Abstract
Atypical cellular disorders are commonly considered part of the gray zone linking oncology to hematology and immunology. Although these disorders are relatively uncommon, they often represent significant clinical problems, provide an opportunity to understand basic disease mechanisms, and serve as model systems for the development of novel targeted therapies. This chapter focuses on such disorders.
In Section I, Dr. Arceci discusses the pathogenesis of Langerhans cell histiocytosis (LCH) in terms of the hypothesis that this disorder represents an atypical myeloproliferative syndrome. The clinical manifestations and treatment of LCH in children and adults is discussed along with possible future therapeutic approaches based upon biological considerations.
In Section II, Dr. Longley considers the molecular changes in the c-Kit receptor that form the basis of mastocytosis. Based on the location and function of c-Kit mutations, he develops a paradigm for the development of specific, targeted therapies.
In Section III, Dr. Emanuel provides a review of the “mixed myeloproliferative and myelodysplastic disorders,” including novel therapeutic approaches based on aberrant pathogenetic mechanisms. Taken together, these chapters should provide an overview of the biological basis for these disorders, their clinical manifestations, and new therapeutic approaches
I. Langerhans Cell Histiocytosis in Children and Adults: Pathogenesis, Clinical Manifestations, and Treatment
Robert J. Arceci, MD, PhD*
Div. of Pediatric Oncology, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Bunting-Blaustein Cancer Research Bldg., 1650 Orleans Street, Room 2M51, Baltimore, MD 21231-1000
Learning From History: Nomenclature and Classification
George Santayana instructed us that “Those who do not learn from history are doomed to repeat it.” The history of investigative work and treatment of Langerhans cell histiocytosis (LCH) has both ignored and heeded such advice. This paper will discuss our current understanding of LCH as well as the historical roots from which that understanding derives.
Our understanding of the diverse group of disorders generically termed the histiocytoses is closely linked to biological insights about the cells that compose the reticuloendothelial or mononuclear phagocytic system.1 When Metchnikov described the cellular reaction to a rose thorn inserted into a larval starfish in the late 1800s, the first stones in the foundation of a system that would eventually and ironically be referred to as the Tower of Babel were established.2 In the early 1920s, Aschoff introduced the term “reticuloendothelial system (RES),” with “reticulo” referring to the characteristic of cells composing this cellular compartment to form a “lattice or reticulum by cytoplasmic extensions,” and “endothelial” referring to the fact that these cells often are situated near vascular endothelial cells.1,3,4
During this period, the macrophage (“macro” = large and “phage” = to eat) and its ability to ingest and digest large foreign particles, including invading microorganisms as well as cellular debris, took on the central role in this system. Macrophages could thus be easily distinguished from “microphagocytic” polymorphonuclear granulocytes (PMN). Additional experimentation provided evidence that macrophages are also critical stimulators of immune responses through the processing and presentation of antigens to T lymphocytes in the context of the major histocompatibility complex (MHC).
The description of the epidermal “dendritic” cell by Paul Langerhans, in 1868, resulted in the addition of yet another group of cells to the RES or MPS (mononuclear phagocytic system).5 Although Langerhans initially believed this cell to be part of the nervous system because of the dendritic-like processes observed following the administration of gold chloride, he subsequently changed his view, arguing that the “Langerhans cell” was more likely to be of hematopoietic origin.5,6 An understanding of the functional role for dendritic cells and, in particular, Langerhans cells would need to wait until the mid-1900s, when the potent ability of Langerhans cells to process and present antigens (especially viral, cancer-associated, and self-antigens) to T lymphocytes and thus profoundly activate immune responses was delineated.7,8
In addition to the overlapping functional characteristics of macrophages and dendritic cells, including Langerhans cells, they have also been shown to share subsets of cytoplasmic and surface differentiation antigens plus a common hematopoietic cellular origin. Finally, the diseases in which macrophages and dendritic cells are involved may also share both clinical and pathophysiologic characteristics.
Thus, the nomenclature describing the histiocytoses is closely linked to the evolving discoveries concerning the biology of the cells composing the reticuloendothelial or mononuclear phagocytic system. The generic term “histiocyte” derives from the Latin “histion,” meaning “little web,” and “kytos,” meaning “cell.” Thus, histiocytes are resident tissue (i.e., the web) mononuclear phagocytes.1
Based on the biological differences between macrophages and dendritic cells, the general category of “histiocytosis” was gradually subdivided into groups of diseases according to which cell was believed to be the critical cell responsible for specific diseases. In 1987, the Histiocytosis Society reclassified the histiocytoses into 3 major classes.9 Class I, termed LCH, included diseases that had been referred to historically as eosinophilic granuloma, Hand-Schüller-Christian disease, and Letterer-Siwe disease. Class II was termed non-LCH, with the major disorders being infection and inherited forms of hemophagocytic lymphohistiocytoses (HLH). Class III, termed malignant histiocytosis, included disorders such as monocytic leukemia, true histiocytic lymphoma, and the very rare malignant tumors of dendritic cells.
As additional information has become available, other classification schemas have been published that further delineate the diseases of the mononuclear phagocytic system. For example, one schema includes (1) histologically nonmalignant proliferative disorders that are related to dendritic cells (LCH, juvenile xanthogranuloma, dendritic cell histiocytomas); and (2) T-lymphocyte/macrophage activation disorders that are associated with immune deficiency, infection, and malignancies and include such disorders as primary or familial hemophagocytic lymphohistiocytosis, infection-associated hemophagocytic syndrome, and sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Malignant disorders of the mononuclear phagocytic system include acute monocytic leukemia and malignant dendritic cell or macrophage-derived neoplasms.10
Classification schemas, while often short-lived, do have significant importance for patients with these disorders because of differences in the clinical course and types of treatment that are effective. Thus, a definitive diagnosis should be made before initiating discussions with patients and families as well as prior to initiating any treatment. This paper will focus on LCH.
What Type of Disease Is LCH? Clues from Epidemiology and Biology
While the clinical manifestations and course of LCH may differ widely, a definitive diagnosis can only be made on a biopsy specimen, which must demonstrate characteristic histological and immunohistochemical features such as mixed cellular infiltrates with accumulations of CD1+ Langerhans cells. The pathologic findings include the usual preponderance of Langerhans cells that are more rounded than their normal counterparts but have a characteristic reniform nucleus. There are also other immunoreactive cell types in such lesions, including eosinophils (giving rise to the term eosinophilic granuloma), neutrophils, macrophages, and lymphocytes. But such a description does not do justice to the enigmatic nature and, thus far, unproven etiology of LCH.
The annual incidence of LCH has been reported to be between 3 to 7 cases per million people.11–,13 Males may be more frequently diagnosed than females. Although most cases of LCH have been reported in children, it is evident that this disease can occur at any age.14 There have been no significant associations of LCH in terms of seasonal variation or geographic or racial clustering, leading to the conclusion that LCH is not caused by an infectious etiology. However, several epidemiological studies have suggested several interesting clinical associations in patients with LCH.15 One case control study revealed a significant odds ratio for postnatal infections, diarrhea, and vomiting, as well as for medication usage in patients with multisystem LCH. Thyroid disease in the patient or in the family of patients was associated with single-system LCH.16 Through retrospective reviews of the literature, a higher association than would be expected by chance has been observed for LCH with various malignancies.17,18 For example, LCH has been observed in association with acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), myelodysplastic syndrome, Hodgkin’s disease, non-Hodgkin’s lymphoma, and a variety of solid tumors. When LCH occurs in patients with leukemia, it is usually observed following treatment for ALL, particularly T-lineage leukemia, while AML has more frequently been reported following treatment for LCH.17,18 These observations have led to the proposal that patients with LCH may have a predisposition for developing both LCH and various malignancies.
Other observations have identified several sets of identical twins who have had LCH.19 These cases usually present when the twins are infants and there is a close concordance of the onset of LCH between the twins. Several examples of fraternal twins have also been identified, but the disease usually occurs at an older age and there is much less concordance of the time of onset of LCH in the twins. The occurrence of LCH in parents and their children as well as among cousins or other relatives has also been observed. The estimated frequency of familial LCH is less than 2% of all cases, although the percentage is not based on a large number of cases.20,21 While LCH in identical twins may be due to transplacental transfer of the disease in utero, similar to that observed in congenital leukemia, an alternative explanation is that there is a common environmental infectious or toxic exposure. The development of LCH in fraternal twins or in other different family members suggests that a common genetic, possibly inherited, predisposition might be responsible, although such observations do not rule out a common environmental factor. Using the model of Knudsen’s hypothesis or “two-hit” model for the development of retinoblastoma, the development of LCH at a very young age would suggest that the individual could have possibly inherited a mutant causative gene and then acquired an inactivation of the other allele. Mutations of both alleles of such a predisposing gene would be acquired in older individuals developing LCH.22
Such data have led investigators to examine the Langerhans cell in LCH more closely, with the intent of determining whether there was evidence that it represented an activated normal Langerhans cell, consistent with the concept of LCH being a reactive disorder, or whether the LCH lesional cell was actually different from its normal counterparts, i.e., an abnormal Langerhans cell. Several approaches have been used to further investigate these possibilities.
Flow cytometry studies of cells from LCH infiltrates or lesions have consistently demonstrated a diploid DNA content.23 However, using methods to assess clonality based on X chromosome inactivation, several reports have shown that the CD1a+ LCH cells from pathologic lesions are clonal, i.e. derived from a common progenitor.24–,27 This observation has held true for patients with isolated eosinophilic granuloma, multifocal bone disease, isolated skin or nodal involvement or in the multisystem form of the LCH. There has been much debate as to whether these results prove that LCH is a malignancy. Clearly, clonality is not sufficient to make the diagnosis of cancer. There are a number of examples, such as dermatological disorders which are clonal but are not considered cancerous.28,29 Furthermore, LCH does not show the histological characteristics of cancer. Nevertheless, these data concerning clonality have suggested the possibility that the lesional LCH cell could have acquired somatic mutations in a gene or genes that regulate cell growth, survival, or proliferation. Unfortunately, traditional cytogenetics have not demonstrated a consistently abnormal karyotype, although one intriguing study reported a t(7;12) translocation from a lesion in a patient with LCH.30 This observation is particularly intriguing in that the Tel gene, originally identified to be involved in a child with chronic myelomonocytic leukemia (CMML), is located in the same region of chromosome 12.31 It remains to be seen whether this observation is made in other cases of LCH or whether specific genes, such as tel, show mutations. More refined approaches to chromosomal analysis using fluorescence in situ hybridization (FISH) have also been used to examine LCH but have not yet detected any consistent chromosomal alterations.32 However, using comparative genomic hybridization (CGH) and molecular methods, a recent study has reported several chromosomal regions that show loss of heterozygosity.33 These data strongly suggest that there is a component of genetic instability in LCH, as observed in some types of neoplasms and myeloproliferative and/or myelodysplastic disorders.
Despite the lack of consistent evidence for genetic alterations in LCH cells, the lesional Langerhans cell demonstrates several phenotypic changes that appear to distinguish it from its normal counterparts. For example, the pattern of staining by the lectin, peanut agglutinin (PNA), is distinct in LCH lesional cells, which demonstrate strong cell surface and perinuclear staining, compared to normal Langerhans cells, which show a low level of diffuse staining.34 Of interest, the lesional LCH cell PNA pattern is similar to that observed in the pathologic Reed-Sternberg cell of Hodgkin’s disease.35 LCH lesional cells also constitutively express very high levels of placental alkaline phosphatase (PLAP) compared to normal Langerhans cells, which transiently induce PLAP expression following activation.34 There is also evidence that the γ-interferon receptor is strongly expressed on LCH lesional cells but is not constitutively expressed on normal Langerhans cells.34 Similarly, LCH cells constitutively express costimulatory receptors such as CD86 and CD80.34,36,37 Relatively high levels of p53 nuclear antigen detection have also been reported, a characteristic commonly observed in tumor cells with p53 mutations or in cells responding to certain genotoxic exposures. However, no mutations have been reported in p53 from LCH lesional cells,38 and the suggestion has been made that the upregulation of wild-type p53 in LCH may be secondary to free oxygen radicals generated in response to increased levels of tumor growth factor (TGF)-beta.39
The antigen expression phenotype of the LCH lesional dendritic cells is thus characteristic in many respects of a constitutively activated Langerhans cell with some aberrant features. When the function of the LCH lesional cells was assessed in terms of antigen presentation and activation of T cells, several investigators reported the surprising results that LCH cells purified from lesions were extremely poor stimulators of T cells.40 Normal Langerhans cells similarly isolated showed potent T-lymphocyte activation capability.34,40 Furthermore, under the stimulation of CD40 ligand, lesional Langerhans cells have been shown to be able to mature in vitro and acquire potent immunostimulatory characteristics.37
The concept of cytokine release by LCH cells as well as the cells recruited into lesions is another important component of this disease. In situ hybridization and immunocytochemical staining methods have been employed. The results show extensive expression of cytokines at high levels in LCH lesions that would be expected to result from or in the activation of T lymphocytes as well as the recruitment of macrophages, eosinophils, and granulocytes.41,42 The accumulation of interleukin (IL)-1 and prostaglandin E2 along with their action on osteoclasts may in part explain the propensity of these lesions to result in significant bone loss.43 Patients with LCH show increased production of immunostimulatory and tissue-damaging cytokines at local sites; it is uncommon for them to have high systemic levels, in contrast to patients with macrophage activation syndromes. The expression of these various cytokines can be used to explain some of the pathological and clinical features of LCH.
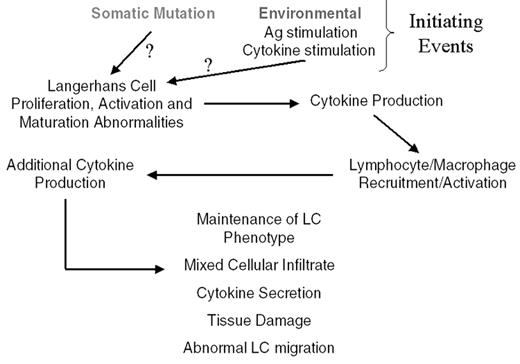
From epidemiologic, genetic, pathological, and clinical data, LCH can be considered to be a “clonal proliferative neoplasm with variable clinical manifestations.”1,44 The multitude of reported alterations of immune function in patients with LCH is more likely a manifestation of the extent to which abnormal Langerhans cells affect immune regulatory pathways, rather than a primary immunodeficiency. A schema is presented in Figure 1 to summarize the possible etiologies and pathophysiology of LCH.
Clinical Presentations of LCH: It’s Not Just for Kids Anymore!
The clinical manifestations of LCH vary considerably and can involve nearly every organ of the body. The historic eponyms of eosinophilic granuloma (usually unifocal LCH), Hand-Schüller-Christian disease (skull lesions, exophthalmos, and diabetes insipidus, or, more commonly, multifocal LCH), and Letterer-Siwe disease (systemic LCH) are all examples of the clinical spectrum of LCH but not specific disease entities.
Localized LCH
Eosinophilic granuloma usually presents as a solitary lesion of bone associated with pain and swelling, characteristically but not exclusively in older children and adults. Presentation is usually that of persistent pain and sometimes swelling. Hematologic manifestations may be a mild leukocytosis and an elevated sedimentation rate. No significant alterations in biochemical parameters are observed. While the calvarium is most commonly affected, other sites include the mandible, long bones, ribs, scapulae, and vertebrae. It is rare to see involvement of the small bones of the hands and feet. Lesions appear as “punched out” holes and sometimes have sclerotic edges, as observed by radiographs. Vertebrae plana often presents with back pain, and x-ray examination shows a collapsed vertebral body.
Patients may also present with localized disease of the skin, manifested by a variety of clinical presentations. The rash may be maculopapular and diffuse, more nodular and eruptive, or even quite erosive when longstanding in the axillary and inguinal regions. Patients commonly present with a “diaper rash” that is refractory to usual treatments. Extensive and persistent skin rash may occur at any age, with adult patients presenting with months to years of severe involvement of scalp, groin, perianal, scrotal, or vaginal areas. Such severely involved areas of skin are potential sites for serious superinfection.
Multifocal and multisystem LCH
Although the clinical triad of skull lesions, diabetes insipidus, and exophthalmos is classically defined Hand-Schüller-Christian disease and has been considered to occur only in young children, these signs do not always occur together, and adult patients may present with indistinguishable features. However, multifocal bone involvement and eczematoid skin rash, usually involving the scalp, axilla, and groin, are characteristic, although not exclusive, of LCH in the young child. There is commonly involvement of the oral cavity and lymph nodes and less commonly the lungs, liver, and brain. The acute changes observed in the lung include the development of micronodular infiltrative disease, bullous formation, and pneumothorax. Acute liver involvement includes elevated transaminases and increased bilirubin and, rarely, sclerosing cholangitis. The most common type of brain involvement is diabetes insipidus, which may occur before any symptoms or signs of LCH appear as well as during or after treatment. The incidence of diabetes insipidus has been reported to be between 5% to 30%, with patients at highest risk being those with extensive cranial bone involvement.45
The eponymous Letterer-Siwe disease classically refers to the infant with diffuse rash, gum disease, hepatosplenomegaly, bone lesions, and not infrequently pancytopenia due to splenic sequestration and bone marrow infiltration. Pulmonary insufficiency due to LCH can develop rapidly and be life-threatening. Patients may also present with failure to thrive and with diarrhea secondary to gastrointestinal involvement. While more common in infants, disseminated LCH can occur at any age. Progression from disease that has limited involvement to severe, systemic disease is rare.
Short and Long-Term Adverse Sequelae
There is a growing realization that patients with LCH may suffer from multiple recurrences of their disease over many years and probably for life. In addition, patients, particularly, but not exclusively, those with multifocal and relapsing disease, appear to have significant long-term sequelae of their disease and/or treatment. In this group of patients, over half will have significant late effects. These sequelae include diabetes insipidus and other hypothalamic/pituitary axis deficiencies leading to stunted growth and failure to achieve sexual maturity. Patients may also have significant neurocognitive and psychological problems as well as neurological complications, particularly in those patients who develop the neurodegenerative pattern of central nervous system (CNS) involvement.46 Other late effects include orthopedic problems, hearing loss, and dental abnormalities. Patients who develop destructive lung disease or hepatic fibrosis (sclerosing cholangitis) may progress such that organ transplantation is required. Patients with LCH may have a lifelong increased risk of pulmonary disease associated with cigarette smoking. The development of secondary and treatment-related malignancies has also been reported in patients with LCH. Long-term follow-up by a multidisciplinary team of caretakers with knowledge of LCH is critical for all patients with LCH.
Diagnostic and Staging Work-Up
A definitive diagnosis of LCH is currently made only with a diagnostic biopsy. Patients should also be evaluated for the extent of disease, which correlates with outcome and helps to direct treatment strategy. Following diagnosis, a careful history, and physical examination, most patients should have a skeletal survey and technetium bone scan; these two studies are complementary in that the former is better for active and older lesions while the latter is more sensitive for early active lesions, which are sometimes missed by the skeletal survey. A complete blood count and differential as well as liver and kidney function tests should be done. While the sedimentation rate often correlates with the extent and activity of disease, it is nonspecific and fluctuates in a fashion similar to that characterizing most acute-phase reactants. A urinalysis with specific gravity should be done to establish that the patient does not have diabetes insipidus. If there is any question, a proper water deprivation test with chemical documentation of serum and urine osmolality and antidiuretic hormone levels should be performed.
A chest radiograph should be obtained as baseline. Magnetic resonance imaging (MRI) with contrast of the brain is being more frequently recommended at the time of diagnosis due to the increasing recognition of the CNS involvement of various types in LCH. Additional diagnostic tests, such as bronchoscopy or chest computed tomography (CT), lumbar puncture, endocrinologic work-up, bone marrow aspirate and biopsy, or gastrointestinal biopsy should be done only when there is clinical evidence or strong suspicion of organ involvement and/or dysfunction.
Treatment Options and Prognosis
Patients with limited involvement of LCH have an excellent prognosis without need for systemic therapy. In contrast, patients with multifocal skeletal involvement, refractory skin involvement, or other organ involvement will nearly always benefit from systemic therapy. This benefit comes in the form of symptomatic relief as well as decreased morbidity and mortality. Nevertheless, even with currently used types of systemic therapy, this group of patients may have a significant number of recurrences of LCH and may be more prone to develop some of the long-term sequelae. Systemic chemotherapeutic regimens have significantly improved the outcome for patients with extensive systemic involvement, including organ dysfunction of the bone marrow, liver, or lung, with approximately a 60% chance of survival at nearly 8 years.49
Limited Disease
Decisions about how to treat LCH patients should be based on the extent of disease, with consideration toward symptomatic relief as well as disease eradication. For example, patients with limited disease may require only diagnostic curettage of an isolated eosinophilic granuloma. However, there may be recurrence at the same site as well as development of new lesions at other sites. With such recurrences, there is rarely a need to repeat surgery or to biopsy an involved new site. For example, asymptomatic recurrences may not need any therapy, as lesions may regress on their own over a period of weeks to months. Symptomatic single lesions that potentially threaten organ function or cosmetic appearance require immediate intervention, usually with relatively low dose (between 400 and 800 cGy) radiation therapy. However, lesions that cause pain but do not threaten vital structures can often be treated with local injection of steroids or a trial of nonsteroidal antiinflammatory agents. The use of chemotherapy, including high-dose steroids and/or vinblastine, may also result in a relatively rapid response. Disease localized to the skin can usually be treated with topical steroids. However, in cases in which there is refractory and/or extensive involvement, the use of topical nitrogen mustard or phototherapy using ultraviolet A (PUVA) therapy has been successfully employed. The use of systemic chemotherapy, as described below, may also be indicated in patients with extensive cutaneous involvement.1,47
Multisystem Disease
The intensity of treatment for patients with multifocal and systemic LCH is currently based on risk group stratification. Several small studies from the 1970s and 1980s established that certain chemotherapeutic agents such as prednisone, vinblastine, vincristine, etoposide, 6-mercaptopurine, and methotrexate had excellent activity against LCH. The larger AIEOP-CNR-HX 83 and the DAL-HX 83/90 trials both demonstrated that complete response rates were 60% to 90% using agents such as vinblastine and etoposide in conjunction with prednisone.48,49 Response rates were greater in patients with multifocal bone disease than in patients with more extensive and/or organ dysfunction. Overall survival was greater than 90% for patients without organ dysfunction and only 46% to 66% in patients with extensive disease involvement and organ dysfunction at approximately 8 years follow-up.49 The recurrence rate was also highest in patients with more extensive disease.
The first international cooperative group study involving a prospective, randomized chemotherapy trial was LCH-I.50 Several important conclusions could be made from this trial. First, no difference in response rate or outcome was observed for patients randomized to receive vinblastine compared to those who received etoposide during the first 6 weeks of therapy. Second, the most predictive prognostic factor for overall survival was the response of patients after 6 weeks of therapy. Third, a incredibly good risk group of patients was identified, characterized by being 2 years of age or older with no pulmonary, hepatosplenic, or hematopoietic involvement. Their response rate was about 90%, and they had a 100% survival rate at an approximately 6-year follow-up. Finally, patients who did not show any significant response to therapy during the first 6 weeks had a particularly poor prognosis, with a mortality of of less than 40% at 5 years.
A comparison of the results from the DAL-HX 83/90 study to those of the LCH-I study suggested that the more aggressive therapeutic approach of DAL-HX 83/90 resulted in a lower recurrence rate as well as a lower incidence of diabetes insipidus. The LCH-II study was designed to test in a randomized trial whether high-risk patients with multisystem LCH benefit by more aggressive treatments, in part to answer this question in a more direct fashion. The final analysis of this trial is pending. A subsequent LCH-III study is addressing several questions, including whether outcome is improved by (1) the addition of intermediate-dose methotrexate to prednisone and vinblastine during initial therapy, or (2) 6 or 12 months of continuation therapy. The LCH-III “risk groups” are defined in Table 1 , and the treatment schema is depicted in Figure 2 (see Color Figures, 519).
These clinical trials as well as a significant number of smaller studies demonstrate that while some patients require very minimal therapeutic interventions, other patients benefit from more aggressive systemic treatment. However, it remains unproven whether more aggressive multiagent chemotherapy is the optimal treatment or whether maintenance therapy significantly reduces the risk of serious, recurrent disease. Another important consequence of these studies is that effective chemotherapeutic treatments have decreased the role of radiation therapy, which is now usually restricted to patients with lesions that could lead to significant adverse sequelae, such as spinal cord compression or localized refractory disease such as is sometimes observed in the mastoid.
At this time, nearly all of the clinical trials have been done in children and adolescents. However, there is a growing realization that many adults suffer from the same types of organ involvement as children do. Although not substantiated by carefully controlled clinical trials, the experience of physicians treating older patients would suggest that adults respond similarly to children and that adults should be treated accordingly. However, some modifications are often needed, such as reducing or eliminating high-dose and chronic exposure to steroids. An important challenge for the future will be to develop clinical trials that include adults, similar to many of the Medical Research Council (MRC) leukemia trials, or to direct trials to some of the types of involvement unique to adults, such as isolated pulmonary histiocytosis. In many regards, adults have become the orphans of this orphan disease.
Treatment of Recurrent Disease
Patients with recurrent disease often respond to the same drugs to which they initially responded. Alternative approaches have been tested for patients with progressive disease while on therapy, including immunomodulatory therapies as well ascytolytic agents.1,47 The responses to immunosuppressive agents, such as cyclosporin or antithymocyte immunoglobulin, have been anecdotal and, at best, transient. Several studies using 2-chlorodeoxyadenosine (2-CdA), including an international Phase II trial, have shown sustained remissions in over a third of patients who had otherwise refractory disease.51–,53 Anecdotal experience has also suggested that some patients with disease that is refractory to 2-CdA may have dramatic responses to the synergistic combination of 2-CdA plus cytosine arabinoside, a regimen with proven benefit in patients with relapsed acute myelogenous leukemia. In addition, drugs designed to reduce inflammatory responses, such as thalidomide or tumor necrosis factor (TNF) inhibitors, are being tested.54,55 Targeted immunotherapy, for example with anti-CD1a antibodies, remains promising but has not yet been definitively tested.56 The application of inhibitors of activated cytokine receptors and their downstream signal transduction pathways also is an important area for future therapeutic trials. The role of hematopoietic stem cell transplantation has been largely unexplored, except in a few case reports, some of which demonstrate prolonged survival without recurrent disease.47,57 Unfortunately, selective reporting of positive results is always a problem in generalizing from single case reports.
For patients with extensive organ dysfunction or progressive disease, alternative therapies are clearly needed. In particular, there remains a significant need to develop strategies for prevention of progressive fibrosis of the lung, sclerosing cholangitis, and fibrosis of the liver as well as the neurodegenerative pattern of CNS involvement. Determining whether agents such as 2-CdA or specific inhibitors of fibrosis will improve the outcome for patients with these complications will require additional clinical trials.
Future Challenges
There has been substantial progress in both the understanding and treatment of LCH over the nearly 150 years since the disorder was first described. Remaining challenges are, however, plentiful. We still do not have a clear understanding of the etiology and pathogenesis of LCH. While there is much information to suggest that LCH is a type of clonal myeloproliferative disorder of the dendritic cell, definitive proof should ultimately include the identification of consistent molecular abnormalities. Another challenge is to accurately determine the incidence of LCH in different regions of the world. This could in part be facilitated if LCH were, like some other disorders, required to be reported to national registries. At least national and regional incidence figures might then be more easily obtained. I expect that the incidence in adults has been largely underreported.
While treatments have been developed that result in improved outcomes for most patients, substantial numbers of patients continue to have problematic recurrent disease; there is also the continued challenge presented by those patients who develop treatment-refractory disease, resulting in a high mortality. Clearly, improved treatments are needed for refractory disease that rapidly progresses or chronically recurs. One hope for the development of more effective initial treatment is that adverse late sequelae of the lung, liver, and CNS can be avoided. While this is a hope, it is uncertain that this will be converted into a reality, as our understanding of the pathogenesis of these sequelae is poor. There is also a growing realization that more adults of all ages are victims of LCH. Early diagnosis, treatment, and close follow-up are as critical for this population of patients as they have been for children. Future clinical trials should either include both children and adults or be developed for specific age groups in order to permit definitive conclusions in terms of treatment efficacy and overall outcomes. Such trials will also help facilitate biological investigations of LCH.
As in the rest of oncology, the greatest progress will likely be made as a result of linking the efforts of laboratory scientists with those of multidisciplinary clinical investigative teams. Such combined approaches are particularly important in relatively rare disorders. In this regard, important lessons should be gleaned from the cooperative group process in pediatric oncology that has so successfully improved our understanding and outcome of childhood cancers.
II. Making Sense of KIT Inhibitors Using Mastocytosis as a Model: Not All KIT Activating Mutations Act Alike
B. Jack Longley, MD*
University of Wisconsin, Dermatology Division, 1 South Park St., Madison, WI 53715
This work was supported by grants from the Leukemia Foundation and the National Institutes of Health. Dr Longley is the recipient of a Sponsored Research Agreement from SUGEN, Inc.
Attempts to predict the sensitivity of individual patient’s neoplastic cells to specific drugs have met with limited success in the past, in part because of a lack of understanding of molecular causes of cellular transformation in individual tumors, and in part because of a lack of drugs that specifically affect known molecular targets. Drugs designed to inhibit individual oncogenic enzymes are becoming more available, and our understanding of the role of these enzymes in individual tumors is expanding at a rapid rate, so the opportunity exists to use in vitro studies of enzyme sensitivities to tailor chemotherapeutic treatment to individual patients, analogous to the way in vitro sensitivities of organisms isolated from individual patients are used to tailor antibiotic selection in infectious diseases. However, in vitro enzyme sensitivity studies are time consuming, expensive, and not widely available. In this paper I describe a strategy designed to predict the sensitivity of tumors in individual patients based on understanding different mechanisms of aberrant activation of the KIT receptor tyrosine kinase in mastocytosis. The crux of this approach is the concept that oncogenic enzymes may be activated in several ways, and that the specific mechanism of activation in a given neoplastic cell may affect its sensitivity to different inhibitors. I will also consider mechanisms by which KIT and other oncogenic enzymes may be activated, the role normal and aberrantly activated KIT plays in the human disease mastocytosis, and finally the implications that different mechanisms of KIT activation have for therapeutic approaches to mastocytosis and other diseases.
How Does the KIT Protein Normally Function?
The KIT protein is a tyrosine kinase encoded by the c-KIT proto-oncogene.1– 3 By definition, tyrosine kinases are enzymes that transfer phosphate from ATP (adenosine-triphosphate) to tyrosines present in substrate proteins. Transfer of phosphate to key tyrosines in a protein may regulate the function of the recipient protein, which in turn may regulate cellular functions such as cell growth and survival. In KIT, the kinase reaction takes place in a “pocket” formed between the two lobes of the intracellular kinase domain.
In the absence of ligand, KIT exists as a transmembrane monomer with an extracellular ligand binding domain and an intracellular kinase domain. The kinase domain, which contains the enzymatic site, is held in a form with minimal enzymatic activity by intrinsic structural components of the monomer. This internal auto-inhibition can be overcome by ligand binding. The KIT ligand, known as mast cell growth factor or stem cell factor (SCF), normally exists as a bivalent dimer that may bind to the extracellular portions of two KIT monomers, inducing their dimerization.4– 9 Dimerization of KIT monomers results in their autophosphorylation, which in turn results in activation of the KIT kinase. Thus, the interaction of ligand with the extracellular portion of the enzyme overcomes the intrinsic autoinhibitory control mechanism. The effects of this extrinsic activation may be further modified within the cell by substrate availability and binding and by dephosphorylation of activated KIT by phosphatases.
How May KIT and Other Enzymes Be Aberrantly Activated in Neoplastic Cells?
Receptor-type tyrosine kinases are transmembrane proteins that are normally activated by physiologically produced ligand molecules that specifically bind to their extracellular portions. However, receptor tyrosine kinases may be aberrantly activated by several mechanisms and aberrant activation may drive the growth of neoplastic cells. The different mechanisms of activation include activation by pathologically produced ligand and activation by mutations that affect the regulation or function of the enzyme portion of the kinase molecule. Specifically, pathologic activating mechanisms include autocrine production of ligand, changes in the intracellular milieu that affect the phosphorylation or substrate binding of the KIT molecule, and mutations of c-KIT itself that result in overexpression or constitutive activation of the enzyme.
We have classified KIT activating mutations as belonging to one of two groups.10 One group consists of mutations that alter the amino acid sequence of regions forming the active kinase “pocket” or enzymatic site. These mutations directly affect the primary and higher order structures of the enzymatic site and for convenience we have called them “enzymatic pocket” or “enzymatic site” type mutations. KIT and a number of other kinases are predicted to have a mobile structure called the “activation loop,” which forms the front of the “pocket” where the enzyme reaction takes place. This activation loop appears to function like a hinged flap that normally restricts access to the rest of the enzymatic site. The activation loop of KIT contains a tyrosine, which when phosphorylated appears to maintain the loop in a relaxed or “open” position, allowing access to the rest of the enzymatic site and allowing enzyme activity. It is presumably the autophosphorylation of this tyrosine that allows activation of KIT when ligand binding induces dimerization of KIT monomers. A common mutation in human mastocytosis affects c-KIT codon 816 and is predicted to alter the charge of the mobile activation loop at the entrance to the active kinase site of KIT, thereby causing constitutive activation of the kinase.
The other general type of mutation involves regulation of an otherwise normal enzymatic site and includes mutations that affect the level of expression of the KIT molecule as well as mutations that alter or destroy regulatory portions of the KIT protein, particularly the portions with autoinhibitory function. We have called this second type of mutation “regulatory type” mutations, and they differ from “enzymatic site” type mutations in that they preserve the normal amino acid sequence of the enzymatic site. For instance, a well-characterized KIT regulatory region is the intracellular juxtamembrane region, encoded by exon 11.11 The secondary structure of this region includes an amphipathic alpha helix that, when intact, suppresses KIT phosphorylation and kinase activity. Mutations disrupting the alpha helix release the inhibitory effects of the juxtamembrane region, resulting in KIT gain of function (constitutive activation). The extracellular portion of KIT also appears to have regulatory function beyond its role in binding ligand, and c-KIT mutations affecting this region may cause spontaneous activation in the absence of ligand. Finally, overexpression of KIT may also cause increased KIT kinase activity. As is the case for activating mutations affecting the extracellular region, the exact mechanism involved in activity with overexpression has not been demonstrated, but an increase in the number of KIT molecules may cause a greater frequency of random collisions with spontaneous dimerization and autophosphorylation. Alternatively, the phenomenon could simply reflect the low basal level of activity of a greater number of KIT molecules. Mutations that cause aberrant expression of KIT in a cell that does not normally express it may be considered a subset of mutations affecting the level of expression of KIT and, together with aberrant stimulation by autocrine production of SCF and other clearly extrinsic mechanisms, can be grouped into the category of aberrant regulatory events causing KIT activation. The distinction between “regulatory” and “enzymatic site” mechanisms appears to be important clinically because the ability of small molecule compounds to inhibit KIT kinase activity depends—in part—on whether KIT is activated by a regulatory event or by an enzymatic site mutation.
How Are Kinase Inhibitors Developed and Why Does It Matter?
The most common kinase inhibitors are small molecules whose size and charge distribution mimic those of ATP. These molecules are typically planar structures with backbones containing multiple hexagonal carbon rings. The molecules compete with ATP for binding at the enzymatic site, but because they lack a transferable phosphate they block the enzyme from its normal function. Because ATP is a ubiquitous source of cellular energy, an inhibitor that perfectly mimicked ATP would be a strong cellular poison and would have limited clinical use. Kinase inhibitors can be made specific for different enzymes or groups of structurally related enzymes by taking advantage of differences in the charge and spatial orientation of the amino acids making up the enzymatic sites of different enzymes. To do this, additional molecules are attached to the hexagonal carbon backbone so that they interact with the amino acids forming the enzymatic site. Addition of particular molecules may promote binding of an inhibitor to one enzyme but not another. Although powerful computer programs exist that allow molecular modeling and prediction of how addition of specific side chains may affect binding of inhibitors, the number of enzymes for which the fine structure of the enzymatic site is known is still limited, and most available inhibitors have been developed by screening potential compounds for activity in vitro. Most such drug development programs involve screening against enzymes with a normal (e.g., wild type) enzymatic site. Thus, specific kinase inhibitors are selected for their ability to interact with the amino acids that make up the normal (wild type) enzymatic site. It follows, then, that mutations that cause enzyme activation by altering the composition of the amino acids of an enzyme’s enzymatic site have a greater chance of affecting the ability of an inhibitor to bind to that site than do regulatory type mutations or events that leave the primary sequence of the enzymatic site intact. The implication is that if the sequence alterations affecting the enzymatic site of an oncogene in the neoplastic cells of a given patient can be detected, that patient can be identified as being at higher theoretical risk of treatment failure with a kinase inhibitor, and more extensive in vitro testing prior to treatment of that individual can be considered.
Clinical Types of Human Mastocytosis
Human mastocytosis can be divided into a small number of clinical types.12 Most cases occur as sporadic disease that is limited to the skin in infants and children. The two most common forms of pediatric mastocytosis are solitary cutaneous tumors called mastocytomas and a more widespread type of cutaneous involvement known as urticaria pigmentosa (UP). The skin lesions of pediatric UP are usually small, lightly pigmented macules (flat lesions) and slightly raised, lightly pigmented papules. Pediatric patients may have any number of skin lesions, from a few to thousands. Pediatric mastocytosis is usually transient, beginning in the first year of life and disappearing or becoming inactive before the end of puberty.
The second most common clinical type of mastocytosis is adult type UP. Adult UP lesions are usually small and flat (macules) and tend to be more heavily pigmented than lesions of pediatric UP. Like pediatric UP, adult UP is almost always sporadic. Most cases occur in young adults, but the disease occasionally arises in adolescence. In contrast to pediatric mastocytosis, sporadic adult type mastocytosis tends to persist and is often progressive, with systemic organ involvement, including the bone marrow, gastrointestinal tract, and lymphoid organs.
A very small number of kindreds have familial mastocytosis, usually inherited in an autosomal dominant fashion. These patients have cutaneous disease that may first occur in infancy or early childhood and that may persist into adulthood with variable systemic involvement. Interestingly, some kindreds with familial mastocytosis also have familial occurrence of gastrointestinal stromal tumors (GISTs). GISTs are derived from the interstitial cells of Cajal, cells that form the autonomic nervous system of the gut and that express KIT. GISTs also occur sporadically, and both the sporadic and familial variants are strongly associated with KIT activating mutations, usually in exon 11 of c-KIT (the exon that encodes the juxtamembrane autoinhibitory region of the KIT protein).
The Role of KIT in Human Mastocytosis
KIT is also known as the receptor for mast cell growth factor, and activation of KIT causes mast cell proliferation and prevents mast cell death by apoptosis. Mutations substituting a valine for aspartate in codon 816 of c-KIT (D816V) lead to constitutive activation of the KIT kinase and are characteristic of sporadic adult mastocytosis and rare atypical cases of pediatric mastocytosis. Inhibition of activated KIT in mast cell lines prevents their proliferation and causes mast cell apoptosis,13– 16 so KIT inhibitors appear to be logical candidates for treatment of human mastocytosis. However, codon 816 mutations directly affect the structure of the KIT kinase enzymatic site, and it appears that no known clinical candidate KIT-inhibiting drugs bind the mutant KIT with sufficient avidity to inhibit the active kinase that causes these forms of mastocytosis. Therefore, treatment of common forms of human mastocytosis awaits the discovery of drugs that effectively inhibit the D816V mutant. Because the mutant enzymatic site may be considerably altered from the wild type site, it is possible that the appropriate drug may not have significant activity against the wild type enzyme. In fact, because KIT activation is involved in a number of normal bodily functions, including hematopoesis and maintenance of the gastrointestinal autonomic nervous system, the best drug would be one that inhibits only the mutant enzyme and shows no activity against wild type KIT or other kinases.
In pediatric mastocytosis, KIT-activating mutations are exceedingly rare. Those that have been reported also affect codon 816, although they may show other substitutions besides the valine typical of adult UP. Pediatric patients with KIT-activating mutations have atypical clinical features; in fact, their disease behaves more like that of the adult forms caused by codon 816 mutations. In addition, rare patients with typical childhood UP have been found to have dominant negative (KIT inactivating) c-KIT mutations. This is a critical observation because it shows that KIT activation is not necessary for mast cell proliferation in at least some patients with pediatric mastocytosis. This implies that the mechanism of oncogenesis in these cases not only does not involve KIT activating mutations but also does not involve KIT signaling such as might be seen with aberrant production of mast cell growth factor/SCF or loss of an intracellular phosphatase. Therefore, KIT inhibitors are unlikely to be effective in these cases.
In contrast to sporadic mastocytosis of all types, patients within one kindred with familial mastocytosis have regulatory type activating c-KIT mutations affecting the juxtamembrane inhibitory alpha helix.17 Interestingly, this kindred also has familial GISTs, tumors known to be associated with regulatory type KIT activating mutations. As predicted by our model, these are regulatory type mutations. In fact, the forms of mutant KIT expressed by these GISTs respond to multiple different KIT inhibitors. Therefore, we would predict that mastocytosis in the patients of this kindred would also respond to KIT inhibitors. Thus, the KIT inhibitors that are currently available appear to have a very limited role in management of the common forms of mastocytosis.
Can This Approach Apply to Other Tumors and Enzymes?
A number of other types of neoplasms besides mastocytosis express KIT, which may contribute to cell proliferation and survival. KIT expression and mutation appear to be an early and perhaps necessary phenomenon in the pathogenesis of GISTs.18,19 As previously mentioned, these tumors mostly express regulatory type mutations and appear to respond well clinically to KIT inhibitors. Additional neoplasms expressing mutated KIT include sinonasal natural killer/T-cell lymphomas, AML, and myeloproliferative disorders (reviewed by Longley et al10 and later by Heinrich et al20). The functional consequences of many of the mutations described in these conditions have not been determined and their significance is unclear, but the expression of KIT, which lacks enzymatic site mutations in some individual cases, would suggest the possibility of treatment of individual patients with KIT inhibitors. In addition, a number of other neoplasms may express KIT, such as carcinoma of the lung, which could be activated by autocrine production of SCF and which should respond to KIT inhibitors.
With regard to other enzymes, a recent report21 described crystallographic studies of the EphB2 receptor tyrosine kinase that showed a juxtamembrane autoinhibitory helix similar to the one found in KIT. The helix was shown to function through direct interaction with the EphB2 kinase domain. This report shows that kinase autoinhibition via secondary protein structures, such as juxtamembrane alpha helices, is a generalizable mechanism of kinase regulation in different receptor tyrosine kinase families. Therefore, other oncogenes have autoinhibitory regions that may be disrupted by “regulatory” type mutations, and we would predict that these types of mutant enzymes would be susceptible to inhibition by inhibitors of the wild type enzyme.
In this vein, in a recent report Gorre et al described diverse mechanisms of imatinib resistance in 9 patients with BCR-ABL-positive chronic myelogenous leukemia.22 Imatinib resistance was associated with acquired mutations of the ABL enzymatic site in 6 patients, and with BCR-ABL gene amplification in the other 3. Therefore, some resistance was due to “enzymatic site” type mutations or—in the case of the gene amplifications (as well as the original translocation forming the BCR-ABL fusion gene)—to “regulatory” type mutations. Gorre et al suggest that the patients with gene amplification might be susceptible to treatment with higher doses of imatinib, but that those with active site mutations may require treatment with a different drug.
Although these speculations need to be tested in clinical studies, it appears likely that activating mutations affecting other enzymes may also be classified as “regulatory” or “enzymatic site” in type, and that this strategy may prove to be generally useful in predicting drug resistance and guiding therapy. I would stress, however, that this paradigm can only be used as a guide to identify those patients who have a higher probability of drug resistance. For instance, if an activated kinase can be identified in a patient’s tumor and no enzymatic site mutation is identified, no further studies may be necessary before treatment with a drug that inhibits the wild type form of the enzyme. On the other hand, if a patient’s tumor expresses a novel mutation affecting the enzymatic site, it may be prudent to express the mutant enzyme in vitro and test its response to various drugs before initiating therapy.
III. Mixed Myeloproliferative/Myelodysplastic Disorders in Adults and Children: Biology and New Therapeutic Approaches
Peter D. Emanuel, MD*
University of Alabama, Division of Hematology, 1824 6th Ave., South WTI 520, Birmingham, AL 35294-3300
Dr. Emanuel has received research support from Johnson & Johnson Pharmaceutical, Novartis, and Berlex.
In the recently proposed new classification schema of hematological malignancies, the World Health Organization introduced a new category of diseases that overlap or “bridge” the myelodysplastic syndromes (MDS) and the chronic myeloproliferative diseases (MPD) groups.1 Four diseases were assigned to this mixed category of MDS/MPD: (1) chronic myelomonocytic leukemia (CMML), (2) atypical chronic myeloid leukemia (aCML), (3) juvenile myelomonocytic leukemia (JMML), and (4) MDS/MPD, unclassifiable. Though it remains to be determined how much these diseases have in common in terms of pathogenetic mechanisms, they are at present grouped together in this category primarily due to similarities in clinical and morphologic presentations. These diseases are, as a general rule, characterized by hyperproliferative effects, with increased numbers of cells at virtually all stages of maturation. However, in addition, morphological and functional dysplasia can be variably observed in many patients. This review will attempt to summarize what is known regarding the pathogenesis of these disorders, as well as review recent attempts for targeted therapeutic approaches.
Chronic Myelomonocytic Leukemia
CMML is a highly heterogeneous disorder that predominantly presents in the elderly.2,3 The French-American-British group initially included CMML in its classification of MDS.4 Later, there was an attempt to classify CMML into either the MPD category if the total white blood cell count was greater than 13,000 × 109/L, or the MDS category if it was less than 13,000 × 109/L.5 Thus, CMML’s categorization into the mixed MDS/MPD category seems appropriate for the present.
CMML pathogenesis
Concurrent with the heterogeneous clinical presentation and course of CMML patients, no consistent genetic abnormality has yet been linked with CMML pathogenesis, although it is clearly a clonal hematopoietic disorder. Karyotypic abnormalities can be demonstrated in a significant number of patients, but most of the abnormalities are also routinely observed in patients with other MDS category diseases. RAS gene mutations are observed in a significant proportion of patients (up to 40%) either at presentation or at some stage during the course of their disease.6 The incidence of RAS mutations in CMML may be higher than in other MDS subtypes. A very small subset of CMML patients have a specific translocation, t(5;12)(q33;p13). This translocation fuses TEL, a member of the Ets family of transcription factors, to the platelet-derived growth factor receptor β (PDGFβR), leading to constitutive activation of the tyrosine kinase domain of the PDGF receptor.7 Multiple signaling pathways appear to be activated by TEL/PDGFβR.8–,11 Complete delineation of the relative contribution of these pathways to the pathogenesis of CMML will require further investigation, including the use of mouse models.12,13 In addition to the activating RAS point mutations and the TEL/PDGFβR gene fusion, a number of studies have investigated the role of specific cytokines in the in vitro growth of CMML progenitors. Tumor necrosis factor (TNF), GM-CSF, Interleukin-3 (IL-3), IL-4, IL-6, and IL-10 have all been implicated as playing potential roles in the pathogenetic hyperproliferative growth pattern of CMML cells in vitro or in vivo in patients.14– 19
Therapy of CMML
For the small subset of patients who demonstrate translocations in their leukemia cells involving PDGFβR, imatinib mesylate (STI571) has been recently reported to produce dramatic and durable responses.20 As most CMML patients are elderly at the time of diagnosis, myeloablative stem cell transplantation is not a viable option for the majority. For those patients who do have a donor and are young enough to tolerate the toxicities, myeloablative stem cell transplantation should be seriously considered, as this remains the only proven curative option for this disease. Nonmyeloablative transplantation strategies are rapidly emerging and may ultimately prove to be a less toxic but effective alternative to myeloablative transplantation. Otherwise, cytokine therapy and low- and high-dose chemotherapeutic options have been attempted, but with limited success. Part of the problem in interpreting many previous trials is that CMML patients have been treated, evaluated, and reported together with all other MDS subtypes, making specific evaluation of the response in CMML problematic. Responses to erythropoietin therapy can be observed in 15-20% of patients.21 CMML patients can also respond temporarily in terms of increases in peripheral blood neutrophil counts to granulocyte colony-stimulating factor (G-CSF) or GM-CSF, but because CMML progenitor cells can also respond to these cytokines, there is a concern for a potentially higher transformation rate to AML.22 Given the toxicities of high-dose chemotherapy in this elderly patient population, chemotherapeutic trials have generally not produced sufficient response rates to justify routine use of high-dose chemotherapy.
Because CMML pathogenesis has been linked to dysregulated signal transduction involving the Ras pathway and to one or more cytokines such as GM-CSF, more targeted therapeutic approaches are now beginning to emerge. The farnesyltransferase inhibitors (FTIs) were developed as specific compounds to block Ras signal transduction by inhibiting Ras binding to the inner plasma membrane.23 Whether this is their true or primary mechanism of action is a matter of ongoing debate, but mounting evidence suggests that response of malignancies to FTIs is independent of RAS mutational status.24–,26 Investigators from Stanford have recently reported preliminary results of the use of one FTI, R115777, in a Phase I/II study of MPD patients, including 2 CMML patients.27 Further results from this and similar trials will be of interest. Blocking the dysregulated cytokine signal transduction operative in CMML, such as by blocking GM-CSF at the cell surface, is also a potentially viable approach to targeted therapy (Figure 3, see Color Figures, 518). Two compounds discussed below, E21R and DT388-GM-CSF, are entering trials in adults and children for various myeloid malignancies and may prove efficacious in CMML. Imatinib mesylate is also being investigated in CMML, but with discouraging early results.
Atypical Chronic Myeloid Leukemia
Atypical CML is, by definition, Philadelphia chromosome negative and BCR/ABL fusion gene negative. Atypical CML affects a truly heterogeneous group of patients, and the exact incidence is not known. Reports of small numbers of these patients indicate that they often have other cytogenetic abnormalities and suffer from short median survival times. Because of the paucity of these patients and their vast clinical heterogeneity, little can be said regarding pathogenesis. Preliminary findings indicate that these patients generally do not respond to imatinib mesylate. Other potential treatment options to consider are a clinical trial, if available, stem cell transplantation, or low-dose chemotherapy such as hydroxyurea.
Juvenile Myelomonocytic Leukemia
Much more is known about the pathogenesis of JMML than about that of CMML and aCML. JMML is a clonal disorder arising from the pluripotent stem cell.28,29 It afflicts infants and young children, with the vast majority of cases presenting when children are ≤ 5 years of age. JMML is generally characterized by marked hepatosplenomegaly, leukocytosis with monocytosis, anemia and thrombocytopenia, and elevated fetal hemoglobin in most patients (even when corrected for age).30 Most patients (> 80%) have a normal karyotype and are, by definition, Philadelphia chromosome negative and BCR/ABL negative.
JMML pathogenesis
More so than any other leukemia or myeloproliferative disorder, JMML cells in vitro nearly uniformly show spontaneous colony formation without addition of exogenous growth factors. This in vitro growth characteristic and the subsequent pathogenesis of JMML have been definitively linked to dysregulated growth factor signal transduction through the Ras pathway.31–,38 This Ras dysregulation results in JMML cells demonstrating a selective hypersensitivity to GM-CSF.31 Either activating RAS point mutations or inactivating NF1 mutations can lead to constitutive signaling of the Ras pathway. JMML patients demonstrate RAS and NF1 gene abnormalities in their hematopoietic cells at estimated rates of 20% and 30%, respectively, and these subsets remain mutually exclusive.33,35,36 Murine hematopoietic cells that are homozygously deleted of Nf1 (Nf1-/-) are also hypersensitive to GM-CSF and, if these cells are transplanted into irradiated recipient mice, they are capable of inducing an MPD reminiscent of JMML.37,38Nf1 mutant murine hematopoietic cells demonstrate hyperactivation to numerous cytokines, including GM-CSF, SCF, and IL-3.39 However, studies by Birnbaum and colleagues with Gmcsf-Nf1 doubly mutant cells have shown that GM-CSF plays a central role in the aberrant growth of Nf1 mutant cells,40 thus providing a compelling rationale for pursuing therapeutic strategies that target the GM-CSF signal transduction pathway in JMML patients.
Therapy of JMML
There is no known, consistently effective therapy for JMML. Single- and multiagent chemotherapy regimens in limited numbers of patients report widely varying response rates but provide little evidence that such therapy improves ultimate outcome. Only allogeneic stem cell transplantation (SCT) has resulted in extended survival.41– 43 Unfortunately, the relapse rate from allogeneic SCT is inordinately high in JMML, ranging from 28-55% in several studies, with 5-year disease-free survival rates ranging from only 25-40%. Although 13-cis retinoic acid can produce a 40-50% overall response rate, complete remissions sustained off therapy are rare.
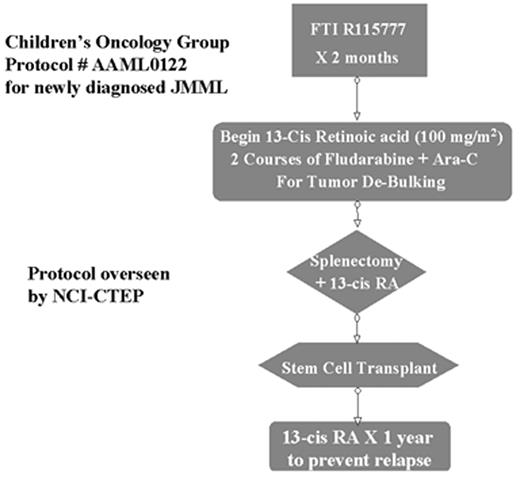
Given the knowledge of JMML pathogenesis, mechanism-based molecularly targeted approaches such as that used for imatinib mesylate in BCR/ABL+ CML now seem justifiable for JMML. However, given that SCT can result in long-term survival, it seemed inappropriate to completely abandon this treatment approach. Therefore, a Phase II window/Phase III trial design for JMML was activated by the Children’s Oncology Group in 2001 (Figure 4 ). There are three appealing aspects to this protocol design. First, it permits the rigorous evaluation of the efficacy of a single molecularly targeted agent in untreated JMML patients who have not yet developed drug resistance. Second, if one phase II agent fails, the protocol is designed to allow for the substitution of a different agent for Phase II testing without disrupting the rest of the study. Finally, the fact that participation in the Phase II window is not required allows patients and their parents who are skeptical regarding experimental agents an opportunity to still enroll in the remainder of the protocol. The first agent to be tested in this format for JMML is the FTI R115777, the same agent being tested in adults with MPD.27 A multitude of other agents (Figure 3, 518) capable of targeting the GM-CSF/Ras pathway (albeit with varying degrees of specificity) are now emerging as potential capable agents that can substitute into the Phase II window of this protocol once R115777 has completed testing.
Peptidomimetics
E21R was created by a single amino acid substitution in the GM-CSF molecule at position 21. This substitution allows E21R to function as a GM-CSF antagonist by binding to the α-subunit of the GM-CSF receptor but preventing its association with the β-subunit, which is critical for downstream signal transduction. At concentrations of 1 μg/mL and 10 μg/mL, E21R induced apoptosis in AML and CML cells,44 and in JMML cells,45 respectively. In a JMML-SCID/NOD mouse model,46 E21R prevented dissemination of leukemic cells and induced remission in animals that had developed JMML-like MPD after injection of human JMML cells. E21R is in Phase II studies in adult AML and CMML in England and Australia. A Phase I trial in relapsed pediatric myeloid malignancies is in the planning stages in the United States.
GM-CSF fused to toxins
Other signaling inhibitors
A DNA enzyme that targets Raf-1 gene expression has shown effectiveness at JMML cell growth inhibition in vitro and in a JMML NOD-SCID mouse model.50 The Nf1 JMML mouse model provides a useful system for testing targeted therapeutics such as the mitogen-activated protein kinase kinase (MEK) inhibitor PD184352.51 These ongoing studies demonstrate the utility of both the Nf1 and NOD/SCID JMML mouse models to not only help elucidate pathogenetic mechanisms but also to test molecularly targeted therapeutics.
Myelodsyplastic/Myeloproliferative Disease, Unclassifiable
For patients who have features of both MPD and MDS but who truly cannot be classified into one of the above three categories, mixed MDS/MPD is listed in this class. Even more than atypical CML, this category appears to be a “catchall” of a mixture of other disorders. Therefore, conclusions regarding pathogenesis and/or treatment recommendations cannot be reached at the present time.
Risk groups as defined by the Langerhans Cell Histiocytosis–III Protocol.
| Group 1: Multisystem “risk” patients |
| Multisystem patients with involvement of 1 or more “risk” organs (i.e., hematopoietic system, liver, spleen, or lungs). Patients with single-system lung involvement are not eligible for randomization. |
| Group 2: Multisystem “low-risk” patients |
| Multisystem patients with multiple organs involved but without involvement of “risk” organs. |
| Group 3: Single-system “multifocal bone disease” and localized “special site” involvement |
| Patients with multifocal bone disease—that is, lesions in 2 or more different bones. Patients with localized special site involvement, such as “central nervous system risk” lesions with intracranial soft tissue extension or vertebral lesions with intraspinal soft tissue extension. |
| Group 1: Multisystem “risk” patients |
| Multisystem patients with involvement of 1 or more “risk” organs (i.e., hematopoietic system, liver, spleen, or lungs). Patients with single-system lung involvement are not eligible for randomization. |
| Group 2: Multisystem “low-risk” patients |
| Multisystem patients with multiple organs involved but without involvement of “risk” organs. |
| Group 3: Single-system “multifocal bone disease” and localized “special site” involvement |
| Patients with multifocal bone disease—that is, lesions in 2 or more different bones. Patients with localized special site involvement, such as “central nervous system risk” lesions with intracranial soft tissue extension or vertebral lesions with intraspinal soft tissue extension. |
Schematic of possible pathophysiologic mechanisms leading to Langerhans cell histiocytosis and subsequent tissue damage.
Abbreviations: Ag, antigen; LC, Langerhans cell.
Schematic of possible pathophysiologic mechanisms leading to Langerhans cell histiocytosis and subsequent tissue damage.
Abbreviations: Ag, antigen; LC, Langerhans cell.
Schema of Children’s Oncology Group (C.O.G.) Protocol #AAML0122 for newly diagnosed JMML patients. Protocol is overseen by NCI-CTEP.
Abbreviations: FTI, farnesyltransferase inhibitor; Ara-C, cytosine arabinoside; RA, retinoic acid; JMML, juvenile myelomonocytic leukemia; NCI-CTEP, National Cancer Institute – Cancer Therapy Evaluation Program.
Schema of Children’s Oncology Group (C.O.G.) Protocol #AAML0122 for newly diagnosed JMML patients. Protocol is overseen by NCI-CTEP.
Abbreviations: FTI, farnesyltransferase inhibitor; Ara-C, cytosine arabinoside; RA, retinoic acid; JMML, juvenile myelomonocytic leukemia; NCI-CTEP, National Cancer Institute – Cancer Therapy Evaluation Program.