it would be expected to, given the DNA damage–p53–Noxa/Puma connection)? What about dexamethasone, thalidomide, proteasome inhibitors, and other agents that are just entering preclinical or early phase clinical trials? Which specific BH3-only pathways do these novel compounds activate in MM cells? It may be possible to unravel the molecular details of how therapeutic agents affect critical regulatory interactions between Bcl-2 family members. Insights into these mechanisms of action would be very valuable for planning future clinical trials in which novel agents will be combined; agents that target different BH3-only family members might be expected to synergize for activation of the mitochondrial pathway of apoptosis. Novel compounds may activate individual BH3-only pathways at subthreshold levels for MOMP; however, if multiple BH3-only members can be activated, inhibition by Bcl-2, Bcl-XL, and Mcl-1 may be overcome, and dramatic apoptosis may ensue (Figure 6 ).
Clinical Trials of Bcl-2 Antisense—Oligonucleotides in MM
Antisense oligodeoxynucleotides (ASO) have been shown to downregulate Bcl-2 expression and induce apoptosis in a number of human tumor models, including mouse xenograft models of melanoma and prostate cancers.50,51 In vitro experiments utilizing ASO suffer from a number of pitfalls, including the requirement for special methods to introduce the ASO into cells that cannot be used in patients (e.g., electroporation, lipofectins, streptolysin-O), the use of concentrations of ASO that are not clinically relevant, and poor experimental designs that lack the minimal controls such as negative control ASO (i.e., those with scrambled or reverse sequence). Nevertheless, preclinical data do indicate antitumor activity for the Bcl-2 ASO G3139 (Oblimersen Sodium, Genasense; 18-mer phosphorothioate ASO directed at codons 1–6 of Bcl-2) and this has been introduced into clinical trials with the goal of sensitizing chemotherapy-resistant cancer cells to apoptosis by downregulating Bcl-2.43,44 In MM, a Phase III study comparing dexamethasone alone with the combination of dexamethasone plus G3139 has completed accrual and the data are pending. Two Phase II studies combining Genasense with active myeloma regimens for the treatment of relapsed/refractory patients have been described in abstract form.52,53 G3139 was administered as a continuous infusion (5–7 mg/kg/day) on days 1–7 repeated every 3 weeks, combined with dexamethasone (40 mg po days 4–7 of each week of G3139) and thalidomide (100–400 mg po per day).52 After 3 cycles, responding patients went on to a maintenance phase with G3139 and pulse dexamethasone given every 5 weeks with continued daily thalidomide. Seventeen heavily pretreated patients have now been enrolled, and 15 patients have completed the induction phase. Responses include 2 CR, 1 near CR, and 5 PR (53% response rate). Some responses were in patients who had previously failed dexamethasone plus thalidomide. Laboratory correlates have thus far failed to demonstrate a significant decrease in the level of Bcl-2 protein expression in BM-derived MM cells; however, these studies are ongoing. A second study added G3139 (7 day continuous infusion at 7 mg/kg/day) to the VAD regimen and 8 patients were reported, including 6 who were refractory to previous VAD chemotherapy.53 Four patients achieved PR with the G3139-VAD combination. Examination of Bcl-2 levels in peripheral blood MM cells using flow cytometry demonstrated a 18% and 17% reduction on days 4 and 7, respectively. For both Phase II studies, the treatment was well tolerated, with G3139 associated toxicities including mild thrombocytopenia, fatigue, and worsening of underlying renal insufficiency. Although these early results are encouraging, further analysis of the data from these ongoing trials will be necessary before conclusions can be drawn about the critical scientific issues they pose. These include whether G3139 actually decreases Bcl-2 protein expression in the MM cells of treated patients, and whether this renders tumor cells more susceptible to combined treatment with regimens that are already known to have high response rates in MM.
Future Drug Development Efforts Targeting the Mitochondrial Pathway of Apoptosis
We believe that Bcl-2, Bcl-XL, and Mcl-1 are valid therapeutic targets in MM, and through the use of structural biology and high-throughput technologies, a number of low-molecular-weight, cell-permeable compounds have been identified that bind to the BH1-3 hydrophobic pocket of Bcl-2 or Bcl-XL and promote apoptosis by blocking the association with BH3-only proteins. Two groups have taken the published nuclear magnetic resonance (NMR) structural data of Bcl-XL and used computer programs to model the 3D structure of Bcl-2.54,55 If the 3D structure of a target protein is known or can be predicted from the structure of a homologous protein, computer screening of chemical databases can identify potential ligands that are predicted to bind to specific regions of the target. Using this technique, a virtual screen of chemical databases led to the identification of compound HA14-1 (MW 409) that was shown to bind to Bcl-2 with an IC50 of 9 μM using a competitive fluorescence polarization assay in which the novel compound was tested for displacement of a fluorescein-labeled Bak BH3 peptide from Bcl-2.55 HA14-1 induced apoptosis of > 90% of HL-60 cells (which express high levels of Bcl-2) when added to cultures at 50 μM for 4 hours. A second group performed a computer screen of 207,000 compounds from the NCI 3D chemical database and identified 7 compounds that bound to Bcl-2 with IC50 values of 1.6 to 14.0 μM.54 One compound (#6) had significant growth inhibitory activity (IC50 4 μM for HL60) and induced apoptosis of cells that expressed high levels of Bcl-2 (such as HL-60, MDA-231), but not low Bcl-2 expressers (T47D, MDA-453). This paradoxical result can be explained by the hypothesis that cells that express high levels of Bcl-2 are dependent on this protein to prevent activation of the mitochondrial pathway. Binding of compound #6 to Bcl-XL was shown by NMR analysis to cause peak shifts corresponding to amino acids located in the BH1-3 hydrophobic groove, thus confirming targeted binding of the compound.54 Using a high-throughput assay based on fluorescence polarization, a screen of a 16,320 compound library yielded two new classes of inhibitors of Bcl-XL.56 NMR analysis demonstrated that these compounds bound to different positions within the Bcl-XL hydrophobic binding pocket and would be expected to compete for binding of BH3 domains from killer proteins, thus inhibiting the antiapoptotic effects of Bcl-XL. In fact these compounds did induce apoptosis of JK cells at 30–300 μM, and apoptosis was blocked by overexpression of Bcl-XL in these and in FL5.12 cells.
Another approach was based on the observation that drugs that inhibit mitochondrial functions such as electron transport often induce apoptosis and bypass Bcl-2 protective effects. Antimycin A, an inhibitor of complex III of the electron transport chain, was shown to induce apoptosis of Bcl-XL-overexpressing hepatocytes by binding to the hydrophobic groove of Bcl-XL.57 Methoxy-antimycin A, a synthetic derivative that no longer inhibited electron transport, maintained activity through its binding to Bcl-XL and inducing loss of mitochondrial transmembrane potential. It was speculated that in cells with high levels of Bcl-XL, antimycin A might activate the latent pore-forming activity of Bcl-XL, thus converting this antiapoptotic protein into a killer.57
Drug discovery efforts to identify small-molecule inhibitors of antiapoptotic Bcl-2 family members are well underway. Once lead compounds are identified, the molecular basis for their interaction with target proteins will be evaluated using NMR or x-ray diffraction, and predictions can be made for structural modifications to increase affinity and specificity. Advances in medicinal chemistry and computer technologies, combined with development of novel high-throughput assays and methods for measuring the affinity of small molecules for target proteins, will permit a rapid, iterative approach for the optimization of lead compounds. Compounds will move from the “wet laboratory” to the virtual world, and back, as each generation of compounds is tested. Given the high degree of homology between the hydrophobic binding pockets of antiapoptotic Bcl-2 family members, this very detailed structural approach will be important for the development of inhibitors that specifically target Bcl-2, Bcl-XL, or Mcl-1. Specific inhibitors will be of great value in determining which antiapoptotic Bcl-2 family members are most important for the survival of MM cells, and whether the functions of antiapoptotic family members are overlapping or serve unique functions in specific tumor cells. The known genetic heterogeneity of MM suggests that responses to these agents will not be uniform, and that alone or in combination with other therapeutic agents, targeted inhibition of Bcl-2 family members should provide a profound reduction of the apoptotic threshold of tumor cells leading to improved therapeutic outcomes. The possibility of inducing apoptosis of normal cells will require that great care be taken in the initial evaluation of these agents. Drugs that simultaneously target all 3 antiapoptotic proteins, while lethal to tumor cells, are likely to be very toxic to the host; specific agents are therefore required.
IV. Improving Disease Control in Myeloma
Bart Barlogie, MD, PhD,*John Shaughnessy, PhD, Joth Jacobson, MS, Joshua Epstein, DSc, and Guido Tricot, MD, PhD
University of Arkansas for Medical Science, Myeloma Institute for Research and Therapy, 4301 West Markham Street, Slot 816, Little Rock AR 72205–7101
Supported in part by CA55819 from the National Cancer Institute in Bethesda, Maryland.
This review of recent progress in the treatment of myeloma addresses high-dose therapy with autologous stem cell support, allogeneic transplants, new drugs, and their roles in disease management. Special scenarios of advanced age, renal failure, and 1° amyloidosis will also be covered. An algorithm for disease management based on prognostic factors will be presented.
Untreated Myeloma
High-dose melphalan autotransplant versus standard therapy
Melphalan 200 mg/m2 (MEL 200) has emerged as the most effective and safest means of applying dose-intensive therapy.1 The Medical Research Council VII trial recently confirmed IFM 90 results2 demonstrating that MEL 200 after C-VAMP induction was superior to ABCM standard combination therapy.3 Stringently defined complete response (CR) was 44% versus 8% (P < .001); median event-free survival (EFS) 32 months versus 20 months (P < .001), and overall survival (OS) 54 months versus 42 months (P = .04) (Table 4 ). The failure of other randomized studies to demonstrate superiority of high-dose over standard-dose therapy may relate to insufficient follow-up, randomization only of responding patients (e.g. after 4 cycles of BVMCP/VBAD4), as well as salvage transplants in the setting of poststandard chemotherapy relapse.
Tandem versus single autotransplant
IFM 94 reported superior EFS and OS with a tandem autotransplant, using MEL 140 followed by MEL 140 + total body irradiation (TBI) 8 Gy, versus a single cycle with MEL 140 + TBI 8 Gy.5 Updated results, recently presented at the IX International Myeloma Workshop in Salamanca, Spain, demonstrate a 7-year postdiagnosis probability of EFS of 20% (confidence interval [CI], 14%–26%) in the tandem transplant arm versus 10% (CI, 5%–15%) in the single transplant arm (P < .03). Similarly, 7-year OS was 42% (CI, 34%–49%) in the tandem transplant arm versus 21% (CI, 13%–29%) in the single transplant arm (P < .01). According to multivariate analysis including all patients, OS was longer with low B2M, younger age, low lactate dehydrogenase (LDH), and with tandem transplant. The main benefit from tandem transplant was seen in patients not yet in CR or near-complete remission (n-CR) after a single transplant.
Another French myeloma cooperative group (Myelome-Autogreffe, MAG) reported on a randomized comparison of VAD × 3 followed by high-dose combination therapy with MEL 140, carmustine, etoposide, cyclophosphamide 60 mg/kg + TBI 12 Gy, that was compared to MEL 140 followed by MEL 140 + etoposide 30 mg/kg + TBI 12 Gy (MAG 95).6 Additional randomization concerned the value of CD34 selection, which was found to give comparable results to unselected stem cell support. There were 230 patients enrolled and 193 randomized (97 single, 96 tandem autotransplants); all were less than 56 years old; the median follow-up is 53 months. No differences were noted in terms of CR and n-CR rates (39% with 1 and 37% with 2 transplants). Likewise, median EFS (31 months vs 33 months) and OS (49 months vs 73 months; P = .14) were similar with single and tandem autotransplants.
The Dutch-Belgian hematology-oncology group (HOVON) evaluated, after 3 to 4 induction cycles of VAD, 2 intermediate doses of MEL 70 (total MEL 140) without stem cell rescue versus the same regimen followed by cyclophosphamide 120 mg/kg plus TBI 9 Gy with peripheral blood stem cell support.7 Interferon was given as maintenance on both arms. With a median follow-up from randomization of 40 months, 81% of patients completed both cycles of MEL 70 and 82% completed the “double intensive therapy” arm. The CR rate was higher in the more intensive arm (28% vs 14%, P = .004). EFS at 48 months from randomization was also superior in the more intensive arm (29% vs 15%, P = .03) whereas OS at 4 years was similar in both arms (50% vs 55%, P = .3). Time to progression was significantly longer in the “double intensive arm” (61% vs 80% at 48 months, P = .003). On multivariate analysis, the more intensive therapy was an independent favorable variable along with level of anemia, lower B2M, and lower LDH. By combining B2M with cytogenetic abnormalities (del 13 and 1 p/q abnormalities), 3 distinct risk groups could be defined with markedly different survival outcomes.
An Italian randomized trial (BOLOGNA 96) evaluated single versus tandem autotransplants with MEL 200 versus an additional second high-dose therapy cycle with MEL 120 m/gm2 plus busulfan 12 mg/kg, both following 4 cycles of VAD and followed by interferon maintenance. Examination of the first 220 patients enrolled between 1996 and 1999 demonstrated superior EFS (median 34 months vs 25 months, P = .05). With a median follow-up of just 38 months, median OS is similar with 1 and 2 cycles of high-dose therapy (56 months vs 60 months).8
According to consensus panel discussions at the recent myeloma workshop in Salamanca, the failure of MAG 95 to demonstrate a difference for all clinical endpoints examined may relate to the difference in the TBI-containing regimens of the 2 study arms (see Table 4 ).
High host risk disease
The dose-limiting toxicity of stem cell–supported high-dose melphalan is stomatitis, which causes greater morbidity and mortality in the setting of renal failure, advanced age, and primary amyloidosis. Melphalan dose reduction to 140 mg/m2 or even 100 mg/m2 virtually eliminates this problem so that such patients are now frequently offered a dose-intensive treatment approach as well. Boccadoro and colleagues had demonstrated superior outcome with MEL 100 in 71 patients aged 60 to 70 years in comparison to a historical standard melphalan-prednisone control.9 These results have since been confirmed in a prospective randomized trial.10 In a comparison of 2 cycles of MEL 100 versus historically controlled pairmates receiving a two (single) cycles of MEL 200, the authors demonstrated comparable OS albeit inferior EFS with the dose-divided regimen.11
The setting of primary amyloidosis, with or without myeloma, is particularly challenging. Recent data from Mayo Clinic, also presented at Salamanca, comprised 125 patients with primary AL involving kidney (66%), heart (48%), peripheral nerves (14%), and liver (17%). The majority received G-CSF for PBSC collection.12,13 Conditioning was mainly with MEL 200 in 59, MEL 140 in 29, and MEL 100 in 11 patients; the remaining 17 patients received MEL 140 + TBI. Reduced dose conditioning was done according to patient risk factors. Hematologic responses (defined as in myeloma) and organ responses (defined as functional improvement) both were higher with MEL 200 and MEL 140 + TBI regimens (74% vs 48%, P = .01). Patient outcome was dominantly affected by serum creatinine at time of transplant and the number of organs involved. Median OS has been reached at 17 months for those patients with more than 2 organs involved by primary AL; organ responses were seen in 64% of patients, a result deemed superior to standard treatment approaches. Dose adjustments allowed for more patients with high-risk primary AL to be treated resulting, however, in a lower response rate. We recommend multiple cycles of high-dose therapy so that treatment can be tailored according to host risk and disease response, as it is now being evaluated in SWOG (S0115): following an initial cycle of MEL 100, the second cycle can be escalated to MEL 140 or MEL 200, in case the first cycle was well-tolerated and an insufficient antitumor effect was noted.
Issues and Non-issues with Autotransplants
Given the current state of the art, CD34 selection does not appear warranted as the level of tumor cytoreduction even with tandem transplants appears insufficient in the majority of patients for this procedure to impact outcome. Moreover, posttransplant immunosuppression appears prolonged, especially in the setting of CD 34+, Thy+, LIN− superselection.14,15 Improving preparative regimens beyond single agent MEL 200 has been difficult. Any reduction in melphalan dose, required to accommodate additional agents (TBI, busulfan, carmustine, cyclophosphamide, etoposide), led at best to equal and often inferior results. This is not to say that additional post- or peritransplant noncytotoxic approaches, such as dexamethasone, thalidomide or Velcade®, might not improve treatment results by interfering with DNA damage repair or suppressing myeloma survival stimuli that originate in the bone marrow microenvironment. Such peri- and intertransplant therapies are now being explored as part of IFM 9916 and at Arkansas.17
The value of intensive remission induction and posttransplant consolidation is currently under investigation in the Arkansas Total Therapy II (TT II) program that also examines the role of thalidomide in a prospective randomized trial design (Figure 7 ).
Results of the first 231 of currently 550 patients enrolled on TT II (accrual goal, 660 patients) were compared with the outcome of all 231 patients previously treated according to TT I. Data are still blinded with regard to thalidomide randomization and are therefore presented for all patients (Table 5 ).
Except for age, TT I and TT II patients were comparable with regard to key prognostic factors such as presence of cytogenetic abnormalities (CA) (TT I, 32%; TT II, 34%), presence of CA 13/hypodiploidy (TT I, 16%; TT II, 20%). Likewise, virtually identical proportions of patients had elevations of B2M ≥ 4 mg/L (29%), C-reactive protein (CRP) ≥ 4 mg/L (51%), and of LDH ≥ 190 IU/L (22%).
Among patients aged < 65 years, 77% completed 2 transplants on TT II and 74% on TT I; among the older age group, 62% of TT II and 48% of TT I patients completed the intended tandem transplant (P < .05). Cumulative treatment-related mortality encompassing the second transplant was lower with TT II than TT I in older patients (9% vs 14%; P < .05). Cumulative CR + n-CR rates were higher with TT II in both younger (80% vs 48%, P < .05) and older (79% vs 42%; P < .05) patients, independent of CA. Four-year EFS was superior with TT II versus TT I (64% vs 34%; P < .001) but not yet for OS (70% vs 62%; P = .13). On multivariate analysis of all 462 patients, TT II was the only favorable variable for both OS and EFS, whereas CA 13/hypodiploidy was the major adverse prognostic feature for both OS and EFS (see Table 5 ). Indeed, patients without CA (72% of TT II and 73% of TT I) faired significantly better with TT II than TT I (Figure 8; see Appendix, page 606). However, OS and EFS were similar with both regimens in case of CA 13/hypodiploidy or other CA.
Thus, further increase of dose intensity as practiced in TT II in comparison to TT I was feasible and safe. While nearly doubling CR + n-CR rates in all patients, regardless of CA, TT II improved EFS and OS markedly only in patients presenting without CA (approximately 70% of patients). Similar survival with TT II and TT I in the CA group, despite higher CR + n-CR rates, may result from lesser degree of cytoreduction beyond the clinical CR detection threshold (i.e., greater drug resistance) and/or more rapid regrowth kinetics in case of CA versus no CA. The contribution of thalidomide in good and poor risk groups is still unknown.
Allogeneic transplants
Standard myeloablative allogeneic transplants have been associated with high mortality often in excess of 50%. European Bone Marrow Transplant (EBMT) registry data, however, indicate marked improvement over the past 5 years so that survival has increased markedly, exclusively due to decreased transplant-related mortality with no reduction in relapse rate.18 Good prognosis was associated with female gender, younger age, low B2M, and responsiveness to prior therapy. An intergroup trial led by Southwest Oncology Group (S9321) evaluated, in patients up to 55 with an HLA-compatible sibling donor, a primary allogeneic transplant using MEL 140 plus TBI 12 Gy after VAD induction.19 The study was closed due to excessive transplant-related mortality after enrolling only 38 patients. Remarkably, however, EFS and OS have stabilized beyond 5 years, whereas there have been continued relapses and disease-related deaths among patients registered on autotransplant and standard treatment arms (Figure 9; see Appendix, page 607).
Nonmyeloablative regimens (mini-allotransplants) permit complete donor hematopoietic engraftment, especially when given after prior melphalan-based autotransplants. Mortality at 100 days has declined markedly to 10%–15% although, eventually, up to 25% to 30% succumb mainly due to complications from chronic graft-versus-host disease. Use of MEL 100 alone20 or in combination with fludarabine21 or TBI 200 cGy plus fludarabine,22 all resulted in only minor acute treatment-related toxicity and afforded prompt hematopoietic engraftment so that a third of patients did not require platelet or red blood cell transfusions. Similar positive results have also been reported for reduced intensity regimens in the context of matched unrelated donor (MUD) transplants.23
Updates of results from Seattle, MD Anderson, and Arkansas revealed superior outcome when mini-allotransplants were conducted as part of a planned tandem transplant strategy, i.e., following a consolidative autotransplant with MEL 200 (Figure 10; see Appendix, page 607).24 Disease recurrence, however, still occurs not infrequently, and it is too early to determine whether such mini-allotransplants, despite their lower acute toxicity, provide sustained disease control previously noted with standard allotransplants.
Prognostic factors
Many prognostic factors, pertinent to standard dose therapy, have also influenced outcome with high-dose therapies. They include B2M, CRP, plasma cell labeling index, LDH, and cytogenetics. Metaphase cytogenetic abnormalities (CA) are usually informative in no more than one third of newly diagnosed patients, although all myeloma cells are aneuploid when examined by interphase molecular analysis. A direct comparison of interphase FISH and conventional cytogenetics as part of TT II revealed that, among those exhibiting FISH-based del 13, only patients with del 13 by conventional cytogenetics had inferior outcome; those with FISH-13 without CA had a survival similar to those without FISH 13; patients with other CA (no FISH 13, CA) had an intermediate prognosis (Figure 11; see Appendix, page 607).25
We recommend that MM with CA of any type be considered a separate “malignant myeloma” entity with a short 3- to 4-year median survival even with tandem transplants that deserve novel treatment concepts (see below),26 including the tandem auto/mini-allotransplant approach (Figure 12; see Appendix, page 608).
Application of gene expression profiling to the majority of TT II patients at diagnosis has revealed unique expression profiles which can distinguish CA versus no CA and, within each subgroup, del 13 versus no del 13.27 Work is in progress to determine whether GEP of bone marrow biopsies together with analyses of highly purified CD138-positive plasma cells can help elucidate whether, in the presence of CA, the bone marrow microenvironment responds with amplified survival and proliferation signals far beyond levels evoked by more benign disease.
Previously Treated Myeloma
Such patients’ initial presentation, treatment details, and response require careful scrutiny with special emphasis on obtaining informative cytogenetics, since survival from relapse after tandem autotransplant is much shorter in case CA is present at relapse (Figure 13; see Appendix, page 608).26 This can be facilitated by computed tomography (CT)-guided fine needle aspirations of magnetic resonance imaging (MRI) or PET scan-identified lesions (Figure 14; see Appendix, page 608).
In case of pancytopenia, the possibility of t-MDS has to be considered, especially in patients treated for more than 1 year with melphalan or other stem cell toxic agents (e.g. carmustine). If cryopreserved PBSC are not available (especially when not previously autotransplanted but also when posttransplant survival exceeds 4–5 years), PBSC collection should be considered. Toward that goal, stem cell–toxic therapy should be avoided and DEX pulsing considered, possibly with the addition of low doses of thalidomide (50–100 mg).
New Agents
Thalidomide
Thalidomide effects responses (≥ 50% myeloma protein reduction [PR]) in one third of patients with advanced and refractory disease, the majority of whom had received prior autotransplants.28 Although not influencing initial response frequency, EFS and OS are poor in the presence of CA (Figure 15; see Appendix, page 609).29
Revimid
Revimid is another immunomodulatory agent that exhibits virtually no sedative and only occasionally neurotoxic side effects. Responses have been reported in one third of patients with advanced and refractory myeloma.30,31 Many of these patients had been previously exposed to thalidomide although true thalidomide resistance was infrequently established. Unlike thalidomide, Revimid® causes myelosuppression which, in the setting of compromised bone marrow reserve due to extensive prior cytotoxic drug exposure, may not be fully reversible. In a Phase III trial for advanced myeloma comparing 2 different schedules of administration (50 mg × 10 doses and 25 mg × 20 doses q 28 days) (Figure 16A and B; see Appendix, page 609), we observed higher response rates with the more prolonged 25-mg dose schedule (Figure 16C; see Appendix, page 609). Grade > 2 thrombocytopenia was linked to pretreatment platelet count < 100,000/μL as a reflection of impaired of hematopoietic reserve (Figure 16D; see Appendix, page 609). A randomized trial is being initiated in SWOG (S0232) for nontransplant candidates, evaluating Revimid alone versus its combination with dexamethasone.
Velcade
The proteasome inhibitor, Velcade®, effected PRs in one third of refractory myeloma patients, most of whom had been exposed previously to thalidomide and many had prior autotransplants.32 Velcade was combined with thalidomide (+ DEX) (VTD regimen) for posttandem transplant relapsed and refractory disease.33 The majority of patients had CA and had been exposed and become refractory to prior thalidomide (Figure 17A; see Appendix, page 610). VTD affected PR in approximately 60% of such patients (Figure 17B; see Appendix, page 610), regardless of the presence of CA (data not shown). Durable disease control, however, was limited to patients exhibiting no CA prior to VTD (Figure 17C and D; see Appendix, page 610).
Gene expression profiling
Studies of gene expression profiling prior to and 48 hours after treatment have been helpful in distinguishing drug-specific and response-specific alterations in gene expression (Figure 18A; see Appendix, page 610).17 Importantly, response to Velcade plus thalidomide could be predicted with high accuracy (Figure 18B; see Appendix, page 610).17
The Arkansas Approach to Therapy
Subjects with MGUS and smoldering multiple myeloma are studied with DNA microarray and immunologically as part of a SWOG trial S0120. Those with smoldering multiple myeloma at high risk for progression to overt disease (single bone lesion, IgA isotype, higher M protein levels)34 are offered thalidomide plus dexamethasone plus bisphosphonates to determine whether such progression can be delayed/prevented (S0231). This trial was developed based on encouraging data with thalidomide plus bisphosphonates observed in nearly 80 patients treated at Arkansas; similar results were noted at MD Anderson35 and Mayo Clinic.36
Toward improving therapy of overt myeloma, a major focus should be on the high-risk entity presenting with CA. We envision the evaluation of Velcade, together with thalidomide + DEX (VTD regimen) in high-risk myeloma up-front, both as induction prior to and for maintenance after melphalan-based tandem autotransplant. Assuming that the bone marrow microenvironment provides critical survival signals that rescue residual myeloma cells during hematopoietic recovery, peritransplant administration of DEX, thalidomide (THAL), or DEX + THAL, eventually together with Velcade, may provide an important adjunct toward extending disease control. Good-risk patients may enjoy a median EFS exceeding 6 to 7 years when treated according to TT II. Given the remarkably high response rate to THAL + DEX for induction, reported recently by Mayo Clinic37 and MD Anderson38 investigators, this approach is currently being tested for induction and maintenance in the context of a standard tandem autotransplant with MEL 200 mg/m2 under the auspices of SWOG (S0115). It is anticipated that such an approach is less toxic than chemotherapy induction and hence may increase the tandem transplant compliance, resulting in disease control comparable to more intensive approaches practiced with TT II.
Perspective and Conclusion
Minor permutations of standard melphalan-prednisone have long hindered advances in myeloma therapy.39 Raising the incidence of CR from previously less than 5% to the 50% level by melphalan dose intensification implies profound tumor cytoreduction well beyond the clinical detection level of 109 tumor cells in a sizeable fraction of patients. Intense investigations of patients with cytogenetic abnormalities, now also readily assessable by GEP, should help identify which of the new agents, targeting both myeloma and the bone marrow stroma, are most promising and can be incorporated into the MEL 200–based backbone of tandem transplants. Advances in such high-risk disease should provide important clues toward further refinement of therapy also for good-risk patients.
Translocation and cyclin D (TC) molecular classification of multiple myeloma.
| Group . | Primary Ig TLC . | Cyclin D . | Hyperdiploid * . | Prevalence, % . | Survival CC, %† . | Survival HDT, %† . |
|---|---|---|---|---|---|---|
| * There is not an absolute correlation of the groups with the presence or absence of hyperdiploidy. | ||||||
| †5-year overall survival with conventional chemotherapy (CC),14 and predicted 5-year overall survival with high-dose therapy (HDT). 23 | ||||||
| ‡ The results likely represent a composite group of TC2 and TC3. | ||||||
| TC1 | 11q13 | D1 | No | 15 | 38 | 88 |
| 6p21 | D3 | No | 3 | |||
| TC2 | None | D1 lo | Yes | 43 | ||
| 30‡ | 50‡ | |||||
| TC3 | None | D2 | ? | 17 | ||
| TC4 | 4p16 | D2 | No | 15 | 10 | 23 |
| TC5 | 16q23 | D2 | No | 5 | 13 | |
| 20q11 | D2 | No | 2 | |||
| Group . | Primary Ig TLC . | Cyclin D . | Hyperdiploid * . | Prevalence, % . | Survival CC, %† . | Survival HDT, %† . |
|---|---|---|---|---|---|---|
| * There is not an absolute correlation of the groups with the presence or absence of hyperdiploidy. | ||||||
| †5-year overall survival with conventional chemotherapy (CC),14 and predicted 5-year overall survival with high-dose therapy (HDT). 23 | ||||||
| ‡ The results likely represent a composite group of TC2 and TC3. | ||||||
| TC1 | 11q13 | D1 | No | 15 | 38 | 88 |
| 6p21 | D3 | No | 3 | |||
| TC2 | None | D1 lo | Yes | 43 | ||
| 30‡ | 50‡ | |||||
| TC3 | None | D2 | ? | 17 | ||
| TC4 | 4p16 | D2 | No | 15 | 10 | 23 |
| TC5 | 16q23 | D2 | No | 5 | 13 | |
| 20q11 | D2 | No | 2 | |||
The incidence of selected oncogenic events in multiple myeloma (MM) tumors and human MM cell lines (HMCL).
| . | MM Tumor . | HMCL . |
|---|---|---|
| *Karyotypic abnormalities of c-myc are 15% in all MM tumors, but the incidence is 47% in 36 advanced MM tumors that generated metaphase chromosomes. | ||
| ≥ 1 IgH translocation | 55%–73% | 92% |
| ≥ 2 IgH independent translocations | < 5 | 50 |
| ≥ 3 IgH independent translocations | < 1 | 13 |
| 5 Recurrent Translocations (combined) | 40% | 89% |
| 11q13 (cyclin D1) | 15 | 29 |
| 6p21 (cyclin D3) | 3 | 3 |
| 4p16.3 (FGFR3 + MMSET) | 15 | 26 |
| 16q23 (c-maf) | 5 | 21 |
| 20q11 (maf b) | 2 | 11 |
| Ectopic cyclin D1 expression [no t(11;14)] | 43 | 0 |
| 8q24 (c-myc) karyotypic abnormality | 15 (47*) | 88 |
| . | MM Tumor . | HMCL . |
|---|---|---|
| *Karyotypic abnormalities of c-myc are 15% in all MM tumors, but the incidence is 47% in 36 advanced MM tumors that generated metaphase chromosomes. | ||
| ≥ 1 IgH translocation | 55%–73% | 92% |
| ≥ 2 IgH independent translocations | < 5 | 50 |
| ≥ 3 IgH independent translocations | < 1 | 13 |
| 5 Recurrent Translocations (combined) | 40% | 89% |
| 11q13 (cyclin D1) | 15 | 29 |
| 6p21 (cyclin D3) | 3 | 3 |
| 4p16.3 (FGFR3 + MMSET) | 15 | 26 |
| 16q23 (c-maf) | 5 | 21 |
| 20q11 (maf b) | 2 | 11 |
| Ectopic cyclin D1 expression [no t(11;14)] | 43 | 0 |
| 8q24 (c-myc) karyotypic abnormality | 15 (47*) | 88 |
Summary of the published placebo-controlled trials of bisphosphonates in patients with multiple myeloma (MM).
| . | Belch35(1991) . | Lahtinen36(1992) . | Berenson37(1996) . | McCloskey38(1998) . | Menssen39(2002) . |
|---|---|---|---|---|---|
| Etidronate: 5 mg/kg/d per os, 24 months per os | |||||
| Clodronate: 2.4 mg/kg/d per os, 24 months (Lahtinen) and 1.6 d/day per os | |||||
| Pamidronate: 90 mg IV, monthly 24 months | |||||
| Ibandronate: 2 mg IV, monthly 12–24 months | |||||
| & Patients with no skeletal fracture at presentation | |||||
| # Patients with inhibition of bone resorption | |||||
| Abbreviations: 0, no effect; +, beneficial effect; −, harmful effect; NA, not assessed. | |||||
| No. evaluable patients | 166 | 336 | 377 | 535 | 214 |
| Bisphosphonates | Etidronate | Clodronate | Pamidronate | Clodronate | Ibandronate |
| Lytic bone lesions | 0 | + | 0 | NA | 0 |
| Pathologic fractures | 0 | 0 | + | + | 0 |
| Bone pain | 0 | 0 | + | + | 0 |
| Bone healing | 0 | NA | 0 | NA | Increase BMD |
| Hypercalcemia | 0 | 0 | + | + (trend) | 0 |
| Survival | – | 0 | + (trend) | + (subset)& | + (subset)# |
| . | Belch35(1991) . | Lahtinen36(1992) . | Berenson37(1996) . | McCloskey38(1998) . | Menssen39(2002) . |
|---|---|---|---|---|---|
| Etidronate: 5 mg/kg/d per os, 24 months per os | |||||
| Clodronate: 2.4 mg/kg/d per os, 24 months (Lahtinen) and 1.6 d/day per os | |||||
| Pamidronate: 90 mg IV, monthly 24 months | |||||
| Ibandronate: 2 mg IV, monthly 12–24 months | |||||
| & Patients with no skeletal fracture at presentation | |||||
| # Patients with inhibition of bone resorption | |||||
| Abbreviations: 0, no effect; +, beneficial effect; −, harmful effect; NA, not assessed. | |||||
| No. evaluable patients | 166 | 336 | 377 | 535 | 214 |
| Bisphosphonates | Etidronate | Clodronate | Pamidronate | Clodronate | Ibandronate |
| Lytic bone lesions | 0 | + | 0 | NA | 0 |
| Pathologic fractures | 0 | 0 | + | + | 0 |
| Bone pain | 0 | 0 | + | + | 0 |
| Bone healing | 0 | NA | 0 | NA | Increase BMD |
| Hypercalcemia | 0 | 0 | + | + (trend) | 0 |
| Survival | – | 0 | + (trend) | + (subset)& | + (subset)# |
Summary of high dose therapy trials in multiple myeloma.
| . | . | . | . | . | . | . | . | . | . | . | EFS . | OS . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author . | Randomization . | Regimen* . | SDT Regimen . | HDT Regimen . | Maintenance . | N . | Age . | Median FU / mos . | % CR . | P . | Median Mos. . | % Yr . | EFS p . | Median Mos. . | % Yr. . | OS P . |
| Abbreviations: SDT, standard dose therapy; HDT, high-dose therapy; IDT, intermediate-dose therapy; IFN, interferon; TBI, total body irradiation; MEL, melphalan; OS, overall survival; MEL, melphalan; CR, complete response; NA, not applicable; G-CSF, granulocyte colony-stimulating factor; DEX, dexamethasone; VAD, vincristine, adriamycin, DEX; VMCP, vincristine, MEL, cyclosphosphamide, prednisone (alternates with VBAP every 3 weeks); BVAP, BCNU (carmustine), vincristine, doxorubicin, prednisone; ABCM, adriamycin (doxorubicin), BCNU (carmustine), cyclophosphamide, MEL; CVAMP, cyclophosphamide, vincristine, adriamycin (doxorubicin), methylprednisolone (prednisone), cisplatin; EDAP, etoposide (VP-16), dexamethasone, Ara-C (cytarabine), cisplatin; FU, follow-up | ||||||||||||||||
| Attal2 | Pre-Rx | SDT | VMCP/BVAP × 18 | IFN | 100 | 58 | 108 | 14 | <.001 | 18 | NA | .01 | 44 | 20 (7 yr) | .03 | |
| HDT | VMCP/BVAP × 4–6 → CTX | MEL 140 + TBI 8Gy | IFN | 100 | 57 | 38 | 28 | NA | 57 | 35 (7 yr) | ||||||
| Child3 MRC VII | Pre-Rx | SDT | ABCM × 4–12 | IFN | 200 | 56 | 42 | 8 | <.001 | 20 | 16 (4 yr) | <.001 | 42 | 46 (4 yr) | .04 | |
| HDT | CVAMP × 3 → CTX | MEL 200 | IFN | 201 | 55 | 44 | 32 | 36 (4 yr) | 54 | 55 (4 yr) | ||||||
| Bladé4 PETHEMA | Responders to Iinduction | SDT | VBMCP/VBAD × 12 | IFN + DEX | 83 | 56 | 66 | 11 | .002 | 34 | NA | NS | 67 | NA | NS | |
| HDT | VBMCP/VBAD × 4 | MEL 200 | IFN + DEX | 81 | 56 | 30 | 43 | NA | 65 | NA | ||||||
| Attal5 IFM 94 | Pre-Rx | HDT ×1 | VAD × 3–4 → G-CSF | MEL 140 + TBI 8 Gy | IFN | 199 | 52 | 75 | 42 ≥ n-CR | 0.1 | 25 | 10 (7yr) | .03 | 48 | 21 (7 yr) | .01 |
| HDT ×2 | VAD × 3–4 → G-CSF | MEL 140 →MEL 140 + TBI 8Gy | IFN | 200 | 52 | 50 ≥ n-CR | 30 | 20 (7 yr) | 58 | 42 (7 yr) | ||||||
| Cavo8 BOLOGNA 96 | Pre-Rx | HDT ×1 | VAD × 4 → CTX | MEL 200 | 110 | 53 | 38 | 21 | NS | 25 | NA | .05 | 56 | NS | ||
| HDT ×2 | VAD × 4 → CTX | MEL 200→MEL 120 + Busulfan | 110 | 53 | 24 | 34 | NA | 60 | ||||||||
| Fermand6 | Pre-Rx | HDT ×1 | DEX × 2 → CTX → | MEL 140 + BCNU + | 97 | 50 | 53 | 39 | NS | 31 | NA | NS | 49 | NA | .14 | |
| MAG95 | VAD × 3–4 | VP16 + CTX + TBI 12Gy | ||||||||||||||
| HDT ×2 | DEX × 2 → CTX → VAD × 3–4 | MEL 140→MEL 140 + VP16 + TBI 12Gy | 96 | 50 | 37 | 33 | NA | 73 | NA | |||||||
| Sonneveld1 HOVON | After VAD +/- Response | IDT | VAD × 3–4 → CTX | MEL 70 × 2 | IFN | 129 | 55 | 40 | 14 | .004 | NA | 15 (4 yr) | .03 | NA | 55 (4 yr) | .3 |
| IDT/HDT | VAD × 3–4 → CTX | MEL 70 × 2 → CTX + TBI 9Gy | IFN | 132 | 56 | 28 | NA | 29 (4 yr) | NA | 50 (4 yr) | ||||||
| Barlogie17 TTI vs SWOG | Historical Controls | SDT | VMCP(P) / VBAP(P) / VAD | IFN | 152 | 52 | 114 | NA | 16 | 5 (10 yr) | <.0001 | 43 | 15 (10 yr) | <.0001 | ||
| HDT ×2 | VAD × 2–3 → CTX → EDAP | MEL 200 × 2 (< PR, MEL 140 + TBI 8.5 Gy) | IFN | 152 | 52 | 41 | 37 | 15 (10 yr) | 79 | 33 (10 yr) | ||||||
| . | . | . | . | . | . | . | . | . | . | . | EFS . | OS . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author . | Randomization . | Regimen* . | SDT Regimen . | HDT Regimen . | Maintenance . | N . | Age . | Median FU / mos . | % CR . | P . | Median Mos. . | % Yr . | EFS p . | Median Mos. . | % Yr. . | OS P . |
| Abbreviations: SDT, standard dose therapy; HDT, high-dose therapy; IDT, intermediate-dose therapy; IFN, interferon; TBI, total body irradiation; MEL, melphalan; OS, overall survival; MEL, melphalan; CR, complete response; NA, not applicable; G-CSF, granulocyte colony-stimulating factor; DEX, dexamethasone; VAD, vincristine, adriamycin, DEX; VMCP, vincristine, MEL, cyclosphosphamide, prednisone (alternates with VBAP every 3 weeks); BVAP, BCNU (carmustine), vincristine, doxorubicin, prednisone; ABCM, adriamycin (doxorubicin), BCNU (carmustine), cyclophosphamide, MEL; CVAMP, cyclophosphamide, vincristine, adriamycin (doxorubicin), methylprednisolone (prednisone), cisplatin; EDAP, etoposide (VP-16), dexamethasone, Ara-C (cytarabine), cisplatin; FU, follow-up | ||||||||||||||||
| Attal2 | Pre-Rx | SDT | VMCP/BVAP × 18 | IFN | 100 | 58 | 108 | 14 | <.001 | 18 | NA | .01 | 44 | 20 (7 yr) | .03 | |
| HDT | VMCP/BVAP × 4–6 → CTX | MEL 140 + TBI 8Gy | IFN | 100 | 57 | 38 | 28 | NA | 57 | 35 (7 yr) | ||||||
| Child3 MRC VII | Pre-Rx | SDT | ABCM × 4–12 | IFN | 200 | 56 | 42 | 8 | <.001 | 20 | 16 (4 yr) | <.001 | 42 | 46 (4 yr) | .04 | |
| HDT | CVAMP × 3 → CTX | MEL 200 | IFN | 201 | 55 | 44 | 32 | 36 (4 yr) | 54 | 55 (4 yr) | ||||||
| Bladé4 PETHEMA | Responders to Iinduction | SDT | VBMCP/VBAD × 12 | IFN + DEX | 83 | 56 | 66 | 11 | .002 | 34 | NA | NS | 67 | NA | NS | |
| HDT | VBMCP/VBAD × 4 | MEL 200 | IFN + DEX | 81 | 56 | 30 | 43 | NA | 65 | NA | ||||||
| Attal5 IFM 94 | Pre-Rx | HDT ×1 | VAD × 3–4 → G-CSF | MEL 140 + TBI 8 Gy | IFN | 199 | 52 | 75 | 42 ≥ n-CR | 0.1 | 25 | 10 (7yr) | .03 | 48 | 21 (7 yr) | .01 |
| HDT ×2 | VAD × 3–4 → G-CSF | MEL 140 →MEL 140 + TBI 8Gy | IFN | 200 | 52 | 50 ≥ n-CR | 30 | 20 (7 yr) | 58 | 42 (7 yr) | ||||||
| Cavo8 BOLOGNA 96 | Pre-Rx | HDT ×1 | VAD × 4 → CTX | MEL 200 | 110 | 53 | 38 | 21 | NS | 25 | NA | .05 | 56 | NS | ||
| HDT ×2 | VAD × 4 → CTX | MEL 200→MEL 120 + Busulfan | 110 | 53 | 24 | 34 | NA | 60 | ||||||||
| Fermand6 | Pre-Rx | HDT ×1 | DEX × 2 → CTX → | MEL 140 + BCNU + | 97 | 50 | 53 | 39 | NS | 31 | NA | NS | 49 | NA | .14 | |
| MAG95 | VAD × 3–4 | VP16 + CTX + TBI 12Gy | ||||||||||||||
| HDT ×2 | DEX × 2 → CTX → VAD × 3–4 | MEL 140→MEL 140 + VP16 + TBI 12Gy | 96 | 50 | 37 | 33 | NA | 73 | NA | |||||||
| Sonneveld1 HOVON | After VAD +/- Response | IDT | VAD × 3–4 → CTX | MEL 70 × 2 | IFN | 129 | 55 | 40 | 14 | .004 | NA | 15 (4 yr) | .03 | NA | 55 (4 yr) | .3 |
| IDT/HDT | VAD × 3–4 → CTX | MEL 70 × 2 → CTX + TBI 9Gy | IFN | 132 | 56 | 28 | NA | 29 (4 yr) | NA | 50 (4 yr) | ||||||
| Barlogie17 TTI vs SWOG | Historical Controls | SDT | VMCP(P) / VBAP(P) / VAD | IFN | 152 | 52 | 114 | NA | 16 | 5 (10 yr) | <.0001 | 43 | 15 (10 yr) | <.0001 | ||
| HDT ×2 | VAD × 2–3 → CTX → EDAP | MEL 200 × 2 (< PR, MEL 140 + TBI 8.5 Gy) | IFN | 152 | 52 | 41 | 37 | 15 (10 yr) | 79 | 33 (10 yr) | ||||||
Univariate and multivariate regression results for Total Therapy I (TT I, n = 231) combined with the first 231 patients on Total Therapy II (TT II).
| . | % of Patients . | Overall Survival . | . | Event-free Survival . | |
|---|---|---|---|---|---|
| Abbreviations: HR, hazard ratio; CI, confidence interval; P, regression model P-value; NS, not significant; CA, cytogenetic abnormalities; CRP, C-reactive protein; LDH; B2M, beta-2 microglobulin | |||||
| TT I / TT II | HR (95% CI) | P | HR (95% CI) | P | |
| Univariate | |||||
| Total Therapy II | — | 0.8 (0.6, 1.1) | .167 | 0.5 (0.3, 0.6) | <.001 |
| Age ≥ 65 yrs | 9/21 | 1.9 (1.4, 2.8) | <.001 | 1.6 (1.2, 2.2) | .003 |
| Any CA | 32/34 | 2.1 (1.6, 2.8) | <.001 | 1.7 (1.3, 2.2) | <.001 |
| CA13 / hypodiploid | 16/20 | 2.8 (2.0, 3.9) | <.001 | 1.9 (1.4, 2.6) | <.001 |
| CRP ≥ 4 mg/L | 47/56 | 1.8 (1.3, 2.3) | <.001 | 1.6 (1.3, 2.1) | <.001 |
| LDH ≥ 190 IU/L | 21/22 | 2.0 (1.5, 2.7) | <.001 | 1.6 (1.2, 2.1) | |
| B2M ≥ 4 mg/L | 30/29 | 1.9 (1.4, 2.5) | <.001 | 1.8 (1.4, 2.4) | <.001 |
| Multivariate | |||||
| Total Therapy II | 0.6 (0.4, 0.9) | .012 | 0.4 (0.3, 0.5) | <.001 | |
| Age ≥ 65 yrs | 1.7 (1.2, 2.6) | .005 | 1.7 (1.2, 2.4) | .002 | |
| CA13 / hypodiploid | 2.6 (1.9, 3.6) | <.001 | 1.9 (1.4, 2.6) | <.001 | |
| CRP ≥ 4 mg/L | 1.7 (1.3, 2.3) | <.001 | 1.6 (1.2, 2.1) | <.001 | |
| B2M ≥ 4 mg/L | NS | 1.5 (1.2, 2.0) | .003 | ||
| LDH ≥ 190 IU/L | 1.5 (1.1, 2.1) | .010 | NS | ||
| . | % of Patients . | Overall Survival . | . | Event-free Survival . | |
|---|---|---|---|---|---|
| Abbreviations: HR, hazard ratio; CI, confidence interval; P, regression model P-value; NS, not significant; CA, cytogenetic abnormalities; CRP, C-reactive protein; LDH; B2M, beta-2 microglobulin | |||||
| TT I / TT II | HR (95% CI) | P | HR (95% CI) | P | |
| Univariate | |||||
| Total Therapy II | — | 0.8 (0.6, 1.1) | .167 | 0.5 (0.3, 0.6) | <.001 |
| Age ≥ 65 yrs | 9/21 | 1.9 (1.4, 2.8) | <.001 | 1.6 (1.2, 2.2) | .003 |
| Any CA | 32/34 | 2.1 (1.6, 2.8) | <.001 | 1.7 (1.3, 2.2) | <.001 |
| CA13 / hypodiploid | 16/20 | 2.8 (2.0, 3.9) | <.001 | 1.9 (1.4, 2.6) | <.001 |
| CRP ≥ 4 mg/L | 47/56 | 1.8 (1.3, 2.3) | <.001 | 1.6 (1.3, 2.1) | <.001 |
| LDH ≥ 190 IU/L | 21/22 | 2.0 (1.5, 2.7) | <.001 | 1.6 (1.2, 2.1) | |
| B2M ≥ 4 mg/L | 30/29 | 1.9 (1.4, 2.5) | <.001 | 1.8 (1.4, 2.4) | <.001 |
| Multivariate | |||||
| Total Therapy II | 0.6 (0.4, 0.9) | .012 | 0.4 (0.3, 0.5) | <.001 | |
| Age ≥ 65 yrs | 1.7 (1.2, 2.6) | .005 | 1.7 (1.2, 2.4) | .002 | |
| CA13 / hypodiploid | 2.6 (1.9, 3.6) | <.001 | 1.9 (1.4, 2.6) | <.001 | |
| CRP ≥ 4 mg/L | 1.7 (1.3, 2.3) | <.001 | 1.6 (1.2, 2.1) | <.001 | |
| B2M ≥ 4 mg/L | NS | 1.5 (1.2, 2.0) | .003 | ||
| LDH ≥ 190 IU/L | 1.5 (1.1, 2.1) | .010 | NS | ||
Two pathways of generating monoclonal gammopathy of undetermined significance (MGUS)/multiple myeloma (MM) tumors.
Tumors can be generated either with (+) or without (−) the occurrence of a primary translocation, the former being mainly nonhyperdiploid and the latter mainly hyperdiploid. Dysregulation of a cyclin D gene appears to be an early event in each case. Secondary translocations can occur in both kinds of tumors. As indicated, tumors in the former pathway selectively generate stromal independent MM cell lines, but the dashed arrows suggest the possibility that the latter pathway might generate cell lines.
Two pathways of generating monoclonal gammopathy of undetermined significance (MGUS)/multiple myeloma (MM) tumors.
Tumors can be generated either with (+) or without (−) the occurrence of a primary translocation, the former being mainly nonhyperdiploid and the latter mainly hyperdiploid. Dysregulation of a cyclin D gene appears to be an early event in each case. Secondary translocations can occur in both kinds of tumors. As indicated, tumors in the former pathway selectively generate stromal independent MM cell lines, but the dashed arrows suggest the possibility that the latter pathway might generate cell lines.
Biological mechanisms involved in the excessive bone resorption in multiple myeloma (MM).
(A) MM tumor induces enhanced osteoclast differentiation and activity directly and through stromal cells/osteoblasts and (B) decreases bone formation.
Biological mechanisms involved in the excessive bone resorption in multiple myeloma (MM).
(A) MM tumor induces enhanced osteoclast differentiation and activity directly and through stromal cells/osteoblasts and (B) decreases bone formation.
Structural domains of Bcl-2 family members.
Transmembrane (TM) domains mediate insertion into the mitochondrial outer membrane. The Bcl-2-homology (BH) domains 1, 2, and 3 of antiapoptotic family members form a hydrophobic binding pocket for BH3-only proteins. Mcl-1 encodes a unique amino terminal domain of 180 amino acids, which may be involved in functions that are unique to Mcl-1. The multidomain killers Bax and Bak can oligomerize to form pores in the outer mitochondrial membrane in a process regulated by BH3-only family members.
Structural domains of Bcl-2 family members.
Transmembrane (TM) domains mediate insertion into the mitochondrial outer membrane. The Bcl-2-homology (BH) domains 1, 2, and 3 of antiapoptotic family members form a hydrophobic binding pocket for BH3-only proteins. Mcl-1 encodes a unique amino terminal domain of 180 amino acids, which may be involved in functions that are unique to Mcl-1. The multidomain killers Bax and Bak can oligomerize to form pores in the outer mitochondrial membrane in a process regulated by BH3-only family members.
The induction of apoptosis by diverse death stimuli occurs through the transcriptional or posttranscriptional regulation of BH3-only proteins.
Levels of Noxa and Puma are transcriptionally upregulated by p53 in response to DNA damage. Cytokines and growth factors act as survival factors by inhibiting the proapoptotic activities of Bad and Bim through changes in the state of protein phosphorylation. Loss of cell adherence to extracellular matrix can lead to a form of apoptosis termed “anoikis,” and this is regulated by BH3 proteins that associate with actin filaments (Bmf) or microtubules (Bim). The caspase 8-mediated cleavage of Bid to form tBid acts as a link between the extrinsic and intrinsic pathways of apoptosis. BH3-only proteins translocate to the mitochondria, where they can bind to antiapoptotic (Bcl-2, Bcl-XL, Mcl-1) or proapoptotic (Bax, Bak) Bcl-2 family members to regulate mitochondrial outer membrane permeability (MOMP) and the release of cytochrome c, apoptosis-inducing factor (AIF), and SMAC/Diablo (see Figure 6). Bax is cytosolic until death signals induce a conformational change followed by its insertion into the outer mitochondrial membrane with formation of oligomers. Recent studies demonstrate that the level of Ca+2 stores in the endoplasmic reticulum (ER) and subsequent uptake of Ca+2 by the mitochondria play an important role in determining the threshold for apoptosis. This cyclical ER-mitochondrial flux of Ca+2 is regulated in part by Bax, Bak, Bcl-2 and other family members that are localized to these organelles. Abbreviations: IMS, inter-membrane space; SMAC, second mitochondrial activator of caspases; PKA, protein kinase A
The induction of apoptosis by diverse death stimuli occurs through the transcriptional or posttranscriptional regulation of BH3-only proteins.
Levels of Noxa and Puma are transcriptionally upregulated by p53 in response to DNA damage. Cytokines and growth factors act as survival factors by inhibiting the proapoptotic activities of Bad and Bim through changes in the state of protein phosphorylation. Loss of cell adherence to extracellular matrix can lead to a form of apoptosis termed “anoikis,” and this is regulated by BH3 proteins that associate with actin filaments (Bmf) or microtubules (Bim). The caspase 8-mediated cleavage of Bid to form tBid acts as a link between the extrinsic and intrinsic pathways of apoptosis. BH3-only proteins translocate to the mitochondria, where they can bind to antiapoptotic (Bcl-2, Bcl-XL, Mcl-1) or proapoptotic (Bax, Bak) Bcl-2 family members to regulate mitochondrial outer membrane permeability (MOMP) and the release of cytochrome c, apoptosis-inducing factor (AIF), and SMAC/Diablo (see Figure 6). Bax is cytosolic until death signals induce a conformational change followed by its insertion into the outer mitochondrial membrane with formation of oligomers. Recent studies demonstrate that the level of Ca+2 stores in the endoplasmic reticulum (ER) and subsequent uptake of Ca+2 by the mitochondria play an important role in determining the threshold for apoptosis. This cyclical ER-mitochondrial flux of Ca+2 is regulated in part by Bax, Bak, Bcl-2 and other family members that are localized to these organelles. Abbreviations: IMS, inter-membrane space; SMAC, second mitochondrial activator of caspases; PKA, protein kinase A
Model for the regulation of the mitochondrial pathway of apoptosis by Bcl-2-family members.
1. In the absence of apoptotic stimuli, Bak molecules reside in the outer mitochondrial membrane but do not oligomerize to form pores; Mcl-1 may associate with Bak to block Bak self-association.
2. Apoptosis-inducing signals promote the movement of BH3-only proteins to the outer mitochondrial membrane (OMM), where some BH3-only family members (e.g., Bid, Bim) transiently associate with Bak or Bax to induce a conformational change resulting in formation of homo-oligomers and induction of apoptosis.
3. Antiapoptotic proteins such as Mcl-1 and Bcl-XL (or Bcl-2, not shown) may be overexpressed in multiple myeloma (MM) cells and sequester BH3-only proteins and prevent their interaction with Bak or Bax, thus overcoming cell death signals. Inhibition of expression of antiapoptotic proteins in MM cells could overcome this tumor survival mechanism.
4. Antiapoptotic effects of Mcl-1 and Bcl-XL can be overcome by excess BH3-only proteins.
5. Resistance to apoptosis may be reversed by the development of low molecular weight, cell-permeable drugs (D) that bind to the hydrophobic BH1-3 pocket of Mcl-1 and/or Bcl-XL, thus preventing the sequestration of BH3-only proteins. The latter are then free to interact with Bak and Bax with the subsequent formation of pores and induction of apoptosis. Efforts are in progress to identify novel compounds that specifically target Mcl-1 (D1) or Bcl-XL (D2).
Model for the regulation of the mitochondrial pathway of apoptosis by Bcl-2-family members.
1. In the absence of apoptotic stimuli, Bak molecules reside in the outer mitochondrial membrane but do not oligomerize to form pores; Mcl-1 may associate with Bak to block Bak self-association.
2. Apoptosis-inducing signals promote the movement of BH3-only proteins to the outer mitochondrial membrane (OMM), where some BH3-only family members (e.g., Bid, Bim) transiently associate with Bak or Bax to induce a conformational change resulting in formation of homo-oligomers and induction of apoptosis.
3. Antiapoptotic proteins such as Mcl-1 and Bcl-XL (or Bcl-2, not shown) may be overexpressed in multiple myeloma (MM) cells and sequester BH3-only proteins and prevent their interaction with Bak or Bax, thus overcoming cell death signals. Inhibition of expression of antiapoptotic proteins in MM cells could overcome this tumor survival mechanism.
4. Antiapoptotic effects of Mcl-1 and Bcl-XL can be overcome by excess BH3-only proteins.
5. Resistance to apoptosis may be reversed by the development of low molecular weight, cell-permeable drugs (D) that bind to the hydrophobic BH1-3 pocket of Mcl-1 and/or Bcl-XL, thus preventing the sequestration of BH3-only proteins. The latter are then free to interact with Bak and Bax with the subsequent formation of pores and induction of apoptosis. Efforts are in progress to identify novel compounds that specifically target Mcl-1 (D1) or Bcl-XL (D2).
Treatment schema.
Abbreviations: VAD, vincristine, adriamycin, dexamethasone; EDAP, etoposide (VP-16), dexamethasone, Ara-C (cytarabine), cisplatin; DCEP, dexmethasone, cyclophosphamide, etoposide (VP-16), cisplatin; CAD, cyclophosphamide, adriamycin, dexamethasone; HD, high-dose therapy; CTX, chemotherapy; MEL, melphalan.
Treatment schema.
Abbreviations: VAD, vincristine, adriamycin, dexamethasone; EDAP, etoposide (VP-16), dexamethasone, Ara-C (cytarabine), cisplatin; DCEP, dexmethasone, cyclophosphamide, etoposide (VP-16), cisplatin; CAD, cyclophosphamide, adriamycin, dexamethasone; HD, high-dose therapy; CTX, chemotherapy; MEL, melphalan.