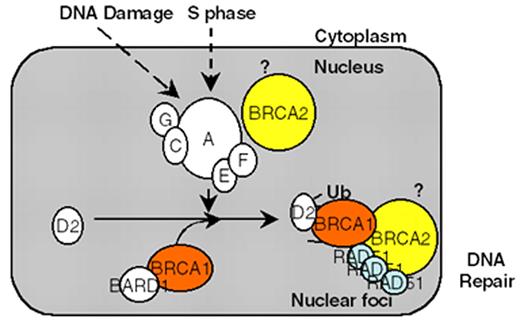
D’Andrea Figure 1 (in D’Andrea et al). Schematic representation of the Fanconi anemia (FA) pathway.
DNA damage-inducible or S phase–specific monoubiquitination of FANCD2 requires the FA protein complex (A,C,G,E,F complex). Loss of any one of these five FA protein subunits results in instability and degradation of the other subunits. Monoubiquitination targets D2 to DNA repair foci containing BRCA1, BRCA2, and RAD51. The BRCA2 protein may function upstream in this pathway by promoting D2 monoubiquitination and/or downstream in the pathway by promoting homologous recombination repair. The interaction of BRCA2/FANCD1 with the RAD51 protein strongly supports a role of this pathway in the regulation of DNA repair activity. Disruption of this pathway leads to chromosome breakage, pancytopenia, and cancer predisposition.
D’Andrea Figure 1 (in D’Andrea et al). Schematic representation of the Fanconi anemia (FA) pathway.
DNA damage-inducible or S phase–specific monoubiquitination of FANCD2 requires the FA protein complex (A,C,G,E,F complex). Loss of any one of these five FA protein subunits results in instability and degradation of the other subunits. Monoubiquitination targets D2 to DNA repair foci containing BRCA1, BRCA2, and RAD51. The BRCA2 protein may function upstream in this pathway by promoting D2 monoubiquitination and/or downstream in the pathway by promoting homologous recombination repair. The interaction of BRCA2/FANCD1 with the RAD51 protein strongly supports a role of this pathway in the regulation of DNA repair activity. Disruption of this pathway leads to chromosome breakage, pancytopenia, and cancer predisposition.
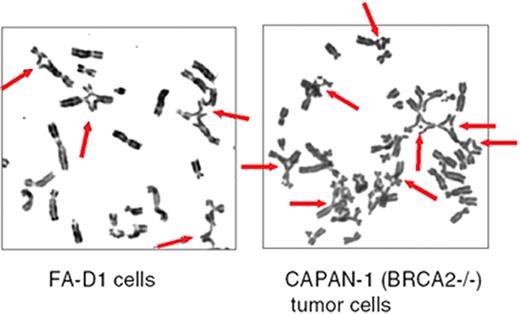
D’Andrea Figure 2 (in D’Andrea et al). Mitomycin C–induced chromosome breaks and radial forms in Fanconi anemia (FA) cells and in BRCA2 (-/-) tumor cells.
The indicated cells were exposed to mitomycin C (40 ng/mL) for 48 hours. Thereafter, the cells were examined for chromosome breakage.
D’Andrea Figure 2 (in D’Andrea et al). Mitomycin C–induced chromosome breaks and radial forms in Fanconi anemia (FA) cells and in BRCA2 (-/-) tumor cells.
The indicated cells were exposed to mitomycin C (40 ng/mL) for 48 hours. Thereafter, the cells were examined for chromosome breakage.
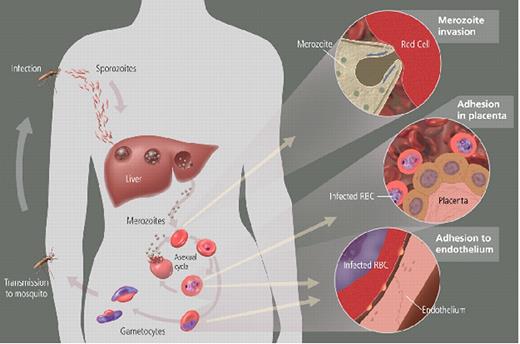
Miller Figure 2 (in Weatherall et al). Parasite life cycle and pathogenesis of falciparum malaria.
The molecular and cellular events during the life cycle influence disease severity. Disease only occurs as a result of the asexual blood stage after the parasite leaves the liver and begins to invade and grow inside red cells (RBCs). All human Plasmodium spp. invade by the same mechanism, but P. falciparum reaches high parasitemia because of greater flexibility in receptor pathways that it can use to invade all RBCs. RBCs infected with P. falciparum must bind to endothelium or placenta for the parasite to avoid spleen-dependent killing mechanisms, but this binding also leads to much of the pathology (see Miller Figure 3F4).
Miller Figure 2 (in Weatherall et al). Parasite life cycle and pathogenesis of falciparum malaria.
The molecular and cellular events during the life cycle influence disease severity. Disease only occurs as a result of the asexual blood stage after the parasite leaves the liver and begins to invade and grow inside red cells (RBCs). All human Plasmodium spp. invade by the same mechanism, but P. falciparum reaches high parasitemia because of greater flexibility in receptor pathways that it can use to invade all RBCs. RBCs infected with P. falciparum must bind to endothelium or placenta for the parasite to avoid spleen-dependent killing mechanisms, but this binding also leads to much of the pathology (see Miller Figure 3F4).
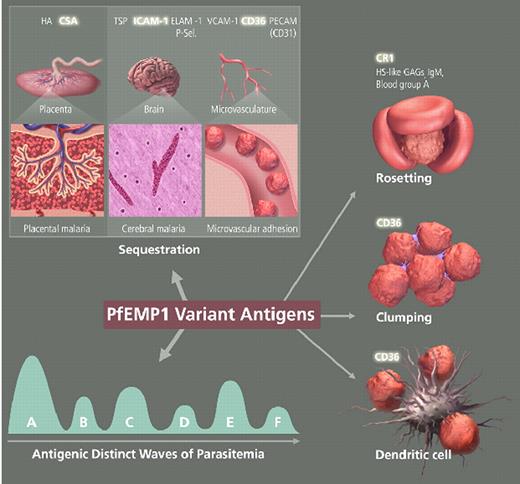
Miller Figure 3 (in Weatherall et al). The variant antigen family of PfEMP1 is central to host-parasite interaction and pathogenesis.
PfEMP1 expressed on the surface of mature P. falciparum-infected RBCs is involved with clonal antigenic variation and can bind to many host receptors through its multiple adhesion domains. The different properties of PfEMP1sequestration for evading spleen-dependent killing and antigenic variation for evasion of antibody-dependent killingcontribute to the virulence and pathogenesis of P. falciparum and are essential for the survival of the parasite. Parasite sequestration in the brain and placenta contribute to the complications of cerebral malaria and placental malaria, respectively. Simultaneous binding to several receptors, binding of uninfected erythrocytes (rosetting), and clumping of infected erythrocytes through platelets are associated with malaria pathogenesis. Parasite-infected RBCs binding to dendritic cells downregulate the host immune response.
Abbreviations: HA, hyaluronic acid; TSP, thrombospondin; ELAM-1, endothelial/leukocyte adhesion molecule 1; P-sel., P-selectin; VCAM-1, vascular cell adhesion molecule 1; CR1 complement receptor 1; HS-like GAGs, heparin sulfate-like glycosaminoglycans; IgM, immunoglobulin M.
Miller Figure 3 (in Weatherall et al). The variant antigen family of PfEMP1 is central to host-parasite interaction and pathogenesis.
PfEMP1 expressed on the surface of mature P. falciparum-infected RBCs is involved with clonal antigenic variation and can bind to many host receptors through its multiple adhesion domains. The different properties of PfEMP1sequestration for evading spleen-dependent killing and antigenic variation for evasion of antibody-dependent killingcontribute to the virulence and pathogenesis of P. falciparum and are essential for the survival of the parasite. Parasite sequestration in the brain and placenta contribute to the complications of cerebral malaria and placental malaria, respectively. Simultaneous binding to several receptors, binding of uninfected erythrocytes (rosetting), and clumping of infected erythrocytes through platelets are associated with malaria pathogenesis. Parasite-infected RBCs binding to dendritic cells downregulate the host immune response.
Abbreviations: HA, hyaluronic acid; TSP, thrombospondin; ELAM-1, endothelial/leukocyte adhesion molecule 1; P-sel., P-selectin; VCAM-1, vascular cell adhesion molecule 1; CR1 complement receptor 1; HS-like GAGs, heparin sulfate-like glycosaminoglycans; IgM, immunoglobulin M.
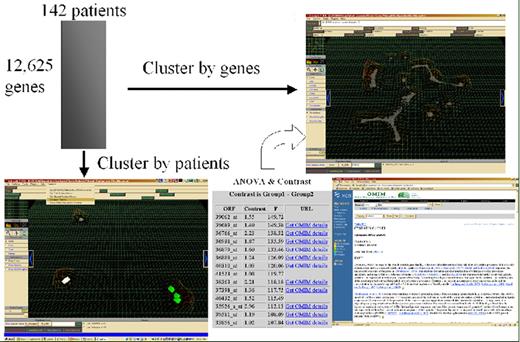
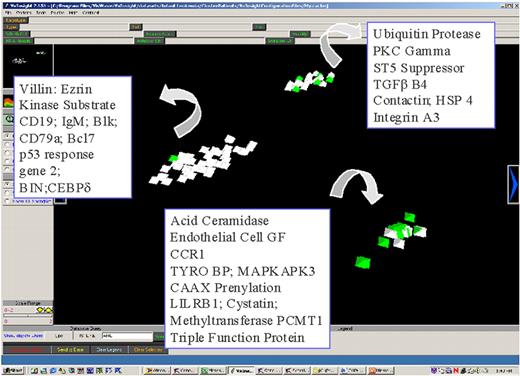
Willman Figure 6 (in Giles et al). Multidatabase knowledge mining.
Willman Figure 6 (in Giles et al). Multidatabase knowledge mining.
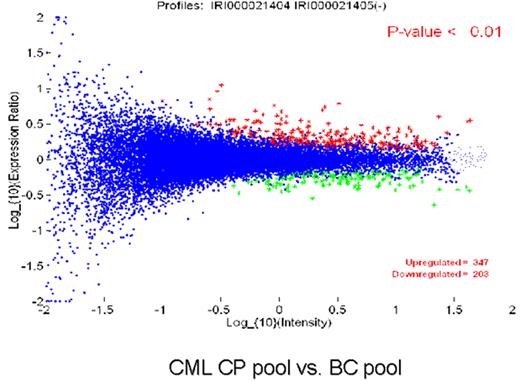
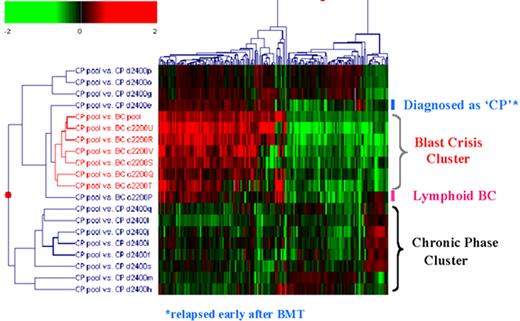
Radich Figure 8 (in Druker et al). Gene expression differences between chronic and blast phase CML.
Figure 8A compares the expression of 25,000 genes between a pool of blast crisis and a pool of chronic phase CML RNA. The Y axis is the relative expression of a gene in blast phase v. chronic phase. The X axis is the intensity of each “spot.” The blue dots represent all genes that are not significantly different between chronic and blast phase. The red dots are genes up-regulated in blast compared to chronic phase (n = 347 genes), and green dots are genes down-regulated in blast compared to chronic phase (n = 203 genes). Figure 8B is a “dendrogram,” which demonstrates the clustering of genes and disease states. Blast crisis cases tend to have gene expression patterns quite similar to other blast crisis cases, but different than chronic phase cases, whose patterns are also fairly similar to one another. Note that a patient with the diagnosis of chronic phase demonstrated a blast crisis gene expression pattern. This patient relapsed early after transplantation.
Radich Figure 8 (in Druker et al). Gene expression differences between chronic and blast phase CML.
Figure 8A compares the expression of 25,000 genes between a pool of blast crisis and a pool of chronic phase CML RNA. The Y axis is the relative expression of a gene in blast phase v. chronic phase. The X axis is the intensity of each “spot.” The blue dots represent all genes that are not significantly different between chronic and blast phase. The red dots are genes up-regulated in blast compared to chronic phase (n = 347 genes), and green dots are genes down-regulated in blast compared to chronic phase (n = 203 genes). Figure 8B is a “dendrogram,” which demonstrates the clustering of genes and disease states. Blast crisis cases tend to have gene expression patterns quite similar to other blast crisis cases, but different than chronic phase cases, whose patterns are also fairly similar to one another. Note that a patient with the diagnosis of chronic phase demonstrated a blast crisis gene expression pattern. This patient relapsed early after transplantation.
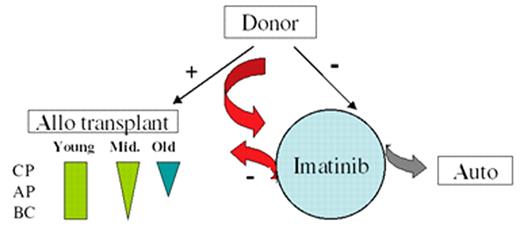
Radich Figure 9 (in Druker et al). A possible modern treatment algorithm for CML.
Patients with a related or unrelated donor could be considered for early transplantation. Young patients could be offered a full allogeneic transplant for any phase from a related or unrelated doror (green rectangle, where the width of the rectangle suggests the enthusiasm for transplant). “Middle age” patients (tactfully defined) are offered transplantation with diminished enthusiasm for advanced disease; elderly patients are offered a nonmyeloablative transplant (blue arrow). Patients with a donor can be tried on imatinib, but sent to transplant if they do not obtain a complete cytogenetic response (CCR), or if they do achieve a CCR, show molecular evidence of progression. Patients without a donor can receive imatinib. If they do not respond, investigation therapy with an autologous transplant can be considered (grey arrow). If they do obtain a CCR, stem cell storage can be considered.
Radich Figure 9 (in Druker et al). A possible modern treatment algorithm for CML.
Patients with a related or unrelated donor could be considered for early transplantation. Young patients could be offered a full allogeneic transplant for any phase from a related or unrelated doror (green rectangle, where the width of the rectangle suggests the enthusiasm for transplant). “Middle age” patients (tactfully defined) are offered transplantation with diminished enthusiasm for advanced disease; elderly patients are offered a nonmyeloablative transplant (blue arrow). Patients with a donor can be tried on imatinib, but sent to transplant if they do not obtain a complete cytogenetic response (CCR), or if they do achieve a CCR, show molecular evidence of progression. Patients without a donor can receive imatinib. If they do not respond, investigation therapy with an autologous transplant can be considered (grey arrow). If they do obtain a CCR, stem cell storage can be considered.
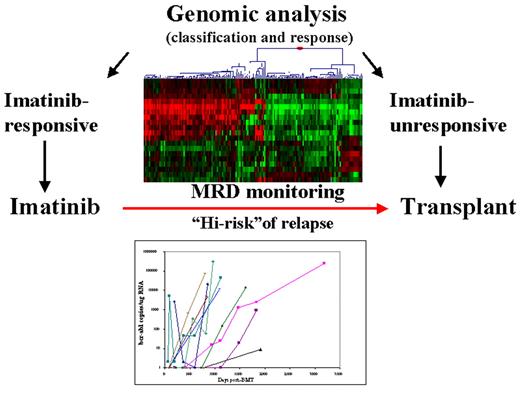
Radich Figure 10 (in Druker et al). CML treatment in the future.
Genomic analysis may suggest the proper treatment option; molecular monitoring may give early insight as to when treatment can be discontinued, or modified.
Radich Figure 10 (in Druker et al). CML treatment in the future.
Genomic analysis may suggest the proper treatment option; molecular monitoring may give early insight as to when treatment can be discontinued, or modified.
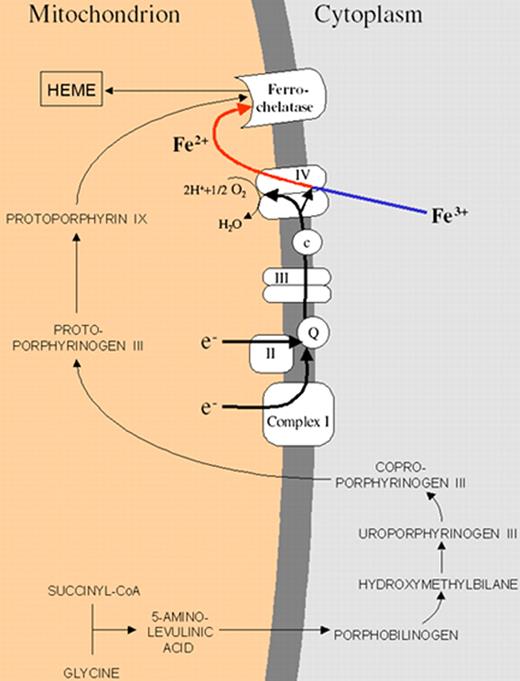
Gattermann Figure 4 (in Greenberg et al). Schematic representation of the heme biosynthetic pathway and its connection with the electron transport chain of the inner mitochondrial membrane.
The mitochondrial respiratory chain provides electrons needed to convert Fe3+ into Fe2+. Only Fe2+ can pass through the inner mitochondrial membrane. The respiratory chain creates the membrane potential needed to carry Fe2+ through the inner mitochondrial membrane. Iron must be provided in the right chemical form, because ferrochelatase can use only Fe2+ for heme synthesis.
Gattermann Figure 4 (in Greenberg et al). Schematic representation of the heme biosynthetic pathway and its connection with the electron transport chain of the inner mitochondrial membrane.
The mitochondrial respiratory chain provides electrons needed to convert Fe3+ into Fe2+. Only Fe2+ can pass through the inner mitochondrial membrane. The respiratory chain creates the membrane potential needed to carry Fe2+ through the inner mitochondrial membrane. Iron must be provided in the right chemical form, because ferrochelatase can use only Fe2+ for heme synthesis.
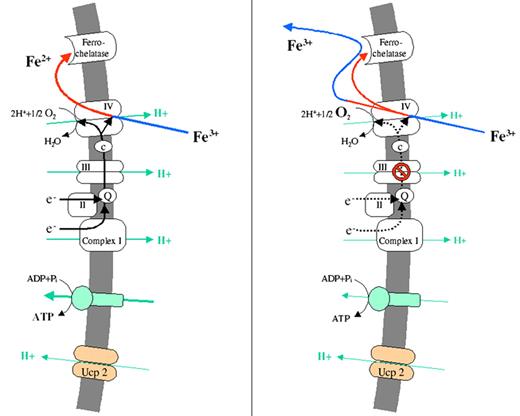
Gattermann Figure 5 (in Greenberg et al). Pathogenesis of mitochondrial iron overload as a consequence of respiratory chain dysfunction.
(A) Uncoupling protein-2, which is expressed during erythroid differentiation, diminishes the mitochondrial protein gradient. This stimulates the respiratory chain to run faster to restore the gradient. Accordingly, oxygen consumption is increased and a low-oxygen environment is created, which is needed to protect Fe2+ from reoxidation.
(B) A respiratory chain defect will slow down oxygen consumption and will thus leave more oxygen in the mitochondrial matrix. Therefore, some of the imported Fe2+ will become reoxidized. Fe3+ will be rejected by ferrochelatase and will thus accumulate in the mitochondrial matrix.
Gattermann Figure 5 (in Greenberg et al). Pathogenesis of mitochondrial iron overload as a consequence of respiratory chain dysfunction.
(A) Uncoupling protein-2, which is expressed during erythroid differentiation, diminishes the mitochondrial protein gradient. This stimulates the respiratory chain to run faster to restore the gradient. Accordingly, oxygen consumption is increased and a low-oxygen environment is created, which is needed to protect Fe2+ from reoxidation.
(B) A respiratory chain defect will slow down oxygen consumption and will thus leave more oxygen in the mitochondrial matrix. Therefore, some of the imported Fe2+ will become reoxidized. Fe3+ will be rejected by ferrochelatase and will thus accumulate in the mitochondrial matrix.
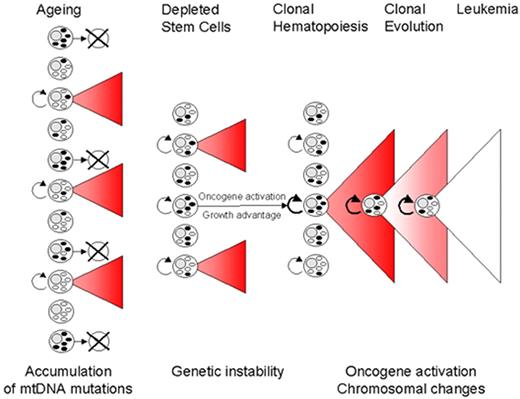
Gattermann Figure 6 (in Greenberg et al). Proposed model of MDS pathogenesis including mitochondrial pathology.
With advancing age, hematopoietic stem cells accumulate mutations of mitochondrial DNA (mtDNA). If a stem cell with mutant mtDNA becomes transformed by additional mutations in the cell nucleus, a clonal bone marrow can be established and the clinical picture of MDS can arise. MtDNA mutations may play a major role in shaping the phenotype of the MDS clone, as exemplified by the formation of ringed sideroblasts. In addition, by contributing to genomic instability, mtDNA mutations may facilitate the transforming event that initiates an MDS clone and may also promote further chromosomal changes which drive the clonal evolution towards leukemia.
Gattermann Figure 6 (in Greenberg et al). Proposed model of MDS pathogenesis including mitochondrial pathology.
With advancing age, hematopoietic stem cells accumulate mutations of mitochondrial DNA (mtDNA). If a stem cell with mutant mtDNA becomes transformed by additional mutations in the cell nucleus, a clonal bone marrow can be established and the clinical picture of MDS can arise. MtDNA mutations may play a major role in shaping the phenotype of the MDS clone, as exemplified by the formation of ringed sideroblasts. In addition, by contributing to genomic instability, mtDNA mutations may facilitate the transforming event that initiates an MDS clone and may also promote further chromosomal changes which drive the clonal evolution towards leukemia.
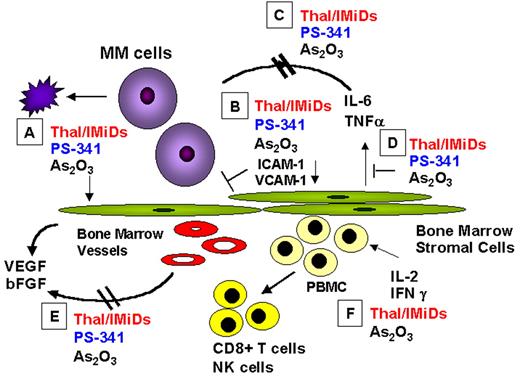
Anderson Figure 5. Mechanisms of action of novel biologically based therapies targeting multiple myeloma (MM) cells and the BM microenvironment include the following: (A) MM cell G1 growth arrest and apoptosis; (B) decreased MM-BMSC binding; (C) decreased cytokine activity; (D) decreased cytokine production in BM; (E) decreased angiogenesis; (F) induced host anti-MM immunity.
Abbreviations: BM, bone marrow; BMSC, BM stromal cell; IMiD, immunomodulatory analog; NK, natural killer; VEGF, vascular endothelial growth factor.
Anderson Figure 5. Mechanisms of action of novel biologically based therapies targeting multiple myeloma (MM) cells and the BM microenvironment include the following: (A) MM cell G1 growth arrest and apoptosis; (B) decreased MM-BMSC binding; (C) decreased cytokine activity; (D) decreased cytokine production in BM; (E) decreased angiogenesis; (F) induced host anti-MM immunity.
Abbreviations: BM, bone marrow; BMSC, BM stromal cell; IMiD, immunomodulatory analog; NK, natural killer; VEGF, vascular endothelial growth factor.
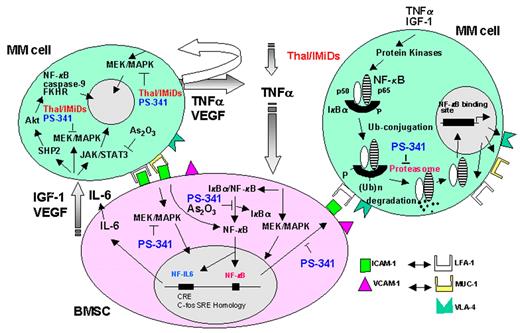
Anderson Figure 6. Signaling pathways triggered by cytokines and their inhibition by novel therapeutic agents in multiple myeloma (MM).
Abbreviations: BMSC, bone marrow stromal cell; IGF, insulin growth factor; IL, interleukin; VEGF, vascular endothelial growth factor.
Anderson Figure 6. Signaling pathways triggered by cytokines and their inhibition by novel therapeutic agents in multiple myeloma (MM).
Abbreviations: BMSC, bone marrow stromal cell; IGF, insulin growth factor; IL, interleukin; VEGF, vascular endothelial growth factor.
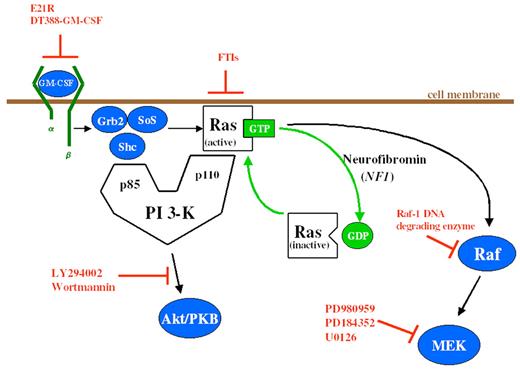
Emanuel Figure 3 (in Arceci et al). Schema of GM-CSF signal transduction through the Ras signaling pathways. Potential inhibitors of this pathway are depicted in orange. E21R is a GM-CSF antagonist analogue. DT388-GM-CSF is a GM-CSF molecule/diphtheria toxin fusion construct.
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; FTIs, farnesyltransferase inhibitors; GTP, guanosine triphosphate; GDP, guanosine diphosphate; PI 3-K, phosphatidylinositol 3-kinase; PKB, protein kinase B; MEK, mitogen-activated protein kinase kinase.
Emanuel Figure 3 (in Arceci et al). Schema of GM-CSF signal transduction through the Ras signaling pathways. Potential inhibitors of this pathway are depicted in orange. E21R is a GM-CSF antagonist analogue. DT388-GM-CSF is a GM-CSF molecule/diphtheria toxin fusion construct.
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; FTIs, farnesyltransferase inhibitors; GTP, guanosine triphosphate; GDP, guanosine diphosphate; PI 3-K, phosphatidylinositol 3-kinase; PKB, protein kinase B; MEK, mitogen-activated protein kinase kinase.
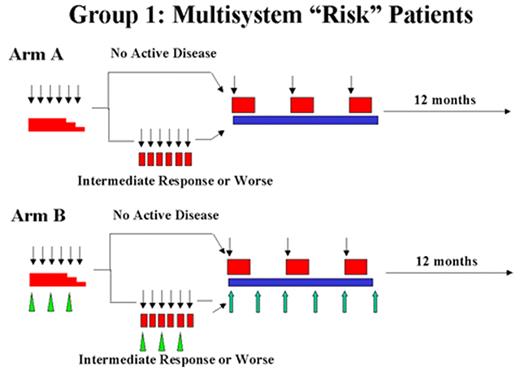
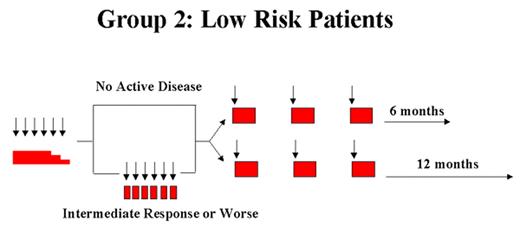
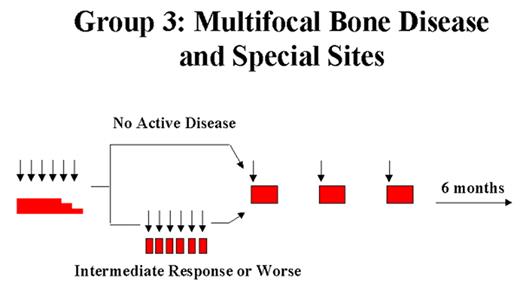
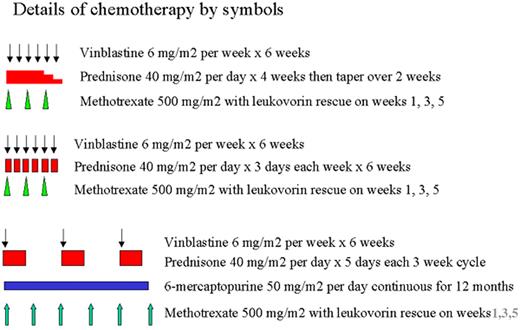
Arceci Figure 2. Langerhans Cell Histiocytosis–III clinical trial. Schema and doses from LCH III Protocol.
Arceci Figure 2. Langerhans Cell Histiocytosis–III clinical trial. Schema and doses from LCH III Protocol.
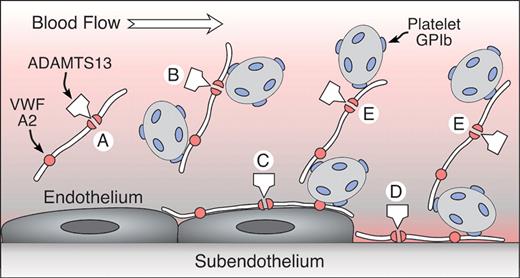
Sadler Figure 2 (in George et al). A model for the role of UL-VWF and ADAMTS13 in TTP.
UL-VWF is released from endothelial cells that enter the circulation (A,B) or adhere to the endothelial surface (C). VWF also binds to exposed connective tissue at sites of injury (D). Platelets adhere to VWF in solution (B) or on surfaces (C,D) through platelet glycoprotein Ib (GPIb). VWF also may recruit platelets to previously adhering platelets (E). ADAMTS13 cleaves the A2 domain of the VWF subunit, severing the multimer. This reaction is slow for VWF in solution (A) but occurs rapidly when platelets adhere to VWF under high fluid shear conditions in suspension (B) or on surfaces (C,D,E), presumably as a consequence of conformational changes induced by tensile force on the VWF multimer. Failure of this mechanism appears to cause TTP.
Adapted with permission from Sadler JE: A new name in thrombosis, ADAMTS13. Proc Natl Acad Sci USA 2002;99:11552-11554 (Copyright 2002 National Academy of Sciences, U.S.A.).
Sadler Figure 2 (in George et al). A model for the role of UL-VWF and ADAMTS13 in TTP.
UL-VWF is released from endothelial cells that enter the circulation (A,B) or adhere to the endothelial surface (C). VWF also binds to exposed connective tissue at sites of injury (D). Platelets adhere to VWF in solution (B) or on surfaces (C,D) through platelet glycoprotein Ib (GPIb). VWF also may recruit platelets to previously adhering platelets (E). ADAMTS13 cleaves the A2 domain of the VWF subunit, severing the multimer. This reaction is slow for VWF in solution (A) but occurs rapidly when platelets adhere to VWF under high fluid shear conditions in suspension (B) or on surfaces (C,D,E), presumably as a consequence of conformational changes induced by tensile force on the VWF multimer. Failure of this mechanism appears to cause TTP.
Adapted with permission from Sadler JE: A new name in thrombosis, ADAMTS13. Proc Natl Acad Sci USA 2002;99:11552-11554 (Copyright 2002 National Academy of Sciences, U.S.A.).
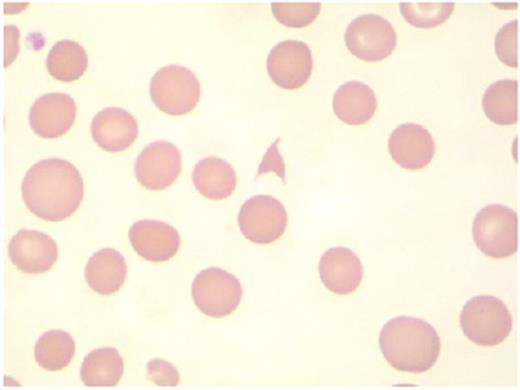
Hambleton Figure 4. The presence of schistocytes on peripheral blood smear suggests the diagnosis of microangiopathy.
Courtesy of Dr Timothy Hamill, Department of Laboratory Medicine, University of California, San Francisco.
Hambleton Figure 4. The presence of schistocytes on peripheral blood smear suggests the diagnosis of microangiopathy.
Courtesy of Dr Timothy Hamill, Department of Laboratory Medicine, University of California, San Francisco.
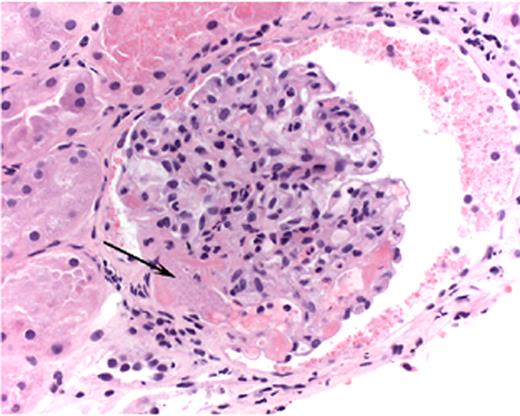
Hambleton Figure 5. Light micrograph of a glomerulus with fibrin-platelet thrombus in afferent arteriole (arrow) as it enters the glomerulus.
Note red blood cell fragments within thrombus (H&E).
Courtesy of Dr. Jean Olson, Department of Pathology, University of California, San Francisco.
Hambleton Figure 5. Light micrograph of a glomerulus with fibrin-platelet thrombus in afferent arteriole (arrow) as it enters the glomerulus.
Note red blood cell fragments within thrombus (H&E).
Courtesy of Dr. Jean Olson, Department of Pathology, University of California, San Francisco.
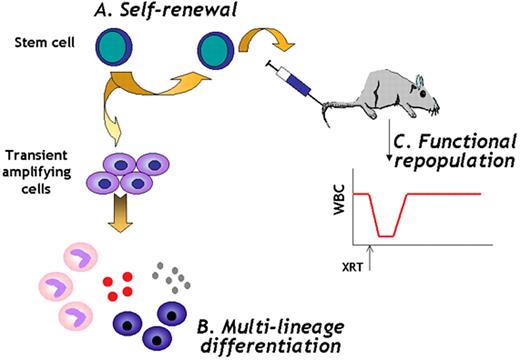
Verfaillie Figure 5. Stem cell definition.
Stem cells are cells (A) that have extensive self-renewal ability, (B) that have multilineage differentiation ability, and (C) that can rescue a lethally damaged tissue in vivo for the lifetime of the recipient.
Abbreviations: WBC, white blood cells; XRT, lethal irradiation.
Verfaillie Figure 5. Stem cell definition.
Stem cells are cells (A) that have extensive self-renewal ability, (B) that have multilineage differentiation ability, and (C) that can rescue a lethally damaged tissue in vivo for the lifetime of the recipient.
Abbreviations: WBC, white blood cells; XRT, lethal irradiation.
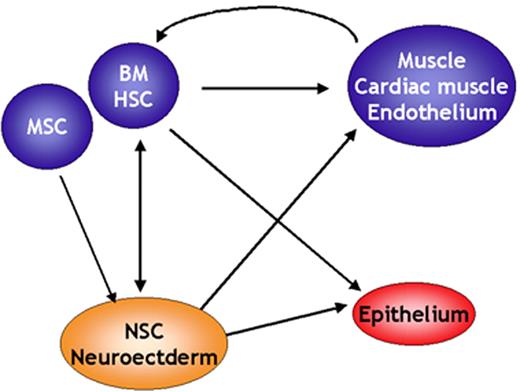
Verfaillie Figure 6. Stem cell plasticity.
Over the past 5 years, at least 50 papers have been published demonstrating that cells or stem cells of one tissue can give rise to cells with phenotypic, morphologic, and in some instances functional characteristics of differentiated cells of a second tissue. Some of these apparent lineage switches occur between tissues that are derived from a different germ layer.
Abbreviations: BM, bone marrow; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; NSC, neural stem cells.
Verfaillie Figure 6. Stem cell plasticity.
Over the past 5 years, at least 50 papers have been published demonstrating that cells or stem cells of one tissue can give rise to cells with phenotypic, morphologic, and in some instances functional characteristics of differentiated cells of a second tissue. Some of these apparent lineage switches occur between tissues that are derived from a different germ layer.
Abbreviations: BM, bone marrow; HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; NSC, neural stem cells.
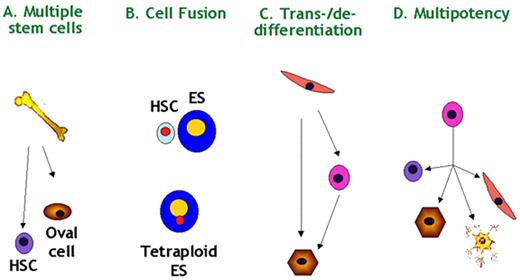
Verfaillie Figure 7. Stem cell plasticity—possible mechanisms.
Four possible hypotheses exist to explain stem cell plasticity: (A) multiple stem cells may coexist in multiple tissues; (B) donor cells may fuse with host cells and acquire characteristics of the host cells; (C) cells may undergo de- and redifferentiation, or trans-differentiate; (D) multipotent stem cells “akin” to ES cells may persist past initial lineage specification. Scientific evidence exists for all possible explanations.
Abbreviations: ES, embryonic stem; HSC, hematopoietic stem cells.
Verfaillie Figure 7. Stem cell plasticity—possible mechanisms.
Four possible hypotheses exist to explain stem cell plasticity: (A) multiple stem cells may coexist in multiple tissues; (B) donor cells may fuse with host cells and acquire characteristics of the host cells; (C) cells may undergo de- and redifferentiation, or trans-differentiate; (D) multipotent stem cells “akin” to ES cells may persist past initial lineage specification. Scientific evidence exists for all possible explanations.
Abbreviations: ES, embryonic stem; HSC, hematopoietic stem cells.
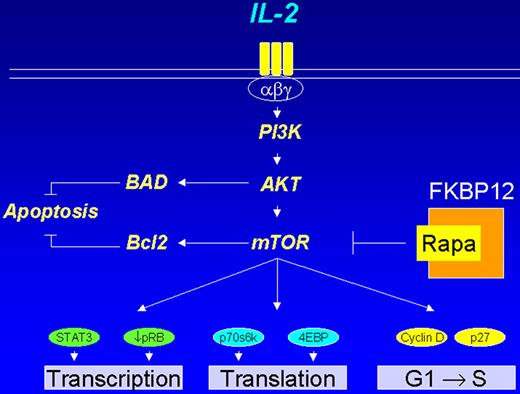
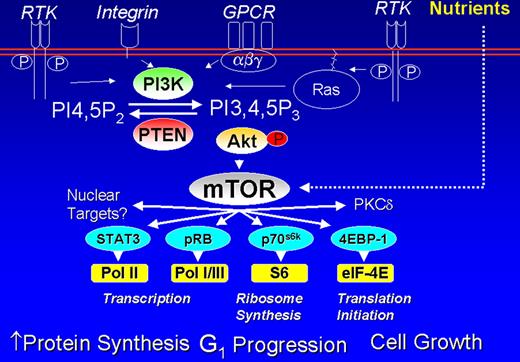
Cheson Figure 1 (in Vose et al). PI3 kinase pathway in lymphoid cells.
Cheson Figure 1 (in Vose et al). PI3 kinase pathway in lymphoid cells.
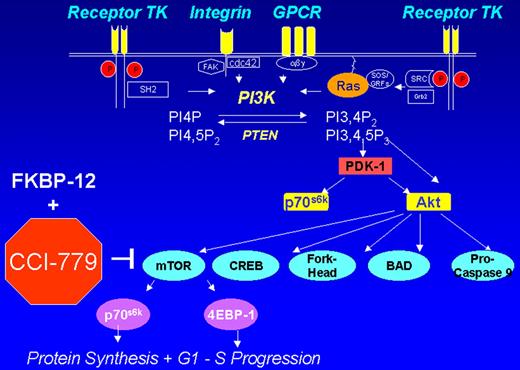
Cheson Figure 3 (in Vose et al). CCI-779 signal transduction pathway.
Cheson Figure 3 (in Vose et al). CCI-779 signal transduction pathway.
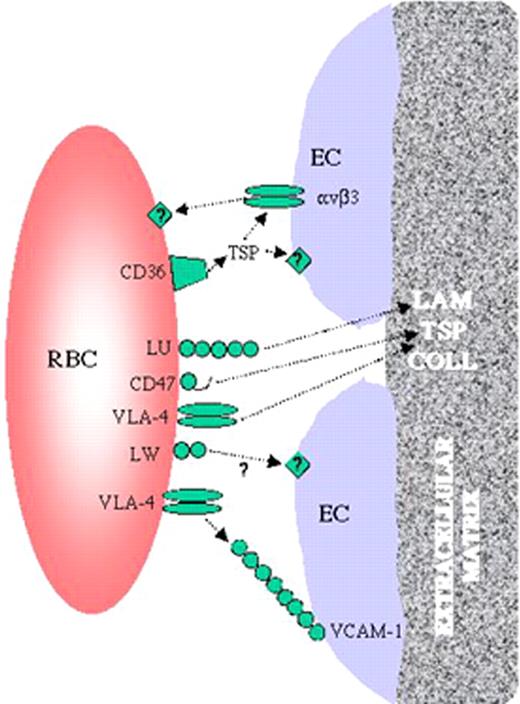
Telen fig 1 (in Garratty et al). Interactions between sickle red cells and the endothelium.
Sickle red cells have been shown to bear an array of adhesion receptors. Some of these, such as CD47, VCAM-1, and the Lutheran blood group protein (LU), are capable of binding extracellular matrix components such as thrombospondin (TSP) and laminin (LAM). Others, including CD36, VLA-4 and LW, appear to mediate red cell binding to endothelial cells, possibly through intermediary linking molecules such as soluble plasma TSP in some cases. Collagen (COLL) is also a major component of the subendothelial extracellular matrix. Some investigators have theorized that endothelial cell damage in sickle cell disease results in exposure of subendothelial matrix to circulating blood cells between retracted endothelial cells.
Telen fig 1 (in Garratty et al). Interactions between sickle red cells and the endothelium.
Sickle red cells have been shown to bear an array of adhesion receptors. Some of these, such as CD47, VCAM-1, and the Lutheran blood group protein (LU), are capable of binding extracellular matrix components such as thrombospondin (TSP) and laminin (LAM). Others, including CD36, VLA-4 and LW, appear to mediate red cell binding to endothelial cells, possibly through intermediary linking molecules such as soluble plasma TSP in some cases. Collagen (COLL) is also a major component of the subendothelial extracellular matrix. Some investigators have theorized that endothelial cell damage in sickle cell disease results in exposure of subendothelial matrix to circulating blood cells between retracted endothelial cells.