Abstract
Blood group antigens (BGAs) can act as functional molecules but also can evoke autoantibodies and alloantibodies, causing autoimmune hemolytic anemia, hemolytic disease of the newborn and hemolytic transfusion reactions.
In Section I, Dr. Marilyn Telen discusses physiologic and pathologic functions of RBC BGA-bearing molecules. She reviews some associations of BGAs with RBC membrane integrity and hemolytic anemia; association of BGAs with enzymatic and transport functions; and adhesion molecules expressed by RBCs, especially with reference to their pathophysiological role in sickle cell disease.
In Section II, Dr. Lawrence Petz discusses the problems of providing blood for patients who have RBC autoantibodies. He provides an algorithm for excluding the presence of “hidden” alloantibodies, when all units appear to be incompatible due to the autoantibody. He emphasizes that clinicians should be aware of these approaches and not accept “the least incompatible unit.”
In Section III, Dr. George Garratty describes two processes, in development, that produce RBCs that result in RBCs that can be described as “universal” donor or “stealth” RBCs. The first process involves changing group A, B, or AB RBCs into group O RBCs by removing the immunospecific sugars responsible for A and B specificity by using specific enzymes. The second process involves covering all BGAs on the RBC surface using polyethylene glycol (PEG). Results of in vitro and in vivo studies on these modified RBCs are discussed.
I. Erythrocyte Blood Group Antigens: Physiologic and Pathologic Functions of Red Cell Antigen-Bearing Molecules
Marilyn J. Telen, MD*
Division of Hematology, Department of Medicine, Duke Comprehensive Sickle Cell Center, and Transfusion Service, DUMC 2615, Duke University Medical Center, Durham, NC 27710
Supported in part by grants HL58939 and HL63409 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Erythrocyte Blood Group Antigens: Functions of Red Cell Antigen-Bearing Molecules
When blood bankers first began rapidly expanding our knowledge of human erythrocyte blood group antigens during the second half of the twentieth century, they may have had little expectation that their work would lead to discoveries of functionally important and sometimes even critical membrane components. Instead, they focused on being able to identify the hundreds of polymorphisms that have now been shown to influence the immune response to allogeneic transfusion. Nevertheless, their work and the subsequent work of membrane biochemists and molecular geneticists, as well as cell biologists, have led to multiple discoveries of functionally interesting and important membrane proteins expressed on the surface of red cells.
Many authors have subdivided the membrane proteins of the erythrocyte into structural or functional groups. Thus, proteins can be classified by structure, such as whether or not they are integral membrane proteins (and thus whether their N- or C-termini are extracellular, or whether they traverse the membrane several times, as is typical for transport molecules). Or, proteins can be classified functionally; they may be important to the structural integrity of the cell, serve as transporters, be active as enzymes, or act as receptors for a wide variety of ligands. However, we now know that several membrane proteins fall into a number of functional categories simultaneously. Table 1 provides a list of gene loci important to the expression of both carbohydrate and protein-based blood group antigens, as well as the known functions of the erythrocyte membrane proteins involved in blood group antigen expression.
Erythrocyte Blood Groups on Molecules Important to Membrane Integrity
When instances of congenital hemolytic anemia are investigated and found to be due to membrane defects, most are variants of hereditary spherocytosis and elliptocytosis. And the majority of these are not associated with unusual alterations in blood group antigens. The single, albeit rare exception to this is the Leach phenotype. The Leach phenotype is the absence of all Gerbich antigens, and this phenotype is caused by total deficiency of glycophorins C and D (GPC, GPD), which are encoded by the GYPC locus. GPC and GPD are the products of a single gene and arise from the use of alternative in-frame translation initiation sites, producing two protein products whose N-termini differ by 21 amino acids but which are otherwise identical. It is believed that the Leach phenotype is associated with hereditary elliptocytosis because the cytoplasmic domains of GPC/D bind to the cytoskeletal protein band 4.1 and provide a critical link between the cytoskeleton and the plasma membrane.1– 5 Total deficiency of GPC/D is associated with about a 25% decrease in expression of band 4.1.
The other blood group antigen-bearing protein whose alteration is associated with hereditary hemolytic anemia due to membrane defects is the anion channel protein (AE1). However, in the case of AE1, the alteration associated with hereditary spherocytosis resides in the cytoplasmic domain normally responsible for interaction with ankyrin, and thus no effects on Diego and Wright blood group antigen expression are seen.6–,9 However, some of these defects are also associated with distal renal tubular acidosis, as AE1 is also normally expressed in renal tubules.10,11
Other Blood Group Antigen Phenotypes Associated with Hemolytic Anemia
Null phenotypes in the Rh and KX blood group systems are also associated with hemolytic anemia, although the mechanisms involved are not understood. While five Rh antigens—D and the antithetical antigen pairs C/c and E/e—are usually considered, the Rh blood group system comprises more than 50 antigens. Two genes, RHD and RHCE, encode highly homologous Rh proteins, which are non-glycosylated multiple membrane spanning proteins of approximately 32,000 Daltons.12 Although Rh proteins have been proposed to be transport proteins, their function remains unproven. The Rh null syndrome arises when both the RH proteins are absent from the membrane.13 Most often, this occurs due to a defect in the RHAG gene, whose gene product is thought necessary for surface expression of RH proteins14 and which has recently been shown to be an ammonium transporter.15–,17 The Rh null syndrome arising from a defect in RHAG is said to be of the “regulator” type, as a gene outside the RH locus is determining nonexpression of RH genes.17–,19 In rare cases, the Rh null syndrome arises due to absence of functional RH genes (“amorph” type).18,20 In either case, red cells have a stomatocytic morphology and shortened half-life. Erythrocytes lacking all Rh protein expression demonstrate a complex set of additional abnormalities, including abnormal cation transport, relative deficiency of membrane cholesterol, and deficient expression of non-Rhrelated blood group antigens, including LW, Ss, U, and Fy5.13
In the McLeod phenotype, the protein product of the XK gene (Kx protein) is absent.21 This produces acanthocytic red cells as well as a mild hemolytic anemia.22,23 Perhaps most importantly, however, defects in the XK gene cause neuroacanthocytosis, associated with a progressive late-onset muscular dystrophy. Since the XK gene resides on the X chromosome, females who carry one normal and one null allele have two populations of red cells, one acanthocytic and the other normal, due to lyonization. Erythrocytes lacking the Kx protein and antigen also have markedly weakened expression of Kell blood group antigens. However, genetic defects that cause the Kell null phenotype (with normal Kx antigen expression) do not produce red cells with shortened half-lives or abnormal morphology.24
Proteins with Complement-Related Functions
At least three well investigated erythrocyte membrane proteins are involved in the regulation of complement and the clearance of immune complexes. The C3b/C4b receptor, also known as complement receptor type 1 (CR1), was the first to be identified and was later given the designation CD35. CR1 bears the Knops/McCoy blood group antigens25 and is believed to be important in immune adherence.26 Recently, the presence of a naturally occurring low-expression polymorphism of CR1 has been associated with the occurrence of more severe malaria.27 The proteins decay accelerating factor (DAF, CD55) and membrane inhibitor of reactive lysis (MIRL, protectin, CD59) were identified later, largely due to investigation of the complement regulatory defect of red cells in paroxysmal nocturnal hemoglobinuria (PNH).28,29 Both CD55 and CD59 are attached to the membrane by glycosylphosphatidylinositol anchors and are thus absent from PNH red cells, in which the synthesis of such anchors is defective.30–,32 CD55 carries the Cromer blood group system, whose null phenotype is associated with a subclinical defect in membrane complement regulation.29 No blood group antigens have thus far been identified on CD59.
Proteins with Enzymatic or Transport Functions
At least three blood group systems reside on proteins that are active enzymatically. The more than 20 Kell blood group antigens reside on a member of the neprilysin subfamily33 of zinc-binding neutral endopeptidases, which include the common lymphocytic leukemia antigen (CALLA). The Kell protein is most closely related to the endothelin-converting enzymes (ECE-1 and ECE-2) and is itself able to cleave big endothelin-3 to endothelin-3 (ET-3) and to a lesser extent big ET-1 and big ET-2 to ET-1 and ET-2 respectively.34 ET-3 is a potent bioactive peptide with a panoply of biological roles. The Cartwright blood group system resides on erythrocyte acetylcholinesterase.35 On erythrocytes, this enzyme is a glycosylphosphatidylinositol-linked protein due to the use of a single exon not used in nonerythroid tissues. It has been postulated but not proven that the presence of acetylcholinesterase on red cells counters diffusion of the neurotransmitter acetylcholine away from its sites of action at neuromuscular junctions. The Dombrock antigens reside on another glycosylphosphatidylinositol-linked protein that is a member of the adenosine 5′-diphosphate (ADP)-ribosyltransferase ectoenzyme gene family.36 However, predicted amino acid sequencing shows that this protein also contains a single RGD sequence, thus raising the possibility that the Dombrock protein may also function in cell adhesion.
Three erythrocyte blood group antigens are known to reside on membrane transporters—Diego on band 3, or the anion channel (AE1), Colton on the water channel aquaporin (AQP-1),37,38 and Kidd on the urea transporter (UT1).39 Deficiency of band 3 in humans and animals is associated with severe hemolysis,11 although it is unclear whether this is due solely to lack of transport function or whether the other role of AE1 as a link between the lipid bilayer and the cytoskeleton is a more important contributor to red cell instability. On the other hand, deficiency of either the water or urea transporters causes no clinically apparent red cell abnormality, although subtle defects in the function of other organ systems (especially kidney) can be identified.40,41
Adhesion Receptors Expressed by Erythrocytes
Perhaps surprisingly, erythrocytes express a rather long list of adhesion molecules (Table 2 ). Among proteins belonging to the IgSF superfamily, three are now well documented to mediate red cell adhesion. CD47 is a thrombospondin (TSP) receptor on both RBC and on other cells. TSP is an extracellular matrix protein found both in basement membranes as well as in a soluble form in plasma. CD47 has also been shown to bind to SIRPα and thus allow erythrocytes to be recognized as “self,” despite their lack of HLA antigens.42,43 Lutheran proteins function as laminin receptors on both sickle red cells as well as many epithelial cancers. The LW protein (ICAM-4) is capable of binding several forms of integrins commonly expressed by leukocytes and other tissues. Other IgSF proteins whose functions are not yet known include CD108, which has one IgSF domain, and CD147, which has recently been shown to be necessary to the ability of erythrocytes to traverse the splenic bed and return to the circulation.
The best characterized non-IgSF erythrocyte adhesion molecule is CD44, which is a hyaluronan receptor.44 On erythroid progenitors, CD44 also cooperates with VLA-4 as a fibronectin receptor.45 CD99 is also known to mediate RBC-lymphocyte interactions, although the physiological role for this interaction remains undefined.
The Pathophysiological Role of Erythrocyte Adhesion Molecules in Sickle Cell Disease
Recently, work on the possible contribution of red cell adhesion to vaso-occlusion in sickle cell disease has led to increased interest in the adhesion molecules carried by both normal and abnormal red cells. Erythrocytes that express predominantly hemoglobin S (SS RBC) adhere to both endothelial cells as well as to extracellular matrix proteins to a degree far greater than do normal red cells (Figure 1, see Color Figures, page 523). Among the erythrocyte adhesion molecules known to be involved are CD36 and VLA-4 (the α4β1 integrin), both of which are only expressed by reticulocytes but not by mature red cells,46 CD47,47 the Lutheran blood group proteins, and perhaps the LW protein.48 However, SS RBC show different binding characteristics to different adhesion targets. For example, SS reticulocytes bind best to thrombospondin (TSP). CD47 is a SS RBC TSP receptor but is expressed by both immature and mature SS RBC.47 It now appears that TSP can bind to CD47 and stimulate a signaling cascade that ultimately activates VLA-4,49 which is only present on reticulocytes. Thus, it is probably VLA-4 that provides the highest affinity interaction of SS RBC with TSP.
In contrast, it is the dense SS RBC that adhere best to the extracellular matrix protein laminin under flow conditions. The red cell laminin receptor has been identified as the protein that bears Lutheran blood group antigens (sometimes also identified as B-CAM/LU). Lutheran proteins are overexpressed by SS RBC in general and particularly by low density (generally younger) SS RBC.50 Low density SS RBC (and reticulocytes in general) bind more soluble laminin than do mature SS RBC.50 However, it is paradoxically the denser SS RBC, which are generally enriched in older cells, that adhere best to immobilized laminin when subjected to flow. Again, multiple lines of evidence have now shown that the Lutheran protein can undergo activation that increases its ability to mediate adhesion to laminin.51,52
Adhesion of SS RBC to endothelial cells has been demonstrated in a variety of in vitro and ex vivo systems. Using an ex vivo system, Kaul and colleagues demonstrated that SS RBC bind primarily to the integrin αVβ3 of endothelial cells first exposed to platelet activating factor.53 These experiments did not utilize plasma, suggesting that there may be an erythrocyte receptor capable of binding directly to endothelial cell αVβ3. However, as endothelial cells themselves produce several potential “linking” or intermediary molecules, including TSP and von Willebrand factor, this hypothesis remains to be proven.
Finally, the LW protein has also been described as being expressed more strongly by SS RBC.48 LW is a known receptor for leukocyte integrins.54 It again remains to be proven whether SS RBC LW is involved in significant adhesive interactions.
In all, current work on the adhesive interactions of erythrocytes and the membrane proteins that mediate these interactions is especially important because of the possibility that this work may open up new modalities of therapy to prevent or ameliorate vaso-occlusive events in sickle cell disease. One can imagine that the future may hold such new therapies as antibodies or peptides that can interfere with red cell adhesion, similar to the multiple anti-platelet reagents now available for the treatment of coronary artery disease. Or, we may be able to offer patients in the future specific inhibitors of signaling processes that augment red cell adhesion, much like we now offer patients with chronic myelogenous leukemia an inhibitor of bcr-abl tyrosine kinase function.
Summary
Both immature as well as mature red cells express blood group antigen-bearing proteins with a diversity of biological functions. Many of these proteins are capable of serving adhesive functions, and some have been proven to do so abnormally in the context of sickle cell disease. We have learned a great deal about specific adhesive interactions of SS RBC and are beginning to discover what regulates the activity or inactivity of red cell adhesion molecules. Now that many of the molecules implicated in adhesion have been well characterized at a molecular level, the effort to use these interactions as therapeutic targets can begin.
II. Transfusing Patients with Autoimmune Hemolytic Anemia: How Should a Clinician Deal with “Least Incompatible” Units?
Lawrence D. Petz, MD*
Medical Director, StemCyte, 400 Rolyn Place, Arcadia, CA 91007
Transfusing patients with autoimmune hemolytic anemia (AIHA) presents a unique set of problems. When a broadly reactive autoantibody is present, as is generally true, all units of red blood cells (RBC) will be incompatible. Nevertheless, with appropriate precautions, survival of transfused RBC is generally about as good as survival of the patient’s own RBC and transfusion generally causes temporary benefit. Indeed, transfusion should never be denied a patient with a justifiable need, even though it may be impossible to find compatible blood.1– 3 In other words, the decision to transfuse should depend on an evaluation of the patient’s clinical status and not on the compatibility test (crossmatch test) findings.
Communication with the transfusion service
Notification of the transfusion service about the potential need for transfusion should occur as soon as transfusion is considered.4,5 The clinician must consider the time that is required by the transfusion service to perform the often complex serologic tests that are necessary to ensure that the optimal red cell product is obtained for transfusion. In a large majority of cases, adequate time can be taken to complete appropriate antibody identification studies and compatibility tests.
The principles of compatibility testing in AIHA
Physicians should be familiar with the following principles of compatibility testing in patients with AIHA so that they may communicate effectively with the transfusion service personnel.
In the day-to-day operation of a hospital’s transfusion service, compatibility testing is performed to determine if units of RBC can be transfused without risk of a hemolytic transfusion reaction. Compatible blood will not react with red cell antibodies in the patient’s serum. The most important antibodies are anti-A and anti-B, since these cause the most severe hemolysis. Hemolysis caused by anti-A and anti-B is easily avoided by providing blood of identical or compatible ABO type. Determining the correct ABO type of the patient is not a major problem in patients with AIHA.
The most important technical problem faced by the transfusion service regarding patients with AIHA relates to other red cell alloantibodies, which are capable of causing hemolytic transfusion reactions. These alloantibodies are developed as a result of previous transfusions or pregnancies and may be directed against antigens of a number of blood group systems, such as Rh, Kell, Kidd, and Duffy. Published data indicate that alloantibodies were detected in 209 of 647 sera (32%) of patients with AIHA,6 clearly indicating the need for a method to detect these antibodies to prevent alloantibody-induced hemolytic transfusion reactions. Indeed, undetected alloantibodies may be the cause of increased hemolysis following transfusion, which may be falsely attributed to an increase in the severity of AIHA.1
These alloantibodies are ordinarily detected and identified by testing the patient’s serum against a panel of red cells of known phenotypes. For example, anti-Jka (an antibody in the Kidd blood group system that can cause serious hemolytic transfusion reactions) is identified by the fact that the patient’s serum will react with Jka+ RBC and will not react with Jka RBC. However, in warm antibody AIHA, the autoantibody in the patient’s serum will generally react with all RBC tested, thus masking the presence of the anti-Jka.
Approaches to Selecting Donor Units of RBC in Patients with Warm Autoantibodies
A number of approaches are available for selecting donor RBC units in patients who have warm autoantibodies.7 These include routine testing of the patient’s serum against a red cell panel; diluting the patient’s serum before doing compatibility testing; performing adsorption tests, which will remove the autoantibody but not alloantibodies from the patient’s serum; and supplying phenotypically matched RBC units.
Testing the patient’s serum against a red cell panel
If a weakly reactive autoantibody and a strongly reactive alloantibody are present, the differences in the strength of the reaction of various cells of the panel will make this evident. However, there is no assurance that a patient’s alloantibody will react more strongly than the autoantibody, so additional tests are necessary.
Dilution technique
A serum dilution, such as 1-in-5, may be selected arbitrarily for compatibility testing in the hope that this will dilute out the autoantibody but not the alloantibody.8 Although the procedure will detect some alloantibodies, Leger and Garratty7 reported that only 19% of potentially clinically significant alloantibodies were identifiable. It appears preferable to select a dilution of the patient’s serum that reacts 1+ against donor RBC and then test that dilution against a panel of RBC. The dilution techniques are easy and rapid. However, they are unreliable, so other, more effective procedures should be performed except in very urgent situations.
Adsorption Procedures
Warm autoadsorption technique.
The optimal adsorption technique for detecting alloantibodies in the presence of a broadly reactive autoantibody is the warm autoadsorption procedure. In this technique, some of the autoantibody is eluted from the patient’s RBC, and then these cells are used to adsorb the autoantibody from the patient’s serum at 37°C. The serum can then be tested for alloantibodies, since alloantibodies will not be adsorbed onto the patient’s own RBC.
A problem faced by transfusion services is that the patient’s severe anemia may preclude obtaining a large enough volume of RBC for the autoadsorption procedure. Physicians should provide as many RBC as may be reasonable because the autoadsorption procedure is the most effective method for detecting alloantibodies in patients with warm autoantibodies. The warm autoadsorption test is not useful in patients who have been transfused recently (within about the last 3 months) because even a small percentage of transfused cells may adsorb the alloantibody during the in vitro adsorption procedure, thus invalidating the results.9
Allogeneic adsorption.
When autoadsorption tests are not feasible, the optimal procedure is the allogeneic adsorption techniquethat is, adsorption of autoantibody from the patient’s serum using several samples of allogeneic red cells of varying phenotypes. For example, performing an adsorption by using a Jka− cell of a serum containing a warm autoantibody and an anti-Jka alloantibody will remove the autoantibody but not the anti-Jka. By selection of 2 or 3 samples of RBC of various phenotypes for the alloadsorption procedure, alloantibodies that are responsible for almost all clinically important hemolytic transfusion reactions can be detected. However, the technique is labor intensive and is unpopular in transfusion service laboratories.
Transfusion of phenotypically matched RBC
When extended phenotyping of the patient’s RBC is performed, it is possible to determine which alloantibodies a patient could develop as a result of previous transfusions or pregnancies. For example, if a patient is Jka+, it is impossible to develop an anti-Jka alloantibody. Transfusion of RBCs that are selected on the basis of the patient’s extended phenotype can provide a significant measure of safety,10,11 but some caveats and precautions must be stressed.
To provide adequate safety, typing must be performed for numerous RBC antigens (e.g., D, C, E, c, e, K, Jka, Jkb, Fya, Fyb, S, and s). However, determining the extended phenotype is technically difficult when the patient has a positive direct antiglobulin (Coombs’) test and may be impossible in a significant percentage of patients with warm antibody AIHA even when attempted by the most skilled technologists. Those transfusion services that may prefer to select blood for transfusion on the basis of extended phenotyping rather than adsorption tests must keep in mind that adsorption tests will be necessary for those patients for whom an extended phenotype cannot be determined.
Partial phenotypinge.g., for Rh, K, and Jka antigenswould not provide protection against alloimmunization by antigens of other blood group systems that can cause hemolytic transfusion reactions,12– 14 and therefore would not preclude the necessity of pretransfusion adsorption studies.
Whether implementation of this approach is cost-effective and feasible at many hospitals and blood centers has not been determined. If the intention is to emphasize providing phenotype-matched units, it must be determined that the blood supplier could reliably provide such units, and it must be recognized that adsorption studies will be required in cases where the patient’s RBCs cannot be phenotyped.
Compatibility testing in cold antibody AIHA
Compatibility testing in cold antibody AIHA is less labor intensive than in warm antibody AIHA. In cold agglutinin syndrome, the autoantibody does not often react up to a temperature of 37°C, whereas clinically significant RBC alloantibodies will react at this temperature. Accordingly, the compatibility test can be performed strictly at 37°C. If the transfusion service is not able to perform testing strictly at 37°C, 1 or 2 cold autoadsorptions should be done, which will not remove a high titer cold agglutinin completely but is likely to eliminate reactions that occur at 37°C. Even though the specificity of cold agglutinins is frequently anti-I, providing RBC negative for the I antigen is not practical because of their rarity, and their use may not be beneficial.
Similarly, in paroxysmal cold hemoglobinuria (PCH), the autoantibody will not react at 37°C. In this disorder, the autoantibody is unusual in that it very often has specificity for the P antigen. Although the routine crossmatch test may appear to be compatible with P+ red cells since the antibody reacts only in the cold (usually < 15°C), there are some suggestions that p or pk red cells (lacking the P antigen) will survive better.15,16 However, these RBC are available from only rare donor files, and patients are likely to require transfusion before the RBCs are available. Generally, transfusion of RBC of common P types should be provided since patients with PCH often have severe hemolysis, and waiting for availability of p or pk RBC is likely to delay a needed transfusion. Successful transfusion of patients with PCH has been reported by numerous authors and, almost certainly, the transfusions were of P-positive blood.17
Use of warm blood for patients with cold antibody AIHA
Eminent authorities offer sharply differing opinions concerning the need for warm blood when transfusing patients with cold antibody hemolytic anemias, and there are few data indicating the effectiveness of such a policy.12,16–,20 In the absence of extensive data, logic must prevail. The use of an in-line blood warmer would appear indicated if the patient has severe cold antibody–induced hemolysis. It is also logical to keep the patient warm, even if the efficacy of such a maneuver has not been proven. If blood is to be warmed, it must be done properly. Unmonitored or uncontrolled heating of blood is extremely dangerous and should not be attempted. Red cells heated too much are rapidly destroyed in vivo and can be lethal.18 In-line blood warmers that are simple, efficient, and safe to use are available.21
“Least incompatible” units
“Least incompatible unit” is not an official term in transfusion medicine and is not defined in the medical literature. In general, it appears to mean the selection of a unit of blood that gives weaker reactions in the compatibility test than other incompatible units. That is, crossmatch tests can be performed using a number of donor units that are ABO and Rh matched with the patient, and the one that reacts least strongly can be selected. The rationale for using “least incompatible” units appears to be that the stronger reactions could be caused by an alloantibody. The use of this term apparently lingers on from the days before effective and practical serologic tests were devised to identify alloantibodies in the presence of autoantibodies that react with all RBCs.
Selecting least incompatible units must not be considered an acceptable alternative to the techniques described above for selecting donor units for transfusion. Relying on least incompatible units instead of performing appropriate serologic studies is a dangerous practice and should be abandoned except in extremely urgent settings in which there is not time to perform adequate serologic tests.
Some transfusion services appear to use “least incompatible” unit in another context. They select donor units for transfusion after adequately accounting for RBC alloantibodies or selecting units on the basis of extended phenotyping. Nevertheless, any unit selected will, of course, be incompatible with the patient’s autoantibody. Transfusion services may choose to crossmatch the selected units with the patient’s serum, although all such crossmatches will be incompatible. While choosing the least incompatible unit from among those that have been selected may provide a level of comfort to the transfusion service personnel and can do no harm, this choice provides no known benefit to the patient. This is true since some variability in reactivity caused by an autoantibody can be expected to occur when a number of units are crossmatched with the patient’s serum, which is simply because of the limitations of precision of serologic reactions.
“Least incompatible” unit should be placed in the garbage heap of serologic terminology for the following reasons: it is not defined in transfusion medicine nomenclature; it is undoubtedly used differently by various transfusion services; and its use does not convey information regarding the extent of compatibility testing performed. Moreover, employing the term at all implies that using least incompatible units is an acceptable alternative to performing adequate serologic evaluation prior to transfusion in patients with AIHA, when in fact it is not an acceptable alternative.
Summary of major points regarding compatibility testing
Understanding the above concepts should allow for effective communication between the transfusion service and the physician ordering the transfusion. The following points should be kept in mind: (1) if the patient has a broadly reactive autoantibody, as usually occurs in AIHA, all units will be incompatible; (2) methods are available to circumvent the potential of a hemolytic transfusion reaction caused by an alloantibody, and these serologic studies should be performed to provide optimal safety; (3) there are no data available indicating that selecting “least incompatible” units is of value. The term is not defined in the transfusion medicine literature and should be discarded.
Other Important Aspects of Transfusion for Patients with AIHA
Posttransfusion hemoglobinemia and hemoglobinuria
Hemoglobinemia and hemoglobinuria following transfusion of a patient with AIHA have generally been attributed to an increased rate of hemolysis, although they may more commonly occur as a result of the increase in the total mass of erythrocytes available for destruction. Indeed, the kinetics of red cell destruction in patients with AIHA always describe an exponential curve of decay, indicating that the number of cells removed in a unit of time is a percentage of the number of cells present at the start of this time interval.18 Thus, the more cells present at zero time, the more cells in absolute number will be destroyed in the unit time span. Indeed, Chaplin indicates that the most common cause of posttransfusion hemoglobinuria in AIHA may not be alloantibody-induced hemolysis but, instead, the quantitative effect of transfusion in increasing the red cell mass subjected to ongoing autoantibody-mediated destruction.22 Such marked posttransfusion hemoglobinemia and hemoglobinuria have the potential for a significant degree of associated morbidity and possible mortality.
In patients with severe anemia, there is a tendency to transfuse large volumes of blood to quickly restore the hemoglobin and hematocrit to near-normal levels. This approach can lead to tragic consequences. Indeed, Gürgey et al23 pointed out that 2 children with warm antibody AIHA who were treated with exchange transfusions died of profound hemolysis shortly after the procedure (although Heidemann et al24 reported successful exchange transfusion in 1 patient).
Patients undergoing severe posttransfusion intravascular hemolysis may develop disseminated intravascular coagulation (DIC), possibly as a result of procoagulant substances present in red cell lysates.25,26 These potential problems have led to strong warnings against overtransfusion of patients with AIHA.1,2,18,22,27 The transfusion of modest volumes of red cells just sufficient to maintain a tolerable hematocrit level appears advisable.18
In vivo compatibility testing
In vivo compatibility testing has been advocated by some28,29 and is, in general, based on testing the survival of an aliquot of red cells from a unit that has been selected for transfusion. However, if a careful serologic study has been done using the techniques and principles already described to select the best possible blood for transfusion, and an in vivo test nevertheless demonstrates a very short red cell survival, there is no logical way to select a different, safer unit.
Some studies have been performed using radiolabeled RBC. Adequate survival (± 70% at 1 hour) of the radiolabeled aliquot was taken as an indication that an acute hemolytic transfusion reaction would not occur following transfusion of the full unit.29 However, survival of an aliquot of RBC over a short period of time does not necessarily provide a good indication regarding the subsequent survival of a full unit of RBC.1,29,30 In vivo compatibility tests using radiolabeled RBC are rarely, if ever, performed on clinical services.
An alternative approach that is used by some transfusion services is to infuse over a period of 20 to 30 minutes an aliquot of ∼50 mL of RBC from the unit to be transfused and observe the patient for symptoms of a hemolytic transfusion reaction and for the development of hemoglobinemia (and hemoglobinuria). The intravascular lysis of as little as 5 mL of red cells will raise the plasma hemoglobin concentration of an adult by about 50 mg/100 mL, an amount easily detected by visual inspection.22 An anticoagulated specimen of blood is obtained after infusion of the aliquot and centrifuged; then the plasma is visually compared with that obtained prior to the infusion. Lack of hemoglobinemia (and hemoglobinuria) suggests that an acute hemolytic transfusion reaction will not occur with infusion of the entire unit of blood. The technique is not applicable if the patient already has hemoglobinemia.
However, an acute hemolytic transfusion reaction serious enough to cause immediate hemoglobinemia is not likely to occur except as a result of ABO incompatibility. It is unlikely that alloantibodies other than those in the ABO blood group system will cause such an acute reaction. Little information, if any, is provided about the presence of other alloantibodies that could cause a delayed hemolytic transfusion reaction. Even if no immediate adverse effects are noted, the survival of the full unit is not likely to be better than that of the patient’s own RBC. Other than detecting ABO incompatibility, the only benefit of this technique is that the test requires evaluation of the patient’s status during the early phase of a transfusion. Close observation of the patient is critical, although it obviously should be carried out in any case. In a modification of this procedure, the transfusion is done slowly until the equivalent of about 50 mL of RBC has been infused, and then a blood sample is obtained for visual inspection of the plasma. The transfusion does not need to be stopped if the patient remains asymptomatic. There is no harm in performing an in vivo compatibility test, but it should not be considered a substitute for adequate compatibility testing.
Use of RBC substitutes
Progress has been made in the development of RBC substitutes, and the availability of O2-carrying therapeutic agents for clinical use could affect the practice of transfusion medicine.31,32 Mullon et al32 described the successful use of a polymerized bovine hemoglobin in a patient with life-threatening AIHA that was refractory to aggressive treatment. However, no RBC substitutes are presently FDA approved.
Use of leukocyte-reduced RBC
It is logical to use leukocyte-reduced RBCs for patients with AIHA in an attempt to decrease the probability of a febrile nonhemolytic transfusion reaction, which might be difficult to distinguish from a hemolytic reaction. A febrile nonhemolytic reaction would lead to cessation of the transfusion and, as a result of the additional technical procedures involved in compatibility testing in AIHA, there may be a significant delay in selecting an alternative unit. On the other hand, if appropriate leukocyte-reduced RBC are not readily available, one should not delay a transfusion just to obtain them.
Use of washed RBC
Previously, washed RBC have been used to reduce the incidence of febrile nonhemolytic transfusion reactions for the same reason that leukocyte-reduced RBC have been used. Washed RBC have also been suggested as possibly being of benefit in patients with AIHA because they are free of complement components, which will be in any plasma-containing blood product. However, the data supporting this justification for using the washed RBC are inconclusive.
Communication between clinicians and the transfusion service
A number of critical questions should be discussed when a patient with AIHA requires transfusion. The clinician should provide the transfusion service personnel with an indication of the urgency of the transfusion, and should inquire about compatibility test procedures undertaken by the laboratory in order to assess the safety of the transfusion. If the transfusion service has attempted to avoid an alloantibody-induced hemolytic transfusion reaction by selecting RBC for transfusion on the basis of adsorption tests or on the basis of matching the extended RBC phenotype of the patient, one can be assured of appropriate safety. If phenotypically matched RBC units are to be used, the clinician should recognize that the RBC should match the extended phenotype of the patient, rather than a partial phenotype. In cold antibody AIHAs, compatibility testing in the warm is generally adequate.
If the transfusion service has selected “least incompatible” units, this should be considered unacceptable unless additional adequate compatibility test procedures have been performed. Even in very urgent situations, there is almost always time to perform at least minimal compatibility test procedures such as using the dilution technique, and perhaps partial RBC phenotyping, which will provide some measure of safety.
In any case, even appropriately selected units of RBC cannot be expected to survive normally but are unlikely to cause an acute hemolytic transfusion. They can be expected to survive about as well as the patient’s own RBC and thereby provide temporary benefit.
III. In Vitro Modulation of the Red Cell Membrane to Produce Universal/Stealth Donor Red Cells
George Garratty, PhD, FRCPath*
American Red Cross Blood Services, Southern California Region, 1130 S. Vermont Avenue, Los Angeles, CA 90006
In Vitro Conversion of Group A and B Red Blood Cells to Group O Red Blood Cells
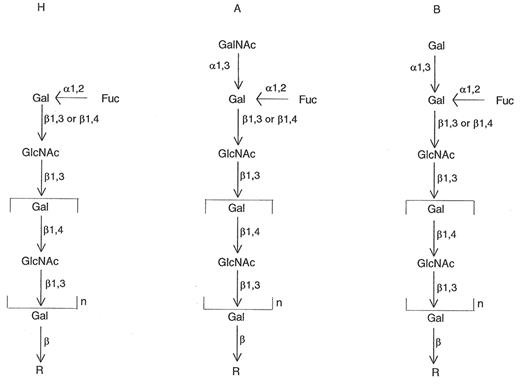
It has been known since the 1930s that certain bacterial extracts would destroy A, B, or H activity.1 During 1947-1961, Morgan and Watkins (in the UK)2,3 and Iseki (in Japan)4 showed that some bacterial enzymes, such as those from Clostridium tertium, C. welchii, Bacillus cereus, and Trichomonas foetus, would specifically destroy A, B, or H antigens. When the red blood cells (RBCs) lost A and B reactivity, they demonstrated increased H activity, and when H activity was lost the RBCs demonstrated reactivity with pneumococcus Type IV antisera. Later, it was shown that degradation of the A and B antigens led to the release of 2 sugars, N-acetylgalactosamine and galactose, respectively; degradation of the H antigen led to release of fucose.5,6 These findings were part of the basis for the elaboration of a biochemical and genetic pathway for the formation of ABH and Lewis antigens on RBCs and in secretions.5,6 The ABH antigens were found to be the result of the interactions of ABO, Hh, Lewis (Lele), and secretor (Sese) genes. The products of A, B, H, and Lewis genes are glycosyltransferases that transfer the immunodominant sugars for A (N-acetylgalactosamine [GalNAc]), B (galactose [Gal]), H (fucose [Fuc]), and Lewis (fucose [Fuc]).
Thus, simply, the ABH antigens are composed of short chains of sugars (Gal, GalNAc, Fuc, and N-acetylglucosamine [GlcNAc]), occurring as glycoproteins or glycolipids. Two chains were described originally. Both chains contain the disaccharide unit β-GlcNAc; the sugars are linked either 1→3 (Type I chains) or 1→4 (Type II chains). Type 1 chains typically are formed in secretions (but can be adsorbed onto RBCs), and Type 2 chains are formed on RBCs. The terminal sugar is the “immunodominant” sugar in the recognition process by specific antibody (e.g., GalNAc for anti-A reactions, Gal for anti-B reactions, and Fuc for anti-H reactions) (see Figure 2 ).
Most individuals inherit an H gene, leading to a fucosyl-transferase, which adds a fucose to the precursor short chain of sugars. This produces the H antigen. Inheritance of A or B genes leads to GalNAc and Gal transferases adding GalNAc and a Gal, respectively, to the H determinant, producing A and B antigens, respectively (Figure 2).5,6
Although it had been known since around 1950 that one could cleave sugars from A and B RBCs to produce H antigen (i.e., group O RBCs), it was not for another 30 years that someone tried to apply it in a practical way.
Conversion of Group B Donor Blood to Group O
During further biochemical studies of the ABH antigens, several investigators degraded the B antigen using nonbacterial enzymes. One successful galactosidase (B-zyme) was made from green coffee beans.7–,9 Goldstein and Lenny, at the New York Blood Center, were the first to successfully apply enzyme conversion of group B blood to group O (ECO RBCs), thus using the coffee bean B-zyme for a practical purpose.10–,15 In 198010 and 198211 the New York Blood Center group reported the results of treating small amounts of group B gibbon and then human RBCs in vitro, and transfusing the RBCs after labeling them with 51Cr into gibbons and humans, respectively.
The ECO RBCs were found to have normal osmotic fragility, acetylcholinesterase, cholesterol, adenosine triphosphate, and 2,3-DPG; methemoglobin formation was minimal, and the P50 value was unchanged. The only change in blood group antigens noted was a loss of B and P1 (P1 activity is also dependent on terminally α-linked galactose) antigens and an increase in H antigen. The ECO RBCs survived normally in group A, B, and O recipients. Following these successful studies, normal group A and group O volunteers received 2 mL of group B ECO RBCs initially, followed 2 weeks later with another 2 mL.12 Then, a final 1 mL of ECO RBCs labeled with 51Cr was administered (i.e., 5 mL of ECO RBCs over a 4-week period). RBC survival was normal. No increase in anti-B was noted in the group A or O recipients, nor did their sera agglutinate or hemolyze ECO RBCs, during a 7-week posttransfusion period.12
In 1991, Lenny et al13 reported the results of transfusing whole units of group B blood that had been converted to group O in vitro, by treating them with the green coffee bean galactosidase. Three group A and 4 group O volunteers received 160–196 mL of ECO RBCs. The ECO RBCs typed as group O using Food and Drug Administration–licensed (FDA-licensed) anti-A and anti-B; routine compatibility tests were negative. The patients showed no clinical signs of a reaction. RBC survival was normal in all cases. Two group O recipients received second transfusions. No increases in anti-B occurred, and RBC survival was normal. In 1994, Lenny et al14 reported the results of transfusing 2 units of ECO RBCs to each of 4 group O volunteers. No clinical or laboratory signs of a reaction were noted, but there was an increase of anti-B. 51Cr-labeled ECO RBCs were administered to 1 subject with increased anti-B; RBC survival was normal. The following year, Lenny et al15 reported results of transfusing multiple and second transfusions of ECO RBCs to group O volunteers. Table 3 shows the protocol. They also studied the use of a recombinant coffee bean galactosidase. The patients showed no clinical or laboratory signs of a reaction. Normal 51Cr RBC survival and the expected hemoglobin/hematocrit results were achieved. Following these transfusions, no increase in anti-B was observed. The recombinant B-zyme appeared to be as efficacious as the native B-zyme. No antibodies to either B-zyme were detected posttransfusion.
In 2000, Kruskall et al16 reported the results of transfusing 21 group A or O patients (rather than the volunteers used in previous studies) with ECO RBCs; 18 patients also received control transfusions. No adverse reactions were observed. The ECO RBCs gave comparable hemoglobin increments, and all but 2 patients had comparable 51Cr RBC survival. One of these patients was bleeding at the time of ECO transfusion, and the other patient’s ECO RBCs were found to be serologically incompatible with the patient’s serum, even though routine antibody screening tests were negative. One patient developed a transient weakly positive direct antiglobulin test without evidence of hemolytic anemia. Two weeks after transfusion, 5 of 19 ECO RBC recipients had increased anti-B titers. The authors found that 20% of group A and 40% of group O sera from random patients agglutinated (often only microscopically), and most often only by polyethylene glycol (PEG) antiglobulin test, the ECO RBCs, but not untreated RBCs. The clinical relevance of this finding is unknown at present.
Conversion of Group A to Group O
One might assume that an A-zyme (a glycosidase capable of cleaving the immunodominant sugar GalNAc) would give similar results to those obtained with the B-zyme. Unfortunately, this has not proven as simple as it was for B to O conversion. A-zymes are not as readily available as B-zymes, so many microbial and animal sources were screened before identifying a suitable source. The New York Blood Center chose avian (chicken) liver as a source for their A-zyme. Using this enzyme, they were able to convert A2 RBCs to O but were only successful in partially reducing the activity of the A1 antigen. It was suggested that this was probably because of repetitive A epitopes attached to the type 2 chain (type 3 chain A) in A1 antigens; these are present in only trace amounts on A2 RBCs (Figure 3 ).17–,19 The New York Blood Center investigators attempted to use a combination of exo- and endoglycosidases in an attempt to remove the internal, in addition to the external, A determinants, but this did not solve the problem.13
The New York Blood Center project was taken over by ZymeQuest (Beverly, MA), which has been actively pursuing new sources of A-zymes that might solve this problem. Kruskall and AuBuchon20 made some strong arguments, including cost-benefit calculations, for the value of being able to convert all group A and B donor units to group O.
Masking RBC Antigens Using PEG
In 1996 and 1997, 4 groups reported that if PEG was covalently bound to RBCs, the RBCs no longer reacted, or gave greatly diminished reactivity, with a range of antibodies to blood group antigens (e.g., ABO and Rh).21– 30
Three of the groups (Taejon, Korea; Albany Medical College, NY; University of Southern California, Los Angeles, CA) initially used a linear 5-kD PEG capped at one end by a methyl group (mPEG) and bonded to RBCs using dichlorotriazine (DT) (otherwise known as cyanuric chloride [CN]). The other group (University of Alabama, Birmingham) initially used a linear 3.4-kD mPEG bonded to RBCs using the hydrosuccinimidyl ester of methoxypolyproprionic acid (SPA). They used much higher concentrations of PEG, ranging from 2.9 to 147mM.
Using these procedures, all 4 groups published promising results. The Korean group (which has not published in this area since its original reports21,23) found decreased agglutination of PEG-RBCs with anti-A, anti-B, and anti-D.21,23 The University of Alabama group found a > 99% reduction in the number of molecules of monoclonal anti-D bound to PEG RBCs. Anti-A titers were reduced from 2048 to < 32 when high concentrations (87-147mM) of PEG were used.22,24,25 No reactions were seen with Duffy, Kidd, and Kell antibodies at a lower concentration (15mM) of PEG. The Albany Medical College group reported that PEG RBCs showed diminished reactions with anti-A and anti-B, even when 5mM PEG was used, and diminished reactions with anti-c, -E, -e, -K, -S, and -s (e.g., 3+ agglutination reduced to 1+) were obtained with PEG concentrations as low as 1.2mM.26–,28 The University of Southern California group reported diminished reactions with anti-A and anti-B, and no reactions with Rh antisera when using a 5-kD PEG.29 The University of Southern California group also prepared custom-synthesized bifunctional PEG-dichlorotriazine [PEG-(DT)2] derivatives of 3.4 and 18.5 kD to examine the relationship between antigen masking and PEG molecular weight. Using the higher-molecular-weight derivative at a moderate concentration (1.3mM), it proved possible to achieve complete inhibition of agglutination with anti-D and all minor blood group antigens, as measured by a standard immediate spin tube test. The A and B antigens were more difficult to mask than the D antigen, but the 18.5-kD PEG-(DT)2 molecule proved more effective than the smaller 5-kD mPEG-DT or 3.4-kD PEG-(DT)2 derivatives.31 A combination of the large (18.5 kD) and small (3.4 kD) PEG-(DT)2 derivatives gave the best results for all blood group antigens tested (A, B, D, C, c, E, e, I, Leb, P1, N, Jka, and Jkb). However, although the PEG-coated RBCs tested negative by direct agglutination, the more sensitive indirect antiglobulin test (IAT) remained positive for many antigens, indicating that the masking was still incomplete. The degree of masking also was found to be donor specific for the D antigen, possibly reflecting the variable number of D antigens present on RBCs of donors with particular Rh phenotypes.31
Problems with PEG-RBCs as Prepared in Initial Reports
Although the reported masking of RBC antigens in these initial studies appeared relatively efficient, it would not satisfy the criteria used by hospital blood transfusion services for pretransfusion compatibility testing. Many of the initial studies used less than optimal procedures for investigating masking. For instance, one study used a platelet aggregometer to measure blood group antigen-antibody reactions,26 and most of the studies used agglutination as an end point (i.e., no reactions using an agglutinating monoclonal anti-D) rather than the antiglobulin test, or flow cytometry, to ensure that there was no uptake of nonagglutinating IgG antibodies. Although the initial data of diminished reactivity with antibodies such as anti-A or anti-B seemed impressive, they would not be acceptable for pretransfusion testing. For instance, a change in anti-A titer from 2048 to < 32 seems efficient masking, but the patient’s serum would still react strongly (e.g., 4+) with group A RBCs when crossmatched.
In 1997, our laboratory had a chance to test PEG RBCs prepared by all the methods initially reported using standard blood bank techniques and flow cytometry. We found that all of the group A PEG RBCs reacted strongly with commercial anti-A; anti-A in group B and group O donor sera also reacted strongly (agglutination, antiglobulin test, and sometimes hemolysis). As reported, most of the PEG RBCs were nonreactive with a wide range of agglutinating antibodies to blood group antigens (e.g., powerful FDA-licensed typing reagents), but we found that they were often reactive by the antiglobulin test (i.e., the RBCs were sensitized with IgG antibodies such as anti-D). This was confirmed by flow cytometry.30,32
Other Problems
When testing PEG-RBCs using some of the original formulations, we detected further problems that would interfere with routine pretransfusion testing and may reflect on in vivo survival.
PEG-RBCs tended to stick to the sides of glass or polystyrene tubes when RBCs were washed. This was overcome by treating the tubes with certain chemicals.
When PEG-RBCs were incubated (30-60 minutes) in vitro with normal compatible plasma, nonspecific uptake of proteins (e.g., IgG and IgM) onto RBCs was detectable by the antiglobulin test and flow cytometry.33 This observation had not been reported by the other groups and seemed important, as such protein-coated PEG-RBCs reacted strongly (48-82% reactivity, adherence + phagocytosis) in a monocyte monolayer assay that has been proven to predict the potential clinical significance of antibodies to blood group antigens (> 3% indicates significance).33,34
In 1997, we presented data showing that some normal sera directly agglutinated PEG-RBCs; cord sera were nonreactive, and reactive normal adult sera no longer reacted following treatment with 2-mercaptoethanol.33 We suggested that our results showed that some sera contain IgM antibodies to PEG or to a neoantigen created by PEG treatment of RBCs. Later, we showed that the agglutination reaction could be inhibited by adding PEG (but not other polymers) to the reactive sera and that PEG-coated beads showed similar results to PEG RBCs, excluding a neoantigen of PEG + RBC membrane elements.35 These results suggested that the agglutination reactions caused by some normal sera were due to PEG antibodies. Although it is often said that PEG is not immunogenic, data in the literature support our conclusions. Richter and Åkerblom36 detected PEG antibodies (predominately IgM) in 3.3% and 0.2% of patients with various allergies and healthy donors, respectively. After hyposensitization with mPEG-modified ragweed extract and honeybee venom, 50% of the patients made PEG antibodies of titers 35-512.
Effect of PEG on RBCs
Using the formulations reported initially (see above), the Korean group found RBC morphology and oxygen equilibrium curves to be normal.23 SDS-polyacrylamide gel electrophoresis showed that PEG was associated with bands 3, 4.5, and PAS-1.23 The University of Alabama group reported that PEG-RBCs showed mild damage microscopically and that fluorochrome-tagged rat PEG-RBCs survived well for 1-3 days but then were rapidly destroyed.24 The Albany group found PEG-RBCs to be morphologically normal and to have normal osmotic fragility and hemoglobin oxygen affinity.26–,28 The PEG-RBCs had normal protein function as evidenced by normal SO4 flux; Na+ and K+ homeostasis was unaffected. No significant lysis was observed following 24 hours of incubation at 37ºC at PEG concentrations of < 6 mM. The Albany group members also showed that PEG bound to band 3. They found normal oxygen binding and cellular deformability at concentrations of 0.6-2.4 mM. Murine PEG-RBCs survived well in vivo only when low concentrations (0.4 mM) of PEG were used.37 Higher concentrations (e.g., 0.6 mM and 0.8 mM) led to poor RBC survival.37 It should be noted that PEG concentrations of 1-5mM were needed to obtain even reasonable antigen masking.
Second-Generation PEG-RBCs
Because of the problems encountered with the original PEG-RBCs in relation to pretransfusion testing and possible poor in vivo survival, various modifications have been tried. The University of Alabama and University of Southern California groups solved many of the problems by using crosslinking and/or branched PEG. The University of Alabama group used a bifunctional PEG-SPA derivative incubated with albumin. This crosslinked PEG-albumin conjugate was called XPEG.39 XPEG-coated RBCs did not react with a wide range of antisera; anti-A and anti-B were detected only weakly by the antiglobulin test. The XPEG-RBCs did not appear to take up proteins nonspecifically. Murine XPEG-RBCs showed better survival than the original PEG-RBCs but still had a half-life reduced by 50% of untreated RBCs. The University of Alabama group later reported on using a 4-branched PEG-(SPA)4 derivative combined with albumin. This product was said to have effective antigen masking and did not react with the anti-PEG in donor sera.40
The University of Southern California group also produced PEG-RBCs using a branched PEG by a different approach (Figure 4 ).31,38 These RBCs were nonreactive with all antisera tested, including anti-A and anti-B, by agglutination, antiglobulin test, and flow cytometry. Unfortunately, lysis of group A PEG-RBCs was still seen with some group O sera containing anti-A hemolysins (G. Garratty and R. Leger, unpublished data, 2000). The Albany Medical Center group has also published an elegant study of classical complement activation related to ABO antibodies and PEG-RBC interactions.41 Minimal echinocytosis of the University of Southern California second-generation PEG-RBCs was noted.31,38 Some (approximately 20%) normal ABO-incompatible donor sera still agglutinated these PEG-RBCs (presumably because of PEG antibodies).31,33–,35,38
Recently, the Alabany Medical Center group reported improving its PEG-RBCs by using a higher molecular weight (20 kD) PEG and benzytriazobyl carbonate (BTC) instead of CN.42 Good antigen masking was said to be achieved. It was difficult to judge the efficiency of the masking of A and B antigens, as the group used only a platelet aggregometer to measure ABO reactions. The investigators did not comment on nonspecific protein uptake (i.e., direct antiglobulin tests using normal ABO-compatible serum) or reactions with donor sera that might represent anti-PEG interactions. Nevertheless, murine BTC PEG-RBCs were claimed to survive normally in mice.42
Alloimmunization
In 1977, Abuchowski et al showed that when mPEG was covalently bonded (using CN) to bovine serum albumin or bovine liver catalase, it lost its immunogenicity when injected into rabbits.43 mPEGs of 1 and 5 kD were effective in rendering the albumin and enzyme nonimmunogenic.43 In addition, PEG-treated albumin did not react in vitro (by immunodiffusion) with rabbit antibovine albumin.43 The rabbits did not make antibodies to the PEG or PEG complex.43 It was suggested that as PEG is known to attract water molecules, each protein molecule becomes coated with a flexible hydrophilic shell of PEG and water molecules; this “shell” effectively masks antigenic sites on the protein.43 This approach has been used commonly to modify proteins (e.g., recombinant proteins), enzymes, drugs, and artificial surfaces. Several such products are in clinical use or are being used in clinical trials (e.g., PEG-adenosine deaminase for treatment of severe combined immunodeficiency syndrome, PEG-conjugated cytokines, and PEG-modified hemoglobin). PEG appears to be nontoxic and is approved for administration to humans.
There are very few data to confirm that the amount of RBC antigen masking achieved so far is sufficient to prevent alloimmunization. The only relevant data are from the Albany group, which injected mPEG-sheep RBCs into mice. There was a “blunted immune response”: approximately 90% less antibody was produced, compared to the immune response in mice that received untreated sheep RBCs.44
Conclusions
Transfusion of ABO-incompatible units is the major cause of transfusion-induced fatalities in the USA and the UK.45,46 Yet much more effort, and many more millions of dollars and pounds, have been invested to prevent transfusion-transmitted infection. A blood system where the majority of units are group O could theoretically be achieved by both of the methods above. Unfortunately, there are still many problems to solve in both areas. Production of ECO-RBCs is well advanced in that clinical trials of B-ECO-RBCs have already been successful. If the new A-zymes are successful, the scaling up of the procedure for blood center production would need to be streamlined.
PEG-RBCs have additional advantages in that masking of ABO and all other blood group antigens of potential clinical significance is theoretically possible. When perfected, PEG-RBCs could be used for any patient, regardless of whether untreated RBCs were incompatible with ABO or other alloantibodies and autoantibodies. Even if ABO antigens cannot be fully masked (e.g., are susceptible to lysis by anti-A/B hemolysins), blood would be readily available for patients usually difficult to transfuse—for instance, patients with autoimmune hemolytic anemia, sickle cell disease, and antibodies to high-frequency antigens. The laboratory problems discussed above must be solved and human studies (e.g., 51Cr RBC survival) still have to be performed, so it will be many years before PEG-RBCs will be available for routine use.
If the new A-zymes are not efficient enough to completely convert A1 to O, and if complete masking of A and B antigens needs more PEG or chemical manipulations than is necessary to retain integrity of the RBCs, then perhaps a combination of both approaches, needing less enzyme and less PEG, could be utilized. In an editorial in Transfusion, Lublin47 suggested such an approach with a cartoon depicting the possible blood center assembly line of 2005: a unit of blood is on an assembly line, where it undergoes pathogen inactivation, leukocyte reduction, treatment with enzymes to cleave A and B sugars from the RBCs, and treatment with PEG to mask all other antigens.
Blood group antigen loci and functional proteins of human erythrocytes.
| Chromosomal Locus . | Locus Name . | Blood Group (Abbreviation) . | Function . |
|---|---|---|---|
| 1p13 | CD58 | None known | Adhesion molecule that binds CD2 |
| 1p36.11 | RH | Rhesus (Rh) | Unknown |
| 1p22.1-p36.2 | SC | Scianna (Sc) | Unknown |
| 1p32-p35 | RD | Radin (Rd) | Unknown |
| 1q22-23 | DARC | Duffy (Fy) | Chemokine receptor |
| 1q32 | CR1 | Knops-McCoy | Complement receptor 1 (complement regulatory protein) |
| 1q32 | DAF | Cromer (Cr) | Decay accelerating factor (complement regulatory protein) |
| 2q14-21 | GYPC | Gerbich (Ge) | Membrane-cytoskeletal interaction |
| 3q13.1-13.2 | CD47 | None known | Integrin-associated signaling, adhesion receptor |
| 4q28-31 | GYPA | MN | Chaperone of the anion channel protein (band 3) |
| 4q28-31 | GYPB | Ss | Unknown |
| 6p21.3 | C4A, C4B | Chido, Rodgers | C4 component of complement |
| 6p21.3 | HLA | Bg | HLA class I |
| 6p21.1-p11 | RHAG | Rh-associated glycoprotein | Ammonium transport |
| 7p14 | AQP1 | Colton (Co) | Water channel protein |
| 7q22.1-22.3 | ACHE | Cartwright (Yt) | Acetylcholinesterase |
| 7q33 | KEL | Kell (K,k,Kp,Js) | Metalloproteinase |
| 9q34.1-q34.2 | ABO | ABO | ABO glycosyl transferases |
| 11p13 | CD59 | None known | Inhibits formation of the complement membrane attack complex (C5-9) |
| 11p13 | CD44 | Indian (In), AnWj | Adhesion receptor |
| 11p15.5 | MER2 | MER2 | Unknown |
| 12p13.2-12p12.1 | DOK | Dombrock | ?ADP-ribosyltransferase |
| 15q22.3-23 | SEMA7A | JMH | Putative adhesion receptor |
| 16p12.3 | UMOD | ?Sda | Tamm-Horsfall protein (uromodulin) |
| 17q12-q21 | EPB3 | Diego (Di), Wright (Wr) | Anion channel protein (band 3, AE1) |
| 18q11-q12 | UT1 | Kidd (Jk) | Urea transporter |
| 19p13.3 | FUT3 | Lewis (Le) | alpha(1,3) fucosyltransferase |
| 19p32.2-pter | OK | Oka | Neurothelin, putative adhesion molecule |
| 19p11-p13 | LW | LW | Adhesion receptor |
| 19q13.3 | FUT2 | Secretor (Se) | alpha(1,2) fucosyltransferase |
| 19q13.3 | FUT1 | Hh | alpha(1,2) fucosyltransferase |
| 19q13.2-13.3 | LU | Lutheran (Lu) | Adhesion receptor |
| 22q11.2-q7er | P1 | P1 | Glycosyltransferase |
| Xp21.1 | XK | Kx | Putative neurotransmitter transporter |
| Xp22.32 | XG | Xg | formerly called PBDX; possible adhesion receptor |
| Xp22.33, Yp11.3 | MIC2 | None known | Mucin, putative adhesion molecule |
| Chromosomal Locus . | Locus Name . | Blood Group (Abbreviation) . | Function . |
|---|---|---|---|
| 1p13 | CD58 | None known | Adhesion molecule that binds CD2 |
| 1p36.11 | RH | Rhesus (Rh) | Unknown |
| 1p22.1-p36.2 | SC | Scianna (Sc) | Unknown |
| 1p32-p35 | RD | Radin (Rd) | Unknown |
| 1q22-23 | DARC | Duffy (Fy) | Chemokine receptor |
| 1q32 | CR1 | Knops-McCoy | Complement receptor 1 (complement regulatory protein) |
| 1q32 | DAF | Cromer (Cr) | Decay accelerating factor (complement regulatory protein) |
| 2q14-21 | GYPC | Gerbich (Ge) | Membrane-cytoskeletal interaction |
| 3q13.1-13.2 | CD47 | None known | Integrin-associated signaling, adhesion receptor |
| 4q28-31 | GYPA | MN | Chaperone of the anion channel protein (band 3) |
| 4q28-31 | GYPB | Ss | Unknown |
| 6p21.3 | C4A, C4B | Chido, Rodgers | C4 component of complement |
| 6p21.3 | HLA | Bg | HLA class I |
| 6p21.1-p11 | RHAG | Rh-associated glycoprotein | Ammonium transport |
| 7p14 | AQP1 | Colton (Co) | Water channel protein |
| 7q22.1-22.3 | ACHE | Cartwright (Yt) | Acetylcholinesterase |
| 7q33 | KEL | Kell (K,k,Kp,Js) | Metalloproteinase |
| 9q34.1-q34.2 | ABO | ABO | ABO glycosyl transferases |
| 11p13 | CD59 | None known | Inhibits formation of the complement membrane attack complex (C5-9) |
| 11p13 | CD44 | Indian (In), AnWj | Adhesion receptor |
| 11p15.5 | MER2 | MER2 | Unknown |
| 12p13.2-12p12.1 | DOK | Dombrock | ?ADP-ribosyltransferase |
| 15q22.3-23 | SEMA7A | JMH | Putative adhesion receptor |
| 16p12.3 | UMOD | ?Sda | Tamm-Horsfall protein (uromodulin) |
| 17q12-q21 | EPB3 | Diego (Di), Wright (Wr) | Anion channel protein (band 3, AE1) |
| 18q11-q12 | UT1 | Kidd (Jk) | Urea transporter |
| 19p13.3 | FUT3 | Lewis (Le) | alpha(1,3) fucosyltransferase |
| 19p32.2-pter | OK | Oka | Neurothelin, putative adhesion molecule |
| 19p11-p13 | LW | LW | Adhesion receptor |
| 19q13.3 | FUT2 | Secretor (Se) | alpha(1,2) fucosyltransferase |
| 19q13.3 | FUT1 | Hh | alpha(1,2) fucosyltransferase |
| 19q13.2-13.3 | LU | Lutheran (Lu) | Adhesion receptor |
| 22q11.2-q7er | P1 | P1 | Glycosyltransferase |
| Xp21.1 | XK | Kx | Putative neurotransmitter transporter |
| Xp22.32 | XG | Xg | formerly called PBDX; possible adhesion receptor |
| Xp22.33, Yp11.3 | MIC2 | None known | Mucin, putative adhesion molecule |
Adhesion molecules expressed by circulating erythrocytes.
| Adhesion Molecule and Alternate Name(s) . | Ligand/Adhesive Function . |
|---|---|
| CD36 (reticulocytes only), platelet glycoprotein IV, Naka (platelets) | Thrombospondin (platelets) |
| VLA-4 (reticulocytes only), α4β1 integrin (CD49d/CD29) | Thrombospondin, VCAM-1, possibly fibronectin |
| CD44, Indian (Ina/Inb), In(Lu)-related p80 | Hyaluronan, possibly also fibronectin |
| CD47, Integrin-associated protein (IAP) | Thrombospondin |
| CD58, lymphocyte associated antigen-3 (LFA-3) | CD2 |
| CD99, MIC2 gene product | Lymphocyte CD99 is necessary for formation of T cell rosettes |
| CD108, JMH, Semaphorin K1 (SEMA7A) | Possible role in adhesion of activated lymphocytes |
| CD147, Neurothelin, Oka | Type IV collagen, fibronectin. laminin in other tissues |
| CD242, ICAM-4, LW | Leukocyte integrins (α4β1, α4β3, αvβ1) |
| CD239, Lutheran, B-CAM/LU | Laminin, possibly also integrins |
| Adhesion Molecule and Alternate Name(s) . | Ligand/Adhesive Function . |
|---|---|
| CD36 (reticulocytes only), platelet glycoprotein IV, Naka (platelets) | Thrombospondin (platelets) |
| VLA-4 (reticulocytes only), α4β1 integrin (CD49d/CD29) | Thrombospondin, VCAM-1, possibly fibronectin |
| CD44, Indian (Ina/Inb), In(Lu)-related p80 | Hyaluronan, possibly also fibronectin |
| CD47, Integrin-associated protein (IAP) | Thrombospondin |
| CD58, lymphocyte associated antigen-3 (LFA-3) | CD2 |
| CD99, MIC2 gene product | Lymphocyte CD99 is necessary for formation of T cell rosettes |
| CD108, JMH, Semaphorin K1 (SEMA7A) | Possible role in adhesion of activated lymphocytes |
| CD147, Neurothelin, Oka | Type IV collagen, fibronectin. laminin in other tissues |
| CD242, ICAM-4, LW | Leukocyte integrins (α4β1, α4β3, αvβ1) |
| CD239, Lutheran, B-CAM/LU | Laminin, possibly also integrins |
Schedule of enzyme conversion (ECO) red blood cell (RBC) transfusions.
| Recipient . | Blood Group . | Initial Transfusion . | Challenge Transfusion . |
|---|---|---|---|
| Information from Lenny et al.15 | |||
| 1 | O | 2 units | 1 unit at 52 weeks |
| 2 | A | 3 units | |
| 3 | O | 3 units | 3 units at 21 weeks |
| 4 | O | 3 units | 2 units at 21 weeks |
| 5 | O | 1 unit | |
| Recipient . | Blood Group . | Initial Transfusion . | Challenge Transfusion . |
|---|---|---|---|
| Information from Lenny et al.15 | |||
| 1 | O | 2 units | 1 unit at 52 weeks |
| 2 | A | 3 units | |
| 3 | O | 3 units | 3 units at 21 weeks |
| 4 | O | 3 units | 2 units at 21 weeks |
| 5 | O | 1 unit | |
A model for the structural differences between antigens constructed by the A1 and A2 subgroup transferases.14
A1 and A2 subgroup α(1,3)N-acetylgalactosaminyltransferases can form the type 2 A moieties (at left) characteristic of the A2 phenotype. These molecules may then serve as precursors for the action of a β(1,3) galactosyltransferase (β1,3Gal transferase) that synthesizes a type 3 precursor (type 3 Gal A) also characteristic of the A2 phenotype. The H locus α(1,2)fucosyltransferase (H transferase) then modifies this precursor, yielding a type 3 H antigen. Type 3 H determinants are then efficiently utilized as substrates by the A1 transferase (but not by the A2 transferase) to form type 3 A molecules that maintain repetitive A-reactive units, and that are proposed to be responsible for the A1 phenotype. R indicates the glycoconjugate substructure that consists of N-linked, O-linked, or lipid-linked glycoconjugates.
A model for the structural differences between antigens constructed by the A1 and A2 subgroup transferases.14
A1 and A2 subgroup α(1,3)N-acetylgalactosaminyltransferases can form the type 2 A moieties (at left) characteristic of the A2 phenotype. These molecules may then serve as precursors for the action of a β(1,3) galactosyltransferase (β1,3Gal transferase) that synthesizes a type 3 precursor (type 3 Gal A) also characteristic of the A2 phenotype. The H locus α(1,2)fucosyltransferase (H transferase) then modifies this precursor, yielding a type 3 H antigen. Type 3 H determinants are then efficiently utilized as substrates by the A1 transferase (but not by the A2 transferase) to form type 3 A molecules that maintain repetitive A-reactive units, and that are proposed to be responsible for the A1 phenotype. R indicates the glycoconjugate substructure that consists of N-linked, O-linked, or lipid-linked glycoconjugates.
Illustration of the potential advantages of crosslinking.
(A) Monofunctional polyethylene glycol (PEG) gives a “hairy” coating that may still expose antigens; (B) a multifunctional PEG crosslinked with albumin forms a “shell,” giving better masking for fewer sites of attachment; (C) the procedure of (B) can be repeated to build up additional layers.31
Illustration of the potential advantages of crosslinking.
(A) Monofunctional polyethylene glycol (PEG) gives a “hairy” coating that may still expose antigens; (B) a multifunctional PEG crosslinked with albumin forms a “shell,” giving better masking for fewer sites of attachment; (C) the procedure of (B) can be repeated to build up additional layers.31