Abstract
The concept of utilizing enhanced immunosuppression rather than myeloablative cytotoxic conditioning has allowed the engraftment of allogeneic stem cells from related and unrelated donors with lower early transplant-related mortality (TRM) and morbidity. This approach shifts tumor eradication to the graft-vs-host immune response directed against minor histocompatibility antigens expressed on tumor cells. This is not without risk, as the long-term effects of graft-versus-host disease (GVHD), it’s treatment, or resulting complications and immunodeficiency may be life threatening. However, this approach does allow the application of a potentially curative procedure to elderly or medically infirm patients who would not tolerate high-dose conditioning regimens.
Section I, by Dr. Sandmaier, describes the current use of nonmyeloablative regimens and matched related or unrelated donors for the treatment of patients with CLL, CML, acute leukemia, MDS, lymphoma, and myeloma.
In Section II, Dr. Maloney discusses the use of cytoreductive autologous followed by planned non-myeloablative allografts as treatment for patients with myeloma or NHL. This tandem transplant approach has a lower TRM than conventional high dose allografting. The nonmyeloablative allograft may allow the graft-versus-tumor (GVT) immune response to eradicate the minimal residual disease that causes nearly all patients with low-grade NHL or myeloma to relapse following autologous transplantation.
In Section III, Dr. Mackinnon discusses the risks and benefits of T cell depletion strategies to prevent acute GVHD, while retaining GVT activity by planned donor lymphocyte infusions.
Finally, in Section IV, Dr. Shizuru discusses the relationship between GVHD and GVT activity. Future studies, employing a greater understanding of these issues and the separation of GVHD from GVT activity by immunization or T cell cloning, may allow nonmyeloablative allogeneic transplantation to be safer and more effective.
I. Transplantation from Related and Unrelated Donors: Outcomes for Hematologic Malignancies
Brenda M. Sandmaier, MD*
Clinical Research Division, Fred Hutchinson Cancer Research Center and University of Washington, 1100 Fairview Avenue North, D1-100, Seattle, WA 98109
In patients with marrow-based diseases, such as leukemias and B-cell malignancies, treated with allogeneic hematopoietic stem cell transplantation (HSCT), conventional conditioning regimens included high doses of chemotherapy with or without total body irradiation (TBI). This was based on the original hypothesis that marrow-ablative doses of chemotherapy and TBI would overcome the host’s immune response while eradicating the underlying disease.1 However, because of toxicities from the intensive conditioning regimens to non-marrow organs such as gut, liver, lung, and heart, conventional high-dose transplants have been restricted to patients younger than 50-55 years of age who are in good medical condition. Such restrictions based on age are problematic in that many hematologic malignancies typically present after the age of 50, thus making the majority of patients ineligible for allogeneic HSCT.2
The concept that dose intensification in itself is necessary for eradication of malignancy came under question early in the history of HSCT. The observation that relapse-free survival was associated with both acute and chronic graft-versus-host-disease (GVHD) dates back over three decades3,4 and emphasizes the potent role of donor hematopoietic elements in the achievement of a cure. Further evidence supporting a graft-versus-tumor (GVT) effect includes lower relapse rates among patients receiving allogeneic HSCT as compared to autologous grafts;5,6 greater incidence of relapse in recipients of a syngeneic7 or T cell-depleted allograft;8 and the ability to induce durable remissions by donor lymphocyte infusions (DLI) for relapse after allogeneic HSCT.9,10 It has become clear that hematologic malignancies cannot always be cured by dose-intensive regimens, even when regimens have been intensified to levels at which non-marrow toxicities have been fatal.
On the basis of these observations, several groups of investigators have developed regimens that exploit the GVT effects while reducing the intensity of conditioning to minimize regimen-related toxicities. This would expand the treatment options for patients including those previously thought to be too old or medically infirm to qualify for conventional HSCT. The strategies used to reduce regimen-related toxicities fall into two broad categories: (1) reduced intensity regimens and (2) minimally myelosuppressive regimens. The reduced intensity regimens rely on the cytotoxic conditioning regimen to eliminate the host-versus-graft (HVG) reactions in addition to maintaining a level of antitumor effect. This comes at a cost of retaining regimen-related toxicities, albeit to a lesser degree as compared to conventional high-dose transplants. In contrast, the minimally myelosuppressive regimens rely on pretransplant and posttransplant immunosuppression to overcome HVG reactions and allow donor engraftment. Other differences exist between these two categories of transplant including the tempo of establishment of complete donor chimerism, and the degree of aplasia that would occur should the patient reject their graft. In general, mixed chimerism exists early on in recipients of minimally myelosuppressive regimens as the regimen itself does not eradicate host hematopoiesis and an allogeneic graft-versus-host (GVH) effect occurs in which the donor cells eradicate the normal host hematopoietic elements in addition to the malignant cells. This is unlike reduced intensity regimens in which the recipients uniformly become aplastic before engrafting with donor hematopoiesis. The benefit of a reduced intensity regimen versus a minimally myelosuppressive regimen may depend on the underlying disease and status of a patient. In patients with diseases such as CML, CLL, and low grade lymphomas, an immunosuppressive regimen may be sufficient to ensure engraftment as these diseases are more sensitive to GVL effects. However, in diseases such as high-grade lymphomas, Hodgkin disease, multiple myeloma, and AML not in remission, where GVL is less potent or the disease too rapidly progressive, a certain amount of cytoreduction may be necessary to minimize residual disease.
This section will discuss the clinical results of both strategies of nonmyeloablative transplants with focus on the minimally myelosuppressive regimens including the preclinical studies on which the clinical studies were based.
Preclinical Studies
The two immunological barriers to overcome in major histocompatibility complex (MHC) identical HSCT included the HVG reaction and the GVH reaction. The HVG reaction can result in graft rejection and is thought, in the MHC-identical setting, to be caused by T lymphocytes, which are similarly thought to also cause GVHD. Therefore, it was hypothesized that agents that control GVHD after HSCT might also be exploited in their ability to control HVG reactions, thus minimizing the need for high-dose chemoradiotherapy given for immunosuppression of the host. Many of the clinical HSCT protocols used in Seattle were developed in a random bred dog model over the past 3 to 4 decades.11–,13 TBI was an integral part of many of the early conventional high-dose conditioning regimens and has played an important role in the development of newer nonmyeloablative transplantation approaches. In the canine model, a single dose of 400 cGy TBI delivered at 7 cGy/min is lethal in the absence of a stem cell graft despite intensive supportive care.14 At 200 cGy TBI, the animals had spontaneous hematopoietic recovery, with only 5% of animals dying with aplasia. While a dose of 920 cGy was sufficiently immunosuppressive to result in 95% engraftment of MHC-matched littermate marrow, a dose of 450 cGy led to rejection in the majority of dogs who either had autologous hematopoietic recovery (36%) or died of marrow aplasia (23%).15 Postgrafting immunosuppression with prednisone did not enhance engraftment, while cyclosporine (CSP) for 5 weeks after HSCT led to engraftment of all the dogs.15 Further reduction of the total body irradiation (TBI) dose to 200 cGy resulted in transient engraftment when CSP alone was given as posttransplant immunosuppression, followed by autologous recovery.16 The combination of methotrexate with CSP failed to increase sustained donor engraftment. In contrast, the use of a newer immunosuppressive agent, mycophenolate mofetil (MMF), was successful in establishing long-term grafts after 200 cGy TBI when given in combination with CSP.17 Reducing the TBI dose to 100 cGy resulted in only transient engraftment, suggesting that further reductions in radiation would require more immunosuppressive agents.
Because of the concern that even low doses of radiation or chemotherapy may increase the risk of secondary malignancies, additional approaches have been taken to further reduce TBI. Investigative approaches included the use of an alpha-emitter, Bi-213, conjugated to an anti-CD45 monoclonal antibody (mAb), which obviated the need for any TBI conditioning and facilitated stable mixed hematopoietic chimerism in MHC-identical littermate grafts.18 The alpha-emitting radionuclides, such as Bi-213, have a very high energy and deposit this energy over only a few cell diameters (40–90 mm); have a short half-life (46 min.), thus making them an attractive alternative to beta-emitting radionuclides for targeting hematopoietic cells without affecting other organs; and may be relatively easy to use in the outpatient setting. The fact that radiation is highly specific for the cells targeted allowed us to further investigate the question of whether the TBI that is now given in the nonmyeloablative regimens is primarily for immunosuppression rather than for the creation of marrow space. Preliminary data indicate that, in studies utilizing Bi-213 conjugated to an anti-TCRab mAb where the majority of the radiation is presumably delivered to T cells, stable engraftment of marrow from MHC-identical littermates occurred. These data support the observation made when dogs conditioned with 450 cGy irradiation to the center lymph node chain from the neck down to the upper abdomen, before allogeneic HSCT, followed by MMF/CSP, had donor engraftment found in non-irradiated marrow spaces and in non-irradiated lymph nodes.19
In the MHC-nonidentical setting, the barriers to engraftment are more complex and difficult to overcome. Whereas in the MHC-identical setting T cells mediate both HVG and GVH reactions, in the MHC-nonidentical setting, natural killer (NK) cells are also important effectors of graft rejection. As the availability of HLA-matched donors is limited and most patients have HLA-haploidentical family members who could serve as HSCT donors, we have been focusing toward developing a nonmyeloablative HSCT regimen for MHC-haploidentical recipients using our preclinical canine model. With unrelated MHC-nonidentical marrow donors, engraftment was uniformly seen after conditioning with 1800 cGy TBI, was the exception after 920 cGy, and was not seen with lower TBI doses.20 Similar results were observed with MHC-haploidentical littermate studies. When cytokine-mobilized peripheral blood stem cells (PBSC) were substituted for marrow, all MHC-haploidentical littermates had successful allogeneic engraftment after 920 cGy.21 This result is similar to those found previously in MHC-mismatched recipients given marrow supplemented by peripheral blood buffy coat cells.20 Additional approaches to enhance engraftment in this model include the use of an anti-CD44 mAb, S5. CD44 is a cellular adhesion molecule expressed on both hematopoietic and nonhematopoietic cells. The mAb was shown to promote engraftment of marrow in MHC-mismatched unrelated dogs not given additional buffy coat infusions or postgrafting immunosuppression after 920 cGy TBI.22–,24 In recipients of PBSC grafts from MHC-haploidentical littermates, rejection uniformly occurred when the radiation dose was lowered from 920 to 450 cGy. When the anti-CD44 mAb S5 was given pretransplant combined with MMF/CSP after 450 cGy, all of the animals engrafted, with 10 to 12 having sustained engraftment.25 Current studies with reduction of the radiation dose to 200 cGy indicate that successful engraftment using the antibody S5 with MMF/CSP occurs, though data indicate that prolonged immunosuppression may be necessary to prevent late graft rejection.
Based on results in the preclinical model in the MHC-identical setting, clinical studies have been initiated in the setting of both malignant and non-malignant diseases. Preliminary data from preclinical studies in the MHC-nonidentical setting will allow extension of this application to patients who do not have MHC-identical donors in future clinical studies.
Clinical Results with Nonmyeloablative Transplant Regimens
Several groups have investigated methods of reducing the regimen-related toxicities of allotransplants while exploiting GVT effects (Table 1 ). The conditioning regimens, while differing between groups, typically included combinations of a purine analog, such as fludarabine, an alkylating agent, or low-dose TBI. The purine analogs (fludarabine, cladribine, or pentostatin) have activity against a wide range of hematological malignancies and are immunosuppressive, which contributes to engraftment in allogeneic HSCT. Many studies have chosen drugs that have some activity against the target malignancy in order to prevent disease progression while waiting for the GVT effect to occur. Giralt et al initially reported on the use of a purine analog-based chemotherapy as a preparative regimen for patients with advanced myeloid malignancies, showing that patients can be successfully engrafted with achievement of complete remissions, of which some appear to be durable.26 The nonmyeloablative combination of fludarabine, cytarabine and idarubicin, while sufficient for engraftment in patients with AML, was not immunosuppressive enough to allow consistent engraftment in patients with CML.27 Using the reduced intensity melphalan/purine analogue combinations (primarily fludarabine), there was consistent engraftment and durable remissions, particularly in patients without bulky or refractory disease.28 Khouri et al, at M.D. Anderson Cancer Center, reported on results in patients with lymphoid malignancies who received allografts from HLA-identical siblings after conditioning with fludarabine and cyclophosphamide. This regimen was effective in achieving engraftment and remissions in patients with both chemosensitive and refractory or untreated disease.29 Slavin et al used a regimen consisting of fludarabine, busulfan (8 mg/kg), and antithymocyte globulin.30,31 While this regimen was less intense than a conventional transplant regimen, it produced marked myelosuppression. All patients achieved complete chimerism or stable partial donor chimerism. Childs et al reported from the National Cancer Institute the use and tolerability of a chemotherapy-based regimen using cyclophosphamide and fludarabine.32 This regimen was successful in achieving full donor chimerism and showed disease responses in patients with a variety of hematological malignancies and in patients with metastatic renal cell carcinoma. The approach taken by the group at the Massachusetts General Hospital in Boston, MA, included a reduced-intensity regimen consisting of cyclophosphamide, antithymocyte globulin, and thymic irradiation, with planned prophylactic DLI to improve chimerism. Complete remissions were seen both in patients who received prophylactic DLI as well as those who did not.33 In an attempt to reduce the incidence of GVHD following nonmyeloablative stem cell transplantation, Campath was used as part of the conditioning regimen in addition to fludarabine and melphalan. This regimen was effective at preventing grades III-IV GVHD in recipients of both HLA-identical sibling donor or matched unrelated donor PBSC allografts. Additionally, a significant portion of the patients achieved a CR.34
While the chemotherapy-based reduced-intensity regimens often have fewer regimen-related toxicities than conventional allogeneic HSCT does, the patients still experience cytopenias, and most require hospitalization.
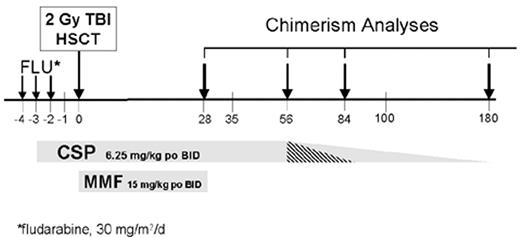
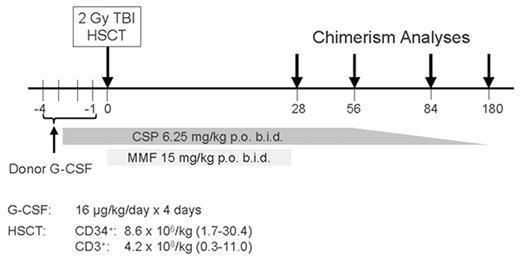
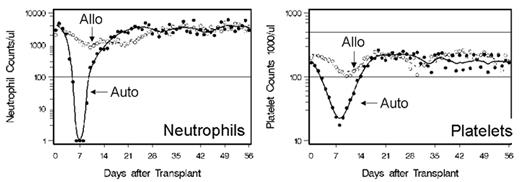
In an attempt to further reduce the toxicities and to be able to perform allogeneic HSCT in the outpatient setting, a low-dose TBI-based conditioning regimen was evaluated in a multi-institutional study. This transplant regimen was identical to that developed in the dog model and consisted of 2 Gy TBI given as a single fraction on day 0, with postgrafting immunosuppression of MMF (15 mg/kg given orally twice a day from day 0 to 27) in combination with cyclosporine (6.2 mg/kg given orally twice a day from day -1 to +35). The source of stem cells was G-CSF-mobilized PBSC collected over 2 days from HLA-identical related donors.35 Participating Centers included Fred Hutchinson Cancer Research Center, University of Washington, Veterans Administration Medical Center, Seattle, WA; University of Leipzig, Leipzig, Germany; Stanford University Hospital, Stanford, CA; City of Hope National Medical Center, Duarte, CA; Baylor University, Dallas, TX; University of Torino, Torino, Italy; and University of Colorado Health Sciences Center, Denver, CO. All patients enrolled in the trial were either too old to be eligible for a conventional transplant or, if younger, had significant medical contraindications to conventional HSCT. The transplant regimen was well tolerated, with the majority of patients who were eligible having their transplant therapy in the outpatient clinic setting. Myelosuppression was mild, with most patients not developing severe neutropenia nor thrombocytopenia. All patients had initial donor engraftment. However, 9 (20%) of the first 45 patients had graft rejection between 2 and 4 months after transplant, which coincided with discontinuation of the MMF/CSP immunosuppression. The pretransplant factors predictive of graft rejection were lack of intensive preceding therapy and, additionally, low-donor T-cell chimerism at day 28. The rejections were non-fatal, with the patients having autologous reconstitution of counts.
The conditioning regimen was modified to prevent graft rejection by the addition of fludarabine 30 mg/m2/day on days 4, 3, and 2, to the 2 Gy TBI conditioning regimen. Additionally, because of development of GVHD once the immunosuppression was discontinued on day 35, cyclosporine administration was prolonged through day 56 (Figure 1 ). In an update on the results of the initial cohort of patients with hematologic malignancies, 192 patients (inclusive of the first 45 patients) with a median age of 55 years received PBSC transplants from HLA-matched related donors.36 With the addition of fludarabine pretransplant, only 3% of all subsequent patients failed to engraft, and all rejections were non-fatal. The patients who had fludarabine added to their TBI conditioning regimen had higher donor T-cell chimerism on day 28 compared to the TBI group (P < 0.001). Grades II, III and IV GVHD occurred in 33%, 11%, and 5%, respectively, of stably engrafted patients. There were no significant differences in the rates of grades II–IV or grades III–IV GVHD when the patients who received fludarabine/TBI were compared to those who received TBI only. Myelosuppression was mild, with the median numbers of days ANC < 500/mL and platelets < 20,000/mL 2 and 0, respectively. In a separate retrospective cohort study, recipients of this nonmyeloablative transplant regimen had significantly reduced red blood cell and platelet transfusion requirements compared to conventional HSCT recipients.37 Only 33% of the patients required platelet transfusions and 64% required red blood cell transfusions. There were significantly fewer bacterial infections than seen after conventional HSCT,38 and the incidence of cytomegalovirus (CMV) disease was reduced in the first 100 days.39 With a median follow-up of 289 (range 100–1,177) days, the overall day 100 non-relapse mortality was 6%, and 59% of the patients were alive, while 18% died of causes related to relapse and progression of their disease, and 22% died of non-relapse causes. The projected 2-year survival and progression-free survival were 50% and 40%, respectively.
Unrelated HLA-matched HSCT
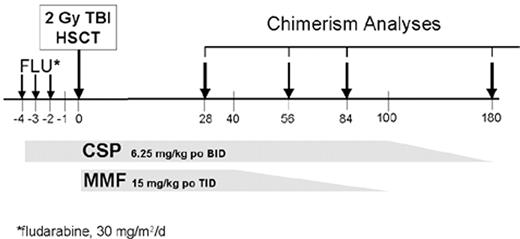
Based on the success of the nonmyeloablative HSCT with HLA-matched related donors, a multi-institutional study of 10/10 HLA-antigen matched unrelated donors was initiated.40 The transplant regimen used fludarabine, 30 mg/m2/day × 3, and 2 Gy TBI on the day of unrelated donor HSCT, followed by postgrafting immunosuppression with an extended course of CSP and MMF (Figure 2 ). The patients were serologically matched for HLA-A, -B, and -C, and allele-level matched for HLA-DRB1 and -DQ with their unrelated donors. The 77 patients entered on trial were ineligible for conventional HSCT because of age and/or comorbid conditions, and had a median age of 53 (range 4–69) years. While the preferred source of stem cells was PBSC, 60 patients received PBSC and 17 patients received marrow. Initial donor T-cell engraftment was documented in 89% of patients with median donor T-cell chimerisms on days 28, 56, and 84 being higher in PBSC recipients as compared to marrow recipients. The granulocyte and marrow donor chimerism values were not significantly different at time points. Sustained donor engraftment occurred in 50 out of 60 PBSC recipients and 10 out of 17 marrow recipients. Rejection was associated with stem cell source (P = 0.03), along with patient diagnosis (P = 0.003). Preceding conventional HSCT or preceding chemotherapy reduced the risk of rejection. With a median follow-up of 6 (range 0.6–19) months, the Kaplan-Meier estimates of survival and progression-free survival at 1 year for recipients of PBSC vs. marrow were 49% vs. 17% and 43% vs. 8%, respectively. Based on the inferior results with bone marrow as a source of stem cells after this minimally myelosuppressive regimen, only PBSC are being used in subsequent trials using low-dose TBI. In addition, the posttransplant immunosuppression has been modified to increase the dose of MMF from b.i.d. to t.i.d. in order to minimize the risk of late graft rejections, and early results have been promising.
Nonmyeloablative Allografting After Failing a Conventional Transplant
Outcomes with conventional allografting following a failed autologous or allogeneic transplant are typically poor, primarily related to a high nonrelapse mortality that occurred early after transplant.41 As nonmyeloablative transplants have reduced regimen-related toxicities, they ideally can be used in situations where patients have failed a conventional allogeneic or autologous transplant due to relapse, rejection, or secondary malignancy. Nagler et al reported using a fludarabine, busulfan, and antithymocyte globulin conditioning regimen for patients with relapsed disease or secondary malignancies following an autologous transplant.42 Other groups have tried this approach with varying degrees of success, though with many of the reduced intensity regimens a significant number of patients still died from nonrelapse causes.
Using the 2 Gy TBI-based regimen, 48 patients who failed a conventional autologous (n = 43), allogeneic (n = 4), or syngeneic (n = 1) HSCT subsequently received an HLA-matched related (n = 29) or unrelated (n = 19) donor allograft.43 The median number of months from conventional transplant was 22 (range 4–26) months. One rejection occurred in a patient receiving an unrelated donor allograft. The day 100 non-relapse mortality was 6%, and overall non-relapse mortality was 15% with a follow-up of 8.4 (range 0.6–31.5) months. The Kaplan-Meier estimate of overall survival, progression-free survival, and non-relapse mortality were 52%, 30% and 13%, respectively. In lymphoma patients who have particularly poor outcome with a second conventional transplant, the Kaplan-Meier estimate of survival was 58% at 1 year, with a transplant-related mortality of 23%, due to multiorgan failure, infection, and/or GVHD.
Summary
The use of a relatively nontoxic, nonmyeloablative, or reduced-intensity preparative regimen results in donor engraftment and may allow the development of a graft-versus-malignancy effect. The use of the fludarabine/TBI regimen ensured engraftment rates similar to conventional allografts. As this approach is potentially curative for susceptible malignancies and has reduced the risk of treatment-related morbidity, this strategy may be extended for use in older patients and those with co-morbidities that preclude using high-dose conventional transplant regimens. Within the broad category of hematologic malignancies, the optimal regimen may ultimately be defined by the nature of the disease being treated. For aggressive fast-growing malignancies such as intermediate/high-grade lymphomas or acute leukemias, cytoreduction by preceding chemotherapy or by a reduced-intensity regimen with disease-specific chemotherapy may be necessary to permit time for adequate GVT to develop. For more indolent diseases such as the low-grade lymphomas and chronic leukemias, a truly nonmyeloablative regimen may be sufficient as the developing GVT immune response may be capable of eradicating all disease.
For nonmalignant diseases, mixed chimerism may be sufficient to provide benefit. However, for malignant diseases, conversion to full donor chimerism is likely necessary. The role of DLI needs to be explored in this process both to achieve full donor chimerism and to treat persistent or relapsed disease after nonmyeloablative HSCT. These effects, however, are often associated with significant GVHD.
Current challenges include minimizing severe GVHD (which results in prolonged immunosuppression) while allowing a GVT effect to occur. Presently in many studies, the rate of immunosuppressive taper is based on whether the patient is at a low versus high risk of relapse or progression of disease. Infections occurring during the postgrafting immunosuppression needed to control GVHD continue to contribute significantly to non relapse mortality in all of the regimens.
With these approaches, significant antitumor responses have been noted, with complete remissions being achieved in many patients with a wide variety of hematologic malignancies. Unlike conventional high-dose transplants, complete remissions may take months, or even longer than a year, to occur—especially with the lower intensity regimens. Only with longer follow-up will it be known whether these remissions will be durable. Future challenges include determining the duration of posttransplant immunosuppression to allow optimal GVT effect without causing excessive GVHD. Ultimately, nonmyeloablative HSCT may be used as a platform for adoptive immunotherapy for the infusion of cytotoxic T cells that are tumor specific, thus potentially augmenting GVT without GVHD. In the future, nonmyeloablative transplants may become the procedure of choice for younger “good risk” patients at an earlier stage of disease for both malignant and nonmalignant indications.
II. Tandem Autologous Followed by Nonmyeloablative Allografts for Lymphoma and Myeloma
David G. Maloney, MD, PhD*
Fred Hutchinson Cancer Research Center, D1-100, 1100 Fairview Ave, North, Seattle, WA 98104
Autologous Transplantation for Myeloma and Lymphoma
High-dose therapy followed by autologous peripheral blood stem cell (PBSC) rescue is frequently used in the treatment of eligible patients with non-Hodgkin’s lymphoma (NHL) and multiple myeloma. In some cases, such as chemosensitive aggressive NHL, this treatment results in long-term disease-free survival (DFS) and is better than salvage chemotherapy.1 These benefits are largely restricted to patients with chemotherapy-sensitive disease at the time of autologous PBSC transplantation (PBSCT). For myeloma, autologous PBSCT is associated with disease cytoreduction and appears to prolong DFS compared with standard therapy; however, there is a continuous risk of relapse, and plateaus are not apparent on DFS curves.2 This appears also to be true with the use of autologous transplantation for low-grade lymphoma.3 The reason that high-dose therapy is not curative in these situations is tumor contamination of the collected PBSCs, inability of the high-dose preparative regimens to eradicate all of the tumor cells in the patient, or both. While purging methods may reduce contamination, these strategies have not yet made any appreciable impact on survival4–,6 and in cases of CD34 selection have resulted in the loss of normal immune cells, leading to immunodeficiency and increased risk of infections.7,8 Approaches using “in vivo purging” with anti-CD20 antibodies have also demonstrated the ability to decrease tumor cell contamination of the PBSC graft. To date, however, none of these approaches have appreciably reduced the risk of relapse following autologous PBSCT, suggesting that resistant and refractory disease in the patient is more likely to contribute to relapse in this setting.
Despite these limitations, high-dose therapy with autologous PBSCT has been associated with a low transplant-related mortality (TRM) of generally less than 10% and has been routinely offered to patients without significant comorbid diseases up to ages 65 to 70. Engraftment is usually rapid (10-12 days), and once patients have recovered from conditioning regimen toxicity, they generally return to a high quality of life. While long-term complications are generally infrequent, patients do have an increased risk of myelodysplasia and marrow failure. A number of posttransplant treatments have been used in attempts to decrease the high risk of relapse in these patients, including thalidomide, monoclonal antibodies, idiotype vaccination, and new phase I agents (reviewed by Anderson9 and Barlogie et al10). While promising, none of these strategies has yet provided randomized clinical trial evidence of efficacy in the posttransplant setting.
Allogeneic Transplantation for Myeloma
Myeloablative conditioning regimens and allogeneic HSCT have been used in the treatment of relatively small numbers of patients with myeloma, as reviewed by a number of different researchers.11–,16 Advantages include the use of a graft free from tumor cells and the potential for a GVT immune response.17 Evidence for GVT has consistently been demonstrated by the lower incidence of relapse for patients with NHL and myeloma following allografting and by the demonstration of antitumor activity in patients who have relapsed following conventional allografting when treated with additional DLI.18–,20 In comparison with autologous transplants, there is a higher rate of molecular remissions associated with the allografts and a lower risk of disease progression.21 The major risk associated with non-T-cell-depleted allogeneic HSCT has been a higher TRM, in part due to the risk of acute and chronic GVHD. Because of these increased toxicities and risks, allogeneic HSCT has been applied to a small number of patients and has been generally limited to younger patients without comorbid diseases. This is in contrast to the peak incidence of these malignancies, which steadily increase with age. The majority of transplants have utilized matched related donors, which again favors younger patients with living donors. In general, myeloablative transplants with matched unrelated donors have not been offered to patients with lymphoma or myeloma who are over the ages of 50 to 55 because of an unacceptably high TRM that has ranged from 20% to 50%. Thus, despite a significant decrease in the relapse rate and evidence of GVT effects, allografts have been associated with inferior survival in nearly all comparisons, largely because of a high peritransplant TRM and the continued late complications of chronic GVHD and infections.22,23 This high TRM does not allow effective GVT immune reactions for more than a minority of patients. More recent trials suggest that with a recent lower TRM associated with allografting, the lower risk of disease progression may eventually lead to a superior outcome.24,25
Allogeneic Transplantation as Salvage for Relapse Following Autologous HSCT
The prognosis for patients who relapse or develop progressive disease following high-dose therapy and autologous HSCT is poor. Nearly all patients will eventually die from disease progression. A number of groups have published limited data on the use of standard high-dose allogeneic HSCT in patients with NHL and myeloma who have relapsed following a prior autologous transplant.26 In general, these have been associated with unacceptable toxicity, largely limiting this to the pediatric population. Even in this situation, the risk of disease relapse remains high. The dose intensity of the conditioning regimen used in the earlier transplant regimen also contributes to the toxicity and success/failure of the subsequent regimen. A lower success rate has been observed in patients treated with a TBI conditioning regimen during their autologous conditioning. Thus, the opportunity to salvage patients with a potentially curative allograft once they have developed progressive disease using conventional high-dose approaches is limited.
Reduced Dose Conditioning and Allogeneic HSCT as Salvage for Relapse Following Autologous HSCT
In contrast to the use of high-dose conditioning, a number of investigators have evaluated the use of reduced-dose conditioning, including nonmyeloablative conditioning, to allow allogeneic HSCT in elderly patients and in patients at high risk of TRM because of comorbid disease or prior high-dose therapy (reviewed in Section I). When a variety of regimens are used, these approaches appear feasible with a much lower risk of regimenrelated mortality (compared to high-dose regimens) and in some cases have been associated with dramatic antitumor effects even in patients at high risk of TRM.27
A number of “nonmyeloablative” regimens have been used. The Seattle group has explored the use of 200 cGy TBI and fludarabine (90 mg/m2) followed by immunosuppression with MMF and cyclosporine.28 This regimen is nonmyeloablative and is minimally myelosuppressive. As a consequence, the conditioning regimen does not provide a significant degree of tumor cytoreduction, especially for rapidly progressive or refractory disease. The regimen has a low early nonrelapse mortality (7%) and reliably allows donor cell engraftment. In contrast to more intensive regimens, the burden for antitumor activity is shifted from the cytotoxic therapy to the immunologic effects of the GVT effect. Unfortunately, in patients with large-volume relapsed/refractory progressive myeloma or NHL, the tumor may outgrow the establishment of an effective GVT immune response. Success with this regimen in this group of patients who have progressed following autologous transplantation has been generally limited to patients who have had cytoreduction of disease prior to the nonmyeloablative allograft. Exceptions include diseases that appear more susceptible to GVT killing, such as low-grade NHL and chronic lymphocytic leukemia (CLL), where dramatic tumor regressions may be observed following the induction of GVT activity. As discussed in Section I, this regimen can be used safely in patients who have failed prior autografts.
Similar regimens have been published. Dreger et al reported on 7 patients with low-grade NHL, CLL, or myeloma who either had failed an autograft or were unable to be mobilized who were treated with fludarabine (150 mg/m2) and cyclophosphamide (2500 mg/m2) and matched allografts (6 sibling donors).29 Patients recovered ANC by day 13. There were 2 treatment-related deaths, and 5 patients are alive and in complete response (CR) 14 months (8-18) posttransplant. Full donor chimerism was observed following the tapering of cyclosporine and usually was associated with GVHD.
Garban et al reported on the use of fludarabine (125 mg/m2), antithymocyte globulin (12.5 mg/kg), and busulfan (4 mg/kg) prior to matched sibling PBSCT.30 GVHD prophylaxis was with cyclosporine alone. Twelve patients, ages 45 to 61, were treated. All had progressed less than 1 year following an autologous transplant. With the allograft, 11 of 12 patients had a partial response (PR) or CR (n = 4). CMV reactivation was observed in 5 patients and caused 1 death. Fifty percent of patients had acute grade II to IV GVHD, and 7 of 9 patients developed chronic GVHD that was extensive in 2. There were 2 early deaths, and 6 patients died < 6 months posttransplant. Overall, 8 patients have died: 3 from disease, 2 GVHD, 1 CMV, and 2 multiorgan failure. Four of 12 patients are alive at 6, 13, 13, and 15 months. This regimen is now being used in high-risk patients following their primary therapy rather than waiting for progressive-resistant disease.
The University of Arkansas group has also used reduced-intensity regimens for the treatment of 31 patients who had previously had 1 or 2 prior instances of autologous HSCT.31,32 Melphalan (100 mg/m2) was used alone for patients with related donors, and 250-cGy TBI and fludarabine (60 mg/m2) were added for patients with unrelated donors. GVHD prophylaxis was with cyclosporine alone for related donor recipients and cyclosporine and prednisone for unrelated donor recipients. DLI were planned at days 21, 42, and 112 for conversion to full donor chimerism or disease persistence/progression. Thirty-one patients, median age 56 (range 38-69), were treated. Thirteen patients had had 1 prior instance of autologous HSCT, and 17 had had 2 prior transplants. Fourteen of the patients had responsive disease (6 had a primary response, 8 had a response to salvage therapy). Twenty-one patients had abnormal chromosome 13 cytogenetics.
By day 100, 87% had full donor chimerism and 2 patients had rejected their grafts. Acute GVHD was seen in 58% of patients and chronic GVHD in 10 patients (extensive in 4). At a median of 6 months follow-up, the CR rate was 61%. Twelve patients have died, including 3 from progressive disease and 6 from late treatment-related toxicity. Three deaths were before day 100. Most late deaths were due to GVHD or associated infections. Median overall survival was 15 months. The estimated 1- and 2-year survival was 71% and 31% respectively. In this series, patients with 1 prior instance of autologous HSCT had a better outcome than did patients with 2 prior instances of autologous HSCT. The estimated 1-year survival was 86% versus 48%, and 1-year event-free survival was 86% versus 31% for patients with 1 prior versus 2 prior autologous transplants. Some of these patients were transplanted with responding disease following autologous HSCT (planned tandem auto-/allotransplants), which may have contributed to the improved outcome in patients having received 1 prior autograft. These results are still premature, and longer follow-up is necessary to determine the impact of GVHD and disease relapse. However, they demonstrate the powerful graft-versus-myeloma effect and the ability to accomplish allogeneic engraftment with a lower TRM than associated with the use of full-dose conditioning regimens in patients who have had a prior instance of autologous HSCT.26
Rationale for Tandem Autologous Followed by Nonmyeloablative Allogeneic HSCT
As discussed above, high-dose therapy and autologous transplantation are frequently used for the treatment of patients with low-grade NHL and for myeloma, despite the nearly universal risk of disease progression. The ability to salvage patients with full-dose conditioning regimens and allografts following relapse is poor and the risk of disease progression high. This fact has led investigators to propose tandem transplants, utilizing high-dose therapy with an autologous PBSC rescue to cytoreduce the disease followed by the use of a reduced-intensity or nonmyeloablative allograft (done prior to disease progression post-autologous-HSCT) to provide GVT and possibly eradicate the malignancy and prevent relapse, all with a lower overall TRM.
Planned Tandem Auto/Allo HSCT for Myeloma
For myeloma patients, most investigators agree that high-dose regimens and autologous HSCT provide disease-free and overall survival advantages compared with continued conventional chemotherapy. Controversy remains as to the optimal conditioning regimen, the timing of the transplant in relationship to response to prior therapy (early in remission or later at the time of relapse), the use of 1 or 2 transplants, and the use of additional posttransplant treatments to delay the recurrence of disease. In most studies, high-dose melphalan with or without additional agents or TBI has been used. Posttransplant treatments include the use of corticosteroids, interferon, and thalidomide as well as a number of new investigational agents active for the treatment of myeloma.9,10
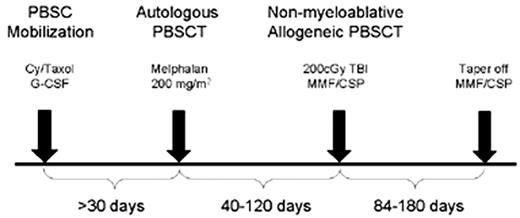
In a series of patients treated in a consortium of institutions coordinated through the Fred Hutchinson Cancer Research Center—including the City of Hope, Stanford University, the University of Colorado, the University of Torino, Italy, and the University of Leipzig, Germany—54 patients with advanced myeloma have been treated using a planned tandem auto/allo approach outlined in Figure 3 . Newly diagnosed patients with stage II/III disease requiring therapy were recommended to have induction chemotherapy with VAD (vincristine, adriamycin, dexamethasone) chemotherapy (4 cycles recommended) followed by autologous PBSC harvesting using high-dose cyclophosphamide (with or without Taxol) and G-CSF. Relapsed or refractory patients were also eligible and entered the study for or following PBSC collection. The first 5 patients had stem cells processed for CD34 selection, but this was discontinued following a patient death due to CMV pneumonia and the realization that CD34 selection did not improve outcome in randomized clinical trials.4,5,8 High-dose therapy with single-agent melphalan (200 mg/m2) was used for tumor cytoreduction with autologous PBSC support. Following recovery from transplant toxicities, 40 to 120 days later, patients underwent nonmyeloablative allografting using the schema shown in Figure 4 . Conditioning was with a single fraction of 200-cGy TBI administered at a low dose rate. Matched sibling donor PBSCs, harvested from 2 phereses following stimulation with G-CSF, were infused immediately following TBI. Patients received postgrafting immunosuppression with cyclosporine and MMF.
Preliminary data from this trial were presented at the plenary session of American Society of Hematology 2001. Updated results include the treatment of 54 patients with this planned tandem approach. Toxicity following the autologous PBSCT included medians of 6 days of neutropenia and 7 days of hospitalization. One patient who had received CD34-selected PBSCT died from CMV pneumonia. Fifty-two of the 54 patients received the planned nonmyeloablative transplant, with a median time between autologous and allogeneic transplant of 62 days. The allografts was well tolerated, with medians of 0 days of hospitalization, neutropenia, and thrombocytopenia. The granulocyte and platelet nadirs were 760 cells/μL and 95,000 cells/μL, respectively. Comparison of the hematologic changes during autologous and allogeneic transplantation is shown in Figure 5 . All patients developed donor engraftment, with medians of 90% donor T-cell chimerism by day +28 that increased to 96% by day +84. One patient died before day 100 from disease progression. Acute GVHD was seen in 38% of patients and was grade II in all but 4 cases. Forty-six percent of patients developed chronic GVHD requiring therapy. Tumor responses occurred slowly, and thus far 57% of patients not in CR at the time of treatment have achieved CR and 26% have achieved PR. Of 28 patients with responsive disease at study entry, 3 have died from transplant-related complications and 2 have relapsed. Sixteen remain in continuous CR and 5 in ongoing PR, and 2 patients have stable disease. Patients with relapsed/refractory disease have had greater TRM, with 5 of 26 patients dying from complications of acute and chronic GVHD infections. In this group, 11 of 26 patients achieved CR and 4 of 26 achieved PR following the allograft. One responding patient has relapsed. Three additional patients have died from myeloma (n = 2) and 1 from lung cancer. Thus, patients treated with responsive disease have had less TRM and improved overall and disease-free survival. With a median follow-up of all surviving patients of 18 months, 79% of patients are alive. These results have been used to plan a comparative trial in the BMT Clinical Trials Network that will evaluate the use of this approach in a multicenter setting for the treatment of patients with stage II/III myeloma.
A similar tandem approach using a more aggressive allogeneic conditioning regimen has been reported by Kroger et al in myeloma patients with stable disease or better following induction or salvage chemotherapy (12 patients in PR).33 Seventeen patients, median age 51 (range 32-64), received melphalan (200 mg/m2) and autologous HSCT. At a median of 119 days post-autologous-transplant (range 60-210), patients underwent conditioning with fludarabine (180 mg/m2), melphalan (100 mg/m2), and ATG (30 mg/kg) followed by allogeneic HSCT. Eight of the patients received matched unrelated grafts. GVHD prophylaxis was with cyclosporine and methotrexate. The allograft regimen did lead to at least 8 days of neutropenia (median time to leukocytes >1 × 109/L was 16 days, range 11-24). All patients required RBC and platelet transfusions. All patients developed full donor chimerism by day 30 to 40 posttransplant. The day 100 mortality was 11%. Acute GVHD (38%) included 4 grade II and 3 grade III disease. Forty percent developed chronic GVHD, but only 1 case was extensive, requiring further therapy. Overall, 18% of patients had CR following the autograft, and 73% had CR following the allograft. With a median follow-up of 13 months following the allograft, 13 patients were alive and 12 free of progression or relapse. The estimated 2-year overall survival was 74% and DFS 56%. Two patients relapsed following allografting. None of the patients entering the study with less than PR from their prior therapy (prior to the autograft) had CR following the allograft. Longer follow-up is required to determine if this regimen, which appeared better tolerated than a fully ablative regimen, will ultimately result in long-term DFS.
Planned Tandem Auto/Allo HSCT for NHL
A similar approach has been used for the treatment of patients with either low-grade NHL or refractory aggressive lymphoma who would not likely be long-term disease-free survivors when treated with autologous HSCT alone. The addition of the allogeneic GVT response may improve the overall response rate and decrease the risk of relapse. In these settings, GVHD remains a double-edged sword, as it contributes to tumor eradication and causes much of the TRM associated with allogeneic transplantation.
Carella et al reported on the use of conditioning with BEAM followed by nonmyeloablative allogeneic transplantation from matched siblings.34 Fifteen patients with Hodgkin’s disease (n = 10) or NHL (n = 5) who were thought unlikely to be cured by the autologous transplant regimen alone, then received fludarabine (90 mg/m2) and cyclophosphamide (900 mg/m2) and HLA-identical allografts. Postgrafting immunosuppression was with cyclosporin and methotrexate. Thirteen patients developed full donor chimerism, 7 following planned DLI. Eleven of the 13 patients had CR postallografting (including 9 with PR from the autologous transplant). Of the 7 patients given DLI, 6 developed GVHD. Overall, 7 patients developed grades II to IV acute GVHD, and 2 patients developed chronic extensive GVHD. Two patients have relapsed. Overall, 10 are alive and 5 are in CR between 210 and 340 days (median 270 days) postallografting. Five patients died, 2 from progressive disease and GVHD, 2 from GVHD/infection, and 1 from disease progression. The allograft improved remissions in the majority of patients but also contributed to mortality, as 2 patients died in CR from complications of GVHD.
A similar approach is ongoing at the Fred Hutchinson Cancer Research Center in Seattle. Patients with relapsed or refractory NHL or with large-volume low-grade NHL not thought to be curable using autologous transplantation alone are treated with consolidative allografts following autologous PBSCT. Patients who have not had dose-limiting radiation therapy undergo treatment with cyclophosphamide and 12-Gy TBI followed by autologous PBSCT. Patients with prior dose-limiting XRT receive the BEAM regimen. Following recovery from the toxicities associated with the autologous conditioning regimen (resolution of mucositis, off of IV antibiotics, without active infection), 40 to 120 days later patients receive a nonmyeloablative transplant regimen using a single fraction of 200-cGy TBI as outlined above for the myeloma trials. Engraftment has been rapid and uniform in these patients, and responses have been observed in refractory patients. At this time, limited long-term data utilizing this approach are available and patients are continuing to accrue to this trial.
Conclusions and Future Studies
In both myeloma and lymphoma, it is clear that there is the potential for a powerful allogeneic GVT immune response. This has been observed through the use of DLI and by the responses to nonmyeloablative allogeneic HSCT. For some sensitive malignancies, or for patients in remission, a nonmyeloablative HSCT may provide enough antitumor activity to be curative. For patients with myeloma or relapsed, chemosensitive aggressive NHL where high-dose therapy with an autologous PBSCT is the standard of care, it is more difficult to substitute a truly nonmyeloablative allogeneic regimen that relies on only GVT for antitumor activity. As most patients with myeloma will eventually relapse following autologous PBSCT, this is a logical patient group for studying tandem transplantation. Data from pilot studies demonstrating 75-90% 1-year survival postallografting are encouraging. Randomized trials are needed to test these approaches to determine if the addition of a nonmyeloablative allograft following a planned cytoreductive autograft will provide long-term DFS that is not offset by the complications of GVHD. However, the logistical reality of accomplishing a truly randomized study among patients with matched sibling donors is unrealistic. Alternative trials are under way. A clinical trial is planned to start in the fall of 2002 within the BMT Clinical Trials Network that will evaluate this approach in multiple centers across the US. Patients with newly diagnosed myeloma requiring therapy will undergo induction therapy (VAD recommended) and proceed to autologous PBSC harvest (following a high-dose cyclophosphamide regimen) prior to study entry. All patients will then receive high-dose melphalan (200 mg/m2) and autologous PBSCT. Sixty to 120 days later, following recovery from transplant-related complications, patients with matched sibling donors will receive 200-cGy TBI, allogeneic PBSCT, and postgrafting immunosuppression with cyclosporin and MMF. Patients without sibling donors will receive dexamethasone and thalidomide post-autologous-transplant. While not a randomized study, this trial should determine in a multicenter approach the feasibility, toxicity, and outcomes of these two approaches. Further trials comparing the contribution of nonmyeloablative allografting following standard therapy for lymphoma, AML, and CLL are either being planned or ongoing in this and other cooperative groups.
Ultimately, these approaches attempt to harness the benefits of allogeneic GVT activity while minimizing TRM. In non-T-cell-depleted transplants, GVHD and/or complications relating to the treatment or prevention of GVHD (infections) remain the biggest challenge. As discussed in Section IV, separation of GVHD from GVT may be possible using a number of strategies. In addition, as discussed in Section III, T-cell-depletion strategies can result in decreased GVHD but increase the risk of immunodeficiency and disease relapse. DLI decrease these risks but are subsequently associated with GVHD. In the long term, a better understanding of the molecular nature of the targets associated with GVHD and GVT will allow a more refined approach. These preliminary data, however, demonstrate that tandem autologous HSCT followed by planned nonmyeloablative allografts can be performed with a lower early TRM and can provide additional antitumor activity. Longer follow-up is required to determine if this approach will ultimately be curative. These studies are now being extended to the use of matched unrelated donors, which will increase the number of patients potentially eligible (see Section I). Thus, the future looks bright, but it will remain a challenge to position the expanded role of nonmyeloablative HSCT with the multitude of new agents that are entering the clinic as promising therapy for patients with NHL and myeloma.9,10
III. The Role of Alemtuzumab (Campath in -1H)Reduced-Intensity Conditioning for Allogeneic Stem Cell Transplantation
Stephen Mackinnon, MD*
Department of Hematology, Royal Free and University College London School of Medicine, 98 Chenies Mews, London WC1E 6HX, United Kingdom
The Antibody and Pharmacokinetics
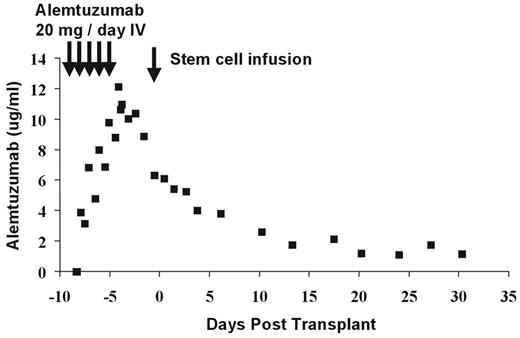
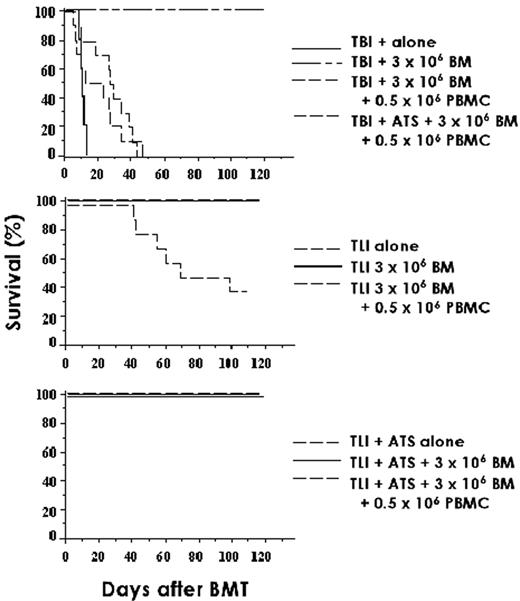
Alemtuzumab (Campath-1H) is a humanized IgG1 monoclonal antibody directed against the CD52 antigen, which is widely expressed on all human lymphoid cells except terminally differentiated plasma cells. It is also expressed on eosinophils, monocytes, dendritic cells (DCs), and macrophages.1 Current transplant protocols using alemtuzumab are based on previous studies, which used the homologous rat IgG2b Campath-1G, where the lymphocytic activity is known to be similar2 but the pharmacokinetics may be quite different. Delayed clearance of the monoclonal antibody may impair immune reconstitution, affect rates of viral reactivation, and limit efficacy of the donor T-cell-mediated graft-versus-leukemia (GVL) effect, whether derived from the graft itself, early adoptive immunotherapy, or later DLI. By administering the antibody to the recipient as part of the conditioning regimen, it will result in T-cell, B-cell, and DC depletion of the recipient. There is effective recipient DC depletion in the peripheral blood,3 but it remains unknown whether alemtuzumab leads to depletion of tissue DCs that might initiate GVHD. In addition, if sufficient antibody is circulating on the day of transplantation, this will result in T-cell depletion of the graft, thereby potentially reducing the incidence and severity of GVHD. The half-life of alemtuzumab in humans is dependent on the amount of target CD52 antigen in the patient. Following an in vivo dose of 20 mg/day for 5 days (–8 to –4) prior to allogeneic transplantation, there is persistence of alemtuzumab in vivo past day 0 sufficient to cause T-cell lysis by complement fixation and antibody dependent cellular cytotoxicity (ADCC) (Figure 6 ).4 With this dose schedule, significant levels of antibody persist through day +28 posttransplant. The optimal dose of antibody to prevent GVHD and minimize posttransplant immune suppression is currently unknown.
Reduced-Intensity Regimens Incorporating Alemtuzumab
The most commonly used regimen combines alemtuzumab with fludarabine and an alkylating agent, usually melphalan or busulfan.5,6 Alemtuzumab has also been added to the BEAM regimen and used in reduced-intensity conditioning for lymphoma.7 A partial list of reported regimens containing alemtuzumab or Campath-1G is shown in Table 2 . These regimens vary in their myeloablative and immunosuppressive properties, and it is currently not known which regimen is optimal for any given clinical scenario.
Toxicity
Reduced-intensity conditioning regimens that include alemtuzumab have been generally well tolerated. Although many of the regimens containing melphalan or busulfan result in 5–7 days of neutropenia, the avoidance of methotrexate for GVHD prophylaxis minimizes mucositis and allows most patients to continue to eat relatively normally. There is, however, infusional toxicity related to alemtuzumab. This is secondary to a cytokine release syndrome that can cause fever, skin rashes, hypotension, and occasionally bronchospasm. This risk of toxicity can be reduced by gradually increasing the doses of the antibody infused starting at a dose of 3 mg/day as per the package insert. Alternatively, a pre-med of methylprednisolone 2 mg/kg IV can be administered prior to the first 20-mg dose of alemtuzumab. This is very effective at preventing the cytokine release. Occasional patients may require corticosteroids following the first day of infusion.
Engraftment and Chimerism
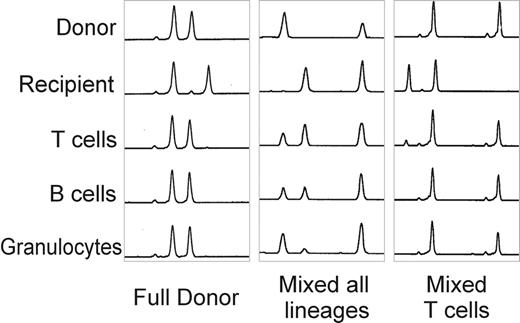
The largest experience has used alemtuzumab in combination with fludarabine and melphalan. With this regimen, the median time to recover an absolute neutrophil count of 0.5 × 109/L was 13 days (8-23) and > 1.0 × 109/L, 17 days (8-47). The median time to achieve platelets > 20 × 109/L was 13 days (3-96) and > 50 × 109/L, 17 days (8-118).5 The incidence of graft rejection was < 3% using peripheral blood stem cells from sibling donors and 6% with bone marrow from unrelated donors.5,12 Lineage-specific chimerism studies have been performed using microsatellite polymerase chain reaction (PCR) in patients following this regimen. Three patterns of chimerism have been documented using this regimen (Figure 7 ):
Fully donor in all lineages
Mixed chimera in all lineages
Fully donor myeloid chimerism with mixed T-cell chimerism
More recently, detailed kinetic studies of engraftment with this regimen have shown that most patients are full donor chimeras as early as day 7 following the transplant and that some patients develop stable mixed chimerism between 1 and 3 months posttransplant.13
As this approach to transplantation is heavily dependent on GVL or GVT effects, the development of mixed T-cell chimerism following reduced-intensity stem cell transplantation (SCT) could be associated with a higher incidence of disease relapse. Therefore, if mixed chimerism persists once immunosuppression has been discontinued, attempts to promote full donor chimerism and GVL activity using DLI might reduce disease recurrence. Most patients with stable mixed chimerism will achieve full donor chimerism following the administration of DLI (see below).
GVHD
Perhaps the most impressive effect of alemtuzumab as part of a reduced-intensity conditioning regimen is in the prevention of GVHD. Published results of sibling donor SCT using other nonmyeloablative conditioning regimens have shown a 38% to 60% incidence of grade II-IV acute GVHD that is the primary cause of death in some patients.14–,17 However, when alemtuzumab has been used as part of the conditioning regimen using HLA-identical siblings, most patients do not develop any GVHD and the reported incidence of acute grade II–IV GVHD following HLA-identical sibling transplantation was 5%.5 When transplants using unrelated donors are assessed, the effects of alemtuzumab in not only preventing GVHD but also in limiting TRM are particularly impressive. For reduced-intensity regimens that do not include alemtuzumab, the reported experience of unrelated donor SCTs using a fludarabine + melphalan protocol observed high rates of severe GVHD, with 1 in 4 patients dying as a direct result of GVHD.15 In contrast, a similar regimen containing alemtuzumab was associated with a low incidence of GVHD despite significant HLA disparity in many of the transplants. Only 6% of patients had grade III to IV acute GVHD, and only 15% developed grade II acute GVHD.12 No patients have yet developed chronic extensive GVHD, and no patients have died as a consequence of GVHD. As pharmacokinetic studies have shown (see above), significant circulating antibody was circulating when the unmanipulated donor PBSC or bone marrow was infused into the recipient and may have resulted in a degree of in vivo T-cell depletion. It is also possible that depletion of other cells (such as DCs) expressing CD52 by Campath-1H could be relevant to the reduced incidence of GVHD.3,18
Disease-Specific Outcomes
Acute myeloid leukemia
Twenty-four patients with acute leukemia/MDS have recently been reported using an alemtuzumab-based regimen.19 All were considered too high risk for conventional myeloablative allogeneic transplantation due to age, disease stage, previous autografting, or lack of a sibling donor. Disease status at transplant was CR1 (n = 3), CR2 or beyond (n = 12), and refractory (n = 2) in those with acute leukemia, and refractory anemia in all 5 MDS patients. Median follow-up was only 418 days. Five patients died of transplant-related causes, giving a 1-year TRM of 19%. Seven of the remaining 19 patients relapsed. Five of these died, including both patients transplanted with refractory AML. Two are currently in CR following salvage chemotherapy. Twelve patients remain alive and progression free. Actuarial 18-month overall survival (OS) and progression-free survival (PFS) rates are 53% and 44%, respectively, for the entire cohort. This compares to 62% and 57% for the 15 patients with AML transplanted in CR. In summary, the results show that this approach has a relatively low TRM compared to conventional allografting given the nature of the cohort treated. Similar results have been obtained in patients with myelodysplasia using a regimen containing alemtuzumab, fludarabine, and busulfan.6 The answer to whether reduced-intensity transplantation with or without alemtuzumab can achieve results superior to those of chemotherapy alone in AML will require longer follow-up and ultimately a randomized trial comparing both treatment modalities.
Hodgkin’s disease
The therapeutic options for patients with Hodgkin’s disease (HD) who relapse soon after completion of first-line chemotherapy or have failed an autologous transplant are very limited. The efficacy of an allogeneic myeloablative transplant in HD remains controversial, and possible benefit was eliminated by very high nonrelapse mortality using TBI-based conditioning regimens.20,21 An alemtuzumab-based reduced-intensity conditioning regimen has been reported in 24 patients with HD.22 At transplant 6 patients were in CR, 13 were in partial remission (PR), and 6 had refractory disease. Two patients died from toxicity secondary to the transplant procedure. Three patients with refractory disease at transplant progressed and died within 3 months posttransplant. Five patients relapsed 19, 18, 17, 9, and 6 months posttransplant. Three patients received salvage chemotherapy to control disease progression, followed by DLI. Two patients received only DLI. Four of these 5 patients achieved and maintain remission. The actuarial survival, PFS, and current PFS rates following DLI ± chemotherapy are 72%, 45%, and 57%, respectively. Longer follow-up will be needed to assess the impact of this protocol on disease control and ultimately on cure.
Non-Hodgkin’s lymphoma/ chronic lymphocytic lymphoma
Results in 70 patients with NHL/CLL who received transplantation using an alemtuzumab, fludarabine, and melphalan regimen were recently reported.23 The median age was 48 years. Forty-one patients had less aggressive histology (CLL [n = 10], mantle cell lymphoma [n = 10], low-grade NHL [LG-NHL] [n = 21]), and 29 had high-grade NHL (HG-NHL). Twenty-nine patients had failed a previous autograft. At transplant 17 patients were in CR, 45 were in PR, and 8 had refractory or progressive disease. Median follow-up was 17 months. Thirteen patients died from regimen toxicity. Fifteen patients have relapsed, and 10 of those have died from disease progression. Two patients with LG-NHL have responded clinically to DLI. Overall survival was 85% for less aggressive lymphomas and 35% for HG-NHL (P = 0.004). PFS for LG-NHL is 65% and for HG-NHL is 22% (P < 0.001). For patients transplanted with refractory disease the PFS was significantly worse (P < 0.003). Similar outcomes have been reported in a smaller number of patients using a regimen containing Campath-1G + BEAM conditioning.7 In conclusion, alemtuzumab-containing regimens were associated with relatively low TRM and low incidence of GVHD. This therapeutic approach has been associated with results that were less good in patients with HG-NHL but with good results in patients with LG-NHL. The long-term benefit of this therapy in LG-NHL is likely to be dependent on the GVL effect of DLI, and initial results in this group of patients are encouraging.
Multiple myeloma
A reduced-intensity conditioning regimen containing alemtuzumab, fludarabine, and melphalan has been used as part of front-line treatment in 17 patients with chemosensitive myeloma (1 CR, 15 PR, 1 minimal response).24 None had previously had an autograft. Median age at transplant was 48 years. Ten received matched sibling and 7 received unrelated grafts. Median follow-up from transplantation was 432 days. Five of 14 evaluable patients developed acute GVHD (3 grade I, 2 grade II). There were 2 early transplant-related deaths. Thirteen patients were evaluable for disease response at 6 months. Compared to pretransplant staging investigations, 4 achieved PR, 3 minimal response, 2 no change, and 3 progressive disease, and 1 remained in CR. Ten patients received DLI for residual disease from 6 months posttransplantation at an initial T-cell dose of 1 × 106/kg, increasing incrementally every 3 months in the absence of GVHD (up to 3 × 107/kg). Ten patients were given DLI and 9 were evaluable for GVHD and disease response. Four developed GVHD, and all of these had further disease response, though only 1 achieved CR. Of the 5 without GVHD, 2 had subsequent disease progression and 3 had stable disease. Actuarial 18-month OS and PFS for the entire cohort were 80% and 53%, respectively. In summary, the use of a Campath-1H-containing conditioning regimen in patients with myeloma resulted in low TRM and GVHD, allowing subsequent DLI in the majority of cases. The conditioning regimen itself had limited antitumor activity in myeloma, and the graft-versus-myeloma effect of posttransplant DLI was disappointing.
Immune Reconstitution and Infectious Complications
Although the adoption of reduced-intensity conditioning regimens has been associated with fewer bacterial, fungal, and viral infections following transplantation, the use of alemtuzumab has been associated with slower immune reconstitution and an increased incidence of viral infections. For example, the pattern and outcome of CMV infections monitored by PCR-based assays and treated preemptively in 101 patients following nonmyeloablative conditioning containing in vivo alemtuzumab has recently been reported.25 Fifty-one patients (50%) had a CMV infection at a median of 27 days posttransplant with a probability of 84.8% in patients at risk of CMV infection (donor or recipient CMV seropositive). The probability of recurrence of CMV infection was more common in unrelated donor transplant recipients. In spite of the higher incidence of CMV infection there was no significant difference in the incidence of overall survival and nonrelapse mortality between CMV-infected and uninfected patients. There also appears to be an increased incidence of adenovirus, respiratory syncytial virus, and parainfluenza virus in alemtuzumab-treated patients; however, many of these infections are not associated with serious clinical sequelae.26 The use of alemtuzumab was associated with a low incidence of GVHD but a high incidence of CMV infections and prolonged immuneparesis. The median time to a CD4+ T cell > 200/mm3 was 9 months in patients studied.
Donor Leukocyte Infusion
Following reduced-intensity conditioning, the presence of mixed chimerism or residual tumor is a risk factor for disease recurrence. DLI can promote full donor chimerism and GVL but are associated with a high risk of GVHD early after transplant. Although DLI with a T-cell content of 1 × 107/kg are relatively safe if administered > 1 year following transplant,27 there are few data on the effective and safe dose of DLI that can be given early after transplant. Recently, a trial of DLI following a reduced-intensity alemtuzumab-containing regimen was reported.28 Thirty-three patients (21 matched sibling, 12 unrelated) were given escalating doses of donor lymphocytes from 6 months posttransplantation for treatment of residual disease or mixed chimerism. Initial cell doses were administered at a median of 239 days posttransplant at a starting dose of 1 × 106 T cells/kg or higher for overt clinical relapse (3 × 106108 T cells/kg). Three patients with bulky progressive disease received chemotherapy prior to DLI. In the absence of GVHD, incremental doses of T cells were administered every 3 months until full donor chimerism or eradication of disease. GVHD occurred in only 2 of 19 evaluable sibling transplants at doses of ≤ 1 × 107/kg but in 8 of 11 evaluable unrelated recipients (P = 0.001). Fourteen of 21 cases in which change in chimerism status was evaluable at ≥ 3 months post-DLI switched to full donor chimerism. Graft-versus-tumor responses in patients with myeloma were often limited; however, complete remissions were documented in patients with LG-NHL, HD, and CML. In summary, escalating dose DLI from 6 months post-reduced-intensity transplantation results in a low incidence and severity of GVHD in sibling allografts but a significantly higher incidence and severity in unrelated allografts. Alternative strategies such as CD8-depleted DLI or tumor-specific DLI are warranted, especially in recipients of unrelated transplants and in patients with a diagnosis of multiple myeloma.29
Summary
Alemtuzumab reduces the incidence of acute and chronic GVHD following reduced-intensity conditioning SCT and reduces GVHD-related mortality. There is a delay in immune reconstitution and an increased incidence of viral infections with the use of alemtuzumab; however, many of these infections are asymptomatic, and at least in the case of CMV, do not adversely affect TRM. For most diseases (with the exception of multiple myeloma), the follow-up is still too short to determine whether alemtuzumab will improve PFS when compared to other reduced-intensity conditioning regimens.
IV. Chronic Graft-Versus-Host Disease: A Double-Edged Sword
Judith A. Shizuru, MD, PhD*
Stanford University Medical Center, Department of Medi-cine, Division of Bone Marrow Transplantation, 300 Pasteur Drive, H1353, MD: 5623, Stanford, CA 94305-5623
Chronic GVHD (cGVHD) is the most common late complication of allogeneic HCT. This syndrome is associated with a high mortality contributing to ~50% of non-relapse-related deaths after allogeneic HCT.1 The growing experience in HCT patients treated with nonmyeloablative or dose-reduced conditioning demonstrates that cGVHD remains a major problem. The therapeutic principle that underlies nonmyeloablative HCT is to establish engraftment of allogeneic hematopoietic cells in order to confer GVT effects. Unfortunately, the donor immune cell populations that confer GVT activity are also those that mediate cGVHD. Furthermore, these donor immune cells augment allogeneic hematopoietic cell engraftment and contribute to immune reconstitution.
In this section the clinical aspects and pathophysiology of cGVHD following conventional allogeneic HCT will be reviewed. Strategies to reduce cGVHD without loss of the beneficial effects conferred by allogeneic grafts will be discussed in the context of nonmyeloablative transplantation.
cGVHD Following Conventional Myeloablative Allogeneic HCT
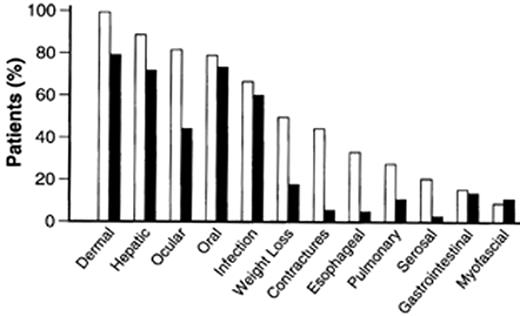
For patients who undergo conventional myeloablative therapies followed by allogeneic HCT, the distinctions between acute GVHD (aGVHD) and cGVHD are based upon both the time of onset and the pattern of clinical manifestations. aGVHD generally occurs < 100 days posttransplantation, coinciding with time of engraftment of donor hematopoietic cells, while cGVHD usually arises > 100 days and rarely later than 500 days post allogeneic HCT.2 Whereas aGVHD primarily involves the skin, gastrointestinal tract, and/or liver in a rapidly progressive pathological process, cGVHD is a more diverse clinical syndrome involving multiple organs and can be slower and more insidious in onset. Risk factors that predispose patients to cGVHD are listed in Table 3 . The clinical features of cGVHD resemble certain autoimmune diseases such as progressive systemic sclerosis, systemic lupus erythematosus, Sjögren’s syndrome, eosinophilic fasciitis, rheumatoid arthritis, and primary biliary cirrhosis. Among the many target organs that cGVHD can affect, the most commonly involved are the skin, liver, eyes, and/or oral-pharynx (Figure 8 ). Weight loss and immunodeficiency are hallmarks of disease severity. Criteria and staging of cGVHD are based upon the number of sites involved and the severity of the abnormality at each site. Table 4 presents a summary of the clinicopathologic classification of cGVHD3 and is a simple staging system that divides patients into those with limited versus extensive disease. Patients with extensive cGVHD have significantly poorer prognosis and require treatment with systemic immunosuppression, while the outcome for patients with limited disease is more favorable: they either do not require treatment or can be treated with localized topical therapy.4 Multivariate analyses have shown that thrombocytopenia (< 100,000 platelet/mm3), progressive onset of cGVHD (directly following from aGVHD), and extensive skin involvement (> 50% body surface area) are risk factors for poor outcome.2,5,6
The degree of HLA disparity directly correlates with the risk of developing cGVHD. cGVHD occurs following bone marrow transplantation in ~40% of patients that receive HLA-identical sibling unmanipulated grafts, >50% of patients that undergo HLA-nonidentical related transplantation, and ~70% of patients that receive grafts from matched unrelated donors (MUD).7 The incidence is even higher for patients who receive transplants from HLA-mismatched MUD. Age also correlates with the risk of developing cGVHD. This risk is 13% in patients younger than 10 years and rises to 46% in those older than 20 years.7 Other risk factors reported include a shortened duration of cyclosporine (CSP) prophylaxis, transfusion of donor lymphocytes, and latent herpes infections in either the donor or the recipient.2
Pathophysiology of cGVHD
The pathophysiology of cGVHD is not well understood. T lymphocytes are the principal mediators of antigen-specific immune responses, and T cells interact with MHC (in human HLA) molecules that present peptides to antigen-specific T-cell receptors (TCRs). Taken together with the knowledge that T cells induce aGVHD as well as autoimmune syndromes, the fact that reduction of T cells in hematopoietic grafts significantly reduces the incidence of both aGVHD and cGVHD8 leads to the conclusion that donor T cells are responsible for mediating cGVHD. Although T cells mediate both processes, the events that induce and perpetuate aGVHD and cGVHD are thought to be distinct. aGVHD occurs in the setting of recent tissue injury from the host conditioning regimen, and thus immune cell populations are exposed to proinflammatory conditions.9 Furthermore, it is possible (although not yet proven) that recipient-derived professional antigen present cells (APCs) such as dendritic cells remain in the recipients’ tissues in the months immediately following transplantation. These APCs can theoretically present recipient endogenous minor peptide antigens directly to donor T cells,10 resulting in a high frequency of donor T-cell clones that can be activated against host minor antigens. In contrast, cGVHD occurs following recovery of tissue injury, and we have shown that the turnover from host to donor dendritic cells in the blood is relatively rapid, occurring within the first 60 days posttransplantation for patients conditioned with either myeloablative or nonmyeloablative regimens.11 This rapid turnover of dendritic cells in the blood suggests that the kinetics of donor dendritic cell repopulation in the tissues may also be relatively rapid. Thus, in order to definitely state whether residual host-versus-donor APCs are instrumental in cGVHD pathophysiology, it will be important to study dendritic cell turnover in the primary tissue targets of cGVHD.
Two central issues that remain to be clarified are the identity of the antigenic targets for the responding T cells and delineation of the cell populations that mediate the ongoing immune response. The significantly higher incidence of cGVHD in patients who receive transplants from HLA-nonidentical donors shows that the allogeneic mismatched HLA molecules are important targets. In fact, the most widely used rodent models of cGVHD rely upon transplantation of mature T cells from inbred parental strains into F1 offspring wherein the parent donor differs by one MHC haplotype from the F1 recipient.12 Another mouse model exists, termed coisogenic, in which T cells from donor mice are transplanted into recipients that differ by only 3 amino acids in their class II MHC molecules.13 Such transplants also result in a cGVHD-like syndrome.14 Because these situations rely upon antigen presentation by a nonidentical MHC molecule, they favor a central role for donorderived cells (APCs) in mediating the disease. For HLA-identical grafts, allogeneic minor peptides are thought to be the antigenic stimulus, and as discussed above host-derived APCs would be more efficient at such antigen presentation. In either case—MHC-mismatched or MHC-matched transplantation—it is thought that recipient alloantigens provide the stimulus for the graft-derived postthymic T cells (i.e., T cells that have completed selection and maturation in the donor environment).
An alternative hypothesis for cGVHD pathogenesis is that T-cell reconstitution following HCT is flawed. In this view, T cells generated de novo from donor stem cells undergo maturation in the new host environment and thus should be tolerant to host antigens. However, it is thought that improper thymic selection can occur posttransplantation and thus autoreactive cells can be allowed to enter the periphery. In experimental and clinical studies, thymic injury and loss of thymic epithelial secretory function were noted.3 Children recover thymic function more rapidly and completely than adults following intensive chemotherapy,15 which may partially explain the age differential in development of cGVHD between younger and older patients. Perhaps the most compelling experimental data demonstrating that perturbations of the T-cell repertoire can arise posttransplantation are from the studies on autologous GVHD.16,17 Autologous GVHD is inducible following high-dose chemoradiation and rescue with autologous hematopoietic cells by the administration of CSP for a short time period after transplantation (~3 weeks).16 A syndrome develops that has a dermal pathology similar to that of allogeneic aGVHD. Rodent models show that there is clonal expansion of T cells that mediate this process and that the expanded cells recognize MHC class II molecules plus a portion of a peptide designated as CLIP (class II-associated invariant chain peptide).17 CLIP occupies the binding groove of MHC class II molecules during the later stages of their biosynthesis, and CLIP must either dissociate or be displaced to allow peptides to bind and the MHC II-peptide complex to be delivered to the cell surface. In recent studies18 it was reported that, in patients who had autologous GVHD induced in order to obtain antitumor effects, clonal predominance was noted by CDR3 spectratyping, suggesting that similar to the rodents a dominant autoantigen may drive the syndrome. The importance of CSP in altering the T-cell repertoire following allogeneic HCT has not yet been reported. However, it is notable that while the use of CSP has significantly reduced the incidence of aGVHD, it has not lowered the rate of cGVHD.
It is likely that both alloreactivity and an altered lymphocyte pool that permits inappropriate responses against autoantigens contribute to the development and perpetuation of cGVHD. As with autoimmune diseases, immunosuppressive treatment can bring the disease under control, but the pathogenic clones persist, although they are quiescent. A situation well known to bone marrow transplant physicians is that allogeneic HCT patients often develop flares in their cGVHD concomitant with the development of localized or systemic infections. Sun exposure may also activate GVHD of the skin. These clinical correlations and the observation that cGVHD is associated with latent viral infections suggest that nonspecific tissue injury and immune stimuli activate latent pathogenic clones.
Further delineation of the cellular interactions that mediate the syndrome has relied upon the parent-into-F1 offspring or coisogenic rodent models described above.12,13 It should be mentioned that these models may be viewed with some reservation since the genetic differences between donor and recipient are not identical to clinical HCT. Nonetheless, certain basic findings are likely applicable. The rodent recipients are immunocompetent and thus more closely resemble nonmyeloablated than myeloablated HCT recipients. When high levels of donor chimerism are achieved, a syndrome resembling aGVHD develops and activated CD8+ T cells12,19 and Th1 cytokines predominate.19,20 In contrast, chronic disease arises when parental engraftment is low and the syndrome is dominated by CD4+ cells and a Th2 cytokine profile.20–,22 In this context, it is notable that studies using the coisogenic mouse model14 showed that cGVHD did not develop in CD4 knockout recipients, whereas CD8 knockout mice and wild-type mice developed severe cGVHD. These data further support the role for residual host immune cells, and CD4+ cells in particular, in exacerbating cGVHD.
Current Approach to Treatment of cGVHD
At present, most allogeneic HCT patients receive unmodified bone marrow or mobilized peripheral blood (MPB) grafts. Thus, the strategy to prevent and/or treat cGVHD entails pharmacologic immunosuppression initiated at the time of transplantation. Unlike solid organ transplantation, HCT can often result in immune tolerance to donor antigens, including hematopoietic specific antigens. Thus, the goal is to taper all allogeneic HCT patients completely off immunosuppressive drugs in the months following transplantation. However, if patients develop extensive cGVHD while on treatment or after taper of immunosuppression, the first-line therapy is high-dose prednisone or prednisone plus CSP. Initial studies demonstrated that early intervention with corticosteroids significantly improves patient survival23 since the survival probability at 3 years posttransplantation was 76% in patients who received early intervention as compared to 26% in patients treated late in their cGVHD course. Before the availability of CSP, a randomized prospective trial comparing prednisone alone versus prednisone plus azathioprine for patients with extensive cGVHD and platelets ≥ 100,000/mm3 (standard risk) showed better 5-year survival (61% versus 47%, respectively) and lower nonrelapse mortality for patients treated with prednisone alone. A recently published report24 compared prednisone alone versus prednisone plus CSP in similar standard-risk patients. The results showed that although prednisone plus CSP reduced the incidence of avascular necrosis as compared to prednisone alone, there was otherwise no difference between the two treatment groups in mortality, recurrent malignancy, need for secondary therapy, or discontinuation of immunosuppression. The impetus for the latter study was the observation that in patients with extensive cGVHD and platelets < 100,000/mm3 (poor risk) the combination of prednisone plus CSP resulted in superior survival of 51%25 as compared to 26% for prednisone alone.6
There is currently no standard salvage treatment for patients who fail their first-line cGVHD therapy. Thus, optimally patients should enter a clinical trial (see also last section). Phase I/II trials for patients with relapsed or refractory cGVHD have been performed. Such patients were included as part of the alternating CSP and prednisone study,25 and therefore it is reasonable to consider changing to this therapy if patients have failed steroids alone or another investigational therapy. Tacrolimus (FK506) has been used for steroid-resistant patients and achieves high concentrations in the liver, even greater than those of CSP. Thus, FK506 is a reasonable choice for patients with liver disease. Thalidomide showed efficacy in the primary treatment of patients with high-risk cGVHD as well as those with refractory disease.26,27 A randomized trial was subsequently performed in patients with high-risk cGVHD to compare thalidomide versus placebo, given in combination with either CSP or FK506 plus prednisone.28 Unfortunately, the side effects of thalidomide, which most frequently were neutropenia and neurologic symptoms, resulted in a large proportion of patients discontinuing treatment before the resolution of their cGVHD. Thus, while thalidomide has demonstrated activity against both high-risk and refractory cGVHD, its application is limited to patients who can tolerate the drug. MMF in combination with FK506 has demonstrated promise for patients with refractory cGVHD.2 Sirolimus (Rapamune), pentostatin, etretinate, and hydroxychloroquine sulfate (Plaquenil) are other pharmacologic agents that are currently being studied. The mechanism of action and details of the clinical trials regarding these drugs have been reviewed elsewhere.29– 31
cGVHD Following Nonmyeloablative HCT
Because the length of follow-up is relatively short, it is difficult to accurately assess the incidence and severity of cGVHD after nonmyeloablative HCT. However, studies from many centers32–,34 have reported that the incidence of extensive cGVHD is at least comparable or higher for patients who undergo nonmyeloablative regimens as compared to conventional myeloablative allografting. Several factors conspire to make nonmyeloablated patients particularly at risk for cGVHD. First, the population that receives these less toxic regimens is generally older. As discussed above, older age is more permissive for development of the syndrome and poor thymic function, which implies that impaired T-cell selection may partially explain the higher prevalence. A second factor is that MPB as compared with bone marrow is the preferred graft source. Studies comparing the outcome of myeloablated patients transplanted with MPB versus bone marrow demonstrated consistent trends toward higher cumulative incidences of cGVHD in recipients of MPB.35–,37 A recent detailed update of one of these studies35 that examined the incidence and severity of cGVHD in patients who received MPB versus bone marrow from HLA-identical siblings found that although the incidence of cGVHD at 3 years was similar in the two groups, cGVHD may be more difficult to control in recipients of MPB.38 The number of successive treatments needed to control cGVHD was higher, and the duration of glucocorticoid treatment was longer, after MPB transplantation than after bone marrow transplantation. The authors concluded that continued long-term assessment of the morbidity associated with cGVHD would be needed to compare the benefits of MPB with those of bone marrow transplantation. MPB is the preferred graft source for nonmyeloablative transplantations because it contains markedly higher numbers of hematopoietic stem and progenitor cells as well as T cells. Both of these cell populations are required to achieve durable and robust allogeneic hematopoietic cell engraftment. Graft content is particularly important for nonmyeloablative regimens because higher resistance to engraftment is encountered. As compared with myeloablated recipients, nonmyeloablated patients retain higher amounts of recipient hematopoietic and lymphoid elements, both of which contribute significantly to resistance to engraftment (see next section).
A third reason that cGVHD may become more prevalent in the nonmyeloablated population relates to the use of DLI. Since these regimens principally rely upon GVT activity to control malignancies, one of the first therapeutic maneuvers considered when a stably engrafted patient has evidence of disease persistence, progression, or relapse is DLI. A prior clinical study examining 140 patients who relapsed following allogeneic HCT showed that cGVHD developed in roughly 60% of patients who underwent DLI as salvage treatment.39 Perhaps of greater significance was the observation that cGVHD occurred in > 80% of DLI patients who responded to treatment.39
A fourth potential and as yet unexplored variable is the effect that residual host populations have on GVHD pathogenesis. As compared with conventional myeloablative regimens, nonmyeloablative conditioning spares more recipient immune cells. Cellular interactions that are likely to be affected by these differences include donor and surviving host T lymphocytes with APCs and lymphocyte regulatory circuits. Studies in mice suggest that such recipient populations drive the pathogenic response, as elimination of host APCs10 or host CD4+ cells14 abrogates the development of GVHD.
A final point regarding the unique aspects of cGVHD in the nonmyeloablative setting relates to the blurring of features that once delineated classical aGVHD from cGVHD. Traditionally, aGVHD is defined as GVHD occurring within the first 100 days following transplantation, with signs and symptoms commonly developing much earlier. Dermal changes typical of acute GVHD are characterized by a maculopapular exanthem or a diffuse erythroderma, while changes representative of chronic GVHD resemble either lichen planus, poikiloderma, morphea, or generalized scleroderma with atrophy, sclerodactyly, and joint contractures. Following nonmyeloablative transplantation, for example, it is not uncommon to observe dermal changes developing beyond day 100 that are in keeping with those described for aGVHD. The reasons for this blurring in the clinical spectrum of GVHD are not known. Perhaps the reduced toxicity of the preparative regimen and diminished cytokine release (cytokine storm) following nonmyeloablative transplantation alters donor and recipient immune responses. Conversion to full donor chimerism is usually delayed as compared with myeloablative transplants and thus residual host cells may alter the alloreactivity of the graft pending conversion to full donor chimerism.9 Furthermore, the immunosuppressive regimens used as prophylaxis often differ from the standard CSP plus methotrexate or CSP plus prednisone, and these more potent combinations may alter GVHD such that it arises primarily during or following the taper of immunosuppression. In our experience in a multicenter consortium using fludarabine and low-dose TBI (200 cGy) followed by postgrafting immunosuppression with CSP and MMF,34 we have extended the taper of immunosuppression in attempts to reduce the risk of GVHD. However, even with an extended taper we are seeing clinical evidence of aGVHD but in the cGVHD time frame. Thus, it is likely that the clinical manifestations of the GVHD syndromes observed following myeloablative allogeneic HCT will differ in patients receiving nonmyeloablative therapies as the pharmacologic regimens are adjusted, and the use of day +100 to delineate between aGVHD and cGVHD will become irrelevant.
Future Strategies to Reduce cGVHD while Preserving Other Essential Graft Activities
Eliminating the problem of cGVHD without altering the beneficial effects of allogeneic HCT remains a major challenge. Strategies to address this issue involve all of the steps that constitute an allogeneic HCT procedure, including altering the preparative regimens, engineering graft content, and changing the immunosuppressive therapies that are used for both prophylaxis and treatment of cGVHD once it has developed.
Alternative Preparative Regimens to Reduce cGVHD
How can the way patients are prepared for transplantation alter the incidence and severity of cGVHD? Most of the current nonmyeloablative protocols make use of doses of chemotherapy that are relatively well tolerated ± low-dose radiation delivered in the few days prior to or on the day of infusion of the hematopoietic graft. The goals of the preparative regimens are to achieve significant lymphoablation with minimal myeloablation. Alternative agents that can reduce both recipient and donor lymphocytes are antibody preparations. Unlike chemotherapy and radiation, antibodies persist in recipient blood and tissues for days to weeks following infusion. The humanized monoclonal antibody Campath-1H was used by a consortium of investigators from the United Kingdom40 as part of a nonmyeloablative regimen. Their data, derived from 46 of 47 patients who received MUD bone marrow grafts and 1 of 47 who received MUD MPB, demonstrated zero incidence of extensive cGVHD with a median follow-up of 344 days. Notably, 20 of 47 grafts were mismatched for HLA class I and/or class II alleles. Campath-1H binds to CD52, which is broadly expressed on thymocytes, T and B cells, monocytes, and granulocytes. The antibody has a prolonged half-life of 15 to 21 days,40 and thus its administration on days –8 to –4 likely results in in vivo depletion of donor T cells. Another possible mechanism of action hypothesized by the investigators is that Campath-1H may also deplete dendritic cells. While these data are very promising, it should be noted that these same investigators observed a very high percentage of CMV reactivation after nonmyeloablative transplantation using Campath-1H.41 They suggest that, while antibody treatment reduces GVHD, there may be profound effects on immune reconstitution, and they have therefore initiated a study of dose de-escalation with the Campath-1H reagent.
Our group at Stanford University developed a different regimen based upon the use of total lymphoid irradiation (TLI) plus rabbit ATG. TLI involves extensive, fractionated irradiation of lymphoid organs with shielding of hematopoietic tissues and is highly lymphoablative. Fractionation of the TLI treatments to ~80 cGy/dose and a minimum interval of 6 hours between fractions reduces the acute and late toxicities associated with this radiation regimen. ATG preparations are polyclonal antibody products derived from immunization of animals with human thymocytes. The rabbit ATG preparation used in our studies (Sangstat, Fremont, CA) results in downmodulation of multiple lymphocyte receptors, including adhesion molecules, and has been shown in primates to deplete peripheral blood, lymph node, and splenic lymphocytes.42 Preclinical rodent models43 demonstrated that the combination of TLI and antithymocyte serum (ATS) is nonmyeloablative and permits allogeneic hematopoietic cell engraftment with significant protective effect against GVHD. Figure 9 shows data from mouse studies comparing TBI ± ATS with TLI ± ATS. The figure demonstrates that (1) at a human equivalent dose of 80 cGy (4080 cGy for mice), TLI is nonmyeloablative, (2) TLI alone as compared with TBI alone demonstrates measurable protection against fatal aGVHD, and (3) the combination of TLI plus ATS gives 100% protection against aGVHD. In the TLI plus ATG group, all mice were donor blood chimeras. TLI but not TBI has been shown to result in increased levels of regulatory “natural suppressor” cells in the peripheral blood and spleen of TLI-treated animals.44 These regulatory cells coexpress both T and NK cell markers and secrete high levels of interleukin-4 (IL-4).43 The addition of ATS to the TLI treatment results in increased percentages of these NK-T cells. Thus, the mechanisms of GVHD protection are thought to be due to the lymphoablative effects of both the TLI and ATG, as well as the induction of regulatory cells.
As of this writing, 8 patients with advanced disease or poor-risk features have been transplanted with the TLI + ATG regimen. Patients received 10 fractions of 80 cGy each on days –11 through –7 and –4 through –1 (total 800 cGy) and rabbit ATG at a dose of 1.5 mg/kg each day on days –11 through –7 (total dose 7.5 mg/kg). Oral CSP was begun on day –3 and MMF on day 0. The MMF was stopped at day +28, and CSP was tapered by 6% per week beginning at ~day +60. The grafts were unmanipulated MPB from HLA-identical siblings or unrelated donors with a goal of infusing > 5 × 106 CD34+ cells/kg recipient weight. Six patients received MPB from HLA-identical sibling donors, and 2 patients were recipients of MUD MPB. All patients developed multilineage chimerism with the majority of blood and bone marrow elements derived from the donor by day +28. The median follow-up was 126 days, and no GVHD was observed. One patient developed CMV enteritis and is recovering.
It is important to once again note that although the primary mechanism of action of the antilymphocyte antibodies is thought to be via reduction of postthymic donor T cells, the animal data also suggest that reduction of residual host T cells and/or dendritic cells may be a second mechanism of action.
Graft Engineering
Technologies now exist that permit the generation of hematopoietic cell grafts with defined cell subsets. Ideally, such grafts should contain purified hematopoietic stem cells (HSCs) plus other cell populations that can facilitate engraftment, confer GVT without GVHD, and augment immune reconstitution.
HSCs are the only cells in a hematopoietic graft that can self-renew and give rise to all blood lineages, and thus are the only cells required to engraft to yield lifelong donor-derived hematopoiesis. The use of CD34 as a selecting marker and the availability of monoclonal antibodies that bind CD34 have allowed many centers to generate grafts that are enriched for stem and progenitor cells. However, such CD34-selected grafts contain significant numbers of T cells (~105 CD3+ cells/kg recipient weight) and other hematolymphoid elements. Methodologies have been developed to further purify HSCs. The human HSC is marked by CD34 as well as the Thy-1 surface molecule,45 and it does not express certain molecules that are found on more mature hematolymphoid populations.45 The T-cell content of these purified grafts is reduced by 6 to 7 logs. Thus, such grafts contain only ~10-100 CD3 cells/kg recipient weight. Purified CD34+Thy-1+ HSCs have already been utilized in clinical trials of autologous HCT for stage IV breast cancer,46 multiple myeloma,47 and NHL.48 The HSC products had either no detectable or significantly reduced levels of tumor contamination. Most important, these studies uniformly demonstrated that it was possible to purify adequate numbers of CD34+Thy-1+ HSCs from MPB that resulted in rapid and sustained engraftment in myeloablated autologous HSC recipients.46– 48
Transplantation of purified CD34+Thy-1+ HSCs has not been performed across allogeneic barriers in humans primarily because of concerns that such grafts will be rejected. From studies in mice and humans, it is known that purified HSCs49 or modified (T-cell depleted) allogeneic grafts8 are more strongly resisted compared to unmanipulated hematopoietic grafts. We have studied this problem in mice49 and determined that resistance to engraftment and graft failure can be overcome in most donor/recipient strain combinations by the infusion of very high doses of HSCs (~4 × 105 HSCs/kg) and/or conditioning of recipients with antibodies directed against specific immune cell subsets in addition to high-dose radiation. Resistance to allogeneic HSC engraftment can also be overcome by adding specific cell populations that facilitate HSC engraftment. An extensive phenotype analysis characterizing the HSC facilitating population was performed in mouse bone marrow50 and demonstrated that the CD8 molecule was the most consistent marker of HSC facilitating cells. However, within the CD8+ population, there was heterogeneity of morphology as well as expression of the α/β TCR, suggesting that conventional T cells and another CD8+ cell type confer facilitating activity. The 2 populations together were more effective at enhancing allogeneic HSC engraftment than either one alone. At the doses of facilitating cells administered, there was no evidence of GVHD. It will be important to determine if a homologous population exists within human bone marrow or MPB. One small study from Germany has reported graft failure following infusion of CD34-selected cells plus defined numbers of T cells (1 × 105 and 2 × 106 CD3 cells/kg) in patients prepared with nonmyeloablative conditioning.51 Until the lowest dose of T cells that will permit engraftment with the current protocols or the human facilitating population is defined, patients receiving nonmyeloablative regimens will continue to receive unmodified hematopoietic grafts.
The purpose of establishing blood chimerism for the majority of patients who receive nonmyeloablative regimens is to permit the lifelong presence of donor cells that provide GVT effects. In the setting of HSC or T-cell-depleted grafts, this activity is lost or diminished, as is protection for infectious agents. Augmentation of GVT activity and immune reconstitution without GVHD are also of central importance for conventional allografts. Thus, this topic is a major area of investigation, and it is beyond the scope of this chapter to review in detail the studies on GVT without GVHD. The reader is referred to Storb et al for more information34 on this topic. One common strategy is to identify the antigenic targets unique to tumor cells and obtain and expand T-cell populations that will exclusively engage and destroy cells that express the defined antigens. TCR specificity can be exploited by engineering cell populations to have enforced expression of receptors that recognize tumor-specific antigens, allowing the generation of large quantities of effector cells. Another promising approach comes from the observation that immune populations other than conventional T cells have potent antitumor properties. We have demonstrated in an allogeneic mouse model that an in vitro expanded lymphocyte population that expresses both NK and T-cell markers demonstrates potent antitumor activity in lymphoma-inoculated mice.52 Clinical studies in haploidentical transplantation demonstrated that in donor/recipient pairs wherein donor antihost NK alloreactivity could be predicted based upon the mismatched HLA alleles, impressive protection from leukemia relapse and graft rejection without GVHD were observed, presumably mediated by NK cells.53
A new area of investigation is the use of multipotent progenitors in augmenting immune reconstitution and possibly GVT. Subsequent to the isolation of mouse HSCs (Thy-1loLin-/loSca-1+c-Kit+), downstream progenitor cells were characterized by similar methodologies of monoclonal antibody labeling and separation by cell sorting.54,55 The common lymphoid progenitor (CLP) can be distinguished from HSCs by a number of surface markers (IL-7Rα+Thy-1.1−Sca-1loc-Kitlo) but primarily by expression of the IL-7α receptor.55 In contrast, the common myeloid progenitor (CMP), which is also identified by a composite phenotype (IL-7Rα−Lin−c-Kit+Sca-1−), is notably IL-7α receptor negative, Sca-1 negative, and Thy-1 negative.54 The branch points of myeloid differentiation were further refined by identification of the granulocyte/monocyte progenitor (GMP) and megakaryocyte/erythroid progenitor (MEP).54 When lethally irradiated mice were rescued with HSCs as compared with HSCs + CLP or HSCs + CMP, both CLP- and CMP-containing grafts resulted in more rapid repopulation in the lymphoid and myeloid lineages, respectively.54,55 The potential functional significance of these progenitor populations was demonstrated in mouse models of infectious diseases. Studies of mice infected with Aspergillus fumigatus and Pseudomonas aeruginosa, two of the most lethal pathogens associated with neutropenia, showed that as compared to HSCs alone cotransplantation of CMPs/GMPs with HSCs was protective.56 Furthermore, cotransplantation of CLPs with HSCs leads to significant protection of mice from lethal challenge with murine CMV as compared with HSCs alone.56 In allogeneic studies, mice cotransplanted with CLP did not develop clinical evidence of GVHD.57 The putative homologous human progenitor populations have been isolated58,59 but have not yet been tested in clinical trials.
Alternative Approaches to Treating cGVHD
Several pharmacologic agents are currently the subject of clinical studies aimed at the treatment of high-risk or refractory cGVHD and have been reviewed elsewhere.2,29–,31 Alternative approaches include monoclonal antibodies and fusion proteins, and photopheresis. Targets for antibody therapy are primarily T-cell-specific molecules, including those involved in TCR engagement and signaling, costimulatory molecules, markers of activation, and cytokines. The IL-2 receptor (IL-2R) has been a focus for antibody therapy because this molecule’s expression is upregulated on activated T cells and is critically involved in T-cell proliferation and activation by IL-2. Promising results have been reported for aGVHD using humanized antibodies directed against the IL-2R.60 Immunotoxins and fusion toxins directed at the IL-2R have been created to obtain better activity against activated T cells.61,62 Ontak is composed of the human IL-2 gene fused to a truncated active portion of the diphtheria toxin gene.61,62 Ontak is being evaluated in pilot studies of both aGVHD and cGVHD.30 Other potential biological agents include anti–tumor necrosis factor-alpha, CTLA-4, and anti-CD40-ligand monoclonal antibodies.
Extracorporeal photopheresis (ECP) is a form of immunotherapy that involves ex vivo exposure of leukapheresed peripheral blood to ultraviolet A (UVA) light in the presence of a photoactivated DNA-intercalating agent, 8-methoxypsoralen (8-MOP). The exposed cells are then returned to the recipient. ECP has resulted in clinical responses to steroid-refractory cGVHD.63 ECP has also been used to effectively treat cutaneous T cell lymphoma (CTCL)64 and to manage patients with scleroderma and solid organ transplants. The immunomodulatory effects of ECP in the treatment of CTCL are thought to be related to induction of apoptosis of circulating tumor cells.65 However, the mechanism of action in the treatment of autoimmune syndromes and GVHD are less clear. Studies in mice have shown that GVHD reactivity can be attenuated if the transferred hematopoietic cells are treated with 8-MOP and UVA,66 and that ECP may induce cell populations that protect against GVHD.67 Studies in humans suggest that ECP results in normalization of skewed CD4/CD8 ratios and in an increase in CD3−/CD56+ NK cells.68 In recent studies,65 the dendritic cell profiles of patients with cGVHD were noted to shift from a type 1 to type 2 dendritic cell predominance following ECP, resulting in a concordant shift from a predominant Th1 to Th2 cytokine profile. These types of observations suggest that cell-based therapy, while currently used primarily for the purpose of activation, may also be used to suppress immune responses in established cGVHD.
Summary
Chronic GVHD has been identified as a major problem associated with nonmyeloablative allogeneic HCT. Reducing this problem in the next generation of nonmyeloablative regimens will significantly diminish the transplant-related morbidity and mortality. Current efforts to prevent and treat cGVHD while still preserving the beneficial effects of allogeneic HCT involve a multidisciplinary approach aimed at the many steps involved in a transplant procedure, including recipient preparation, graft content with cotransplantation of immune cell populations, and GVHD prophylaxis and treatment.
Nonmyeloablative conditioning regimens for stem cell transplantation.
| . | . | . | . | . | . | GVHD . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Transplant Center . | No. Pts. Studied . | Diagnosis . | Conditioning Regimen . | Postgraft Immuno- suppression . | No. Pts. Achieving > 90% Donor Chimerism . | Acute Grade II-IV . | Chronic . | No. Pts. Achieving CR . | Outcome(s) (# or % of Patients) . |
| Abbreviations: CR, complete remission; A, ara-C; AML, acute myelogenous leukemia; ATG, antithymocyte globulin; B, busulfan; C, cisplatin; CLL, chronic lymphocytic leukemia; CSP, cyclosporine; CY, cyclophosphamide; DLI, donor lymphocyte infusion; F, fludarabine; GD, genetic diseases; HM, hematologic malignancies; I, idarubicin; L, lymphoma; M, melphalan; MDS, myelodysplastic syndrome; MP, methylprednisolone; NA, not available; MTX, methotrexate; ST, solid tumors; T, tacrolimus; TBI, total body irradiation; TI, thymic irradiation; 2-CDA, 2-chlorodeoxyadenosine. | |||||||||
| M.D. Anderson26 | 15 | AML/MDS | F+I+A | CSP+MP | 6 | 3 | 0 | 8 | Overall survival: 6 |
| F+I+M | Disease-free survival: 2 | ||||||||
| 2-CDA+A | Median 100 days | ||||||||
| M.D. Anderson29 | 15 | CLL/NHL | F+CY | T±MTX | 8 | 4 | 2 | 8 | Overall survival: 7 |
| F+C+A | 3 after DLI | Median 180 days | |||||||
| M.D. Anderson28 | 86 | HM | F+M | ||||||
| 2- CDA+ M | T+ MTX | 40 of 42 in CR | 34 | 21 | 49 | Overall 2- yr survival: 28% | |||
| 2- yr Disease- free survival: 23% | |||||||||
| 2- yr Non- relapse mortality: 45% | |||||||||
| Jerusalem30 | 26 | HM/GD | F+B+ATG | CSP | 7 | 10 | 9 | NA | Overall survival: 22 |
| 2 after DLI | 2 after DLI | Disease-free survival: 21 | |||||||
| Median 240 days | |||||||||
| Jerusalem31 | 23 | L | F+B+ATG | CSP | 16 | 8 | 4 | NA | Disease-free survival: 10 |
| 2 after DLI | Median 675 days | ||||||||
| NIH32 | 15 | HM/ST | F+CY | CSP | 7 | 10 | 4 | 5 | Overall survival: 8 |
| 3 after stopping CSP | 1 after DLI | 121-409 days posttransplant | |||||||
| 3 after DLI | |||||||||
| Boston33 | 21 | HM | CY+ATG±TI | CSP | NA | 12 | NA | 8 | Overall survival: 11 |
| 7 after DLI | 6 after DLI | Disease-free survival: 7 | |||||||
| Median 445 days | |||||||||
| Seattle36 | 192 | HM | 2 Gy TBI±F | MMF+CSP | NA | 88 | NA | NA | Overall survival: 114 |
| Nonrelapse mortality: 3 | |||||||||
| Relapse mortality: 35 | |||||||||
| Median 289 days | |||||||||
| . | . | . | . | . | . | GVHD . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Transplant Center . | No. Pts. Studied . | Diagnosis . | Conditioning Regimen . | Postgraft Immuno- suppression . | No. Pts. Achieving > 90% Donor Chimerism . | Acute Grade II-IV . | Chronic . | No. Pts. Achieving CR . | Outcome(s) (# or % of Patients) . |
| Abbreviations: CR, complete remission; A, ara-C; AML, acute myelogenous leukemia; ATG, antithymocyte globulin; B, busulfan; C, cisplatin; CLL, chronic lymphocytic leukemia; CSP, cyclosporine; CY, cyclophosphamide; DLI, donor lymphocyte infusion; F, fludarabine; GD, genetic diseases; HM, hematologic malignancies; I, idarubicin; L, lymphoma; M, melphalan; MDS, myelodysplastic syndrome; MP, methylprednisolone; NA, not available; MTX, methotrexate; ST, solid tumors; T, tacrolimus; TBI, total body irradiation; TI, thymic irradiation; 2-CDA, 2-chlorodeoxyadenosine. | |||||||||
| M.D. Anderson26 | 15 | AML/MDS | F+I+A | CSP+MP | 6 | 3 | 0 | 8 | Overall survival: 6 |
| F+I+M | Disease-free survival: 2 | ||||||||
| 2-CDA+A | Median 100 days | ||||||||
| M.D. Anderson29 | 15 | CLL/NHL | F+CY | T±MTX | 8 | 4 | 2 | 8 | Overall survival: 7 |
| F+C+A | 3 after DLI | Median 180 days | |||||||
| M.D. Anderson28 | 86 | HM | F+M | ||||||
| 2- CDA+ M | T+ MTX | 40 of 42 in CR | 34 | 21 | 49 | Overall 2- yr survival: 28% | |||
| 2- yr Disease- free survival: 23% | |||||||||
| 2- yr Non- relapse mortality: 45% | |||||||||
| Jerusalem30 | 26 | HM/GD | F+B+ATG | CSP | 7 | 10 | 9 | NA | Overall survival: 22 |
| 2 after DLI | 2 after DLI | Disease-free survival: 21 | |||||||
| Median 240 days | |||||||||
| Jerusalem31 | 23 | L | F+B+ATG | CSP | 16 | 8 | 4 | NA | Disease-free survival: 10 |
| 2 after DLI | Median 675 days | ||||||||
| NIH32 | 15 | HM/ST | F+CY | CSP | 7 | 10 | 4 | 5 | Overall survival: 8 |
| 3 after stopping CSP | 1 after DLI | 121-409 days posttransplant | |||||||
| 3 after DLI | |||||||||
| Boston33 | 21 | HM | CY+ATG±TI | CSP | NA | 12 | NA | 8 | Overall survival: 11 |
| 7 after DLI | 6 after DLI | Disease-free survival: 7 | |||||||
| Median 445 days | |||||||||
| Seattle36 | 192 | HM | 2 Gy TBI±F | MMF+CSP | NA | 88 | NA | NA | Overall survival: 114 |
| Nonrelapse mortality: 3 | |||||||||
| Relapse mortality: 35 | |||||||||
| Median 289 days | |||||||||
Reported regimens containing alemtuzumab.
| Conditioning Regimen . | Reference . |
|---|---|
| Abbreviations: BEAM, BCNU, etoposide, Ara-C, melphalan; TBI, total body irradiation; ATG, antithymocyte globulin. | |
| Alemtuzumab 100 mg + fludarabine 150 mg/m2 + melphalan 140 mg/m2 | 5 |
| Alemtuzumab 100 mg + fludarabine 150 mg/m2 + busulfan 8 mg/kg | 6 |
| Campath-1G 50 mg + BEAM | 7 |
| Alemtuzumab 100 mg + 2 Gy TBI | 8 |
| Fludarabine 180 mg/m2 + ATG 40 mg/kg + busulfan 6.4 mg/kg + in vitro T-cell depletion with alemtuzumab 20 mg | 9 |
| TBI 450 cGy + fludarabine 120 mg/m2 + alemtuzumab 40 mg | 10 |
| Alemtuzumab 100 mg + fludarabine 120 mg/m2 + cyclophosphamide 2 g/m2 + in vitro T-cell depletion with alemtuzumab | 11 |
| Conditioning Regimen . | Reference . |
|---|---|
| Abbreviations: BEAM, BCNU, etoposide, Ara-C, melphalan; TBI, total body irradiation; ATG, antithymocyte globulin. | |
| Alemtuzumab 100 mg + fludarabine 150 mg/m2 + melphalan 140 mg/m2 | 5 |
| Alemtuzumab 100 mg + fludarabine 150 mg/m2 + busulfan 8 mg/kg | 6 |
| Campath-1G 50 mg + BEAM | 7 |
| Alemtuzumab 100 mg + 2 Gy TBI | 8 |
| Fludarabine 180 mg/m2 + ATG 40 mg/kg + busulfan 6.4 mg/kg + in vitro T-cell depletion with alemtuzumab 20 mg | 9 |
| TBI 450 cGy + fludarabine 120 mg/m2 + alemtuzumab 40 mg | 10 |
| Alemtuzumab 100 mg + fludarabine 120 mg/m2 + cyclophosphamide 2 g/m2 + in vitro T-cell depletion with alemtuzumab | 11 |
Risk factors for development of chronic graft-versus-host disease (GVHD).
| • Prior acute graft-versus-host disease |
| • Older donor/recipient age |
| • HLA mismatch |
| • Unrelated donor |
| • Donor lymphocyte infusion |
| • Latent herpesvirus infection in donor/recipient |
| • Shortened duration of cyclosporine |
| • Prior acute graft-versus-host disease |
| • Older donor/recipient age |
| • HLA mismatch |
| • Unrelated donor |
| • Donor lymphocyte infusion |
| • Latent herpesvirus infection in donor/recipient |
| • Shortened duration of cyclosporine |
Clinicopathological classification of chronic graft-versus-host disease (GVHD).
| Reprinted with permission from Sullivan KM. Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, ed. Hematopoietic Cell Transplantation (ed 2nd). Malden, MA: Blackwell Science, Inc.; 1999:515-536. |
| Limited Chronic GVHD |
| Either or both: |
| 1. Localized skin involvement |
| 2. Hepatic dysfunction due to chronic GVHD |
| Extensive Chronic GVHD |
| Either: |
| 1. Generalized skin involvement, or |
| 2. Localized skin involvement and/or hepatic dysfunction due to chronic GVHD |
| Plus: |
| 3a. Liver histology showing chronic aggressive hepatitis, bridging necrosis, or cirrhosis, or |
| 3b. Involvement of eye (Schirmer test with < 5 mm wetting), or |
| 3c. Involvement of minor salivary glands or oral mucosa demonstrated on labial biopsy, or |
| 3d. Involvement of any other target organ |
| Reprinted with permission from Sullivan KM. Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, ed. Hematopoietic Cell Transplantation (ed 2nd). Malden, MA: Blackwell Science, Inc.; 1999:515-536. |
| Limited Chronic GVHD |
| Either or both: |
| 1. Localized skin involvement |
| 2. Hepatic dysfunction due to chronic GVHD |
| Extensive Chronic GVHD |
| Either: |
| 1. Generalized skin involvement, or |
| 2. Localized skin involvement and/or hepatic dysfunction due to chronic GVHD |
| Plus: |
| 3a. Liver histology showing chronic aggressive hepatitis, bridging necrosis, or cirrhosis, or |
| 3b. Involvement of eye (Schirmer test with < 5 mm wetting), or |
| 3c. Involvement of minor salivary glands or oral mucosa demonstrated on labial biopsy, or |
| 3d. Involvement of any other target organ |
HLA-matched related hematopoietic stem cell transplantation (HSCT).
Abbreviations: TBI, total body irradiation; CSP, cyclosporine, MMF, mycophenolate mofetil.
HLA-matched related hematopoietic stem cell transplantation (HSCT).
Abbreviations: TBI, total body irradiation; CSP, cyclosporine, MMF, mycophenolate mofetil.
HLA-matched unrelated hematopoietic stem cell transplantation (HSCT).
Abbreviations: CSP, cyclosporine; TBI, total body irradiation; mmf, mycophenolate mofetil
HLA-matched unrelated hematopoietic stem cell transplantation (HSCT).
Abbreviations: CSP, cyclosporine; TBI, total body irradiation; mmf, mycophenolate mofetil
Tandem transplant treatment timeline.
Abbreviations: PBSC, peripheral blood stem cell; PBSCT, PBSC transplantation; G-CSF, granulocyte colony stimulating factor; TBI, total body irradiation; MMF, mycophenolate mofetil; CSP, cyclosporine.
Tandem transplant treatment timeline.
Abbreviations: PBSC, peripheral blood stem cell; PBSCT, PBSC transplantation; G-CSF, granulocyte colony stimulating factor; TBI, total body irradiation; MMF, mycophenolate mofetil; CSP, cyclosporine.
Nonmyeloablative allogeneic transplant schema.
Abbreviations: TBI, total body irradiation; HSCT, hematopoietic stem cell transplantation; G-CSF, granulocyte colony stimulating factor; CSP, cyclosporine; MMF, mycophenolate mofetil.
Nonmyeloablative allogeneic transplant schema.
Abbreviations: TBI, total body irradiation; HSCT, hematopoietic stem cell transplantation; G-CSF, granulocyte colony stimulating factor; CSP, cyclosporine; MMF, mycophenolate mofetil.
Hematologic changes following autologous and allogeneic transplantation (n= 40).
Hematologic changes following autologous and allogeneic transplantation (n= 40).
Mean serum alemtuzumab levels in 10 patients who received 20 mg/day intravenously on days –8 to –4.
Mean serum alemtuzumab levels in 10 patients who received 20 mg/day intravenously on days –8 to –4.
Lineage-specific chimerism analyses from 3 separate patients following reduced-intensity transplantation using microsatellite polymerase chain reaction (PCR).
Three separate patterns of chimerism are shown. The left panel shows full donor chimerism following transplant, the center panel indicates mixed chimerism in all lineages, and the right panel shows mixed chimeras in the T-cell lineage alone.Reprinted with permission from Kottaridis P, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyelo-ablative stem cell transplantation. Blood. 2000;96:2419-2425.
Lineage-specific chimerism analyses from 3 separate patients following reduced-intensity transplantation using microsatellite polymerase chain reaction (PCR).
Three separate patterns of chimerism are shown. The left panel shows full donor chimerism following transplant, the center panel indicates mixed chimerism in all lineages, and the right panel shows mixed chimeras in the T-cell lineage alone.Reprinted with permission from Kottaridis P, Milligan DW, Chopra R, et al. In vivo CAMPATH-1H prevents graft-versus-host disease following nonmyelo-ablative stem cell transplantation. Blood. 2000;96:2419-2425.
Features of clinical extensive chronic graft-versus-host disease in patients transplanted before 1980 (open columns,n= 47) and from 1980 to 1999 (shaded columns,n= 145). Data are from a single transplant center.
Reprinted with permission from Sullivan KM. Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, ed. Hematopoietic Cell Transplantation (ed 2nd). Malden, MA: Blackwell Science, Inc.; 1999:515-536.
Features of clinical extensive chronic graft-versus-host disease in patients transplanted before 1980 (open columns,n= 47) and from 1980 to 1999 (shaded columns,n= 145). Data are from a single transplant center.
Reprinted with permission from Sullivan KM. Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, ed. Hematopoietic Cell Transplantation (ed 2nd). Malden, MA: Blackwell Science, Inc.; 1999:515-536.
Nonmyeloablative conditioning with total lymphoid irradiation (TLI) and antithymocyte serum (ATS) protects mice against lethal graft-versus-host disease (GVHD).
The number of mice in each group was 10.
(A) Survival of mice conditioned with lethal total body irradiation (TBI) or TBI plus ATS and engrafted with allogeneic bone marrow (BM) or BM plus peripheral blood mononuclear cells (PBMC). TBI alone was lethal, and grafts containing PBMC resulted in death due to GVHD.
(B) Survival of mice conditioned with 4080 cGy of TLI and engrafted with BM or BM plus PBMC. TLI was nonmyeloablative, and TLI-conditioned mice showed some protection from lethal GVHD.
(C) Survival of mice conditioned with TLI plus ATS. All mice survived the conditioning and were protected from lethal GVHD.
Reprinted with permission from Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J Immunol. 2001;167:2087-2096. Copyright 2001. The American Association of Immunologists, Inc.
Nonmyeloablative conditioning with total lymphoid irradiation (TLI) and antithymocyte serum (ATS) protects mice against lethal graft-versus-host disease (GVHD).
The number of mice in each group was 10.
(A) Survival of mice conditioned with lethal total body irradiation (TBI) or TBI plus ATS and engrafted with allogeneic bone marrow (BM) or BM plus peripheral blood mononuclear cells (PBMC). TBI alone was lethal, and grafts containing PBMC resulted in death due to GVHD.
(B) Survival of mice conditioned with 4080 cGy of TLI and engrafted with BM or BM plus PBMC. TLI was nonmyeloablative, and TLI-conditioned mice showed some protection from lethal GVHD.
(C) Survival of mice conditioned with TLI plus ATS. All mice survived the conditioning and were protected from lethal GVHD.
Reprinted with permission from Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J Immunol. 2001;167:2087-2096. Copyright 2001. The American Association of Immunologists, Inc.
This work was supported by NIH grants CA78902, CA18029, and HL36444.
Dr. Mackinnon has received research support from Schering AG and Amgen.