Abstract
The outlook for patients with sickle cell disease has improved steadily during the last two decades. In spite of these improvements, curative therapies are currently available only to a small minority of patients. The main theme of this chapter is to describe new therapeutic options that are at different stages of development that might result in further improvements in the outlook for patients with these disorders.
Dr. Joseph DeSimone and his colleagues had previously made the important observation that the hypomethylating agent 5-azacytidine can reverse the switch from adult to fetal hemoglobin in adult baboons. Although similar activity was demonstrated in patients with sickle cell disease and β-thalassemia, concern about the toxicity of 5-azacytidine prevented its widespread use in these disorders. In Section I, Dr. DeSimone discusses the role of DNA methylation in globin gene regulation and describe recent clinical experience with decitabine (an analogue of 5-azacytidine) in patients with sickle cell disease. These encouraging studies demonstrate significant fetal hemoglobin inducing activity of decitabine in patients who fail to respond to hydroxyurea.
In Section II, Dr. George Atweh continues the same theme by describing recent progress in the study of butyrate, another inducer of fetal hemoglobin, in patients with sickle cell disease and β-thalassemia. The main focus of his section is on the use of a combination of butyrate and hydroxyurea to achieve higher levels of fetal hemoglobin that might be necessary for complete amelioration of the clinical manifestations of these disorders. Dr. Atweh also describes novel laboratory studies that shed new light on the mechanisms of fetal hemoglobin induction by butyrate.
In Section III, Dr. Ronald Nagel discusses the different available transgenic sickle mice as experimental models for human sickle cell disease. These experimental models have already had a significant impact on our understanding of the pathophysiology of sickle cell disease. Dr. Nagel describes more recent studies in which transgenic sickle mice provide the first proof of principle that globin gene transfer into hematopoietic stem cells inhibits in vivo sickling and ameliorates the severity of the disease.
Although stroke in adult patients with sickle cell disease is not as common as in children, adult hematologists, like their pediatric colleagues, need to make management decisions in adult patients with a stroke or a history of stroke. Dr. Robert Adams has led several large clinical studies that investigated the role of transfusions in the prevention of stroke in children with sickle cell disease. Much less is known, however, about the prevention of first or subsequent strokes in adult patients with sickle cell disease. In Section IV, Dr. Adams provides some general guidelines for the management of adult patients with stroke while carefully distinguishing between recommendations that are evidence-based and those that are anecdotal in nature.
I. DNA Methylation and the Treatment of Hemoglobinopathies
Joseph DeSimone, PhD,* and Yogen Saunthararajah, MD
VA Chicago West Side Division, 820 South Damen Avenue, Chicago IL 60612
Elevated fetal hemoglobin (HbF) has been demonstrated to be beneficial in sickle cell anemia and β-thalassemia, and pharmacological manipulation of HbF levels has been a goal for several decades. The γ-globin gene promoter had been shown to be nonmethylated at a CpG residue during fetal development, and this dinucleotide sequence became methylated (mCpG) postnatally when γ-globin gene expression was silenced.1 Early studies demonstrated that treatment of tissue culture cells with the cytidine analog 5-azacytidine (5-azaC) led to cellular differentiation and DNA hypomethylation.2 Incorporation of 5-azaC into baboon DNA resulted in a 40- to 70-fold increase in γ-globin gene expression in the adult,3 and to hypomethylation of the the γ-globin promoter.4 These early studies led to clinical trials of this analog in sickle cell anemia and β-thalassemia,5– 8 which demonstrated significant increases in HbF levels (10%–25%), F cells (30%–40%), and total hemoglobin (1–3 g/dL) in patients receiving 5-azaC.
Despite these encouraging results, clinical trials were discontinued after publication of a flawed study, using the Fischer rat cancer model, suggesting that 5-azaC was carcinogenic.9 Fischer rats historically have a 20% rate of testicular cancer,10 but no cancers were observed in the control animals. A subsequent paper by this group11 demonstrated that the deoxy form, 5-aza-2-deoxycytidine (decitabine), was not carcinogenic and possibly had tumor suppressor activity. Ten of 49 control Fischer rats developed cancer, whereas no cancers were found in 10 animals treated with decitabine (P = .107). Tumor suppressor activity was also demonstrated in a mouse intestinal neoplasia model, using weekly injections of decitabine. In these genetically susceptible mice, the average number of intestinal adenomas was reduced from 113 in control mice to only 2 in treated mice.12 At the time that these papers were published, trials of hydroxyurea (HU) for the treatment of sickle cell anemia were already under way. The early results suggested that it would be a beneficial therapy, and therefore, further trials with 5-azaC analogs were thought to be unnecessary. However, when the results of the Multicenter Study of Hydroxyurea (MSH) were published demonstrating that only 50% of the HU-treated patients had increased HbF levels following treatment,13 the use of 5-azaC analogs was reevaluated.
DNA Hypomethylation and Globin Gene Expression
When a 5-azaC analog incorporates into DNA as 5-aza-2-deoxycytidine, it covalently binds DNA methyltransferase (DNMT), thereby depleting DNMT concentration.14–,15 This decrease in DNMT results in DNA hypomethylation as DNMT is required for maintenance of the methylation pattern during replication. Patients treated with 5-azaC analogs show global hypomethylation. Both the ε- and γ-globin promoters are equally hypomethylated following treatment, but only the γ-globin gene is expressed, demonstrating that hypomethylation is necessary but not sufficient for gene expression.6 These results are consistent with our present understanding of the molecular requirements for gene expression. Transcription requires an open chromatin configuration dictated by specific patterns of histone modifications, including acetylation, methylation, and phosphorylation of amino acids in amino terminal histone tails, and hypomethylation of CpG dinucleotide residues. For example, histone acetylation is required for an active chromatin configuration, and acetylated histone does not associate with DNA having mCpG dinucleotides.16
The level of acetylation is, to a large extent, dependent on histone deacetylase (HDAC) activity. It was previously thought that histone modification was dependent on DNA methylation.16 The mCpG binding proteins (MeCP1 and MeCP2) help maintain the methylated state. These DNA binding proteins are found in large protein complexes containing DNMT, histone acetylase, and HDAC. Binding of MeCP passively recruits HDAC favoring DNA methylation, histone deacetylation, and gene suppression. When the CpG is not methylated, MeCP does not bind, thus favoring histone acetylation and gene expression.
There is a third level of regulation related to DNA methylation, and that is histone methylation. Histone methylation can have an activating or suppressing effect depending upon which amino acids are methylated, and the number of methyl groups added. The current hypothesis is that histone modifications and DNA methylation may influence each other. It has also been shown that there is a direct connection between the enzymes that methylate DNA and methylate histones.17 Thus DNA methylation appears to be central in transcriptional regulation. This crosstalk suggests that inhibitors of DNMT and HDAC may collaborate to activate transcription.18
The CpG content of the β-globin locus is very low relative to the density seen in CpG islands. However, the 12 kb region surrounding the γ genes is enriched 2- to 3-fold in CpG dinucleotides in comparison with the δ-β-globin region, and the immediate γ-globin promoter region (5 CpG/200 base pair [bp]) is enriched 6-fold. These CpG dinucleotides are conserved in simian primates, which express γ-globin in the fetal stage, but not in prosimians, which express the γ gene only in the embryonic period.19 During fetal development in simian primates, these unmethylated CpG sequences allow expression of the γ-globin gene during fetal development, but methylation leads to suppression during the adult stage.19 It is informative that the high HbF responder baboons (Papio anubis) have 2 fewer CpG dinucleotides in their γ-globin promoters than the human. The loss of these CpG dinucleotides may be responsible for their high HbF response. In support of this hypothesis, reporter genes with low-density CpG promoter regions, which bind MeCP220 and contain less than 0.5–1.0 mCpG/100 bp, are not suppressed when introduced into cells.21
Treatment of Sickle Cell Anemia with 5-azaC
Prior to the discontinuation of the use of 5-azaC, 8 patients with sickle cell anemia were treated with 5-azaC at a dose of 1–2 mg/kg/d intravenously (IV) for 4–7 days.5,7,8,22,23 Seven patients had increases in HbF (0.2–0.7 g/dL) and/or F cells (10%–25%), and total hemoglobin (1–2 g/dL). There were variations in patient response depending on baseline HbF, dose, and injection schedule. Only 1 patient did not respond,22 probably because of cytotoxicity, as there was a marked reduction of reticulocytes during treatment. One of the patients treated was treated for 3 cycles. The first course was continuous IV infusion (2 mg/kg/d × 7 days) followed by subcutaneous injection at the same dose for 4 days (week 10) and for 5 days (week 18). During the initial course, HbF increased from 6% to 13.7%, and after the third cycle to 22% with 80% F cells.5 Additional patients were treated for up to 500 days.22 In 2 patients treated for > 100 days, HbF increased from 1.5% to 7.6% and from 2.9% to 17.5%, while total hemoglobin increased from 8.5 to 12.0 g/dL and 8.0 to 9.2 g/dL, and F cells increased from 8.0% to 42% and from 18% to 64%, respectively. Although there were insufficient patient data for statistical analysis, the frequency of vaso-occlusive crises appeared to decrease in both patients.22 Treated patients demonstrated reductions in red cell density, ferritin, and indirect bilirubin levels.5 A common side effect seen in these studies was a dose-dependent decrease in the absolute neutrophil count. Following these early studies, patients with sickle cell anemia were not treated with 5-azaC analogs for 15 years.
Treatment of β-Thalassemia with 5-azaC
Six patients with severe β-thalassemia have been treated with 5-azaC for periods of up to 2.5 years. In this small group of patients, the most effective dose/schedule was 2 mg/kg/d by continuous IV infusion for 2–5 consecutive d/wk, every 2 to 4 weeks. At this dose and interval, there were increases in hemoglobin concentration of > 3 g/dL. One patient’s hemoglobin concentration increased from 3 g/dL to 6.3 g/dL after 2 cycles and remained elevated for 50 days until the treatment was stopped.24 The increase in hemoglobin concentration was generally associated with an increase in absolute reticulocyte count, suggesting correction of ineffective erythropoiesis.
In an attempt to develop a treatment schedule more suitable for chronic administration, 2 patients were treated at the same dose (2 mg/kg/d) given as a single subcutaneous injection.25 One patient was injected 3 consecutive d/wk for 4 weeks and the other for 6 weeks. In both patients, the hemoglobin concentration did not increase and fell in the absence of transfusion. When given during 2 previous cycles of treatment, at 2 mg/kg/d by continuous IV infusion, this dosage was effective in raising the hemoglobin concentration 2 g/dL in this patient. Although HbF levels were not determined, the γ-globin mRNA increased 2.8-fold (relative to α-globin mRNA) in 1 patient, and 4.8-fold in another.26 Ferrokinetic studies were performed on 1 patient before and after treatment.26 Iron clearance increased (T1/2 38 minutes vs 56 minutes), and the iron utilization improved (16% vs 32%). These results reflect a 1.6-fold increase in overall effectiveness of erythropoiesis. However, it was countered by an offsetting hematopoietic suppression that did not support an increase in hemoglobin concentration. We have recently shown that decitabine suppression of hematopoiesis is not the result of cytotoxicity but rather is caused by preferential megakaryocyte differentiation at the expense of granulocytic, and to a lesser extent erythroid differentiation.27,28 Perhaps, erythropoietin could be beneficial, as it would stimulate erythroid differentiation. In another case report, when oral 5-azaC was given in combination with tetrahydrouridine to inhibit intestinal degradation, there also was no increase in hemoglobin concentration.29 It is noteworthy that all drug delivery procedures are effective in treating patients with sickle cell anemia, where ineffective erythropoiesis is not an important factor in the pathogenesis of the disease.
Recent Studies Using Decitabine to Treat Sickle Cell Anemia
Decitabine, unlike 5-azaC, is not incorporated into RNA, does not affect protein production,30 and therefore, is probably less cytotoxic and a better agent for treating sickle cell anemia. Juttermann et al14 demonstrated that after incorporation into DNA, decitabine binds covalently to DNMT, blocks DNA polymerase progression, and at high levels, adduct formation results in growth arrest and cell death. Therefore, toxicity is the result of DNMT trapping rather than hypomethylation. To limit covalent adduct formation we chose low-dose decitabine therapy to allow efficient excision repair, DNA polymerase progression, and survival of hypomethylated cells.
Eight patients with sickle cell anemia were initially treated in a dose escalation trial.27 Decitabine was given IV at doses ranging from 0.15 to 0.3 mg/kg/d for 5 d/wk for 2 weeks. Five patients were HU nonresponders. HbF, F cell, F/F cell, γ-globin synthesis, complete blood count, and blood chemistry were measured. For all patients, mean HbF increased from 3.55% to 13.5%. In the HU nonresponders HbF levels increased from 2.28% to 2.6% on HU, and 12.7% on decitabine. Total hemoglobin increased by 1 g/dL or more in 6 of 8 patients. There was an average 2.2-fold increase in platelet count, which occurred at the sixth week and returned toward baseline by week 7. This increase in platelets was mirrored by a transient 3-fold decline in neutrophils, reaching a nadir at 6 weeks and returning toward baseline by week 7.
A second trial of decitabine was designed to determine the effect of repeated dosing on HbF levels and toxicity over 9 months.28 All 7 patients had been enrolled in the previous trial,27 and 5 of the 7 were HU nonresponders. Decitabine was administered by IV infusion at a starting dose of 0.3 mg/kg/d, 5 consecutive days per week for 2 weeks. This treatment was followed by a 4-week observation period. If the absolute neutrophil count dropped below 1000, the dose was reduced by 0.05 g/kg/d in the next 6-week cycle. A dose of drug was determined for each patient, which resulted in elevated HbF levels that were maintained for the remainder of the study while the absolute neutrophil count remained above 1500 with no sign of cumulative toxicity. Mean HbF and average maximal HbF measured during the last 20 weeks of treatment increased to 13.93% ± 2.75% and 18.35% ± 4.46%, respectively, from a pretreatment mean of 3.12% ± 2.75%. Mean and peak hemoglobin levels increased from 7.23% ± 2.75% to 8.81 ± 0.42 g/dL and 9.73 ± 0.53 g/dl, respectively. The mean peak F cell number during treatment was 69% ± 10.12%. Although there were periodic depressions in absolute neutrophil counts (ANCs), which occurred 5 to 6 weeks after beginning each treatment cycle, the mean ANC during the last 20 weeks of treatment (4200 ± 1350) was not significantly different from the pretreatment mean (4600 ± 1560).
The ANCs of 2 HU nonresponder patients never fell below 2000 and the nadirs, which occurred at 5–6 weeks of each cycle, generally remained above 3000. As in the previous study, decreases in ANC were probably caused by preferential differentiation into the megakaryocytic pathway at the expense of neutrophils. The absence of cumulative toxicity over the 36-week study period suggested that the interval between treatment cycles could be reduced without an increase in toxicity.
A third trial of decitabine was designed to test the efficacy of subcutaneous administration, and to determine the effect of increased HbF levels on several clinical correlates.31 Eight patients (5 new) resistant or intolerant to HU received decitabine at a dose of 0.2 mg/kg/d for 1–3 consecutive d/wk in 2 cycles of 6 weeks’ duration. Successful induction of HbF was defined as > 80% F cells containing > 20% HbF/F cell. Additional surrogate clinical end points were RBC adhesion to thrombospondin (TSP) and laminin, levels of D-dimers, thrombin-antithrombin (TAT) complexes, prothrombin fragments 1 and 2 (F1+2), C-reactive protein (CRP), soluble VCAM (sVCAM) and von Willebrand factor propeptide (VWFpp).
All patients demonstrated statistically significant increases in F cells (Table 1 ). HU nonresponders had fewer F cells at baseline but demonstrated a rate of increase similar to that seen in HU responders. F cells increased by the second week after initiation of treatment, and they decreased by the second week after discontinuation of treatment. The primary end point of 80% F cells with > 20% HbF was achieved by 4 patients, all of whom were HU responders. The peak HbF levels achieved when these patients were treated with HU were 9%–14%. The peak HbF/F cell was 26%–28% in all patients, which approximates the optimal HbF/F cell estimated by Noguchi et al.32 In addition to the increases in HbF and F cells, there was a mean increase of 2 g/dL in total hemoglobin (7.6 to 9.6 g/dL). Both the absolute reticulocyte count (P = .0006) and total bilirubin (P = .01) decreased during treatment. The absolute reticulocyte count correlated inversely with total hemoglobin (P < .0001), suggesting that the absolute reticulocyte decrease resulted from the increased hemoglobin. This decrease in stress reticulocytes should be beneficial, as these are the most adhesive fraction of RBC.33There were also marked changes in a number of clinical correlates. RBC adhesion to both TSP and laminin decreased (Table 2 ; P < .005). By decreasing HbS polymerization, HbF would be expected to lead to decreased RBC adhesion to TSP and laminin. In multivariate analysis, a significant association was noted between the percentage of F cells and RBC adhesion to TSP and laminin (P = .046 and P = .004, respectively). RBC adhesion to TSP also demonstrated a significant association with the ANC (P = .02). The adhesion molecules sVCAM-1 and VWFpp were released from damaged endothelial cells. The levels of both molecules decreased with treatment (P < .05).
In sickle cell anemia, abnormal exposure of molecules such as phosphatidyl-serine on the RBC surface and adhesion of RBCs to endothelial cells/endothelial damage can trigger coagulation and inflammatory pathways. Increased levels of markers of active coagulation (TAT, F1+2, and D-dimers) were noted at baseline. Treatment decreased D-dimer levels, a measure of fibrinolysis of cross-linked fibrin (P < .04), while markers of thrombin generation, TAT and F1+2, decreased but the increase was not statistically significant. CRP, a marker of inflammation, was elevated at baseline but the decrease with therapy was not statistically significant (P = .18).
For those markers that decreased significantly with treatment (D-dimers, sVCAM, and VWFpp), we looked for correlations with hematological parameters (percentage F cells, Hb concentration, absolute reticulocyte count [ARC], and ANC). SVCAM levels inversely correlated with total hemoglobin (P = .002), VWFpp levels correlated with the ARC (P < .0001). There was no significant correlation between D-dimers and the hematological parameters.
Conclusions and Future Directions for Treatment with Decitabine
Decitabine is clearly a very effective agent for stimulating HbF production for the treatment of patients with hemoglobinopathies. A total of 13 patients with sickle cell anemia, both HU responders and nonresponders have demonstrated markedly elevated HbF levels. Because decitabine is incorporated only into DNA, it may be less toxic than the 5-azaC analog. The only toxicity observed in patients with sickle cell anemia is mild to moderate leukopenia, which is of short duration lasting only a few days. This leukopenia is most likely caused by activation of factors favoring megakaryocytic differentiation at the expense of granulocytic differentiation, and to a lesser extent erythroid differentiation.29–,30 Present data also indicate that the maximum tolerated dose, as defined by this moderate leukopenia, is variable among patients, and that HU nonresponders may have a higher maximal tolerated dose (MTD). In 2 HU nonresponder patients, an MTD has not yet been established but is probably greater than 0.2 mg/kg/d for 2 consecutive d/wk. In a previous study, these patients did not reach their MTD when given IV infusion at 0.3 mg/kg/d, 5 d/wk for 2 weeks.30 Further studies on these patients are required to determine their MTD and whether the increased dose will result in a better response.
The development of low dose decitabine protocols will be important because high doses, which are cytotoxic, would inhibit reticulocyte production and would blunt the HbF response. Even mild cytotoxicity would be detrimental in the treatment of patients with β-thalassemia. Several β-thalassemia patients treated previously with the 5-azaC analog had marked increases in γ-globin mRNA and significant reductions in ineffective erythropoiesis, but this positive effect was counteracted by hematopoietic suppression.26 Although no studies have been reported in which β-thalassemia patients were treated with decitabine, such studies have been initiated, and accurate assessment of hematopoietic suppression will be critical. Decitabine is expected to be superior to 5-azaC, as it has no effect on RNA synthesis, and therefore lower cytotoxicity. If it is found that the dose of decitabine required to correct ineffective erythropoiesis is hemato-suppressive in some patients, then a long-acting erythropoietin might have to be added to the regimen to enhance erythropoiesis.
II. Reactivation of Fetal Globin Genes with Butyrate and Hydroxyurea: New Clinical and Mechanistic Insights
George F. Atweh, MD,* Hassana Fathallah, PhD, and Rona S. Weinberg, PhD
Mount Sinai Medical Center, One Gustave Levy Place, Box 1079, New York NY 10029-6504
The beneficial effects of high levels of HbF in sickle cell disease (SCD) have been recognized for many years. In 1948, Watson et al noted that newborns with sickle cell disease do not manifest significant clinical problems related to their disease in the first 6 months of life until the HbF declines to adult levels.1 It was later shown that most patients with sickle cell disease from certain regions of Saudi Arabia2 and India3 who inherit a genetic determinant for high HbF have a very mild sickling disorder. More recently, the Cooperative Study of Sickle Cell Disease (CSSCD), a large multicenter study of the natural history of SCD, demonstrated an inverse correlation between HbF levels and the frequency of painful crises and early death.4,5 These clinical and epidemiological observations were also supported by laboratory studies that demonstrated a sparing effect of HbF on polymerization of deoxyhemoglobin S.6 This appreciation of the beneficial effects of HbF in sickle cell disease has stimulated a great deal of interest in the development of therapeutic agents that increase HbF production in patients with this disorder.
Hydroxyurea
5-AzaC was the first HbF-inducing agent to be tested as a therapeutic agent in patients with SCD. The rationale for the use of this drug was that its DNA-demethylating activity might lead to hypomethylation of the promoters of the fetal γ-globin genes, resulting in their transcriptional activation.7 Interestingly, controversy over the mechanism of activation of fetal γ-globin gene expression by 5-azaC led to the identification of hydroxyurea as a second therapeutic agent that stimulates HbF production. Some investigators hypothesized that 5-azaC increases HbF production by accelerating erythroid cell differentiation rather than by hypomethylation of DNA. In other words, as the bone marrow recovers from the cytotoxic effects of the chemotherapeutic agent, erythropoiesis is accelerated and HbF is induced. This proposed mechanism of induction of HbF is similar to the mechanism of induction of HbF following significant blood loss. To support this hypothesis, experiments were performed to show that HU, an S-phase specific chemotherapeutic agent with no DNA demethylating activity, could also increase HbF production in phlebotomized baboons8 and in patients with SCD.9 These interesting observations, however, did not completely resolve the controversy about the mechanism of action of 5-azaC and HU. A recent study demonstrated that a new 5-azaC analogue, 2-deoxy-5-azacytidine, could induce HbF in sickle cell patients who are resistant to HU, suggesting that the two drugs have different mechanisms of action.10
In contrast to 5-azaC, HU is an oral agent with a relatively good safety profile and is widely used in the treatment of myeloproliferative disorders. HU was first used in a number of small-scale nonrandomized clinical trials that confirmed its HbF inducing activity in SCD.9,11,12 These studies provided the proof of principle and led to the identification of an effective dose schedule for the use of this drug in the treatment of patients with sickle cell disease. These Phase I/II studies were followed by a large randomized, placebo-controlled MSH that had well-defined clinical efficacy endpoints.13 This study had to be terminated prematurely when interim analysis showed a significant reduction in the frequency of crises and acute chest syndrome in the hydroxyurea treated group compared to the control group. There was also a reduction in the frequency of blood transfusions in the HU treated group but no changes in the frequency of stroke or death during the relatively short duration of the study.13 A recent follow-up study of the same population of patients who were originally enrolled in the MSH trial showed a positive correlation between survival and HbF levels in patients who were treated with HU.14 Thus, HU became the first HbF-inducing drug to be approved by the FDA for the treatment of SCD and is now widely used in the treatment of patients with this disease throughout the world.
Butyrate
In 1985, Ginder et al reported that the administration of butyrate to chickens pretreated with 5-azaC results in the induction of embryonic globin gene expression.15 Shortly thereafter, Perrine et al16 and Bard and Prosmanne17 showed that infants born to diabetic mothers had higher HbF levels at birth than their age-matched controls. Interestingly, the diabetic pregnant mothers had high levels of butyric acid in their plasma. Perrine et al went on to demonstrate that butyrate infusions in utero in sheep fetuses prevented the switch from fetal to adult hemoglobin that is normally seen around the time of birth.18 This was followed by the demonstration that the administration of butyric acid to adult baboons could partially reverse the switch from fetal to adult globin expression.19 These preclinical observations formed the basis for clinical trials that investigated the therapeutic potential of butyrate as an HbF-inducing agent in patients with SCD and β-thalassemia.
Perrine et al20 first evaluated the effects of arginine butyrate on HbF production in 6 patients with β-globin disorders (3 patients with SCD and 3 patients with β-thalassemia). Treatment with butyrate for 2 weeks resulted in an increase in γ-globin chain synthesis in all 6 patients. This initial study was later followed by another study that was conducted by Sher et al21 in which arginine butyrate infusions were administered to 5 patients with SCD and 5 patients with β-thalassemia. A 10-week dose escalation schedule of arginine butyrate administration resulted in a significant increase in HbF levels in 2 of the 5 patients with SCD. The increase in HbF levels, however, was not sustained with continuous high-dose therapy. Moreover, there was no increase in the hemoglobin levels in any of the 5 patients with β-thalassemia who were enrolled in this study.
Sodium phenylbutyrate was also used to induce HbF production in patients with SCD. All 6 patients who received the drug orally showed a rapid increase in the number of circulating F-reticulocytes.22 Two of the 6 patients who received the drug for a period of 5 to 6 months increased their HbF levels from 10.6% to 18% and 10.4% to 16%, respectively. However, since this oral regimen required the intake of 30–40 tablets/day, poor compliance was a major problem that limited the effectiveness of this agent in the outpatient setting.
The potential role of intravenous arginine butyrate in SCD has recently been revisited.23 The first 6 patients who were enrolled in this new study received arginine butyrate infusions for 8 hours a day, 5 days per week. The HbF levels increased in half of these patients but the increase in the HbF levels was not sustained with continuous long-term therapy. These observations and those of Sher et al,21 summarized above, suggested that toxicity resulting from long-term exposure to butyrate might be responsible for the loss of the HbF response. To examine this hypothesis, a regimen in which butyrate was given intermittently to allow recovery of the bone marrow from the well-known antiproliferative effects of butyrate was investigated in 11 patients. The HbF levels of the 11 patients enrolled on this regimen increased from a mean of 7.2% at baseline to a mean of 21.0% on intermittent butyrate therapy. This HbF response to butyrate was sustained in all patients,23 including 1 patient who continued to receive arginine butyrate for more than 6 years.
Interestingly, all 5 patients who did not respond to butyrate in both the continuous and intermittent butyrate studies described above had baseline HbF levels below 2%, whereas all 10 responders had baseline HbF levels of 2% or above.23 Although the mechanism by which butyrate stimulates HbF production has not been fully elucidated, it is believed that butyrate’s effect on HbF production is at least in part mediated by inhibition of histone deacetylases. Inhibition of histone deacetylases results in increased histone acetylation, which opens up the chromatin structure and makes DNA more accessible to transcription factors. This mechanism of action, however, does not provide any insight into the molecular basis for the specificity of γ-globin gene activation by butyrate. More recently, it has been recognized that histone deacetylases exert their effect regionally through their recruitment to DNA in a sequence-specific manner by binding to transcription factor complexes.24,25 This might explain the specificity of the butyrate effect and also the clinical observation that the HbF inducing activity of butyrate requires partially active human γ-globin genes whose regulatory elements may be occupied by transcription factors.
Combination Therapy with Hydroxyurea and Butyrate
Of the 5 patients in the study summarized above who did not respond to butyrate, 3 were treated with HU. All 3 increased their HbF levels above 20% in response to HU.23 The absence of cross-resistance to the HbF inducing activities of HU and butyrate confirms the long-held suspicion that these drugs activate γ-globin gene expression by different mechanisms. This makes it likely that the use of these agents in combination therapy regimens would induce HbF production in an additive or synergistic manner. This hypothesis was recently tested in 3 patients who were enrolled in a combination therapy protocol consisting of HU for several months followed by HU and butyrate. All 3 patients had a marked increase in their HbF levels after butyrate was added to HU. Thus, the combination of butyrate and HU was more effective in these patients than HU alone. In 1 patient, who was totally resistant to the effect of butyrate following both weekly and intermittent therapy, the addition of butyrate after HU therapy resulted in a big increment in the HbF level. This demonstrates that resistance to butyrate is not absolute and could be reversed following pretreatment with HU.26 Similar advantages of combinations of other HbF inducing agents were previously described in studies of HU with erythropoietin (EPO)27 and HU with sodium phenylbutyrate.28
Before embarking on large-scale studies of combinations of HbF-inducing agents, it is important to ask if there is a justification for the use of combinations of 2 or more agents in SCD. The study of the natural history of sickle cell disease had clearly shown that higher HbF levels are associated with less severe clinical outcomes.4,5 This, however, does not preclude the possibility that the clinical benefits of increased HbF levels may plateau when the level exceeds 20%. Even though patients from Saudi Arabia and India whose Hb levels are generally above 20% have a very mild clinical course, they are not always free of all sickling complications.2 Interestingly, Sutton et al26 described a patient with SCD who developed progressive pulmonary hypertension while her HbF level was greater than 20% on HU. Addition of butyrate to HU in this patient resulted in a peak HbF level of 45% and resulted in a marked amelioration of the pulmonary hypertension. Although there is no definitive evidence yet that HbF levels of 30%–45% are significantly better than levels of 20%–25%, laboratory data support the hypothesis that such high levels may be necessary to completely inhibit intracellular polymerization of deoxyhemoglobin S in red blood cells.29,30 Thus, with the current availability of multiple drugs that can activate HbF in an additive or synergistic manner, we suggest that the aim of pharmacological therapy should be to achieve the highest possible HbF level rather than to settle for the traditionally accepted level of 20%.
Mechanism(s) of HbF Induction by Butyrate
As discussed above, the leading hypothesis for the mechanism of induction of HbF by butyrate is that it exerts its effect by increasing histone acetylation and opening up the chromatin structure of the promoter of the γ-globin gene. This results in increased transcription of the γ-globin gene by making its DNA more accessible to transcription factors. This mechanism is suggested by the known enzymatic activity of butyrate as a nonspecific inhibitor of histone deacetylases.31 Although there is considerable circumstantial evidence in support of this hypothesis,32 it is important to note that there is no experimental evidence yet that induction of HbF by butyrate is associated with changes in histone acetylation in the β-globin gene cluster. In the course of our clinical investigation of the activity of butyrate in SCD, we made some observations that are compatible with this hypothesis and others that suggest that this is not the only mechanism of induction of HbF by butyrate. We would like to describe the new laboratory studies that we are conducting to elucidate the mechanism(s) of HbF induction by butyrate.
Mechanism of the rapid HbF response to arginine butyrate
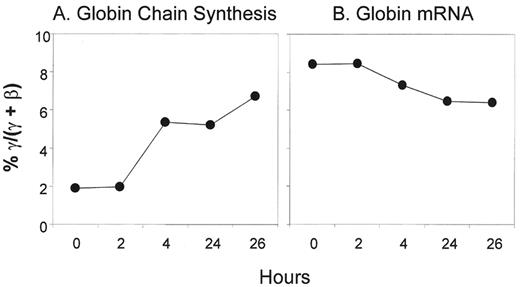
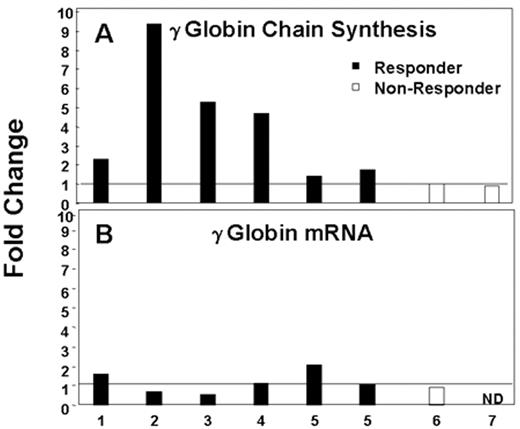
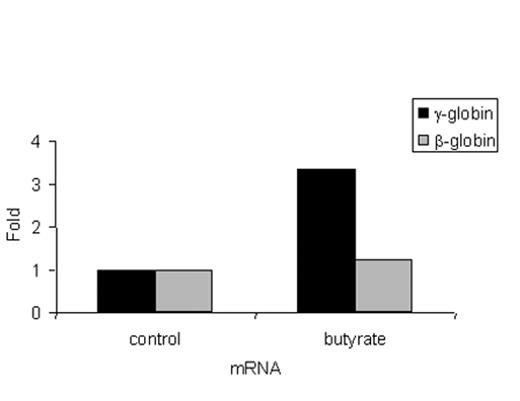
Most patients treated with intermittent arginine butyrate therapy require several cycles to achieve their peak HbF response. However, in some patients, the peak HbF response is reached within a few days. To investigate the kinetics and the mechanism of the rapid HbF response to butyrate, γ-globin chain synthesis and γ-globin mRNA were analyzed in reticulocytes of patients enrolled in our butyrate studies before treatment and at intervals from 2 to 26 hours after the initiation of treatment. Data from a representative butyrate responsive patient are shown in Figure 1 . Reticulocyte γ-globin chain synthesis was 1.9% pretreatment and increased to 6.7% by 26 hours after the initiation of the butyrate infusion. In contrast, no consistent changes were seen in the levels of γ-globin mRNA during the same period. Data from this patient and 6 additional patients with sickle cell disease are summarized in Figure 2 . γ-Globin chain synthesis increased from 1.5- to 5-fold in the 5 patients who went on to have a significant increase in their HbF levels following butyrate therapy in vivo. In contrast, no increase in γ-globin chain synthesis was seen in 2 patients who did not respond by increasing their HbF levels following butyrate therapy. The increase in γ-globin chain synthesis was very rapid, occurring within 2 days of beginning therapy in all patients (data not shown). These increases in γ-globin chain synthesis were not associated with corresponding increases in γ-globin mRNA levels. In the majority of patients enrolled in this study, the HbF level continued to increase gradually during the 4 to 5 months after the initiation of intermittent therapy (data not shown).
These observations allow predictions about the mechanisms that may account for the rapid increase in γ-globin chain synthesis in response to butyrate. If the mechanisms were transcriptional, both γ-globin chain synthesis and γ-globin mRNA levels would be expected to increase. Similarly, posttranscriptional mechanisms (e.g., increase in mRNA stability) would also result in an increase in the levels of γ-globin mRNA and γ-globin chain synthesis. If the mechanism were translational, γ-globin mRNA levels would not be expected to change while γ-globin chain synthesis would be expected to increase. Finally, posttranslational mechanisms would not be expected to result in changes in the levels of γ-globin mRNA and γ-globin chain synthesis. In the studies described above, γ-globin chain synthesis increased but γ-globin mRNA did not change significantly. Thus, the butyrate-induced rapid increase in HbF levels is likely to be mediated by translational mechanisms.
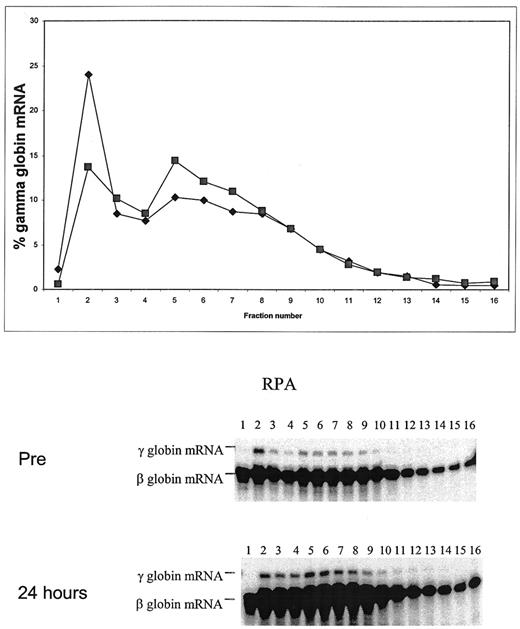
We assessed the efficiency of translation of γ-globin mRNA directly by performing ribosome loading studies using reticulocytes harvested before and after the initiation of butyrate therapy. In the representative study shown in Figure 3 , reticulocyte γ-globin chain synthesis increased 4-fold, from 1.03% pretreatment to 3.96%, within 24 hours of the initiation of therapy. Prior to the butyrate infusion, 25% of reticulocyte γ-globin mRNA was in the prepolysomal messenger ribonucleoprotein (mRNP) fraction. The prepolysomal mRNP fraction decreased to 14% by 24 hours. This was associated with a reciprocal increase in the fraction of γ-globin mRNA in the polysomal region from 29% pretreatment to 37.6% at 24 hours. This analysis shows that exposure to butyrate leads to a rapid shift of γ-globin mRNA from the prepolysomal to the polysomal fraction, which is indicative of an increase in its translational efficiency. The mechanism that may be responsible for the increase in the transitional efficiency of γ-globin mRNA following butyrate infusion remains to be elucidated.
Mechanism of persistence of HbF induction following intermittent exposure to butyrate
Patients who respond to intermittent arginine butyrate therapy maintain their high levels of HbF as long as they receive the drug, even during weeks between cycles of therapy.23 We are now investigating the mechanisms that may be responsible for the persistence of HbF induction during intermittent administration of butyrate. Blood mononuclear cells were harvested from patients before and 24 hours after the initiation of a typical cycle of butyrate therapy. The mononuclear cells were cultured in methylcellulose in both the presence and absence of butyrate. Before the initiation of butyrate infusions, mean γ-globin chain synthesis in erythroid colonies was 29.4% ± 12.6% when the cells were cultured in vitro in the absence of butyrate and 45.7% ± 8.5% when the cells were cultured in vitro in the presence of butyrate (P = .03) (Figure 4A ). In contrast, γ-globin synthesis in colonies derived from progenitors harvested 24 hours after the initiation of butyrate infusions did not increase in response to the addition of butyrate in vitro (42.5% ± 6.7% in the absence of butyrate and 42.6% ± 12.1% in the presence of butyrate) (P = .98) (Figure 4B ). Interestingly, in the absence of butyrate exposure in vitro, γ-globin chain synthesis was significantly higher in blast-forming units erythroid (BFU-E) cultured from cells harvested 24 hours after the initiation of butyrate infusions (45.1% ± 3.9%) than in BFU-E cultured from cells harvested before the initiation of butyrate infusions (29.4% ± 12.6%) (P = .01) (Figure 4C ). Finally, it is important to note that the mean γ-globin chain synthesis in pretreatment erythroid colonies cultured in vitro in the presence of butyrate (45.7% ± 8.5%) was similar to γ-globin chain synthesis in post-treatment erythroid colonies grown in vitro in the absence of butyrate (42.5% ± 5.7%) (P = .72).
These experiments demonstrate that once erythroid progenitors are exposed to butyrate in vivo for as little as 24 hours, the colonies that are generated by these cells in vitro in the absence of butyrate express high levels of HbF. In other words, the progeny of these progenitor cells continue to express high levels of HbF in vitro as a result of butyrate exposure in vivo. Since BFU-E derived colonies are initiated from a single progenitor cell that undergoes multiple rounds of cell division in vitro, these observations suggest that the effect of butyrate on γ-globin gene expression is heritable and can persist following multiple rounds of cell division. The fact that additional exposure to butyrate in vitro does not result in a further increase in γ-globin chain synthesis suggests that the activation of the γ-globin genes is maximal following a brief exposure to butyrate in vivo. Thus, at least some of the effects of butyrate on γ-globin gene expression must be mediated by epigenetic changes that persist after multiple cell divisions. These experiments provide the basis for an excellent experimental model for the study of the persistence of the butyrate effect on γ-globin expression during intermittent butyrate therapy. These laboratory observations also provide an explanation for the clinical observation that successive cycles of intermittent butyrate therapy result in additive augmentation of HbF production and a gradual increase in HbF levels in peripheral blood red cells of patients with SCD.23
Effects of the butyrate on histone acetylation in the β-globin gene cluster
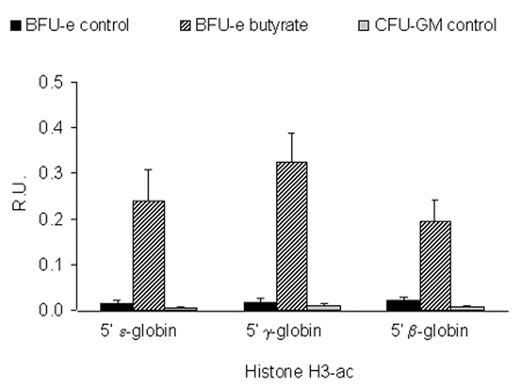
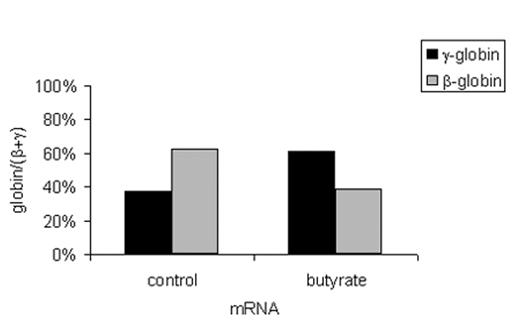
Until recently, experimental tools were not available to assess the acetylation of histones in specific regions of the genome of eukaryotic cells. The development of the chromatin immunoprecipitation (ChIP) assay made it possible to investigate the effects of butyrate on histone acetylation within the β-globin gene cluster.33 We have used this assay to analyze the effects of butyrate exposure on the acetylation of histones in the β-globin gene cluster in BFU-E colonies cultured from patients with sickle cell disease in either the presence or absence of butyrate as described above. In the absence of butyrate, measurable low level acetylation of histone H3 was seen in the promoters of the ε-globin, γ-globin, and β-globin genes in erythroid cells (Figure 5 ). Interestingly, there was no significant acetylation of histone H3 in any of the promoters of the β-like globin genes in colony-forming units granulocyte-macrophage (CFU-GM) cultured from the same patients. Exposure to butyrate in culture increased acetylation of histone H3 in the promoter of the ε-globin gene by 16-fold, the γ-globin gene by 20-fold, and the β-globin gene by 9-fold. Interestingly, in the same cells, γ-globin mRNA levels increased by more than 3-fold while the level of β-globin mRNA did not change. When the mRNA levels are expressed as ratios, γ/β+ γ increased from 37.1% to 61.4% while β/β+ γ decreased from 62.9% to 38.6%. This represents a switch from predominantly β-globin gene expression to predominantly γ-globin gene expression. Thus, these experiments demonstrate that the transcriptional activation of the γ-globin gene by butyrate is associated with a marked increase in the acetylation of histone H3 in its promoter. They also demonstrate that although exposure to butyrate results in increased histone H3 acetylation in the promoter of the β-globin gene, this exposure does not result in its transcriptional activation. This should not be surprising since histone acetylation is clearly not the only factor that regulates the level of gene expression in eukaryotic cells.
Concluding Remarks
It took more than 50 years after the discovery of the molecular basis of SCD to develop specific drug therapy that reduces its morbidity and its mortality. Fortunately, the rate of progress in the development of new therapeutics has recently accelerated and the future is considerably more promising than the past. There are a number of pharmacological agents at different stages of development that can induce HbF production. These agents induce HbF by different mechanisms and their use in combinations has resulted in additive and at times synergistic activation of γ-globin expression. Whether the very high levels of HbF that can be achieved with combination therapy would be sufficient to prevent all the complications of this disease remains to be determined. New therapeutic agents are also being developed to target the adhesive interactions of sickle cells with the endothelium or to prevent the dehydration of sickle cells. Once the activity of these drugs as single agents is confirmed, the stage will be set for testing combinations consisting of agents that induce HbF with agents that target other pathways in the pathogenesis of sickle cell disease. After many years of frustration due to the limitations of existing supportive therapies that merely ameliorate some of the complications of sickle cell disease, we can look forward to an era of multiple effective therapeutics that target different aspects of its pathogenesis. This will undoubtedly lead to a brighter outlook for all patients who suffer as a result of this dreadful disease.
III. Sickle and Thalassemic Transgenic Mice and Gene Therapy
Ronald L. Nagel, MD,* and Mary E. Fabry, PhD
Albert Einstein College of Medicine, 1300 Morris Park Avenue, Ullman Building, Room 921, Bronx NY 10461-1975
The first concept that needs to be emphasized when considering the experimental use of transgenic mouse models is that they are never a perfect reproduction of human disease. First, because the genome of mice and men are similar but sufficiently different.1,2 Second, because even in monogenic mutations such as sickle cell and thalassemia, the phenotype can be modified by secondary loci introduced with the genetic background (“modifier” or also called, “epistatic” genes),3,4 resulting in hemolysis, adhesion of young sickle cells, urinary concentration defect, extent of the HbF up-regulation, etc. This phenomenon explains the intense inter-individual variation in phenotypic expression (and consequently severity) among sickle cell anemia patients and to a lesser extent thalassemia patients. Animal models, having the advantage of being congenic or nearly congenic, have a somewhat different set of modifier genes when compared to humans.
Sickle Cell Anemia Transgenic Models
First and second generations of sickle mice
It is often asked, which is the best sickle model for testing therapeutics including gene therapy relevant to sickle cell anemia? The answer to this simple question is, none of the models are perfect, and they contain various dissimilarities, for the reason given above. Hence, the best answer would be to use more than one model and tailor the selection to the problem at hand.
Sickle transgenic mice with a mixture of endogenous globin chains and various sickle genes
Early transgenic mouse models failed to produce a full sickle cell anemia phenotype because of the absence of human α, low expression of βS and/or because mouse α-chains are as effective an inhibitor of polymerization as human γ-chains.5
Introduction of human α-globin4 and deletion of the mouse βMajor 5,6 increased intracellular polymer formation generated by the NY1-transgene6,7 (see Table 3 ). Sickling occurs in 95% of erythrocytes after slow deoxygenation, with intracellular polymer fiber formation but not fascicles of fiber. Magnetic resonance imaging (MRI) at 9.4 tesla revealed that some transgenic animals had enlarged kidneys with prolonged relaxation time, consistent with increased organ weight and water content.6 The glomerular filtration rate (GFR) was elevated, which is characteristic of young sickle cell patients. Furthermore, exposure to hypoxia resulted in significantly decreased hematocrit, increased erythrocyte density, and induced a urine-concentrating defect.7
The introduction of dominant sickle globin genes (producing hyper-sickling hemoglobins, that are stronger than native HbS) S-Antilles8 or the recombinant construct β-SAD9 containing a sickle mutation (S), HbLos Angeles mutation (A) and a D Punjab mutation (D), represented a further advance. The enhanced effect on polymer formation in erythrocytes of mice expressing the hyper-βS globins are due to two features: reduction in oxygen affinity that shifts the conformation of the hemoglobin to the T-state and favors polymer formation and reduced solubility which suggests that new contact sites in the polymer have been created. HbS-Antilles has, in addition to the βS mutation at β-6 (Glu→Val), a second mutation in the same chain at β-23 (Val→Ile). The second mutation results in a lower oxygen affinity and lower solubility under deoxygenated conditions than HbS. In contrast to patients heterozygous for HbS who are well, patients who are heterozygous for HbS-Antilles have significant clinical disease. The HbD Punjab mutation at β-121 (Glu→Gln) results in a severe sickle syndrome in the compound heterozygote state due to the very low solubility of the deoxygenated hybrid tetramer α2βSβD.
Mouse globins and hyper-sickling globins can complicate the interpretation of the effect of anti-sickling globins used in gene therapy efforts. The complications derive from four aspects of these hyper-hemoglobin S containing mice: 1) They express mouse α, which, surprisingly, is an anti-sickling globin.10 2) They introduce high or low oxygen affinity which alters the probability that βS will deoxygenate at venous pO2. 3) They alter the sickle nucleation process,9 which is likely to affect the polymerization delay time and hence, affects the probability of vaso-occlusion. 4) They may affect the red cell membrane in a way similar to HbOArab, with which the SAD hemoglobin has something in common: the increased positive overall charge, that might lead to binding cytoskeletal proteins.
A long-standing objection to all of the early transgenic mice described up to this point is that they exhibited either no anemia or very mild anemia9,11 and the anemia seen was most evident during the neo-natal period. In human SCD there is a well-established correlation in individual patients between elevated Hct and painful crisis, probably due to increased blood viscosity pari pasu with Hct elevation, increasing the probability of slow flow, deoxygenation, and vaso-occlusion. Therefore, the absence of anemia in these models might contribute to increased pathology rather than the reverse.
Nevertheless, highly informative results in pathophysiology and therapeutics have been obtained from these models.
Sickle transgenic mice offer a unique opportunity to study the mechanisms of cation transport in sickle cell disease. One of the most exciting observations of the sickle transgenic mouse was the discovery that the deoxy potassium efflux first described by Tosteson,12 which is unique to human sickle cells, is also found in the red cells of transgenic mice expressing high levels of HbS.13 The properties of the calcium-stimulated potassium channel appear to be similar in the mouse and the human red cell.14 This property has been used to advantage by Stocker et al15 to demonstrate the beneficial effects on red cell density of inhibition of the potassium channel by clotrimazole and its derivatives.
The human and mouse K:Cl cotransporter found in kidney and other tissues has been cloned from red cells.16,17 There was 89% identity of the mouse K:Cl with the human form, which translates to 96% identity at the amino acid level. Volume and pH stimulated K:Cl are low in most sickle mouse models that express residual murine hemoglobins. However, active K:Cl is found in sickle mice expressing exclusively human globins and in mice expressing HbC, rendering them credible models for cation transport in SCD.18
The kidney is a major source of pathology in humans with SCD. Glomerular sclerosis and elevated blood urea nitrogen and proteinuria have been found in the SAD mouse.11 Mice expressing human α and βS-globin on a homozygous mouse βmajor deletional background (NY1DD) have an enhanced GFR and a urine concentrating defect when exposed to hypoxia7 while the same investigators found that S+S-Antilles mice have a spontaneous urine concentrating defect.19 NYIDD mice18 have elevated levels of nitric oxide synthase (NOS) in the kidney which become even higher when the mice are exposed to hypoxia and the authors speculated that this could explain the elevated GFR and could lead to other forms of renal damage as well as explain other symptoms of SCD.
The role of NOS in the pathogenesis of some aspects of sickle cell disease has now been appreciated,20–,22 although it had been noted before that arginine, the precursor of nitric oxide (NO), is depleted in the plasma of sickle cell patients.23 In patients with arginine depletion, the production of NO would be expected to fall and the production of O2− and peroxynitrite would be expected to rise.24 Other symptoms traceable to elevated NOS activity such as low systemic blood pressure have been found in both sickle cell patients25 and transgenic mice expressing HbS and HbS-Antilles.26
The retina of NY1DD transgenic sickle mice was the first animal model of preretinal neovascularization and has many of the features of the retina in SCD.27 Lutty et al have found pathology in about 30% of the mice over 1 year of age.26 Pathology consists of drop-out of vessels in the retina, structures reminiscent of black sunbursts which are due to invasion of pigmented epithelial cells from the choroid, and loss of photoreceptors in regions where the underlying choroid has been destroyed. Lutty el al28 also observed choroid destructive pathology in sickle mouse retinas that had not previously been appreciated. Stimulated by this observation, human eyes from autopsies of sickle cell patients where examined, and choroidal damage was found that closely resembles that seen in mice.29 This is a case of reverse pathology resulting from the study of sickle mouse retina: a pathological event found first in mice that spurred the successful search for similar pathology in humans. This was an unexpected result of the study of animal models. The reason for the delay in detecting this phenomenon in patients was the lack of a simple clinical technique for routine examination of the choroid.
Microcirculation has been one of the most interesting areas of study in the sickle transgenic mouse. Adhesion of sickle cells to the endothelium and its potential role in SCD was first proposed by Hebbel;30 however, it was not observed in a microcirculatory bed until Kaul et al31 first studied the phenomena in an ex-vivo rat mesoappendix preparation and later in vivo in a cremaster muscle preparation of the S+S-Antilles mouse.32 This is the first model that allowed the study in vivo of this important aspect of the pathophysiology of sickle cell anemia and it has led, more recently, to important explorations to the inhibition of sickle cell adhesion,33,34 a central event in sickle vasoocclusion, which has been demonstrated to occur by adhesion in the small post-capillary venules followed by the trapping of the dense rigid sickle cells.31 Recent studies by Turhan et al35 in BERK mice that express exclusively human HbS (but generated by transplantation and hence not subjected to a life with sickle symptomatology) in mice prepared with TNF-α indicate that adherent leukocytes might similarly facilitate mechanical trapping of elongated sickle red cells. Also, in infections and inflammation response it is possible that leukocytes may contribute predominantly to vasoocclusion. Given the profound differences in hematological characteristics and inflammation among different sickle mouse models of varying severity, a given mouse model may be more useful than another transgenic line to dissect the role of a given factor in leukocyte pathophysiology.
In all sickle mouse models described to date, the liver is a significant site of pathology. The liver suffers from repeated infarcts that are increased when the mice are made hypoxic36 and occur with a greater frequency as judged by both histology and elevated levels of the serum enzyme alanine transaminase in more severe mouse models such as the S+S-Antilles mouse.13 Elevated levels of eNOS and iNOS have also been found in the livers of transgenic mice, which has been attributed to hypoxic damage and/or the effect of shear stress due to the effectively higher viscosity of sickle blood and possibly damage by adhesion of red cells.34 Elevated levels of eNOS and iNOS are probably playing separate roles in these animals. From the experiments cited above, it is clear that eNOS is responsible for the vascular responses observed. The other isozyme, iNOS, is potentially capable of producing much higher levels of NO, but may play its role in disease primarily through tissue damage, possibly mediated in part through the action of peroxynitrite and subsequent formation of nitrotyrosine. Elevated levels of nitrotyrosine have been detected in sickle transgenic mice in kidney with a localization similar to that observed for elevated levels of iNOS.20
The spleen is enlarged in most of the more severe transgenic models that have been reported to date and is a major site of both erythropoiesis and red cell destruction. The lung in transgenic mice with more severe pathology shows thickened septa,11 fibrosis, and pulmonary infarct.9 The brain is relatively uncharacterized in transgenic sickle mice; however, occasional red neurons and rare pyknotic neurons were observed in S+S-Antilles mice.11 The older animals had neuronal dropout, pyknotic neurons and supporting cells, and pyknotic Purkinje cells in the cerebellum, all of which are compatible with intermittent ischemia.
Sickle transgenic mice with exclusively human globins
Sickle transgenic mice expressing exclusively human α and βS are a desirable target for testing antisickling strategies. Several transgenic sickle knockout models expressing exclusively human globins have been generated.38– 41
Both the NY1KO41 and the BERK38 mouse with very low levels of adult HbF expression have severe pathology characteristic of sickle cell disease that includes reticulocytosis, anemia, loss of urine-concentrating ability, splenomegaly, erythroid hyperplasia in bone marrow, liver damage characteristic of ischemia, iron deposition, and evidence of chronic organ damage in multiple tissues. All of the mice described here are protected by high levels of HbF expression during the fetal period.
The NY1KO and the BERK models differ in MCH (which is low in thalassemia) and the ratio of newly synthesized β- and α-globin chains (which is 0.8 or less for β-thalassemia). NY1KO mice have normal to near normal values for both MCH and the β/α ratio for chain synthesis. In contrast, the BERK mouse has low MCH and a β/α ratio similar to that seen in THAL mice. Both of these models, as well as those described by Ryan et al39 and Chang et al,40 are suitable for the study of pathology associated with SCD; however, the BERK and the Chang models have some characteristics of β-thalassemia, which may affect not only red cells, but also other organs. For example, the simultaneous presence of β-thalassemic-like characteristics and HbS may result in an environment that is highly susceptible to oxidative damage, since both syndromes are associated with free radical production. In addition, the lack of HbF expression in the adult mouse reduces its fidelity to the human disease, in which adults have heterogenous distribution of HbF with a pattern and degree of expression that varies from individual to individual.42
Why do these KO models express a thalassemia-like phenotype? The best explanation is that sickle cell anemia in full phenotypic expression is lethal in mice.43 This hypothesis is buttressed by the considerable difficulty in generating these mice, taking about 300 crosses before the proper genotype was generated. In effect, there are two features of mice that suggest the actual mechanism for this apparent lethality. One is the hyperosmolarity of mouse plasma (330 mOsm vs 290 mOsm in humans), and the second prolethality is the increased in red cell 2,3DPG to almost double the concentration compared to human red cells. Intraerythrocytic 2,3DPG has been found to be a pro-sickling allosteric effector.44
In spite of all of this, if more than one model is utilized, significant information can be gleaned from experiments involving the models described above.
The second generation of KO transgenic mice
One avenue to improve the KO mice described above is to eliminate their thalassemic-like features. Fabry et al41 have generated sickle transgenic mice expressing exclusively human globins with 3 levels of HbF expression using their previously described sickle constructs (cointegrated human miniLCRα2 and miniLCRβS 6) mouse α- and β-globin-knockouts,38,39 and 3 different human γ-transgenes. At all 3 levels of HbF expression, these mice have balanced chain synthesis, nearly normal mean corpuscular hemoglobin, and, in some cases, F-cells. A strong effect of HbF on pathology was observed: Mice with the least adult HbF expression were the most severe. Progressive increase in HbF from less than 3% to 20% to 40% correlated with progressive increase in hematocrit (22% to 34% to 40%) and progressive decrease in reticulocyte count (from 60% to 30% to 13%). Urine concentrating ability was normalized at high HbF, and tissue damage detected by histopathology and organ weight were ameliorated by increased HbF. The γ-transgene that produces intermediate levels of HbF was also introduced into the knockout sickle mice described by Pàszty and coworkers38 (BERK mice). It was found that the level of HbF required to ameliorate low hematocrit and normalize urine concentrating defect was different for the NY1 and BERK mice.41 The NY1KO mice have sufficiently faithful sickle pathology to serve as a platform for testing antisickling interventions.
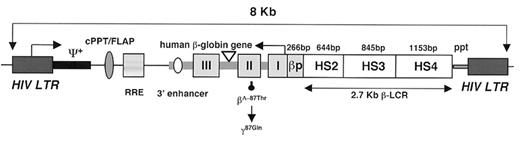
Gene therapy with lentivirus vectors has been successful using sickle transgenic mice. Pawliuk et al45 designed a lentivirus vector that contained a βA globin gene variant with a mutation at a site (β87T→Q) that previously had been shown to prevent HbS polymerization46 and optimized the vector for transfer to hematopoietic stem cells and gene expression in the adult red blood cell lineage (Figure 6 ). Long-term expression (up to 10 months) was achieved, without preselection, in all transplanted mice with erythroid-specific accumulation of up to 52% of the antisickling protein out of the total hemoglobin in 99% of circulating red blood cells. Two mouse transgenic models, BERK and SAD, were used to study the effect of the expression of the antisickling globin β87(T→Q). Inhibition of red blood cell dehydration, the number of irreversible sickle cells (ISCs) and sickling tendency (by counting sickled cells as well as determining the delay time of polymerization in solution) was achieved with correction of hematological parameters, splenomegaly, and prevention of the characteristic urine concentration defect (Figure 7; see Appendix, page598).
Transgenic Models for Thalassemia
Although human thalassemia is due to any one of a myriad of mutational events including small deletions, point mutations, frame shifts, crossing-over, and only occasionally deletions in the coding sequences, all mouse models are due to large deletions or knockouts in the coding sequences. Mouse models with varying severity of thalassemia due to either deletions or knockouts of the α- and β-globin chains are available. Skow et al47 detected a deletion of the mouse βmajor (Hbbth-1) that is nonlethal and can be bred to homozygosity. The resulting mice have a high reticulocyte count, anemia, microcytic cells, low MCH, and a low MCHC with a broad distribution of red cell densities similar to patients with thalassemia intermedia. Three α-chain mutations leading to thalassemia have been described, all of which are due to deletions.45
Mouse models have been advocated as models for thalassemia by several authors who have noted their similarity to human thalassemia48,49 and have drawn parallels between human β-thalassemia and the effect of the homozygous mouse βmajor deletion (Hbbth-1). Normally, the mouse βmajor gene contributes 80% of the β-globin chains, but, in the presence of the deletion, synthesis of βminor increases from 20% of α-globin synthesis to 70%–80% of the α-globin synthesis. The increased synthesis should result in amelioration in the mouse case, but the levels of α-globin in the membrane, decrease in spectrin and oxidation of membrane proteins are comparable to that found in severe human thalassemia intermedia.50 Treatment protocols for mice with deletional thalassemia consisting of administration of erythropoietin (EPO), clotrimazole, and HU have been reported to result in amelioration of the thalassemia.51 Epo cDNA alone has also been reported to completely ameliorate thalassemia in mice with the homozygous mouse βmajor deletion (Hbbth-1) when the cDNA was elecrotransferred into muscle.52
In 1995 both Ciavatta et al53 and Yang et al54 succeeded in generating full β knockouts. A full knockout of the α-gene was generated by Pàszty et al55 in the same year making possible generation of transgenic mice with exclusively human hemoglobins. All of these knockouts are lethal in the homozygous state unless rescued by a transgene.
Gene therapy using lentivirus vectors has been successful using thalassemia transgenic mice. May and coworkers56 reported that long-term synthesis of chimeric hemoglobin (muα2/huβA2) could be achieved in mice with a heterozygous KO of both murine βmajor and βminor, equivalent to human thalassemia intermedia following engraftment with bone marrow cells transduced with a lentiviral vector encoding the human β-globin gene (0.8 proviral copy per genome). Without post-transduction selection, the treated chimeras exhibited durably increased Hb levels over 40 weeks. Ineffective erythropoiesis and extramedullary hematopoiesis regress, as reflected by normalization of spleen size, architecture, hematopoietic colony formation, and disappearance of liver extramedullar hematopoiesis. Hence, the manifestations of ineffective erythropoiesis were corrected by a sustained increase of 3 to 4 g/dL of hemoglobin. Hepatic iron accumulation is markedly decreased in 1-year-old chimeras, indicating persistent protection from secondary organ damage. These results demonstrate, in a moderately severe model, that viral-mediated globin gene transfer of hematopoietic stem cells effectively treats a severe Hb disorder.
The next chapter of this quest came with the demonstration by Imren et al57 of permanent, panerythroid correction of β-thalassemia in mice (equivalent to severe thalassemia intermedia in humans), resulting from a homozygous deletion of the murine βmajor globin gene, by transplantation of syngeneic bone marrow transduced with an HIV-1-derived [human β globin gene/LCR] lentiviral vector. This vector contained the Rev responsive element and the central polypurine tract/DNA flap that help with export of mRNA from the nucleus to the cytoplasm. The viral titers produced were high enough to achieve transduction of virtually all of the hematopoietic stem cells in the graft with an average of three integrated proviral copies per genome in all transplanted mice. The transduction was sustained for > 7 months in both primary and secondary transplants, at which time approximately 95% of the red blood cells in all mice contained human β-globin contributing to 32 ± 4% of all β-like globin chains. Hematological parameters approached complete phenotypic correction, as assessed by Hb levels and reticulocyte and red blood cell counts. All circulating red blood cells became and remained normocytic and normochromic, and their density was normalized. Free α-globin chains were completely cleared from red blood cell membranes, splenomegaly abated, and iron deposit was almost completely eliminated in liver sections. These findings indicate that virtually complete transduction of the hematopoietic stem cell compartment can be achieved by high-titer lentiviral vectors and that position effect variegation can be mitigated by multiple events of proviral integration to yield balanced, panerythroid expression. These results provide a solid foundation for the consideration of human clinical trials in β-thalassemia patients.
More recently, Rivella et al58 have studied the therapeutic potential of gene therapy in the context of a lethal anemia, in an animal model of particularly severe β0-thalassemia major. In this model, mice engrafted with β-globin-null [Hbb(th3/th3)] fetal liver cells succumbed to ineffective erythropoiesis within 60 days and rapidly developed severe anemia (2–4 g/dL), massive splenomegaly, extramedullary hematopoiesis, and hepatic iron overload. Most mice (11 of 13) treated by lentivirus-mediated globin gene transfer were rescued. Long-term chimeras with an average 1.0–2.4 copies of the TNS9 vector in their hematopoietic and blood cells produced up to 12 g/dL chimeric Hb consisting of muα2/huβA tetramers. Pathology indicated that erythroid maturation was restored, while extramedullary erythropoesis and iron overload dramatically decreased. These findings demonstrate the remarkable efficacy of lentivirus-mediated globin gene transfer in treating a fulminant type of thalassemia (even more severe than the human form) and strongly support the efficacy of gene therapy in severe hemoglobinopathies.
It should be noted that all of the models described above used lethal irradiation prior to transplant. The projected use in humans will most likely require a partial ablation of the marrow. Recently Fisher’s group in Paris, working on the gene therapy of immune deficiency syndrome using retroviral vectors, reported that two recipients developed ALL. This mishap has raised the concern that lentiviral constructs could also carry the risk of insertional mutagenesis. However, it has recently been demonstrated that two insulator sequences can be added to the lentiviral construct to successfully prevent upregulation of genes in the proximity of the insertion.59 On a more positive note, the same lentiviral construct that was used in the successful treatment of transgenic models for SCD45 has recently been used to transduce human cord blood and fetal liver cells60 demonstrating the initial transduction of human multipotent cells with in vivo repopulating activity.
These developments, possible only through the existence of animal models, auger well for the future of gene therapy in hemoglobinopathies, although obstacles may still lie ahead.
IV. Stroke in Adults with Sickle Cell Disease
Robert J. Adams, MS, MD*
Medical College of Georgia, 1429 Harper St., HF1154, Augusta GA 30912-3200
Stroke as a complication of SCD has been well studied, but most of the data are from children.1,2 The concept proposed by Powars et al in 1978 that adults are at greater risk for intracranial hemorrhage and children at risk for ischemic stroke3 has been supported by data from the CSSCD.4 As survival prospects continue to improve, more adults with SCD will be encountered who have possible risk factors for stroke more typical of the general population, such as hypertension and abnormal lipids, and little is known about how risk related to SCD interacts with these other factors. The STOP (Stroke Prevention Trial in Sickle Cell Anemia) primary prevention paradigm, with transcranial Doppler (TCD) ultrasound screening and the use of prophylactic transfusion,5 is based on data from children, and there are no systematic data on how useful this approach might be for adults over what will be decades of risk exposure. HU has been used for secondary prevention by at least two groups and appears promising as an alternative to transfusion in older children and young adults.6,7 A number of other options for stroke prevention are available,8 and data are clearly needed on how to use agents such as aspirin and other antiplatelet drugs to prevent stroke in SCD.
Epidemiology
The seminal report by Powars et al on the natural history of stroke included data on 35 patients with stroke, mostly children.3 Of 11 with hemorrhage, the mean age was 25 years (range 14–36) compared to 7 years for cerebral infarction. Powars and colleagues also reported on the phenomenon of later life hemorrhage after childhood cerebral infarction. The CSSCD4 reported that the prevalence of stroke increased with age up to age 50 years in SCD. The lowest incidence of new cerebral infarction (0.04 per 100 patient years) was observed in patients 20–29 years, a group which also had the highest incidence of intracerebral hemorrhage (0.44). Few strokes were recorded in older patients, and most of these were hemorrhages. Whether this represents a peak of hemorrhage risk in early adult life or the vagaries of small sample size is not known. It seems intuitive that the risk of stroke would continue to increase with age in SCD as it does generally, rather than peak in the third decade. Of risk factors for hemorrhage in adults with SCD, only lower steady state Hb and higher leukocyte counts stood out in the CSSCD multivariate analysis.
Mechanism of Stroke
Little is known about the mechanism of stroke that is specific to adults with SCD. A number of causes of stroke in young adults in the general population could be at play in SCD but data are limited.9,10 Some of these are listed below:
Cardiac source of embolus from endocarditis or other valvular disease, paradoxical embolus with patent foramen ovale (PFO) and atrial septal aneurysm
Coagulopathies and thrombophilia, including anticardiolipin antibodies
Arterial dissections from trauma
Use of recreational drugs
Vasculopathy or arterial disease promoted by elevated homocysteine
Familial lipid disorders causing premature atherosclerosis
Small vessel disease from diabetes and advanced hypertension
Among these only elevated homocysteine and coagulopathies/thrombophilia have been reported in SCD. Nsiri et al reported elevated antiphosolipid antibodies in a small series of mostly children, but levels were not particularly high (17 + 9 GPL/mL for IgG) and were not related to painful crises.11 A study by Westerman et al12 compared 20 HbSS with 17 HbSC patients on measures of thrombophilia. They found that antiphospholipid antibodies (particularly IgG antibodies to phoshatidylserine, which is exposed with red cell membrane damage) were elevated and that protein C and S activities were lower in HbSS than in HbSC. These findings were related to each other and with other measures of coagulation activation, which were abnormal in HbSS but not HbSC subjects. However, neither of these studies was particularly targeted to adults with SCD. Factor V Leiden is probably not an important cofactor,13 but a number of other possible risk factors for complications (not necessarily stroke) in children have been reported in small series including reduced protein C and S14 and other coagulation inhibitors,15 elevated homocysteine16 and endothelin-1.17 Again, none of these studies looked specifically at adults.
CSSCD data also suggested that males with elevated blood pressure were at risk for stroke, although the blood pressures of all the patients with SCD were lower than gender- and race-matched values of non-SCD patients in studies such as NHANES (National Health and Nutrition Evaluation Survey) I and II.18 The authors of this substudy suggested special attention be given to patients with elevated blood pressure, whether children or adults.18 In what is probably the most comprehensive study of stroke in young adults, the Baltimore Washington Cooperative Young Stroke Study, Kittner and colleagues included strokes due to SCD (19.8% of their patients had “hematologic and other” etiologies).10 Cigarette smoking and hypertension were two important risk factors for stroke, especially in young black patients, and both can be modified.19 These data reinforce the importance of traditional risk factor assessment and modification in young adults with SCD.
In the absence of specific data, it must be assumed that most adults with SCD and brain infarction probably have a late onset of the large vessel vasculopathy that has been well described in children,20 and the patients should be approached accordingly. Why some patients don’t develop symptoms until after childhood is not known.
Prevention and Treatment Options
Ischemic stroke
There is little specific information on prevention and treatment of ischemic stroke in adults with SCD, and the clinician must decide whether to approach an SCD patient with transient ischemic attacks (TIAs) or stroke in a manner similar to children with SCD, or along guidelines established for adults without SCD.8,21,22 As the life expectancy of patients with SCD increases, more adults presenting with central nervous system (CNS) symptoms can be anticipated. The interaction of SCD-specific risk factors with risk factors for stroke seen in adults without SCD has not been determined, although high blood pressure was identified as a stroke risk in the CSSCD.18 Specifically, the role of chronic transfusion is unclear. The recommendations that follow are based primarily on current recommendations for treatment and prevention in patients without SCD.
Treatment of hyperacute ischemic stroke in adults is accomplished using tissue plasminogen activator (TPA).22 It is not clear whether TPA, which has a significant risk of bleeding, is appropriate for patients with SCD as no experience with its use has been reported. However, there is no clear reason to exclude SCD patients from TPA therapy, and it remains the only FDA-approved therapy for treatment of ischemic stroke. In this setting the risk of symptomatic intracerebral hemorrhage is at least 6%, the reported rate from adults without SCD, and might be higher. However, its use is recommended according to established guidelines (see http://216.185.102.50/Whats_News/AHA_Science_Advisories/tpa-ok.html).23
In addition, TPA should not be given unless emergency care and appropriate facilities are available. Caution is advised before giving TPA to patients with severe stroke, and careful explanation of the risk of bleeding to the patient and family is advised. There is a 6.4% risk of symptomatic brain hemorrhage, and about half of these are fatal. If a hemorrhage occurs, the guidelines suggest preparing for blood transfusion (for extracranial bleeds) and administering 4–6 U of cryoprecipitate or FFP and 1 U of single donor platelets. Surgical removal of intracranial hemorrhage should be considered. TPA can be given to patients on antiplatelet agents, but these drugs, as well as heparin, should not be given for the first 24 hours after using TPA. There is evidence that aspirin (325 mg one-time dose) within the first 48 hours after stroke onset has a small beneficial effect and is recommended if TPA is not used.
The acute evaluation of the patient requires a non-contrast computed tomography (CT) scan to rule out hemorrhage. After the decision is made regarding TPA, a magnetic resonance study of the brain is often performed to better delineate the area of ischemia/infarction. Diffusion-weighted imaging (DWI) can be used to determine very early ischemic damage and to sort out cases where the diagnosis of TIA or stroke is not clear.
Prevention of stroke in patients with TIAs or previous stroke is accomplished with use of either antiplatelet agents or warfarin based on the likely cause of the symptoms.8,21 Carotid endarterectomy is reserved for those with high-grade symptomatic carotid stenosis (> 70% lumen diameter stenosis) and in some cases patients with asymptomatic stenosis.24 The prevalence of carotid disease in SCD is unknown but appears to be very low. The prevalence of intracranial stenosis with stroke is likely to be much higher in adults with SCD than in those without SCD, in whom it accounts for about 10% of all strokes, but as many as 25% in non-Caucasian patients.25 TCD ultrasound has not been systematically applied to adults with SCD, and the primary prevention guidelines outlined above using transfusion do not apply to adults with SCD. A pilot study performed at our center did not suggest a high prevalence of occlusive disease among adults without a stroke history, but more data are needed (unpublished observations).
In adults without SCD who have significant intracranial stenosis (> 50%), either warfarin or antiplatelet agents are used, and a trial is ongoing to determine which is best. In patients with a cardiac source of embolism, especially valvular abnormalities and atrial fibrillation, long-term anticoagulation is indicated.8 Other patients are treated with antiplatelet agents, and risk factors such as smoking, high cholesterol and high blood pressure should be addressed.26
The following work-up is recommended for adults presenting with TIA or ischemic stroke: CBC with differential and platelet count, electrocardiogram (EKG), transthoracic echocardiogram with consideration of transesophageal echocardiogram, partial thromboplastin time (PTT), a brain study to include MRI, DWI, and magnetic resonance angiography (MRA) and/or TCD and carotid duplex ultrasound or CT angiography to determine the status of the intracranial and extracranial vessels. Testing of blood for protein C and S deficiency, homocysteine elevation and anticardiolipin antibodies should be considered if the cause of stroke is not clear. Etiologies of stroke seen in young patients without SCD, including CNS infection, illicit drug use, and arterial dissection, should be considered.
There are currently three options for antiplatelet therapy for secondary stroke prevention in non-SCD patients. The American Stroke Association recommended guidelines regarding these agents are shown in Table 4 . These guidelines are similar to those for prevention of stroke after completed brain infarction.
An alternative approach in the SCD patient is to use chronic blood transfusion as applied in children with SCD. In children, regular transfusions are given with a customary target of reducing Hbs to ≤ 30% of total hemoglobin. Either simple or exchange methods can be used.27,28 Some centers have discontinued transfusion after the 18th birthday if 3 or more years of therapy have been given.29 HU has now been used in uncontrolled series for secondary prevention with encouraging results.6,7 Despite a reported 17% recurrence in a study that treated older children and young adults with HU,6 Ware et al report that overlapping transfusion and HU therapy for some months may result in prevention of the early recurrences seen in the first study. HU is an attractive option since it is already indicated for some adults to reduce other complications of SCD.
Recently, surgical bypass has been reported in a patient with SCD and intracranial thrombosis. Surgical procedures that have been developed to treat moyamoya syndrome may be considered due to the similarity in anatomic location of the arterial disease in moyamoya and SCD.30 These procedures may be last resort options for patients who cannot be otherwise treated or who continue to have brain infarction despite medical therapy. However, risk and benefit in this setting have not been established, and no recommendation can be made.
Intracranial hemorrhage
The clinical presentation of intracranial hemorrhage is usually more dramatic that that of ischemia and may include severe headache, vomiting, stupor or coma. Hemiparesis may be present, especially with intraparenchymal bleeding. A child with such a presentation requires rapid but careful evaluation to rule out mimics such as meningitis, sepsis, hypoxemia, drug intoxication, or other metabolic derangements. A non-contrast cranial CT should be performed as soon as possible. Intracranial hemorrhage should be managed based on the location of the blood on the CT scan.
Subarachnoid hemorrhage (SAH).
The usual cause is rupture of a berry aneurysm. The aneurysm may not be seen on CT but can sometimes be inferred by the location of blood. No cause may be identified for minor subarachnoid hemorrhage even when angiography is performed, but an angiogram is recommended to identify aneurysms or arteriovenous malformations (AVM)18 if surgery is being considered. This is clinically relevant because aneurysms may re-bleed, SCD patients may have multiple aneurysms that require management,31 and surgical clipping, as well as AVM removal, have been successfully performed in many cases with SCD. While aneurysms can be identified using MR, MRA is not a definitive test for this vessel abnormality unless special techniques are used.33
Initial treatment of subarachnoid hemorrhage is stabilization in a neurological intensive care unit and intravenous normotonic fluids to avoid dehydration. The effect of transfusion on the course and outcome of hemorrhage in SCD is not known. However, since viable cases should go on to angiography, and preparation for angiography involves reduction of Hb S to less than 30% total Hb, transfusion is recommended in a manner similar to treatment of acute ischemic stroke. Nimodipine, a calcium antagonist that improves outcome after SAH by counteracting delayed arterial vasospasm, is indicated in adults with SAH.33 Use in this setting with young children is not approved but is reasonable on an empiric basis. The adult dosage of 60 mg p.o. every 4 hours for 21 days should be adjusted by weight.
Intraparenchymal hemorrhage.
If the CT shows blood primarily confined to the parenchyma, the cause may still be an AVM particularly if there is also subarachnoid bleeding. Intraparenchymal bleeding may be associated with large vessel vasculopathy especially if there is moyamoya formation present or in some cases even if no vessel pathology can be seen on angiography. Evaluation of these patients with MRA may be sufficient if there is no subarachnoid blood because an aneurysm is not likely as the source of bleeding, but better definition of the vasculature can be obtained with conventional angiography.
Initial management depends on the size and location of the bleeding. A rapid search for coagulopathy should be made with a PTT and PT determination, and correction of any coagulopathy initiated. Management of the hematoma includes medical control of intracranial pressure and consideration for surgical removal in selected cases, particularly if there is a large (> 3 cm) cerebellar hematoma. Re-bleeding in this setting in the short term is rare. Giving normotonic fluids and avoidance of hypotension are important.34
Intraventricular hemorrhage.
Intraventricular hemorrhage is unusual but may be seen in the case where fragile moyamoya vessels near the ventricular wall rupture into the ventricular space. In such cases the patient is at risk for acute hydrocephalus and death if ventricular flow is obstructed. These patients should receive prompt neurosurgical evaluation for intraventricular catheter placement for drainage. After acute stabilization, evaluation of the cerebral vessels, best done by conventional angiography, should be undertaken to try to identify the underlying cause.
Prevention of Hemorrhage Recurrence
There is considerable published experience with management of aneurysms in SCD, and now newer catheter based approaches are being applied generally. The recurrence risk of intracerebral hemorrhage is not clear. Since some of these patients have hemorrhage due to moyamoya and/or advanced arterial occlusive disease, transfusion therapy and possibly surgery could be considered after the initial effects of the hemorrhage have abated. Use of long-term transfusion to prevent hemorrhage recurrence has not been studied.
Summary
Risk of stroke in SCD adults is probably elevated compared to the general population and hemorrhage is a particular risk. Specific data to guide treatment and prevention are not available. My recommendations are as follows:
Work up adults for other potential causes, especially those that would lead to use of warfarin such as high risk of cardiac embolization; if echocardiography (TTE) is negative, do a transthoracic echocardiography (TEE).
Evaluate intracranial and extracranial circulation.
If ischemic stroke occurs in the absence of significant intracranial vasculopathy, use antiplatelet agents.
If vasculopathy is present, use chronic transfusion, as well as antiplatelet agents then reevaluate in 1–2 years.
If recurrent symptoms (TIA or stroke) develop in the face of severe arterial disease, consider surgery.
Treat acute ischemic stroke with IV TPA if the patient meets accepted guidelines for treatment within 3 hours of onset.
Approach hemorrhage patients acutely as you would any patient, but perform preangiogram transfusion.
Transfuse chronically for SAH and cases of intracerebral hemorrhage with arterial occlusive disease for at least one year then reevaluate.
Identify and address risk factors for stroke such as hypertension, abnormal lipids, diabetes and smoking.
Changes in F cells and fetal hemoglobin (HbF) following decitabine treatment.
| Patients . | Pre-tx F Cell % . | Max F Cell % . | P Value . | Pre-tx HbF % . | Max HbF % . | P Value . |
|---|---|---|---|---|---|---|
| Abbreviations: Tx, treatment; HU, hydroxyurea. | ||||||
| HU nonresponders (n = 3) | 16.2 ± 10.8 | 55.1 ± 11.1 | 0.002 | 2.4 ± 1.6 | 14.5 ± 2.2 | 0.0006 |
| HU responders (n = 5) | 51.3 ± 6.3 | 81.2 ± 4.4 | 0.0001 | 9.0 ± 2.5 | 23.9 ± 3.1 | < 0.0001 |
| All patients (n = 8) | 38.1 ± 7.6 | 71.4 ± 6.5 | < 0.0001 | 6.5 ± 1.4 | 20.4 ± 2.0 | < 0.0001 |
| Patients . | Pre-tx F Cell % . | Max F Cell % . | P Value . | Pre-tx HbF % . | Max HbF % . | P Value . |
|---|---|---|---|---|---|---|
| Abbreviations: Tx, treatment; HU, hydroxyurea. | ||||||
| HU nonresponders (n = 3) | 16.2 ± 10.8 | 55.1 ± 11.1 | 0.002 | 2.4 ± 1.6 | 14.5 ± 2.2 | 0.0006 |
| HU responders (n = 5) | 51.3 ± 6.3 | 81.2 ± 4.4 | 0.0001 | 9.0 ± 2.5 | 23.9 ± 3.1 | < 0.0001 |
| All patients (n = 8) | 38.1 ± 7.6 | 71.4 ± 6.5 | < 0.0001 | 6.5 ± 1.4 | 20.4 ± 2.0 | < 0.0001 |
Changes in surrogate clinical end points.*
| . | . | Pretherapy . | Post-cycle 1 . | P† . | Post-cycle 2 . | P‡ . | Normal Range . | Multivariate Regression Analysis (P < 0.05) . |
|---|---|---|---|---|---|---|---|---|
| *Statistical analysis of changes in RBC adhesion to thrombospondin (TSP) and laminin, levels of D-dimers, Thrombin-antithrombin (TAT) complexes, prothrombin fragments 1 and 2 (F1+2), C-reactive protein (CRP), soluble VCAM (sVCAM) and von Willebrand factor propeptide (VWFpp). The variables that changed significantly with treatment were analyzed for correlations with the following hematological parameters: percentage F cells, absolute neutrophil counts, total hemoglobin, and reticulocyte counts (multivariate regression analysis); significant associations are shown in the last column. | ||||||||
| Values are mean ± SE; paired 2-tailed t test. | ||||||||
| † P, significance of change from pretherapy to postcycle 1. | ||||||||
| ‡ P, change from pretherapy to postcycle 2. | ||||||||
| Measures of RBC adhesion to endothelium | Adhesion to TSP (RBCs/mm2) | 1570 ± 170 | 690 ± 150 | < 0.001 | 910 ± 160 | < 0.001 | < 60 | F cell % and ANC |
| Adhesion to laminin (RBCs/mm2) | 3470 ± 500 | 1950 ± 300 | 0.004 | 1570 ± 210 | < 0.001 | < 250 | F cell % | |
| Measures of thrombin generation and fibrinolysis | D-dimer (ng/mL) | 490 ± 90 | 320 ± 50 | 0.02 | 300 ± 50 | 0.03 | < 400 | Nil |
| TAT (ug/L) | 7.0 ± 1.7 | 8.6 ± 2.3 | 0.15 | 5.2 ± 0.9 | 0.11 | 1.0 – 4.1 | ||
| F1+2 (nmol/L) | 1.75 ± 0.22 | 1.56 ± 0.16 | 0.23 | 1.41 ± 0.15 | 0.051 | 0.04–1.1 | ||
| Measure of inflammation | CRP (mg/dL) | 1.25 ± 0.27 | 1.19 ± 0.34 | 0.80 | 0.82 ± 0.26 | 0.18 | < 0.7 | |
| Measures of endothelial cell damage | sVCAM (ng/mL) | 1170 ± 140 | 930 ± 100 | 0.01 | 840 ± 100 | 0.02 | 395–714 | Total Hb |
| VWFpp (u/dL) | 196 ± 26 | 156 ± 28 | 0.015 | 144 ± 13 | 0.049 | 74–153 | Absolute reticulocyte count | |
| . | . | Pretherapy . | Post-cycle 1 . | P† . | Post-cycle 2 . | P‡ . | Normal Range . | Multivariate Regression Analysis (P < 0.05) . |
|---|---|---|---|---|---|---|---|---|
| *Statistical analysis of changes in RBC adhesion to thrombospondin (TSP) and laminin, levels of D-dimers, Thrombin-antithrombin (TAT) complexes, prothrombin fragments 1 and 2 (F1+2), C-reactive protein (CRP), soluble VCAM (sVCAM) and von Willebrand factor propeptide (VWFpp). The variables that changed significantly with treatment were analyzed for correlations with the following hematological parameters: percentage F cells, absolute neutrophil counts, total hemoglobin, and reticulocyte counts (multivariate regression analysis); significant associations are shown in the last column. | ||||||||
| Values are mean ± SE; paired 2-tailed t test. | ||||||||
| † P, significance of change from pretherapy to postcycle 1. | ||||||||
| ‡ P, change from pretherapy to postcycle 2. | ||||||||
| Measures of RBC adhesion to endothelium | Adhesion to TSP (RBCs/mm2) | 1570 ± 170 | 690 ± 150 | < 0.001 | 910 ± 160 | < 0.001 | < 60 | F cell % and ANC |
| Adhesion to laminin (RBCs/mm2) | 3470 ± 500 | 1950 ± 300 | 0.004 | 1570 ± 210 | < 0.001 | < 250 | F cell % | |
| Measures of thrombin generation and fibrinolysis | D-dimer (ng/mL) | 490 ± 90 | 320 ± 50 | 0.02 | 300 ± 50 | 0.03 | < 400 | Nil |
| TAT (ug/L) | 7.0 ± 1.7 | 8.6 ± 2.3 | 0.15 | 5.2 ± 0.9 | 0.11 | 1.0 – 4.1 | ||
| F1+2 (nmol/L) | 1.75 ± 0.22 | 1.56 ± 0.16 | 0.23 | 1.41 ± 0.15 | 0.051 | 0.04–1.1 | ||
| Measure of inflammation | CRP (mg/dL) | 1.25 ± 0.27 | 1.19 ± 0.34 | 0.80 | 0.82 ± 0.26 | 0.18 | < 0.7 | |
| Measures of endothelial cell damage | sVCAM (ng/mL) | 1170 ± 140 | 930 ± 100 | 0.01 | 840 ± 100 | 0.02 | 395–714 | Total Hb |
| VWFpp (u/dL) | 196 ± 26 | 156 ± 28 | 0.015 | 144 ± 13 | 0.049 | 74–153 | Absolute reticulocyte count | |
Panoply of sickle transgenic mice: description of the features of the most commonly used transgenic sickle mice.
| . | βs% . | αH% . | γ% > 9 wks . | MCH pg/cell . | Retics % . | Hct . | Urine Conc. mOsm . |
|---|---|---|---|---|---|---|---|
| * 8-hour water deprivation | |||||||
| ** 24-hour water deprivation | |||||||
| Abbreviations: MCH, mean corpuscular hemoglobin | |||||||
| C57BL | — | — | — | 14.5 | 2.2 | 48 | 3147**,1688* |
| NY1DD | 75 | 56 | — | 14.1 | 3.2 | 47.0 | 2821** |
| S+S Antilles | 80 | 58 | — | 14.3 | 11.1 | 44.5 | 1302**,* |
| SAD | 19 | 58 | — | 15.1 | 3.6 | 44.0 | 3277** |
| NY1KO γL | 97 | 100 | < 3 | 14.2 | 63.2 | 22.4 | — |
| NY1KO γM | 80 | 100 | 30 | 13.7 | 30.1 | 34.0 | 1176* |
| NY1KO γH | 60 | 100 | 40 | 14.4 | 12.9 | 41.1 | 3285** |
| BERK | > 99 | 100 | < 1 | 9.3 | 36.5 | 28.7 | 898* |
| . | βs% . | αH% . | γ% > 9 wks . | MCH pg/cell . | Retics % . | Hct . | Urine Conc. mOsm . |
|---|---|---|---|---|---|---|---|
| * 8-hour water deprivation | |||||||
| ** 24-hour water deprivation | |||||||
| Abbreviations: MCH, mean corpuscular hemoglobin | |||||||
| C57BL | — | — | — | 14.5 | 2.2 | 48 | 3147**,1688* |
| NY1DD | 75 | 56 | — | 14.1 | 3.2 | 47.0 | 2821** |
| S+S Antilles | 80 | 58 | — | 14.3 | 11.1 | 44.5 | 1302**,* |
| SAD | 19 | 58 | — | 15.1 | 3.6 | 44.0 | 3277** |
| NY1KO γL | 97 | 100 | < 3 | 14.2 | 63.2 | 22.4 | — |
| NY1KO γM | 80 | 100 | 30 | 13.7 | 30.1 | 34.0 | 1176* |
| NY1KO γH | 60 | 100 | 40 | 14.4 | 12.9 | 41.1 | 3285** |
| BERK | > 99 | 100 | < 1 | 9.3 | 36.5 | 28.7 | 898* |
Use of antithrombotic agents in patients with transient ischemic attacks (TIAs).21
| Event . | Recommended Therapy . | Therapeutic Options . |
|---|---|---|
| Abbreviations: ASA, acetylsalicylic acid (aspirin); ER-DP, extended-release dipyridamole. | ||
| * Neither ER-DP+ASA nor ASA alone is recommended for patients who are allergic to aspirin or unable to take low-dose aspirin. | ||
| **The recommended antithrombotic agents have not been specifically tested in patients who have experienced a TIA during ASA therapy. | ||
| TIA (atherothrombotic) | ASA 50–325 mg/d | ER-DP 200 mg+ASA 25 mg BID Clopidogrel 75 mg/d Ticlopidine 250 mg BID ASA 50–1300 mg/d |
| TIA (atherothrombotic) aspirin intolerant* or if TIA occurs during ASA therapy** | ER-DP 200 mg + ASA 25 mg BID Clopidogrel 75 mg/d | Ticlopidine 250 mg BID Warfarin (INR 2–3) ASA 50–1300 mg/d |
| TIA (cardioembolic) | Warfarin target INR 2.5 (range 2–3) | ASA 50–325 mg/d (if contraindications to warfarin) |
| Event . | Recommended Therapy . | Therapeutic Options . |
|---|---|---|
| Abbreviations: ASA, acetylsalicylic acid (aspirin); ER-DP, extended-release dipyridamole. | ||
| * Neither ER-DP+ASA nor ASA alone is recommended for patients who are allergic to aspirin or unable to take low-dose aspirin. | ||
| **The recommended antithrombotic agents have not been specifically tested in patients who have experienced a TIA during ASA therapy. | ||
| TIA (atherothrombotic) | ASA 50–325 mg/d | ER-DP 200 mg+ASA 25 mg BID Clopidogrel 75 mg/d Ticlopidine 250 mg BID ASA 50–1300 mg/d |
| TIA (atherothrombotic) aspirin intolerant* or if TIA occurs during ASA therapy** | ER-DP 200 mg + ASA 25 mg BID Clopidogrel 75 mg/d | Ticlopidine 250 mg BID Warfarin (INR 2–3) ASA 50–1300 mg/d |
| TIA (cardioembolic) | Warfarin target INR 2.5 (range 2–3) | ASA 50–325 mg/d (if contraindications to warfarin) |
The rapid reticulocyte γ-globin response: kinetics in a representative butyrate responsive sickle cell disease patient.
A: γ-globin chain synthesis; B: γ-globin mRNA.
The rapid reticulocyte γ-globin response: kinetics in a representative butyrate responsive sickle cell disease patient.
A: γ-globin chain synthesis; B: γ-globin mRNA.
γ-Globin response in seven sickle cell disease patients.
(▪) Butyrate responsive patients. (□) Butyrate nonresponsive patients.
A: γ-globin chain synthesis; B: γ-globin mRNA.
γ-Globin response in seven sickle cell disease patients.
(▪) Butyrate responsive patients. (□) Butyrate nonresponsive patients.
A: γ-globin chain synthesis; B: γ-globin mRNA.
Alteration in the polysomal distribution of γ-globin mRNA in reticulocytes as a result of butyrate therapy.
Analysis of γ-globin mRNA from reticulocytes from a representative butyrate responsive sickle cell disease patients (⧫) Before therapy; (▪) 24 hours after therapy. Fractions 1 and 2 are the pre-polysomal region. Fractions 5 to 8 represent the 1 to 3 ribosomal region.
Alteration in the polysomal distribution of γ-globin mRNA in reticulocytes as a result of butyrate therapy.
Analysis of γ-globin mRNA from reticulocytes from a representative butyrate responsive sickle cell disease patients (⧫) Before therapy; (▪) 24 hours after therapy. Fractions 1 and 2 are the pre-polysomal region. Fractions 5 to 8 represent the 1 to 3 ribosomal region.
Blast-forming units erythroid (BFU-E)–derived colony α-globin chain synthesis in cultures from in vivo butyrate responsive sickle cell disease patients.
A: Effect of in vitro butyrate on pretreatment colonies. B: Effect of in vitro butyrate on colonies initiated 24 hours after in vivo butyrate infusions began. C: Comparison of pretreatment colonies and colonies initiated 24 hours after in vivo butyrate therapy began, both cultured in vitro in the absence of butyrate. Open symbols are cultures from individual sickle cell disease patients. Each data point is the mean ± 1 SD of triplicate cultures. Solid line represents the mean of all cultures.
Blast-forming units erythroid (BFU-E)–derived colony α-globin chain synthesis in cultures from in vivo butyrate responsive sickle cell disease patients.
A: Effect of in vitro butyrate on pretreatment colonies. B: Effect of in vitro butyrate on colonies initiated 24 hours after in vivo butyrate infusions began. C: Comparison of pretreatment colonies and colonies initiated 24 hours after in vivo butyrate therapy began, both cultured in vitro in the absence of butyrate. Open symbols are cultures from individual sickle cell disease patients. Each data point is the mean ± 1 SD of triplicate cultures. Solid line represents the mean of all cultures.
Butyrate-induced changes in histone H3 acetylation in sickle cell disease.
A: Acetylation of histone H3 in BFU-E or CFU-GM derived cells from a patient with sickle cell disease. The bar graph shows the results of ChIP analysis of the promoters of the ε-, γ-, and β-globin genes expressed as relative units (RU).
B: mRNA levels of different globins measured by real-time PCR and expressed as fold change between control BFU-E cells and butyrate exposed BFU-E cells.
C: mRNA levels of different globins measured by real-time PCR and expressed as ratios in control BFU-E cells and butyrate exposed BFU-E cells.
Butyrate-induced changes in histone H3 acetylation in sickle cell disease.
A: Acetylation of histone H3 in BFU-E or CFU-GM derived cells from a patient with sickle cell disease. The bar graph shows the results of ChIP analysis of the promoters of the ε-, γ-, and β-globin genes expressed as relative units (RU).
B: mRNA levels of different globins measured by real-time PCR and expressed as fold change between control BFU-E cells and butyrate exposed BFU-E cells.
C: mRNA levels of different globins measured by real-time PCR and expressed as ratios in control BFU-E cells and butyrate exposed BFU-E cells.
The lentivirus vector use for the correction of sickle cell in transgenic mice.
Diagram of the βT27Q-globin provirus.
Abbreviations: HIV LTR, human immune deficiency type-1 virus long terminal repeat; +, packaging signal; cPPT/flap or central polypurine tract/DNA flap; RRE or Rev-responsive element; P, β-globin promoter; ppt, polypurine tract.
The 3′ β-globin enhancer, the 372 bp IVS2 deletion, the βT87Q mutation and sections of DnaseI hypersensitive sites (HS) 2, HS3, and HS4 of the β-globin LCR are indicated.
The cPPT/flap and the RRE element help in the export of the mRNA from the nucleus to the cytoplasma.
This vector integrates with an average of 3 copies per genome that is higher than previous vectors.
This vector has given no indication of silencing over time.
Reprinted with permission from
Abbreviations: LCR, locus control region, 5′ to the β-like gene cluster, involved in the development-appropriate globin gene expression; IVS2, intervening sequence 2.
The lentivirus vector use for the correction of sickle cell in transgenic mice.
Diagram of the βT27Q-globin provirus.
Abbreviations: HIV LTR, human immune deficiency type-1 virus long terminal repeat; +, packaging signal; cPPT/flap or central polypurine tract/DNA flap; RRE or Rev-responsive element; P, β-globin promoter; ppt, polypurine tract.
The 3′ β-globin enhancer, the 372 bp IVS2 deletion, the βT87Q mutation and sections of DnaseI hypersensitive sites (HS) 2, HS3, and HS4 of the β-globin LCR are indicated.
The cPPT/flap and the RRE element help in the export of the mRNA from the nucleus to the cytoplasma.
This vector integrates with an average of 3 copies per genome that is higher than previous vectors.
This vector has given no indication of silencing over time.
Reprinted with permission from
Abbreviations: LCR, locus control region, 5′ to the β-like gene cluster, involved in the development-appropriate globin gene expression; IVS2, intervening sequence 2.