Abstract
The myelodysplastic syndromes (MDS) are characterized by hemopoietic insufficiency associated with cytopenias leading to serious morbidity plus the additional risk of leukemic transformation. Therapeutic dilemmas exist in MDS because of the disease’s multifactorial pathogenetic features, heterogeneous stages, and the patients’ generally elderly ages. Underlying the cytopenias and evolutionary potential in MDS are innate stem cell lesions, cellular/cytokine-mediated stromal defects, and immunologic derangements. This article reviews the developing understanding of biologic and molecular lesions in MDS and recently available biospecific drugs that are potentially capable of abrogating these abnormalities.
Dr. Peter Greenberg’s discussion centers on decision-making approaches for these therapeutic options, considering the patient’s clinical factors and risk-based prognostic category.
One mechanism underlying the marrow failure present in a portion of MDS patients is immunologic attack on the hemopoietic stem cells. Considerable overlap exists between aplastic anemia, paroxysmal nocturnal hemoglobinuria, and subsets of MDS. Common or intersecting pathophysiologic mechanisms appear to underlie hemopoietic cell destruction and genetic instability, which are characteristic of these diseases. Treatment results and new therapeutic strategies using immune modulation, as well as the role of the immune system in possible mechanisms responsible for genetic instability in MDS, will be the subject of discussion by Dr. Neal Young.
A common morphological change found within MDS marrow cells, most sensitively demonstrated by electron microscopy, is the presence of ringed sideroblasts. Such assessment shows that this abnormal mitochondrial iron accumulation is not confined to the refractory anemia with ring sideroblast (RARS) subtype of MDS and may also contribute to numerous underlying MDS pathophysiological processes. Generation of abnormal sideroblast formation appears to be due to malfunction of the mitochondrial respiratory chain, attributable to mutations of mitochondrial DNA, to which aged individuals are most vulnerable. Such dysfunction leads to accumulation of toxic ferric iron in the mitochondrial matrix. Understanding the broad biologic consequences of these derangements is the focus of the discussion by Dr. Norbert Gattermann.
I. Controversies and Therapeutic Options in Myelodysplastic Syndrome: Biologically Targeted Approaches
Peter L. Greenberg, MD*
Professor of Medicine, Hematology Division, Room S161, Stanford University Medical Center, Stanford, CA 94305 and Head, Hematology Section, Palo Alto VA Health Care System, Palo Alto CA 94304.
Dr. Greenberg has received research support from Amgen, Johnson & Johnson, Celgene, and Genentech.
Therapeutic dilemmas abound in myelodysplastic syndrome (MDS) because of the disease’s multifactorial pathogenetic features and heterogeneous stages, and the patients’ generally elderly ages. Underlying the cytopenias and evolutionary potential in MDS are innate stem cell lesions, cellular/cytokine-mediated stromal defects, and immunologic derangements. Given the developing understanding of biologic and molecular lesions in MDS and recently available biospecific drugs that are potentially capable of abrogating these abnormalities, specific targets are being evaluated for possible therapeutic intervention.
Goals of therapy range from symptom management/hematologic improvement (using low-intensity treatment with biologically targeted agents) to attempts at changing the natural history of the disease (generally using high-intensity treatment, including chemotherapy and hemopoietic stem cell transplantation). This review will center on decision-making approaches for these therapeutic options, considering clinical factors such as the patient’s age, performance status, and risk-based prognostic category. The format for this review will be to attempt to respond to questions generally posed by patients to their physicians regarding this problematic disease, about which a great deal of uncertainty and controversy exist:
What is my disease?
How long will I live with my disease? What problems should I anticipate experiencing? What is my chance of developing leukemia?
What treatments are available for my disease? Which treatment(s) should I receive? When should I receive them?
How can I learn more about my illness? Are there clinical trials with which I can and should become involved? How do I find out about them?
What is my disease?
A. Diagnostic Classification
MDS is characterized by hemopoietic insufficiency associated with cytopenias leading to potentially serious morbidity (transfusion-dependent anemia, bleeding manifestations) and mortality (death from infection in the setting of neutropenia), plus the additional risk of leukemic transformation. The disease may arise de novo or may develop following treatment with mutagenizing agents after the patient has been treated with chemotherapy or chemoradiotherapy for other diseases (usually other malignancies). The latter variant is termed secondary or treatment-related MDS. MDS is generally relatively indolent, often with a pace of disease comprising at least several months and with a rate of progression related to a number of defined clinical features.
The French-American-British (FAB) classification initially categorized patients morphologically for the diagnostic evaluation of MDS.1 Of importance for diagnosis is the morphologic finding of dysplastic changes in at least 2 of the 3 hemopoietic cell lines. These include megaloblastoid erythropoiesis, nucleocytoplasmic asynchrony in the early myeloid and erythroid precursors, and dysmorphic megakaryocytes.2 MDS patients have been classified by FAB as having 1 of 5 subtypes of disease:
Refractory anemia (RA): < 5% marrow blasts;
RA with ringed sideroblasts (RARS): < 5% blasts plus ≥ 15% ringed sideroblasts;
RA with excess of blasts (RAEB): 5-20% marrow blasts;
RAEB in transformation (RAEB-T): 21-30% marrow blasts; and
Chronic myelomonocytic leukemia (CMML): ≤ 20% marrow blasts plus monocytosis > 1000/mm3.
CMML has been categorized as MDS, although it often has characteristics of a myeloproliferative disorder (MPD). Some groups have separated these patients into proliferative and nonproliferative/dysplastic subtypes, with prognosis being most dependent on the proportion of marrow blasts. Patients with the dysplastic form have been classified within the FAB subtypes based on their percentage of marrow blasts.
Methods are needed to enhance our ability to stratify patients by their morphologic and biologic features. Such approaches could improve prognostication and treatment for these individuals. Regarding morphologic approaches, a World Health Organization (WHO) panel has recently issued a report with proposals for reclassifying MDS,3,4 although it has not yet been universally accepted because of certain controversial issues.5 In this report, suggestions have been made to modify the FAB definitions of MDS. Although most prior data require at least 2-line dysplasia to diagnose MDS, the WHO guidelines accept unilineage dysplasia for the diagnosis of RA and RARS, so long as other causes of the dysplasia are absent and the dysplasia persists for at least 6 months. Table 1 provides a comparison of the FAB and WHO classifications.
Other categories within the WHO proposal include refractory cytopenia with multilineage dysplasia (RCMD), separating RAEB patients into those with < 10% versus > 10% marrow blasts, 5q syndrome, and MDS Unclassified. MDS/MPD has been proposed for patients who previously had been classified as CMML.
The WHO panel has also suggested excluding RAEB-T patients from MDS (proposing acute myeloid leukemia [AML] to now include patients with ≥ 20% marrow blasts, rather than the previously used 30% cutoff). However, as stated above, MDS is not only a disease related to blast quantitation, but one that possesses a differing pace related to its distinctive biologic features, in contrast to de novo AML. Recent studies have provided conflicting evidence regarding the utility of the WHO proposals.6,7 Further studies will be needed to substantiate the prognostic value of this system.
Additional morphologic advances (e.g., degree of dysplasia, fibrosis) could provide additive information for characterizing MDS by building upon well-established forms of MDS categorization. Regarding biologic advances, as a new understanding of critical molecular, immunologic, immunophenotypic (using flow cytometry) and cytogenetic features of MDS emerges, these parameters will also be added to currently accepted methods as a means to improve the characterization of MDS.
How long will I live with my disease? What problems should I anticipate experiencing with my disease? What is my chance of developing leukemia?
B. Disease Natural History
1. Clinical features
One of the major morbidities of MDS is symptomatic anemia, with associated fatigue, which occurs in the vast majority (∼60-80%) of patients. Other cytopenias may also contribute to the patient’s symptom distress, including neutropenia (∼50-60%) and dysfunctional neutrophils leading to an increased incidence of infections. Thrombocytopenia (∼40-60%) and thrombocytopathy ensue in more advanced forms of MDS, with associated bleeding. Of importance is being alert to the potential postoperative bleeding and infectious complications that may ensue in these patients who possess dysfunctional platelets and neutrophils. Proactive management of patients’ perioperative periods with relevant transfusion and antibiotic support is quite important.
With a moderate degree of variability, RAEB patients and those with RAEB-T generally have a relatively poor prognosis, with a median survival ranging from 5 to 12 months (reviewed in Greenberg8). In contrast, RA patients or RARS patients have median survivals of approximately 3 to 6 years. The proportion of these individuals who transform to AML varies similarly, ranging from 40% to 50% in the relatively high-risk RAEB/RAEB-T patients, and from 5% to 15% in the low-risk RA/RARS group. Regarding time-to-disease evolution, 25% of patients with RAEB and 55% of patients with RAEB-T underwent transformation to AML at 1 year; 35% of patients with RAEB and 65% with RAEB-T underwent transformation to AML at 2 years. RAEB patients with ≥ 10% blasts have poorer prognoses than do those with <10% blasts. In contrast, for patients with RA the incidence of transformation was 5% at 1 year and 10% at 2 years, and none of the RARS patients underwent leukemic transformation within 2 years.
In addition to having symptoms related to their cytopenias and need for multiple transfusions, MDS patients have major concerns about the potential for their illness to evolve into acute leukemia. Emotional stress and life-planning issues need to be addressed. All of these features lead to difficulties patients have with their quality of life (QOL).9,10 Assessment of and engagement in the patients’ relevant QOL domains—physical, functional, emotional, social, spiritual—are important for determining and potentially improving the clinical status of these individuals. Several recent studies have demonstrated the positive effects of effective therapy for MDS on patients’ QOL.11,12
2. Prognostic stratification
Despite its value for diagnostic categorization of MDS patients, the prognostic limitations of the FAB classification have become apparent, with quite variable clinical outcomes within the FAB subgroups. The morphologic features contributing to this variability include the wide range of marrow blast percentages for patients with RAEB (5-20%) and CMML (1-20%); lack of inclusion of critical biologic determinants, such as marrow cytogenetics; and the degree and number of morbidity-associated cytopenias. These well-perceived problems for categorizing MDS patients have led to the development of additional risk-based stratification systems.13,14
The International MDS Risk Analysis Workshop developed a consensus risk-based International Prognostic Scoring System (IPSS) for primary MDS (Table 2 ).14 Compared with prior systems, the IPSS has markedly improved prognostic stratification of MDS patients. In the workshop, cytogenetic, morphologic, and clinical data were combined and collated from a relatively large group of patients who had been included in previously reported studies that relied on independent risk-based prognostic systems. FAB morphologic criteria were used to establish the diagnosis of MDS.
Patients with CMML were subdivided into ‘proliferative’ and ‘nonproliferative-dysplastic’ subtypes. Proliferative type CMML patients (those with white blood cell counts > 12,000/mm3) were excluded from this analysis, since these individuals predominantly represented MPD rather than MDS.15 Nonproliferative CMML patients had white blood cell counts ≤ 12,000/mm3 as well as other features of MDS, and were included.
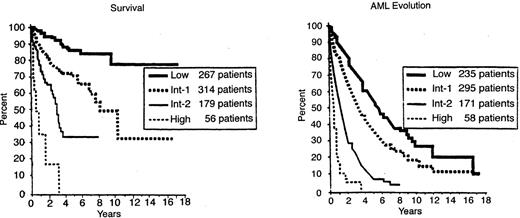
The most significant independent variables for determining outcome for both survival and AML evolution were found to be marrow blast percentage, number of cytopenias, and cytogenetics subgroup (Good, Intermediate, Poor) (Table 2).14 Patients with normal marrow karyotypes, del (5q), del (20q), and –Y (70%), had relatively good prognoses, whereas patients with complex abnormalities (i.e., ≥ 3 anomalies) or chromosome 7 anomalies (16%) had relatively poor prognoses. The remaining patients (14%) were intermediate in outcome. Of the patients in the complex category, the vast majority had chromosome 5 and/or 7 abnormalities in addition to other anomalies.
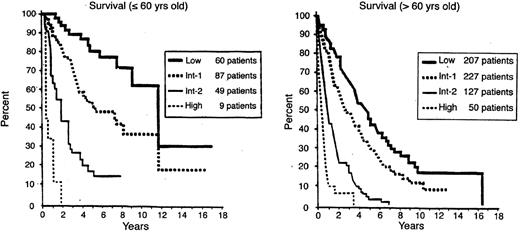
When the risk scores for the 3 major variables were combined, patients were stratified into 4 distinctive risk groups in terms of both survival and AML evolution. These risk groups are Low, Intermediate-1 (Int-1), Intermediate-2 (Int-2), and High (Table 2). Median survivals and risk of MDS evolution were determined, and survival was shown to also be related to age (Table 3 and Figures 1 and 2 ).14 Much less precise discrimination between the 4 subgroups occurred when either cytopenias or cytogenetic subtypes were omitted from the classification. This system separated patients into relatively low-risk (IPSS Low, Intermediate-1 [Int-1]) and high/poor-risk (Intermediate-2 [Int-2] and High) prognostic groups.
Extension of this system was planned to subsequently include certain immunologic, morphologic, and molecular anomalies that would also be shown to have an impact on clinical outcomes. Flow cytometric analysis of blasts from MDS patients has provided a valuable additive prognostic tool, as demonstrated by a recent study from Japan.16 These investigators showed that marrow blasts from most MDS patients possess a specific immunophenotypic signature that is distinct from AML and normal blasts.16 These investigators showed that a high percentage of enriched MDS blast cells had an immunophenotype descriptive of committed progenitor cells (i.e., were positive for CD34, 33, 13, 38, HLA-DR). In addition, differential expression of other surface markers on these blasts correlated with stage of disease and prognosis. Thus, the immature-type CD7 marker was generally positive on blasts from late-stage MDS patients who had poor clinical outcomes, whereas the more mature CD15 marker was generally positive on blasts from MDS patients with earlier stage disease and better prognoses. A shift occurred to a more immature phenotype accompanying disease progression. These investigators also demonstrated that RAEB-T blasts possessed immunophenotypic markers more closely related to MDS than to de novo AML, indicating that there are biologic differences between these entities.16 Incorporation of such analyses into the IPSS system should further refine the ability to provide useful prognostic information in MDS.
The separation of CMML patients into proliferative and non-proliferative/dysplastic subgroups was supported by a recent study of their clinical outcomes.17 An extensive review of CMML patients has also indicated that CMML patients could be subdivided into 4 prognostic risk groups based on a combination of independent predictive factors: level of marrow blasts and degrees of anemia, peripheral blood immature mononuclear cells, and lymphocytes.18 In these patients, the presence of ras mutations, high LDH, and high β2 microglobulin levels were associated with poorer prognoses.
What treatments are available for my disease? Which treatment(s) should I receive? When should I receive them?
C. Therapeutic Options
Data suggest that pathogenetic lesions in MDS relate to ineffective hemopoiesis due to enhanced apoptosis of hemopoietic cells within MDS marrow, particularly in low-risk patients.19–,22 This intramedullary hemolysis is contributed to by increased marrow production of inhibitory cytokines such as tumor necrosis factor-alpha (TNF-α), occurring in part through enhanced angiogenesis.19,22– 24 During disease progression, decreased apoptosis of the hemopoietic precursors occurs with associated expansion of the abnormal clone. Biospecific agents aimed at countering some of these lesions in MDS, which currently are being evaluated in experimental trials, include stimulatory growth factors, antiapoptotic agents, blockers of inhibitory cytokines, antiangiogenesis compounds (mainly for low-risk disease), and ras inhibitors and arsenicals (mainly for higher-risk disease; see below).
The therapeutic options for MDS include supportive care, low-intensity therapy, and high-intensity therapy. The main goal of low-intensity therapy generally is to cause hematologic improvement and is used mainly in low-risk disease, whereas high-intensity therapy generally aims to alter the disease’s natural history (i.e., improve survival, decrease AML evolution) and is used mainly in higher-risk disease.25 Concomitant with both aims is the desire to improve patients’ QOL. Cytogenetic responses are surrogate markers of response that will likely have an impact on disease outcome similar to that found in treatment of other myeloid malignancies.
1. Supportive care
Currently, the standard of care in the community for MDS is ‘supportive care.’ This type of care entails red blood cell transfusions, generally leukocyte reduced, as needed for symptomatic anemia or platelet transfusions for severe thrombocytopenia or thrombocytopenic bleeding. For patients with excessive iron accumulation due to the number of red blood cell transfusions received (generally > 40 units), iron chelation therapy should be instituted, generally with subcutaneous nightly desferrioxamine.26 For this parameter, serum ferritin levels and associated organ dysfunction (e.g., heart, liver, pancreas) should be monitored.
2. Low-intensity therapy
Low-intensity therapy includes use of biologic response modifiers or low-intensity chemotherapy, agents that generally can be administered to outpatients, in addition to supportive care. These agents [except for erythropoietin (Epo), for which much data regarding its efficacy exist] should be administered in the context of clinical trials, because for most of these agents limited information is available regarding their general efficacy, optimal doses, toxicity, or the appropriate selection of patients.
a. Treatment of the anemia of MDS: Epo±G-CSF.
Hemopoietic stimulatory cytokine support should be considered for treating refractory symptomatic cytopenias, particularly the anemia occurring in MDS.27 Much progress has been made in improving the management of the anemia in MDS, with its associated fatigue. The general presentation of the anemia related to MDS is a hypoproductive macrocytic anemia, often associated with suboptimal elevation of serum erythropoietin (Epo) levels.8 Morphologic examination of a bone marrow aspiration is important to determine FAB subtype, iron status, and the presence or absence of ringed sideroblasts. The potentially beneficial use of recombinant human Epo to treat symptomatic anemia in MDS has been well established. Data indicate that Epo given daily or 3 times a week subcutaneously in relatively high doses may be efficacious, generally in doses of 10,000-20,000 units daily, with response rates of 20-30%.28 For responders, drug doses and their frequency may be reduced as tolerated. For patients not responding after 4-6 weeks, the drug could be given in combination with granulocyte colony-stimulating factor (G-CSF), 1 μg/kg/day (or with granulocyte-macrophage colony-stimulating factor [GM-CSF]), which will generally double the response rate (i.e., to 40%-60%).29 This response pattern is particularly true for MDS patients with serum Epo levels < 500 mU/mL and for those with ringed sideroblasts.30,31 Evidence has indicated that G-CSF (and to a lesser extent GM-CSF) has synergistic erythropoietic activity when used in combination with Epo and markedly enhances the erythroid response rates.29– 31 This is particularly evident for patients with ringed sideroblasts or with serum Epo levels < 500 mU/mL.
Iron repletion needs to be verified prior to instituting Epo therapy. Often, despite adequate iron stores, poor utilization of storage iron in many of these patients may make oral iron supplementation helpful. Erythroid response generally occurs within 2 to 3 months of treatment. If no response occurs by that time, this treatment should be considered a failure and discontinued and alternative therapies considered (generally within a clinical trial). These therapies to consider (indicated below) include specific attempts at altering biologic lesions in MDS: anti-angiogenic compounds (anti-vascular endothelial growth factor [VEGF] drugs), anti-tumor necrosis factor (TNF) agents (e.g., Enbrel), thalidomide, 5-azacytidine (AzaC), immunosuppressive type therapies (e.g., antithymocyte globuline [ATG], cyclosporin), singly or in combination. As multiple biologic abnormalities contribute to the cytopenias of MDS, it is likely that combination therapy will be needed to sustain hematologic responses in these patients.
A recent report indicated the presence of neutralizing anti-erythropoietin antibodies associated with pure red cell aplasia and Epo resistance in a small number of patients with renal failure who had received chronic Epo treatment.32 Virtually all such cases have been reported from Europe and may relate to differing Epo preparations or manufacturing processes which differ from those employed elsewhere. Morphologic marrow examination and Epo antibody assays will aid in diagnosis of this iatrogenic problem.
For attempting to enhance erythropoiesis in MDS by using less frequent dosing, a modified form of erythropoietin (darbepoetin) with a 3-fold longer biologic half-life than standard Epo has shown good efficacy with less frequent dosing for patients with anemias induced by renal failure or chemotherapy.33 Trials are ongoing to assess the role of this agent in MDS.
b. Treatment of neutropenia.
Although low neutrophil levels have been demonstrated to be improved with either recombinant human G-CSF or GM-CSF, data have not shown decreased infections or improved infection management with these agents in MDS. A Phase III randomized trial in high-risk MDS that compared chronic administration of G-CSF to observation and demonstrated no difference in the incidence or pace of development of AML or of infections in the 2 study arms.34 For those patients with RAEB, a shortened survival was noted in the G-CSF arm. Thus, this agent is not recommended for chronic prophylactic use alone in MDS. Rather, a suggested approach is to employ these cytokines in neutropenic MDS patients with recurrent or antibiotic-refractory infections.
c. Treatment of thrombocytopenia.
For treating thrombocytopenia in MDS, relatively low doses (10 μg/kg/d) of IL-11 have recently been shown in a Phase I/II trial to be more effective than higher doses of the drug.35 Further studies are ongoing with this agent. Occasional efficacy of danazol has also been reported in such patients. Studies are warranted to determine the potential efficacy of thrombopoietin for the thrombocytopenia present in MDS.
d. 5-azacytidine.
As a form of low-intensity chemotherapy, recent encouraging data in MDS have been reported with the hypomethylating agent AzaC.36 In a randomized Phase III trial, AzaC decreased the risk of leukemic transformation; it also improved the survival of a portion of the patients.36 This trial evaluated 171 MDS patients, comparing AzaC (75 mg/m2/d subcutaneously for 7 days every 28 days, predominantly as an outpatient) with supportive care. Patients in the supportive care arm whose disease worsened were permitted to cross over to AzaC. Responses occurred in 60% of patients on the AzaC arm (7% complete response, 16% partial response, 37% improved) compared with 5% (improved) receiving supportive care. Median time to leukemic transformation or death was 21 months for AzaC versus 13 months for supportive care. Transformation to AML occurred in 15% of patients on the AzaC arm and in 38% receiving supportive care. Eliminating the confounding effect of early crossover to AzaC, a landmark analysis after 6 months showed median survival of an additional 18 months for AzaC and 11 months for supportive care. QOL assessment found significant major advantages in physical function, symptoms, and psychological state for patients initially randomized to AzaC.12 Thus, AzaC treatment resulted in significantly higher response rates, improved QOL, reduced risk of leukemic transformation, and improved survival compared with supportive care. This agent appears to provide a new treatment option that is superior to supportive care for patients with MDS. Further trials with this agent are ongoing to attempt to confirm and extend these findings.
e. Immunosuppressive therapy.
The biologic response modifiers potentially capable of altering immune medicated subtypes of MDS which are now available include ATG and cyclosporine37– 40 and will be discussed in detail by Dr. Neal Young in Section II. However, these data, though encouraging for specific subtypes of MDS (mainly hypoplastic MDS patients with normal cytogenetics and evidence of a paroxysmal nocturnal hemoglobinuria [PNH] clone and HLA-DR2 subtype), require further evaluation in more extended clinical trials.
f. Anti-TNF, anti-angiogenesis agents.
Numerous Phase I/II clinical trials evaluating the roles of specific biologic response modifiers have been used to treat MDS. Relatively small pilot trials with anti-TNF fusion protein (etanercept, Enbrel) indicated erythroid responses in about 30% of patients in one trial, and lower levels of responses in another.41,42
Clinical trials assessing the role of antiangiogenesis compounds [e.g., thalidomide, anti-VEGF agents] are ongoing. Although thalidomide has multiple mechanisms of action, its role as an antiangiogenic or anti-TNF agent may be important in its therapeutic effects. Results of treatment with thalidomide have been reported in 2 separate trials, with a 19% response rate in one and 56% in the other, including erythroid and trilineage responses.43,44 In the latter trial, relatively high doses of thalidomide (i.e. > 300 mg/day) were tolerated, an unusual finding for this elderly population. Additional studies, including those with less toxic metabolites of thalidomide, are ongoing to better define the role of these agents in MDS.
Use of a number of biologic response modifiers (amifostine, pentoxyphylline, interferon, low-dose AraC, retinoids, vitamin D analogues, butyrates) has previously been shown to have quite limited efficacy in Phase I-II trials with MDS patients.8,45 Other ongoing clinical trials include the use of agents capable of inhibiting neoplastic growth, such as anti-ras type compounds [farnesyl transferase inhibitors (FTIs)] and arsenic trioxide. Recent preliminary studies have shown efficacy of FTIs in treating poor-risk AML, CMML, and MPDs.46,47
3. High-intensity therapy
High-intensity therapy includes intensive induction chemotherapy or hemopoietic (marrow or peripheral blood) stem cell transplantation (HSCT).8,48 Although these approaches have a greater chance of changing the natural history of the disease, they also have an attendant increased risk of regimen-related morbidity and mortality. The management of patients with relatively high-risk (i.e., IPSS Int-2, High) MDS or MDS-AML is particularly problematic and, as indicated above, must involve consideration of their age and performance status. These patients are generally elderly, often with major co-morbidities, and have a higher proportion of biologic abnormalities of their HSCs. The marrow precursors of high-risk MDS and MDS-AML patients are often associated with multilineage dysplasia and possess concomitant features of primitive marrow stem cells, poor-risk marrow cytogenetic abnormalities, and a higher degree of cellular MDR than do those from de novo AML.49,50
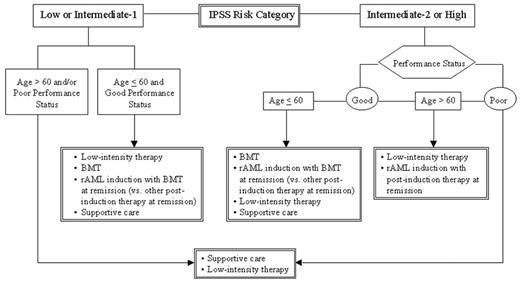
For individuals < 60 years old with relatively high-risk disease and good performance status, preference should be given to high-intensity therapy (Figure 3 ).51,52 In this clinical setting, using an HLA-matched donor for allogeneic HSCT is the preferred high-intensity therapy option. For those lacking a matched donor, induction chemotherapy (intensive or with experimental biologic agents), AzaC, or experimental trials are preferred. For high-risk patients > 60 years old with good performance status, AzaC or high-intensity experimental trials are preferred (Figure 3).
a. Chemotherapy.
Responses to standard chemotherapy in MDS or MDS-related AML are lower than in de novo AML.8,53 This disparity is due to both the generally elderly age of MDS patients and the differing biology of the MDS stem cell and marrow stroma, including poor-risk cytogenetics, earlier stem cell phenotype, and increased expression of multidrug resistance (MDR) markers. Recent comparative studies have not demonstrated benefit of several different intensive chemotherapy regimens in MDS.54 Trials of intensive chemotherapy in MDS have established that advanced age (> 45-50 years) is an unfavorable prognostic factor. Furthermore, MDS patients with karyotypic abnormalities have lower complete remission and survival rates compared to patients with normal cytogenetics.
A high degree of MDR-1 (p170 glycoprotein) occurs in advanced MDS precursors with associated decreased responses and response durations with many standard treatment regimens of induction chemotherapy.50 Numerous compounds, including quinine, cyclosporin A, and the cyclosporin A analog PSC-833 (Valspodar), have been tested for their ability to reverse the MDR phenotype. The French Cooperative Leukemia Group reported the results of a Phase III randomized study that showed that quinine plus chemotherapy significantly increased the complete remission rate and survival in MDR-1-positive MDS patients compared to chemotherapy alone.55 This positive experience has generally not been reproduced with other MDR-1 modulating agents.56 Additional MDR modulating agents plus chemotherapy are being evaluated for treating patients with advanced MDS. Given the multiple possible resistance mechanisms extant in MDS/leukemic cells, after such lesions are identified, it may be useful to try combinations of MDR blockers.
Another hypomethylating agent with activity and mechanism of action similar to AzaC is decitabine (5-aza-2′-deoxycytidine). With doses requiring hospitalization of patients, encouraging results have been shown treating high-risk MDS patients, some showing cytogenetic conversion.57 The overall response rate was 49%, with 64% responses in patients with a high-risk IPSS. The median response duration was 31 weeks, and the median survival from the start of therapy was 15 months (14 months for the high-risk IPSS patients). In several patients, normalization of clonal cytogenetic abnormalities (often high-risk) was seen. An ongoing phase III study aims to establish whether these responses translate into prolonged overall survival for high-risk patients.
Clinical trials are now ongoing using agents targeted to block neoplastic cell proliferation, including use of FTIs. This predominantly outpatient-based orally administered regimen has recently been shown to have encouraging efficacy for poor-risk AML patients as well as for MPD and CMML, and it is less toxic than intensive chemotherapy.46,47 These features are potentially valuable for the generally elderly high-risk MDS patient population, whose members are often ineligible for intensive chemotherapy. Further evaluation of these trials in comparison to standard therapy will be necessary to establish their relative efficacy.
b. Hemopoietic stem cell transplantation (HSCT).
Numerous studies have provided information regarding the potential prolonged survival and possible curative role of HSCT for a portion of MDS patients. Allogeneic sibling and matched unrelated donors are suggested for many patients < 60 years old who have good performance status. However, such treatment for MDS patients, particularly those who are elderly, comes with a relatively high attendant risk of transplant-related morbidity and mortality. Further, given the age and performance status restrictions, along with limited donor availability, many MDS patients are not eligible for such treatment. For those who are eligible, institutions use their individual age cutoffs, based in part on their track record and on the competing protocols.
Several large studies from the Fred Hutchinson Cancer Research Center, Seattle, and from the European Bone Marrow Transplant Group (EBMTG) using allogeneic HSCT from an HLA-matched sibling donor have indicated 3-5 year disease-free survival (DFS), transplant-related mortality (TRM), and relapse rates of 41/34%, 42/44%, and 17/39%, respectively (numbers refer to FHCRC/EBMTG percentages).58,59 Relapse rates generally increased from 4% in the low-risk patients to 27% in the higher-risk Seattle patients (i.e., those with RAEB-II, RAEB-T, or IPSS-2, High). DFS also diminished with increased IPSS risk categories: 3-year DFS was 60%, 36%, and 28% in IPSS Int-1, -2, and High, respectively. In a Vancouver study, DFS was particularly related to marrow cytogenetics, with DFS of 51%, 40%, and 6% in IPSS Good, Intermediate, and Poor risk categories, respectively.60
Results for allogeneic HSCT from matched unrelated donors (MUD) for MDS have generally been less successful than those from matched sibling donors. Data from the National Marrow Donor Program regarding these MUD transplants demonstrated 29% 2-year DFS and 54% TRM but relapse rates of only 14%.61 Graft failure and GVHD were particular problems in these transplants. Improved DFS was independently associated with less advanced MDS subtype, higher cell dose, recipient cytomegalovirus (CMV) seronegativity, shorter interval from diagnosis to transplantation, and transplantation in recent years. Higher TRM was independently associated with older recipient and donor age, HLA mismatch, and recipient CMV seropositivity. However, recent studies from Seattle have demonstrated improved results for these patients using targeted busulfan levels plus cytoxan in preparative regimens.62 Allogeneic HSCT with related/unrelated matched donors in 109 patients had 3 year DFS, TRM, and relapse rates of 56/59%, 28/30%, and 16/11%, respectively. In addition, relapse-free survival was improved in those receiving peripheral blood compared to marrow stem cells.
Non-myeloablative HSCT regimens63 with improved patient tolerance, particularly for the elderly MDS patients, are important experimental options currently being evaluated. In certain research settings, autologous HSCT are performed after chemotherapy-induced remission.64 Comparative clinical trials are needed to establish whether such transplants should be performed before or after achievement of remission following induction chemotherapy. Major progress is being made in transplant biology and clinical approaches, with specific focus for MDS. Such advances should provide improved therapeutic options for treating these patients within the foreseeable future.
Autologous HSCT has been used for treating some patients in complete remission after induction chemotherapy. Although the transplant-related mortality is low with this approach, it is attended by a relatively high relapse rate.
4. Factors determining treatment approaches
Based on the above biologic and clinical considerations, deliberations by the U.S. National Comprehensive Cancer Network (NCCN) Panel for MDS Practice Guidelines have suggested51 that management approaches utilizing an algorithm composed of the patient’s: (a) IPSS prognostic subgroup categorization; (b) Age; and (c) Performance status.
This approach provides a useful risk-based method to stratify therapeutic options and permits individualized prioritization of low- versus high-intensity therapy for patients in differing risk categories. Careful monitoring for disease progression and the patient’s desires play major roles in the timing and decision to embark on specific treatment. It is useful to stratify patients into age groups > 60 or < 60 years old, as this is often the age of eligibility for certain intensive therapeutic options, particularly for HSCT. Medical centers use differing age cutoff points, and thus biologic rather than chronologic age may be used to establish patients’ protocol eligibility.
Low-intensity treatments (e.g., with biologic response modifiers) are generally preferred for MDS patients in the lower-risk categories (IPSS Low and Int-1), whereas high intensity (e.g., intensive chemotherapy or HSCT) is used for the higher-risk patient group (IPSS Int-2, High), depending on the patient’s age, performance status, and personal preferences51 (Figure 3 and ref. 52).
A finite period of observation (at least 4-6 weeks) is needed to determine the degree of clinical stability for MDS patients. Individuals with unstable disease (i.e., those with evidence of clinical progression, including a decrease in blood counts or increasing transfusions) need follow-up evaluation, which should include a repeat marrow examination. If the patient remains in the Int-1 or Low-risk group, the treatment options indicated for those patients (Table 3) should be continued. If, however, the patient progresses to the Int-2 or High-risk groups (or the patient had initially been in one of these risk groups), the treatment path for these higher-risk categories should be used.
For patients with poor performance status in either risk category, only supportive care or low-intensity treatment is suggested. This proposal relates to their concurrent co-morbidities, rendering them unlikely to tolerate high-intensity treatments.
How can I learn more about my illness? Are there clinical trials with which I can and should become involved? How do I find out about them?
D. Resources
Many institutions have health libraries with medical books, some focused intended for the lay public, that review the hematologic malignancies. Stocking such books in these libraries could be useful for MDS patients and their families, particularly if the information/jargon is “translated” in concert with health care professionals. Both the MDS Foundation (www.mds-foundation.org) and the Aplastic Anemia-MDS Foundation (www.aamds.org) have developed websites and published booklets providing information for patients, physicians, and other health care professionals regarding MDS. These websites describe ongoing clinical trials and indicate national and international institutions that are involved in these trials and with physicians who possess special expertise in evaluating and treating MDS. Patient and physician contact with physicians at these institutions and their involvement in these trials will be central for progress to occur in developing appropriate therapeutic directions for this disease.
II. Role of the Immune System in the Pancytopenia of MDS and Immunosuppressive Therapies
Neal S. Young, MD*
Chief, Hematology Branch, Building 10, Room 7C103, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892-1652
The concept of MDS originated in the merger of two ill-defined but clearly different hematologic processes— “preleukemia” and “refractory anemia”—and the FAB classification attempted to collect several disparate diagnostic categories by shared histologic features, mainly the appearance of dysmorphic marrow cells. This organizational scheme is now fracturing: on one side, the more obviously malignant categories—RA with excess blasts and chronic myelomonocytic leukemia—have been ceded to classifications of the acute leukemias and myeloproliferative processes; on the other, the indistinct boundary between hypocellular MDS and aplastic anemia (AA), largely ignored by the FAB classification, has been enlarged with the recognition of similar pathophysiologic mechanisms and common responses to therapies extending to some pancytopenias associated with frankly cellular bone marrows.
Indeed, pancytopenia, not leukemia, is the proximate cause of death in patients who die as a result of MDS. Why hematopoiesis fails, and especially the explanation for the development of bone marrow hypocellularity that accompanies this failure, is not clearly understood. Nevertheless, recent successful therapeutic pilot studies and novel results of laboratory studies, reviewed here and elsewhere,1,2 suggest that MDS is closely related to diseases in which the pathophysiology of bone marrow failure is mediated at least in part by the immune system.
A. MDS and AA
For the clinician, MDS and AA can be difficult to distinguish. AA has been defined historically by extremely low bone marrow cellularity on biopsy. A significant proportion of MDS also shows marrow hypocellularity, with large series averaging about 20% of cases; hypocellular MDS has not been reported to be markedly different in clinical characteristics like age, FAB subtype, or prognosis from the more common cellular disease.3,4 However, between AA and MDS the differential diagnosis often relies on “soft” histologic criteria, such as myeloid cells lacking granules (an appearance that can also arise from technical staining artifact), dyserythropoiesis (with megaloblastoid changes frequently seen in aplasia), or dysmorphic megakaryocytes (but these cells are usually scanty in a hypocellular aspirate smear). Bone marrow cell cytogenetic abnormalities are more objective evidence of MDS, but some authorities regard findings such as trisomy 6 or 8 as simply delineating a subset of otherwise typical AA. Both AA and MDS are associated with expansion of clones of PIG-A mutated stem cells, lacking cell surface expression of the glycosylphosphoinositol-linked proteins diagnostic of paroxysmal nocturnal hemoglobinuria (PNH), leading to diagnoses of AA/PNH or MDS/PNH.
The distinction between MDS and AA becomes further blurred because some AA patients, usually after immunosuppressive therapy, appear to evolve to MDS, with recurrent pancytopenia associated with bone marrow dysplasia or chromosomal aberrations. In our series of 122 patients with severe AA treated at NIH with antithymocyte globulin (ATG) and cyclosporine, 13 had evolved at 5 years; in the European Group for Bone Marrow Transplantation experience, the risk for evolution to MDS was estimated at about 12% at 12 years posttreatment.5,6 There are stereotypical patterns of chromosome changes:7 monosomy 7 is most frequent, associated with refractory pancytopenia and leukemic conversion in patients who have not responded to immunosuppressive treatment; in contrast, trisomy 8 typically is observed in patients whose adequate blood counts often require continued cyclosporine therapy.
AA is a more uniform clinical entity than is MDS: blood count findings are usually striking, the bone marrow morphology is unambiguous, and the response to therapy is relatively predictable. The essential pathophysiology of acquired AA also has been delineated, at least in outline,8 as an efficient and specific immune system attack on hematopoietic stem and progenitor cell targets, leading to the absence of these cells on morphologic, phenotypic, and functional assays. T cells of Th1/Tc1 cytokine profile, producing interferon-γ and tumor necrosis factor (TNF), induce apoptosis in their marrow targets through activation of the Fas receptor, leading to destruction of the hematopoietic cell compartment. At the time of presentation with severe pancytopenia this process is advanced; surrogate stem cell assays suggest that the stem cell pool is reduced to a few percentage points or less of normal. Most patients do respond with hematologic improvement to immunosuppressive therapies, but both relapse of pancytopenia and a requirement for continued cyclosporine administration to maintain adequate blood counts are common.
B. The Origin of Hematopoietic Failure in MDS
1. MDS hematopoiesis in tissue culture
The highly varied pattern of hematopoiesis in tissue culture in MDS should be contrasted with a monotonous picture in AA. Cell proliferation rates are often elevated, as measured by in vitro nucleotide incorporation,9 autonomous colony formation,10 or expression of specific cell markers like MIB-1,11 reflective of the cellular character of most MDS bone marrows. Hematopoietic progenitor-derived colony formation is variable: early and late erythroid progenitors (CFU-E and BFU-E, respectively) are usually diminished, consistent with the prevalence of anemia, while myelopoiesis from granulocyte-macrophage colony forming cells (CFU-GM) may be near normal in the majority of the same cases.12 More primitive multipotential progenitors (CFU-GEMM) generally have been reduced,13 as have long-term culture-initiating cells (LTC-IC) (secondary colony formation was usually low, but a quarter of patients nevertheless retained normal numbers;14 others found blast colony-forming normal in number but deficient in secondary erythroid colonies).15 Entirely normal colony formation, as occurs in a significant proportion of patients, is found in good prognostic subtypes, such as 5q and ringed sideroblast anemia. Conversely, patients with low CFU-GM numbers, aberrant myeloid maturation in vitro, predominant cluster over colony formation, and abnormal immunophenotypes of progenitors (all findings seen in acute myelogenous leukemia) have an expectedly poor prognosis.12 The response of progenitors to hematopoietins in vitro is poor, even with high concentrations of purified or recombinant factors, alone or in combination. Tissue culture studies have not been especially predictive of therapeutic outcomes in clinical interventional trials, but pharmacologic concentrations of erythropoietin, G-CSF, or GM-CSF do increase lineage-specific colony growth, at least in a subset of patients.16 Purified CD34 cells from MDS patients have generally normal, if variable, response profiles to early acting growth factors such as stem cell factor, interleukin (IL)-3, and IL-6,17,18 but primitive colony formation, when low, has been unaffected by addition of exogenous factors.19
2. Cell death in the dysplastic marrow
The occurrence in MDS of pancytopenia despite normal to increased numbers of marrow precursor cells led to the inference that cell death in the hematopoietic cell compartment is dominant over cell proliferation (for reviews, see refs. 20–,22). That the histologic abnormalities of MDS might reflect not only a process of intramedullary cellular destruction but specifically apoptosis or programmed cell death was first proposed by Clark and Lampert,23 and more recent, quantitative studies have supported this conclusion: for example, an elevated apoptotic index of 3% in MDS compared to normal marrow values of 1%, as based on morphological and ultrastructural changes on thin section biopsies.24 Poor growth of colonies and dominance of clusters may reflect a high initial rate of growth followed by failure of both proliferation and differentiation.25 However, cultured MDS cells also manifest much more striking degrees of apoptosis than may be seen in fresh cells, a potential source of artifact.26 In biopsy specimens, single cells can be examined for evidence of apoptosis, but their exact lineage usually cannot be confidently determined, and not only hematopoietic precursors but also stromal cells, endothelium, and fat may score positive.27
Other more specific measurements of apoptosis have been abnormally elevated in MDS in comparison with normal and also leukemic marrow: in situ end labeling (ISEL) detection of DNA strand breaks; terminal deoxynucleotide transferase incorporation of nucleotides on 3′ ends of DNA (TUNEL); detection of subgenomic DNA in histograms of cell populations subjected to fluorescence-activated flow cytometry; binding of annexin-V to exposed phosphatidylserine of cell membranes, also assessed by flow cytometry; and the detection of apoptosis-related proteins like bcl-2 in individual permeabilized cells. Mechanistically, low bcl-2 expression has correlated with high apoptotic rates,10,28,29 and enhanced bcl-2 expression has been linked to leukemic transformation; sequential caspase activation consistent with appropriate death signal transduction also has been reported.30 Improvements in blood counts with growth factor therapy31 and anti-inflammatory agents like pentoxifylline32 have correlated with lowered rates of apoptosis. However, the MDS patient populations examined have been very heterogeneous, and the range of results broad; for example, in one large study of 175 patients by ISEL, 71 showed high and 43 low levels of apoptosis, and 61 were normal.33
More detailed conclusions have proven controversial. First, one appealing hypothesis proposed that programmed cell death dominated early in the pancytopenic phase of MDS, and that leukemic transformation represented escape from the apoptotic (intracellular) process or (extracellular) environment. Higher rates of apoptosis have been observed in RA and RAEB compared with transforming MDS or acute leukemia,28,34–,36 while others have found increased rates in late stages of disease.37 Second, a question remains as to the extent of apoptosis in MDS, with quantitative inferences ranging from minimally or modestly increased26 to massive destruction,38 arguments confounded by significant levels of apoptosis in normal marrow specimens, and often relatively small differences between MDS and normal. Third, is apoptosis restricted to certain hematopoietic cells in MDS? Evidence from flow cytometric analysis of individual cells supports apoptosis in the CD34 cell compartment.34,36,39 Others have suggested that cell death is far more frequent among mature cells (of high compared with low density)38 and rare by TUNEL measurements in CD34 cells within biopsies.40
3. Pro-inflammatory cytokines and immune triggering of apoptosis in MDS
Similar mechanisms of hematopoietic cell destruction have been hypothesized to operate in MDS as for AA. Early observations included measurements of overexpression by cultured MDS patients’ blood41 and marrow cells42 of lymphocyte type I cytokines, TNF, and interferon-γ. TNF levels have been elevated in MDS marrow biopsies30,43 and in plasma and sera,44,45 as has circulating soluble TNF receptor.46 Levels have declined on treatment with anticytokine therapies.47,48 Multiplex polymerase chain reaction has shown elevated marrow messenger RNA (mRNA) for TNF among other cytokines in a subset of MDS patients.49 MDS bone marrows have evidence of downstream effects of TNF activity such as caspase activity,30 induction of nitric oxide synthase,50 and intracellular redox changes leading to DNA damage.51 As with levels of apoptosis, different groups have reported variable correlations: TNF expression has been elevated with RA45 or disease transforming to leukemia,52 heralded a poor prognosis,53 or been found entirely unrelated to MDS subtype.54
The source of TNF in MDS marrow has been assumed to be the macrophage;52 in one large study of marrow biopsies, TNF, transforming growth factor (TGF), and monocyte/macrophage numbers were highly correlated.55 Stromal elements56–,58 also have been implicated. Our group identified T lymphocytes functionally inhibitory of autologous marrow progenitor cells, with appropriate class I histocompatibility restriction and with diminished activity after successful immunosuppressive therapy (see below).59 Additionally, MDS patients may have increased cytotoxic T cells60 and a skewed T cell repertoire, as assessed by T cell receptor Vβ type.59
TNF expression has been studied in relationship to induction on hematopoietic target cells of Fas, a cell surface protein and member of the tumor necrosis factor receptor family; Fas triggering initiates programmed cell death. Flow cytometry has been used to measure increased Fas and Fas-ligand surface expression on MDS CD34 cells; in functional assays, monoclonal antibody-mediated blockade of Fas, Fas ligand, or TNF enhanced MDS hematopoiesis in long-term bone marrow colony culture and progenitor assays.45 Addition of anti-Fas ligand antibody in other experiments also decreased apoptosis in MDS marrow samples.61 TNF-related apoptosis inducing ligand (TRAIL) also may be involved in MDS: multiple members of this family and its receptors were overexpressed in patient marrows, and TRAIL addition has modulated hematopoietic colony formation by MDS but not normal marrow, with its effects varying with disease stage.62,63 Other in vitro experiments have linked Fas to ineffective erythropoiesis of MDS: in differentiating erythroid cells the rate of apoptosis in MDS CD34-cell derived culture was high, Fas was overexpressed in fresh and cultured cells, and the peak of apoptosis correlated with Fas ligand expression.64 Flow cytometric results for Fas and Fas-ligand expression have been confirmed by amplification of complementary DNA (cDNA) from mRNA45,65 and immunohistochemistry.35,65 Fas expression usually has not correlated to the degree of apoptosis in clinical specimens.66,67 Fas expression has been more striking in RA than in other MDS subtypes45 and may decrease in the MDS marrow during transformation to leukemia.35 An important clue as to the identity of a target of Fas-mediated cytotoxicity has been suggested by experiments using marrows from patients with different cytogenetic abnormalities: trisomy 8 cells express Fas and markers of apoptosis and are susceptible to Fas triggering in vitro, in contrast to cells from patients with monosomy 7 or other chromosome changes.68
C. Clinical Immune Dysfunction in MDS
Global peculiarities of immune system function have included decreased NK cell activity, antibody dependent cell killing, mitogenic response, and diminished CD4 cell numbers, and for B cell function altered immunoglobulin levels, monoclonal gammopathies, and various autoantibodies (reviewed in ref. 69). In a large Japanese single institution collection of 153 patients, 63% had some abnormal immunological test: hypergammaglobulinemia was most common followed by hypoglobulinemia, and the presence of antinuclear antibodies, rheumatoid factor, anti-DNA antibodies, and positive Coombs test.70 Frank autoimmune diseases may be increased in patients with MDS.69 In the series from Hyogo, Japan, described above, 12% of 153 patients developed some autoimmune disorder.70 Of 30 patients from the University of Minnesota, 18 showed systemic acute autoimmune phenomena: various combinations of vasculitis, arthritis, pleuritis, pericarditis, myositis, and neurologic symptoms.71 Many cases of lymphoid and plasma cell neoplasms occurring with MDS have been collected.72 Of special importance for pathophysiology as well as treatment, expanded populations of histologically recognizable large granular lymphocytes (LGL) in the marrow also can coexist with MDS. In a survey of 83 recent referrals we found 9 patients with MDS who also had a clonal expansion of circulating CD8+, CD57+, CD56+ LGL cells consistent with the diagnosis of LGL leukemia.73 Individual patients may show evidence of MDS with AA, PNH, and LGL.73,74
D. Immunosuppressive Therapies in MDS
1. Prednisone
An early study showed that prednisone alone improved low blood counts in a minority of patients with MDS and that the response could be predicted in vitro by enhancement of CFU-GM growth.75 Subsequent to this report, some investigators used prednisone to treat MDS, but low response rates, transient responses, and increased risk of infection make corticosteroids unattractive agents in MDS, and their mode of action remains unclear.
2. ATG
Because of its similarity to aplastic anemia, hypoplastic MDS with severe cytopenia was occasionally treated with ATG: a summary of individual cases and small series reveals hematological responses in 8 of 13 hypoplastic MDS patients.76,77 Based on these reports, other unpublished observations, and the hypothesis that a T cell-mediated process may cause pancytopenia, we evaluated ATG as immunosuppressive treatment to improve marrow function in MDS.78 The completed study79 involved 61 patients who were red cell- or platelet-transfusion dependent; 37 had RA, 14 RAEB, and 10 RARS (23 had hypocellular marrow biopsies). Most had failed previous treatment with single or multiple agents. Patients received ATG at 40 mg/kg/day for 4 days. Twenty-one (34%) patients became red cell transfusion-independent within 8 months of treatment (median, 75 days). Transfusion-independence was maintained in 76% of responding patients for a median of 32 months (range, 20-58). Twenty-three of 41 (56%) severely thrombocytopenic patients had sustained platelet count increases between 25,000 and 290,000/μL and 18/41 severely neutropenic patients achieved sustained neutrophil counts > 1000/μL. At last follow-up 39 patients were alive with an actuarial survival of 64% at a median of 34 months. Of the 21 responders, 20 survive and 1 has died following leukemic progression. In the others, no significant alteration in the bone marrow appearance or cellularity was observed and cytogenetic abnormalities, present in 4, persist. Three relapsed to transfusion-dependence but 1 regained red cell transfusion-independence after a second course of ATG. Of the 40 non-responders, 21 died, 15 from cytopenia and 7 from progression to leukemia. Response conferred significant survival benefit (at 4 years, 95% versus 38%). In the subset of 41/61 patients with an Intermediate-1 prognostic score, responders had 100% survival at 3 years and no disease progression versus 45% survival and 51% probability of disease progression in non-responders. In a multivariate analysis of 82 patients treated with either ATG or cyclosporine, younger age, shorter duration of red cell transfusion dependence, and the presence of HLA DRB1 15 predicted a response to immunosuppression. The presence of an expanded PNH clone also is a favorable marker.80 (Abnormalities in the T cell receptor Vβ chain repertoire were common, occurring both in responders and non-responders, and were thus of no prognostic value.)
3. Cyclosporine
Cyclosporine also had been occasionally used in MDS.83 Jonasova and co-workers systematically treated 16 patients with RA and 1 with RAEB with standard doses of cyclosporine for 5-31 months: substantial hematological responses were observed in 14, mostly occurring around 3 months.84 Transfusion-independence was achieved in all 12 patients requiring red cells before cyclosporine administration, and significant increases in leukocyte and platelet counts also occurred. Responses were unrelated to marrow cellularity or the presence of blasts; among 6 patients with abnormal karyotype, 3 responded (all were 5q). Four of 8 Japanese MDS patients improved with cyclosporine81 and another 4 cases, whose MDS included erythroid hypoplasia and evidence of T cell clonal expansion, became transfusion-independent with cyclosporine treatment;85 in another patient, however, cyclosporine treatment was associated with seeming leukemic progression, which was reversed with drug discontinuation.86
4. Soluble TNF receptor
Excessive amounts of soluble receptor offer a direct and specific mechanism to inhibit TNF’s putative negative effects on hematopoiesis in MDS. In small pilot trials from multiple institutions, the soluble receptor, Enbrel, has been well tolerated, but treatment has produced very modest clinical responses in a minority of patients.87–,89 In one study from Seattle and Stanford, among 12 evaluable patients, 4 showed increased hemoglobin levels, and 2 each also had somewhat higher platelet or neutrophil numbers.87 At NIH, only 1 patient of 15 evaluable became temporarily red cell transfusion-independent (as did a patient in an Italian case report);89 progression to leukemia was seen in 3 cases.88 In both trials, TNF had inconsistent effects on marrow hematopoietic progenitor cell growth pre- and post-treatment, nor was there correlation with TNF plasma levels.
5. Other drugs
Amifostine can protect cells from oxidative stress after exposure to cytokines including TNF and suppress inflammatory cytokine release. Pentoxifylline interferes with the lipid signaling pathway used by the proinflammatory cytokines TNF, TGF, and IL-1. Thus both these drugs may have immunosuppressive activity. Pentoxifylline and ciprofloxacin appeared to lower TNF levels in 14 patients with MDS, without any hematological responses.90 Among thalidomide’s diverse actions is modulation of T helper cells from a Th1 to a Th2 cytokine profile. However, in vitro correlations with clinical responses were stronger for angiogenesis parameters than with cytokine markers.91 Clinical results with these agents in MDS are discussed in detail above.
Conclusions
We know very little about the interaction of the immune system, the marrow microenvironment, and the MDS stem cell. Similarities between MDS and AA, including the response to immunosuppressive treatment, raise questions about the relationship between these two diseases. The origins of MDS remains unclear; as in AA, a viral etiology is possiblefeline leukemia virus infection is associated with MDS in cats and simian retroviruses with dysmorphic changes in monkey marrow! Neither the antigens evoking the T cell response nor the precise mechanism of T cell-mediated myelosuppression is defined; more precise genomic annotation of cytogenetic abnormalities92,93 and chip and microarray gene expression approaches94– 97 should provide clues and even answers. The abnormal cytokine milieu conceivably could be the initiator of genetic instability leading to clonal evolution in AA and leukemia in MDS; if true, immune modulation at an early stage of clonal evolution might help to maintain disease stability.
III. Ring Sideroblast Formation and Its Role in MDS Pathophysiology
Norbert Gattermann, MD, PhD*
Klinik fur Haematologie, Onkologie und Klinische Immunologie, Universitatsklinikum Dusseldorf, Moorenstr. 5, D-40225 Dusseldorf, Germany
Marrow erythroblasts appear as ringed sideroblasts on Prussian blue staining if their mitochondria contain dense deposits of iron. For authentication on light microscopy, three criteria must be met: (1) the Prussian blue-positive iron granules must be abnormally large, (2) they must exceed five or six in number, and (3) they must form an arc extending around at least 30% of the nucleus. If bone marrow ringed sideroblasts exceed 15 percent of erythroblasts in a patient with MDS, the case is usually classified as RARS. The presence of ringed sideroblasts is a common morphological change in MDS, which may be closely connected with a basic pathomechanism of preleukemia. Elucidating the cause of mitochondrial iron overload may therefore shed some light on other riddles of MDS pathology.
A. Prevalence of Mitochondrial Iron Overload in MDS
Ringed sideroblasts are not confined to the sideroblastic type of MDS (RARS according to the FAB classification). They are also found in RA, RAEB, and even in some cases of RAEB-T. Jacobs and Bowen1 stated that in RA, “the number of erythroblasts with ring siderotic granules may vary from 1% to 90% and there is no clear demarcation between ‘sideroblastic’ and ‘non-sideroblastic’ cases.” Juneja et al2 found an astonishing prevalence of ringed sideroblasts when they examined by light microscopy the bone marrow of 133 patients with primary MDS. Ringed sideroblasts ranging from 1% to 86% of cells were found in 57% of cases. Among 46 patients with < 5% blasts in the bone marrow (corresponding to RA and RARS), a great majority (87%) had more than 20% ringed sideroblasts. Among 65 patients with RAEB, ringed sideroblasts were present in 40%. One fifth of the patients with RAEB had more than 20% ringed sideroblasts. Among 22 patients with RAEB-T, 7 had ringed sideroblasts. Only 2 patients with RAEB-T had more than 20% ringed sideroblasts. Juneja et al2 defined ringed sideroblasts as erythroblasts containing five or more Prussian blue-positive granules covering one third or more of the circumference of the nucleus. Still, the criteria may have been softer than usual, since abnormal size of the granules was apparently not required. From the large database of the Düsseldorf MDS Registry, the following data were retrieved: among patients with < 5% blasts in the bone marrow (corresponding to RA and RARS), 35% had more than 20% ringed sideroblasts (41% had more than 15% RS). Among patients with RAEB, 12% had more than 20% ringed sideroblasts (15% had more than 15% RS) (U. Germing, personal communication).
Electron microscopy is more sensitive to detect mitochondrial iron overload. When Maldonado et al3 studied the erythrocytic line in RA (preleukemia) and myelomonocytic leukemia, they found iron overload, including the presence of large numbers of definitely pathologic sideroblasts, in all their patients with preleukemia. There were iron deposits in a significant number of the mitochondria and in a large number of the normoblasts. Often, but not always, the presence of iron in the mitochondria was accompanied by degenerative changes consisting of swelling, vacuolization, and other signs of internal disarray such as rupture or separation of the cristae and formation of myelin figures. Sakura et al4 studied the ultrastructural abnormalities of erythroblasts in 30 patients with RA. Iron-laden mitochondria were found in erythroblasts of intermediate stage maturation in 30% of the patients, and the incidence of erythroblasts with this abnormality in each patient ranged from 0% to 28.6% (mean ± SD = 4.3 ± 8%). Cohen et al5 used transmission electron microscopy to examine bone marrow aspirates from 26 patients with MDS, including 3 patients with RARS. Iron deposits in the mitochondria were often seen, accompanied by marked alterations of the mitochondrial structure. Recently, van de Loosdrecht et al6 also investigated the ultrastructural characteristics of erythroblasts in MDS. Among 22 patients, only 2 had been diagnosed as having sideroblastic anemia (RARS). Nevertheless, 16 of 22 patients (73%) showed iron-laden mitochondria. In 55% of the cases, the mitochondria were enlarged with or without disruption of internal cristae and/or mitochondrial membranes, which was significantly associated with accumulation of iron.
B. Previous Misconception of the Pathomechanism
It has been speculated that an enzyme defect of the heme synthetic pathway (Figure 4, see Color Figures, page 516) leads to a shortage of heme precursors in sideroblastic anemia. Iron, which is imported into mitochondria for heme synthesis, would then lack its reaction partner (protoporphyrin IX) and would therefore accumulate in the mitochondrial matrix. However, such an enzyme defect of protoporphyrin synthesis has been found only in hereditary X-linked sideroblastic anemia, where delta-aminolaevulinic acid synthase, the first enzyme of heme synthesis, is mutated.7 A primary enzyme defect has not been identified in acquired idiopathic sideroblastic anemia. This is not surprising, since Kushner et al reported that in these patients, protoporphyrin IX is elevated rather than reduced.8 This finding virtually excludes an enzyme defect upstream in the pathway. Instead, it suggests that the last step of heme synthesis, namely incorporation of iron into protoporphyrin IX, is disturbed. This step is catalyzed by ferrochelatase, an enzyme of the inner mitochondrial membrane.
However, ferrochelatase is not the culprit in sideroblastic anemia, since it has been demonstrated that increased red cell protoporphyrin concentrations are not correlated with low ferrochelatase activities.9 One is therefore left with the paradox of marked deficiency of heme synthesis despite (a) abundant protoporphyrin IX, (b) more than ample iron in the mitochondrial matrix, and (c) normal ferrochelatase. Why is iron not properly inserted into protoporphyrin IX to make heme?
C. New Pathogenetic Model of Mitochondrial Iron Accumulation
The answer is that iron is probably not in the right chemical form. It has been shown by energy-dispersive x-ray analysis that iron accumulates in sideroblastic anemia in the ferric form (Fe3+), mainly as ferric phosphate.10 Ferrochelatase accepts only ferrous iron (Fe2+) for heme synthesis. If iron is not provided in the right chemical form, it cannot be utilized and will thus accumulate in the mitochondrial matrix. A new pathogenetic model is therefore proposed11 that no longer postulates an elusive enzyme defect of heme synthesis but concentrates on deranged mitochondrial iron metabolism.
1. Evolutionary consideration
Selectivity for Fe2+ is a feature of ferrochelatase that has been conserved during evolution. Ferrochelatase was already involved in the synthesis of heme molecules in photosynthetic bacteria more than 3 billion years ago, when the atmosphere of the earth was virtually free of molecular dioxygen, and all the iron that was available was Fe2+.12 Initially, iron metabolism was anaerobic and exclusively based on Fe2+. An important change occurred when the ancestors of cyanobacteria appeared. These bacteria developed a sophisticated photosynthesis that used water and liberated dioxygen into the atmosphere, thus making aerobic existence possible. Over the next billion years, atmospheric concentration of dioxygen increased, and abundant and soluble Fe2+ was converted into Fe3+. When ferrous iron disappeared, organisms had to find ways to utilize ferric iron instead. Evolution is conservative, taking parts of the old and building upon them to create something new. Accordingly, the principle of Fe2+ uptake through cell membranes was conserved, together with the enzyme ferrochelatase, which uses Fe2+ for heme synthesis. The problem was solved by developing membrane-bound ferrireductases, which bring Fe3+ into the ferrous form. Ferrochelatase was therefore not forced to change its habit of selectively using Fe2+. In eukaryotic cells, ferrochelatase is located in the inner mitochondrial membrane. More than a billion years ago, a eukaryotic cell engulfed a smaller aerobic prokaryotic cell (a particular type of purple bacterium). This was followed by the establishment of a symbiotic relation. Evolution of the prokaryotic endosymbiont, which apparently possessed ferrochelatase, led to the development of mitochondria.
2. Iron uptake into mitochondria
It is still unclear how iron is guided through the cytoplasm toward the mitochondria, but it most likely travels as a ferric iron compound because ferrous iron is extremely unstable in the presence of oxygen. Upon arrival at the mitochondrion, Fe3+ must be converted into Fe2+ because, similar to the uptake of iron through cytoplasmic membranes, iron must be in the ferrous form to cross the inner mitochondrial membrane.13 Mitochondria are capable of catalyzing this conversion, and there is experimental evidence that the electrons necessary for the reaction are provided by the mitochondrial respiratory chain.14,15 It is not known where exactly the reduction of iron occurs, but circumstantial evidence points to respiratory chain complex IV (cytochrome c oxidase). Mitochondrial iron uptake is an energy-dependent process. It does not require adenosine triphosphate (ATP), but it requires a membrane potential over the inner mitochondrial membrane.13 This membrane potential is created by the respiratory chain, which pumps protons out of the mitochondrial matrix and thereby creates a strong electrochemical proton gradient (Figure 5A, see Color Figures, page 516). This proton gradient is also needed for mitochondrial ATP production.
3. Maintenance of iron in the ferrous state
After traversing the inner mitochondrial membrane, iron must be maintained in its ferrous form. If reoxidized, it will become unavailable to ferrochelatase. How is reoxidation of iron prevented? In a recent paper, Orkin’s group 16 identified genes that are upregulated during erythroid differentiation. Among them was the gene for uncoupling protein-2 (Ucp2). This protein may be involved in preventing reoxidation of iron. Uncoupling proteins allow protons to slip back into the mitochondrial matrix, thereby diminishing the proton gradient (Figure 5A, page 516), which stimulates the respiratory chain to run faster to restore the gradient. The faster it runs, the more heat it produces and the more oxygen it consumes. Uncoupling proteins physiologically induce heat production for such things as reviving hibernating animals or protecting newborn babies. In erythropoietic cells, heat production is probably irrelevant; however, increased consumption of oxygen is likely to be important, because it may create a low-oxygen environment, which is needed to protect ferrous iron from reoxidation. Something like this was already suspected 40 years ago by Porra and Jones,17 who investigated ferrochelatase. They found that the enzyme did not work in the presence of oxygen.17 They also noted that “the total inhibition by air of ferrochelatase activity in soluble extracts is interesting since there are many examples of aerobic heme synthesis in avian erythrocytes, crude hemolysates, and liver homogenates. It is possible that secondary oxidative processes in these crude preparations may maintain anaerobic conditions at the site of iron insertion.” This requirement of near-anaerobic conditions (resembling those for iron metabolism 3 billion years ago) explains why Ucp2 is needed in the mitochondria of erythroid cells.
4. The role of mitochondrial respiratory chain defects
The mitochondrial respiratory chain thus plays an important role in mitochondrial iron metabolism: (a) It provides the electrons needed to convert Fe3+ into Fe2+. (b) It provides the membrane potential needed to carry iron through the inner mitochondrial membrane. (c) In collaboration with Ucp2 it removes oxygen from the mitochondrial matrix, thereby providing a low-oxygen environment which protects the delivery of ferrous iron to ferrochelatase.
First evidence of respiratory chain dysfunction in the bone marrow of patients with sideroblastic anemia was provided by Aoki,18 who examined several mitochondrial enzymes. He found that cytochrome c oxidase and oligomycin-sensitive ATPase, both components of the respiratory chain, had reduced activity, whereas citrate synthase, an enzyme of the mitochondrial matrix, was not impaired. The reduced activity of cytochrome c oxidase and oligomycin-sensitive ATPase was not secondary to excess mitochondrial iron, because enzyme measurements were performed using the patients’ granulocytes, which do not show mitochondrial iron loading.
The function of the respiratory chain (RC) can be compromised by mitochondrial DNA (mtDNA) mutations. This is because several RC subunits have their genes on the mtDNA. Altogether, the large multienzyme complexes of the RC are built of about 80 subunits. Most of them are encoded by nuclear DNA, but 13 subunits are encoded by mtDNA. The human mtDNA is a double-stranded ring molecule with a length of about 16,500 bp. The entire sequence has been known since 1981. It contains only 13 protein genes, all of them coding for subunits of the respiratory chain and ATP synthase. In contrast to chromosomal DNA, the mitochondrial genome is extraordinarily compact: it has no introns, making it very likely that mutations will strike a coding DNA sequence. mtDNA has no protective histones and also lacks an effective DNA repair system. Because it is located near the inner mitochondrial membrane, mtDNA is highly exposed to oxygen free radicals generated by the respiratory chain. Altogether, this results in a mutation rate more than 10 times that of chromosomal DNA. Mutations of mtDNA can cause human disease. Hereditary mtDNA diseases are usually characterized by progressive neuromuscular and cognitive dysfunction because neurons and muscle cells are particularly dependent on ATP regeneration by an intact respiratory chain. However, mtDNA mutations can have deleterious effects on almost any organ system, including the bone marrow, as illustrated by Pearson’s syndrome.
Pearson’s syndrome is a rare congenital disorder characterized mainly by permanent lactic acidosis, pancreatic insufficiency, other metabolic derangements, and severe RA, with moderate numbers of ringed sideroblasts in the bone marrow. The anemia is usually accompanied by neutropenia and thrombocytopenia. The bone marrow shows dysplastic changes, including prominent vacuolization of precursor cells. Pearson’s syndrome is caused by large deletions of mtDNA.19 The link between mitochondrial DNA damage and the sideroblastic phenotype in Pearson’s syndrome must involve malfunction of the respiratory chain.
Gradual effects on iron metabolism and energy production can be envisaged, depending on the degree of RC dysfunction (Figure 5B, see Color Figures, page 516). mtDNA mutations causing a mild defect, which only moderately disturbs the flux of electrons through the RC, will slow down oxygen consumption and will thus leave more oxygen than usual in the mitochondrial matrix. Bowen and Peddie20 showed that bone marrow mononuclear cells (MNCs) from patients with MDS consume less oxygen than MNCs from normal controls. Since a mild RC defect will not cause a marked decrease in the mitochondrial membrane potential, ferrous iron will continue to be imported. However, some of it will become reoxidized (Fe2+ → Fe3+) because increased oxygen is available. Fe3+ will be rejected by ferrochelatase and will thus accumulate in the mitochondrial matrix. In order to accumulate large amounts of iron, mitochondria must still be fit enough to convert ferric iron into ferrous iron, which is a requirement for transmembrane passage, and to provide an adequate membrane potential for iron import. Therefore, this scenario may correspond to pure sideroblastic anemia (PSA). The disease is characterized by massive mitochondrial iron overload, but does not show significant dysplasia in nonerythroid cells. The absence of multilineage dysplasia may reflect a mild degree of mitochondrial dysfunction.21
MtDNA mutations with a stronger impact on RC activity will decrease proton pumping and will therefore diminish the mitochondrial membrane potential (ΔΨm). Decreased ΔΨm was found in bone marrow cells, particularly in erythroblasts, of patients with sideroblastic anemia by Matthes et al.22 The membrane potential may still be strong enough to support mitochondrial iron import, but ATP regeneration may now be compromised. Since ATP deficiency can be detrimental to diverse cell functions, such a scenario may correspond to sideroblastic anemia with multilineage dysplasia.
mtDNA mutations producing a severe RC defect can be expected to destroy much of the mitochondrial membrane potential. If, therefore, iron import is no longer guaranteed, the amount of iron entering the mitochondrial matrix will not be sufficient to create a sideroblastic phenotype. Paradoxical though it may appear, severe RC defects may cause less mitochondrial iron overload. Still, heme synthesis will be severely compromised. By strongly interfering with cellular differentiation, severe RC defects may also contribute to the phenotype of advanced MDS, which is characterized by increased blast cells in the bone marrow.
In summary, respiratory chain defects can easily impair mitochondrial iron metabolism and heme synthesis. Since heme synthesis, together with globin chain synthesis, is the main biosynthetic task of red cell precursors, these bone marrow cells are particularly vulnerable to respiratory chain dysfunction. Mitochondrial defects may therefore provide an answer to the question why anemia is an early and usually the predominant feature of myelodysplastic syndromes.
D. Mitochondrial DNA Mutations in Patients with MDS
Since there are no specific chromosomal abnormalities associated with the sideroblastic phenotype in MDS, Pearson’s syndrome provided a welcome hint that mutations of the mitochondrial genome might be involved. Another hint comes from the phenomenon of intraclonal heterogeneity of the sideroblastic phenotype. Not all erythroblasts in sideroblastic anemia are ringed sideroblasts, and even the ringed sideroblasts differ in their degree of mitochondrial iron overload. This has been demonstrated even within single erythroid colonies cultured in vitro.23– 25 In those colonies, cells of the same maturational stage show different degrees of mitochondrial iron overload. This finding is hard to explain in terms of a nuclear gene defect, which should affect all cells of the clone in the same way. An explanation is easier to find if mitochondrial genetics are considered. Because cells contain hundreds of mitochondria, they can simultaneously harbor normal mitochondria and mitochondria with mutant mtDNA. Coexistence of normal and mutant mtDNA is called heteroplasmy. Heteroplasmy is typical of mtDNA diseases. When a heteroplasmic cell divides, the mutant and normal mitochondria are randomly segregated to the daughter cells. This results in a heterogeneous population consisting of nearly normal cells, grossly abnormal cells, and intermediate cells. Applied to sideroblastic anemia, random segregation of mitochondrial DNA could explain the variable expression of the sideroblastic phenotype.
We have not been able to find large deletions of mtDNA in patients with acquired idiopathic sideroblastic anemia or any other type of MDS.26 Because large deletions represent very severe defects of mtDNA, strong selective pressure during incessant clonal proliferation may eliminate hematopoietic progenitor cells carrying detectable amounts of deleted mtDNA. Instead, we have identified numerous point mutations of mtDNA.27– 30 Since disease-related mtDNA mutations are usually characterized by coexistence of mutant and wild-type mtDNA, we are specifically searching for heteroplasmy in the patients’ bone marrow cells. Initially, we employed high-density RFLP (restriction fragment length polymorphism) analysis and, later, TGGE (temperate gradient gel electrophoresis). Recently, we have been using temperature-dependent heteroduplex analysis with automated denaturing HPLC (the WAVE™ system, Transgenomic, Crewe, UK). To cover the whole mitochondrial genome, 67 overlapping segments are amplified by PCR and subjected to heteroduplex analysis. Pathological findings are confirmed by automated fluorescent DNA sequencing. We identified mtDNA mutations that are heteroplasmic, are not present in controls, and affect conserved nucleotides/amino acids. On the basis of 30 patients whose mitochondrial genome has now been scanned completely, we estimate that such clonal mtDNA mutations are detectable in at least one third of MDS patients. In those patients who were available for additional investigations, the clonal mtDNA mutation was found to be present in blood and bone marrow cells, but not in buccal mucosa cells or cultured skin fibroblasts. Most likely, the mutations occurred in multipotent hematopoietic stem cells. Mutations identified to date involve protein genes (cytochrome c oxidase subunits I and II, cytochrome b, NADH dehydrogenase subunit 5, ATPase subunit 8), mitochondrial transfer RNAs (tRNAs for Ala, Leu, Ser, Gly, Lys, Thr, Pro, Ile, Trp), and mitochondrial ribosomal RNAs (16S-rRNA and 12S-rRNA). Mitochondrial tRNA mutations are well established as a cause of mtDNA disease. Through impairment of mitochondrial protein synthesis, they can affect all respiratory chain complexes containing subunits encoded by mtDNA. A similar effect can be postulated for mutations in mitochondrial ribosomal RNAs.
E. Broader Implications of Mitochondrial Dysfunction
Besides impaired heme synthesis and the formation of ringed sideroblasts, there may be other consequences of mitochondrial dysfunction in MDS bone marrow cells.
1. ATP deficiency
Mitochondrial DNA diseases are largely explained by impaired mitochondrial energy production. The performance of differentiated cells, involving pumps and channels and other ATP-dependent “machines,” strongly relies on mitochondrial ATP regeneration. More basic cellular functions may also be affected. In mammalian cells, there is a hierarchy of ATP-consuming processes.31 If energy supply becomes inadequate, cells shut down their metabolic activities in a certain order. Pathways of macromolecule synthesis, i.e., protein and RNA/DNA synthesis, are most sensitive to energy supply.31 This may be pertinent to the proliferation and function of bone marrow cells in MDS. In a study of ineffective erythropoiesis in sideroblastic anemia, Wickramasinghe et al32 showed an accumulation of early polychromatic cells in G2 and the presence of cells that were apparently arrested after a period in DNA synthesis. DNA synthesis was rarely seen in cells with pronounced siderotic deposits. A later study with electron microscope autoradiography corroborated the concept that cells with large quantities of mitochondrial deposits become arrested in their progress through the cell cycle.33 The data also revealed that the accumulation of increasing quantities of iron-containing material within the mitochondria of an erythroblast was frequently (but not invariably) associated with and was possibly responsible for (a) a depression of RNA synthesis and (b) a more marked depression of protein synthesis.
Cell proliferation appears to be particularly compromised in advanced stages of MDS. Jensen and Hokland34 analyzed by multiparameter flow cytometry the proliferative activity of myelopoiesis in myelodysplasia. There was a clear decrease in the immature myeloid cell proliferative activity upon progression within the FAB groups. The authors concluded that the block in differentiation in MDS patients might be coupled to a simultaneous block in proliferation, especially in advanced stages.
ATP deficiency may also contribute to chromosomal instability. Since the mitotic spindle apparatus depends on ATP-consuming motor proteins, cells with inadequate ATP supply may have difficulty in correctly segregating their chromosomes during mitosis. Notably, karyotype anomalies in MDS are characterized by losses or gains of whole chromosomes, or large deletions, rather than specific translocations. Chromosomal instability seems to be virtually absent in patients with pure sideroblastic anemia, for whom only a mild RC defect is proposed. These patients have the lowest frequency of karyotype anomalies, the lowest incidence of leukemic transformation, and the best survival among all MDS patients.35,36 In the case of more severe RC defects, impairment of ATP-dependent DNA repair mechanisms may be another pathway contributing to genomic instability.
2. Apoptosis
Increased apoptosis of bone marrow cells is a major mechanism of ineffective hemopoiesis in MDS. However, it is not clear what triggers apoptosis. Intrinsic defects within the clonal cells may be at least as important as extrinsic apoptosis-inducing signals. In recent years, it has become clear that mitochondria play a central role as regulators and effectors of apoptosis. Mitochondrial dysfunction can lead to necrotic and apoptotic cell death.37 It is thus conceivable that mitochondrial defects predispose bone marrow cells to apoptosis. Hyperactivation of the mitochondria-caspase-9 pathway was seen in low-risk MDS and appears to be more important for the induction of apoptosis than the death receptor-caspase-8 pathway.38 Apoptosis can be induced by specific mitochondrial respiratory chain inhibitors.39 mtDNA mutations may have a similar consequence in MDS bone marrow cells.
While apoptosis is significantly increased in the “early” MDS subtypes RA, RARS, and RAEB (<10% blasts), it is comparable to normal controls in advanced MDS.40,41 This does not contradict the proposal that mitochondrial defects are more severe in advanced MDS. With more severe mitochondrial dysfunction, genomic instability may come into play, providing ample opportunity to acquire chromosomal lesions that help to escape apoptotic control.
3. Pyrimidine nucleotide synthesis
An enzyme necessary for de novo pyrimidine nucleotide synthesis, dihydroorotate dehydrogenase (DHODH), is located in the inner mitochondrial membrane, with its function depending on interaction with the respiratory chain. We have shown that RC inhibition impairs pyrimidine synthesis and thereby affects DNA precursor pool concentrations (Gattermann N, Dadak M, Wulfert M, Hofhaus G, Simmonds A, unpublished data). If cellular deoxyribonucleoside triphosphate levels are perturbed, a wide range of genetic events may follow as a result of aberrant DNA replication or repair.42 In that way mitochondrial dysfunction may again contribute to genomic instability. In addition, impaired pyrimidine nucleotide synthesis may help to explain the megaloblastic changes that are often found in MDS bone marrow cells.
F. Synopsis
Figure 6(see Color Figures, page 517) shows how mtDNA mutations fit into the picture of MDS pathogenesis. With advancing age, hematopoietic stem cells apparently accumulate mtDNA mutations. Age-dependent accumulation of mtDNA mutations has been demonstrated in brain, skeletal muscle, heart, liver, skin, and buccal mucosa cells (see Nekhaeva et al43 and references therein). We have shown that it also occurs in the bone marrow.26 Some mtDNA mutations will be irrelevant;44 some may be severe. Some of the affected stem cells will not be able to cope with their mitochondrial damage and will die, probably by apoptosis. This may contribute to age-related depletion of the bone marrow reserve. Other stem cells harboring mutant mtDNA will continue to proliferate, and their daughter cells may show dysfunction and morphological changes, but this is not MDS, since the bone marrow is not clonal. To avoid any misunderstandings it should be emphasized that mtDNA mutations per se do not cause MDS because they cannot provide the cellular growth advantage that is necessary for clonal expansion. Only if a stem cell carrying mutant mtDNA becomes transformed by additional mutations in the cell nucleus can a clonal bone marrow be established and the clinical picture of MDS arise. However, mtDNA mutations may play a major role in shaping the phenotype of the MDS clone, as exemplified by the formation of ringed sideroblasts. In addition, by contributing to genomic instability, mtDNA mutations may facilitate the transforming event that initiates an MDS clone and may also promote further chromosomal changes that drive the clonal evolution toward leukemia.
Classifications of myelodysplastic syndrome (MDS).
| FAB1 . | WHO3,4 . |
|---|---|
| Abbreviations: MDS, myelodysplastic syndrome; FAB, French-American-British; WHO, World Health Organization; RA, refractory anemia; RCMD, refractory cytopenia with multilineage dysplasia; RARS, RA with ringed sideroblasts; RS, ringed sideroblasts; RAEB, RA with excess of blasts; RAEB-T, RAEB in transformation; AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; MPD, myeloproliferative disorder. | |
| †Requires ≥ 6 months of anemia unrelated to other causes. | |
| ‡< 5% marrow blasts, micromegakaryocytes, thrombocytosis. | |
| §MDS: WBC ≤13,000/mm3; MPD: WBC >13,000/mm3. | |
| RA | RA (unilineage)† 5q− syndrome‡ RCMD |
| RARS | RARS† (unilineage) RCMD (with RS) |
| RAEB | RAEB-I RAEB-II |
| RAEB-T | AML |
| CMML | MDS/MPD§ |
| — | Unclassified |
| FAB1 . | WHO3,4 . |
|---|---|
| Abbreviations: MDS, myelodysplastic syndrome; FAB, French-American-British; WHO, World Health Organization; RA, refractory anemia; RCMD, refractory cytopenia with multilineage dysplasia; RARS, RA with ringed sideroblasts; RS, ringed sideroblasts; RAEB, RA with excess of blasts; RAEB-T, RAEB in transformation; AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; MPD, myeloproliferative disorder. | |
| †Requires ≥ 6 months of anemia unrelated to other causes. | |
| ‡< 5% marrow blasts, micromegakaryocytes, thrombocytosis. | |
| §MDS: WBC ≤13,000/mm3; MPD: WBC >13,000/mm3. | |
| RA | RA (unilineage)† 5q− syndrome‡ RCMD |
| RARS | RARS† (unilineage) RCMD (with RS) |
| RAEB | RAEB-I RAEB-II |
| RAEB-T | AML |
| CMML | MDS/MPD§ |
| — | Unclassified |
International Prognostic Scoring System (IPSS) for myelodysplastic syndrome (MDS).*
| Prognostic Variable . | Survival and AML Evolution Score Value . | ||||
|---|---|---|---|---|---|
| Prognostic Variable . | 0 . | 0.5 . | 1.0 . | 1.5 . | 2.0 . |
| Marrow blasts (%) | < 5 | 5-10 | — | 11-20 | 21-30 |
| Karyotype† | Good | Intermediate | Poor | ||
| Cytopenias‡ | 0-1 | 2-3 | |||
| Prognostic Variable . | Survival and AML Evolution Score Value . | ||||
|---|---|---|---|---|---|
| Prognostic Variable . | 0 . | 0.5 . | 1.0 . | 1.5 . | 2.0 . |
| Marrow blasts (%) | < 5 | 5-10 | — | 11-20 | 21-30 |
| Karyotype† | Good | Intermediate | Poor | ||
| Cytopenias‡ | 0-1 | 2-3 | |||
| Risk Category . | Combined Score . |
|---|---|
| Abbreviations: AML, acute myeloid leukemia. | |
| * Modified from Greenberg P, Cox C, Le Beau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088. | |
| † Good = normal, Y, del(5q), del(20q); Poor = complex (≥ 3 abnormalities) or chromosome 7 anomalies; Intermediate = other abnormalities. | |
| ‡ Neutrophils < 1800/μL, hemoglobin < 10 g/dL, platelets < 100,000/μL. | |
| Low | 0 |
| Int-1 | 0.5-1.0 |
| Int-2 | 1.5-2.0 |
| High | > 2.5 |
| Risk Category . | Combined Score . |
|---|---|
| Abbreviations: AML, acute myeloid leukemia. | |
| * Modified from Greenberg P, Cox C, Le Beau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088. | |
| † Good = normal, Y, del(5q), del(20q); Poor = complex (≥ 3 abnormalities) or chromosome 7 anomalies; Intermediate = other abnormalities. | |
| ‡ Neutrophils < 1800/μL, hemoglobin < 10 g/dL, platelets < 100,000/μL. | |
| Low | 0 |
| Int-1 | 0.5-1.0 |
| Int-2 | 1.5-2.0 |
| High | > 2.5 |
Age-related survival and acute myeloid leukemia (AML) evolution in myelodysplastic syndrome (MDS) patients within International Prognostic Scoring System (IPSS) subgroups.*
| . | . | Median Survival (yr) . | |||
|---|---|---|---|---|---|
| . | No. of Pts . | Low . | Int-1 . | Int-2 . | High . |
| Total pts.: No. (%) | 816 | 267 (33%) | 314 (38%) | 176 22%) | 59 (7%) |
| 5.7 | 3.5 | 1.2 | 0.4 | ||
| Age ≤ 60 yr | 206 (25%) | 11.8 | 5.2 | 1.8 | 0.3 |
| > 60 yr | 611 | 4.8 | 2.7 | 1.1 | 0.5 |
| ≤ 70 yr | 445 (54%) | 9.0 | 4.4 | 1.3 | 0.4 |
| > 70 yr | 371 | 3.9 | 2.4 | 1.2 | 0.4 |
| . | . | Median Survival (yr) . | |||
|---|---|---|---|---|---|
| . | No. of Pts . | Low . | Int-1 . | Int-2 . | High . |
| Total pts.: No. (%) | 816 | 267 (33%) | 314 (38%) | 176 22%) | 59 (7%) |
| 5.7 | 3.5 | 1.2 | 0.4 | ||
| Age ≤ 60 yr | 206 (25%) | 11.8 | 5.2 | 1.8 | 0.3 |
| > 60 yr | 611 | 4.8 | 2.7 | 1.1 | 0.5 |
| ≤ 70 yr | 445 (54%) | 9.0 | 4.4 | 1.3 | 0.4 |
| > 70 yr | 371 | 3.9 | 2.4 | 1.2 | 0.4 |
| . | . | 25% AML Evolution (yr) . | |||
|---|---|---|---|---|---|
| . | No. of Pts . | Low . | Int-1 . | Int-2 . | High . |
| Abbreviations: AML, acute myeloid leukemia; pts., patients; NR, not reached. | |||||
| * Modified from Greenberg P, Cox C, Le Beau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088. | |||||
| Total pts.: No. (%) | 759 | 235 (31%) | 295 (39%) | 171 (22%) | 58 (8%) |
| 9.4 | 3.3 | 1.1 | 0.2 | ||
| Age ≤ 60 yr | 187 (25%) | > 9.4 (NR) | 6.9 | 0.7 | 0.2 |
| > 60 yr | 572 | 9.4 | 2.7 | 1.3 | 0.2 |
| ≤ 70 yr | 414 (55%) | > 9.4 (NR) | 5.5 | 1.0 | 0.2 |
| > 70 yr | 345 | > 5.8 (NR) | 2.2 | 1.4 | 0.4 |
| . | . | 25% AML Evolution (yr) . | |||
|---|---|---|---|---|---|
| . | No. of Pts . | Low . | Int-1 . | Int-2 . | High . |
| Abbreviations: AML, acute myeloid leukemia; pts., patients; NR, not reached. | |||||
| * Modified from Greenberg P, Cox C, Le Beau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088. | |||||
| Total pts.: No. (%) | 759 | 235 (31%) | 295 (39%) | 171 (22%) | 58 (8%) |
| 9.4 | 3.3 | 1.1 | 0.2 | ||
| Age ≤ 60 yr | 187 (25%) | > 9.4 (NR) | 6.9 | 0.7 | 0.2 |
| > 60 yr | 572 | 9.4 | 2.7 | 1.3 | 0.2 |
| ≤ 70 yr | 414 (55%) | > 9.4 (NR) | 5.5 | 1.0 | 0.2 |
| > 70 yr | 345 | > 5.8 (NR) | 2.2 | 1.4 | 0.4 |
Survival (left) and freedom from AML evolution (right) of myelodysplastic syndrome (MDS) patients related to their classification by the International Prognostic Scoring System (IPSS) for MDS: Low, Int-1, Int-2, High (Kaplan-Meier curves). AML indicates acute myeloid leukemia.
Reprinted with permission from Greenberg P, Cox C, Le Beau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088.
Survival (left) and freedom from AML evolution (right) of myelodysplastic syndrome (MDS) patients related to their classification by the International Prognostic Scoring System (IPSS) for MDS: Low, Int-1, Int-2, High (Kaplan-Meier curves). AML indicates acute myeloid leukemia.
Reprinted with permission from Greenberg P, Cox C, Le Beau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088.
Survival, based on ages ≤ 60 years old (left) versus > 60 years old (right), of myelodysplastic syndrome (MDS) patients related to their classification by the International Prognostic Scoring System (IPSS) for MDS: Low, Int-1, Int-2, High (Kaplan-Meier curves).
Reprinted with permission from Greenberg P, Cox C, Le Beau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088.
Survival, based on ages ≤ 60 years old (left) versus > 60 years old (right), of myelodysplastic syndrome (MDS) patients related to their classification by the International Prognostic Scoring System (IPSS) for MDS: Low, Int-1, Int-2, High (Kaplan-Meier curves).
Reprinted with permission from Greenberg P, Cox C, Le Beau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079-2088.
Proposals for therapeutic options in MDS based on patient’s IPSS category, age, and performance status: NCCN MDS Panel Guidelines.51,52 Treatments are listed in order of preference. The term ‘rAML induction’ indicates use of chemotherapeutic induction regimens planned for ‘resistant-type AML.’
Abbreviations: NCCN, National Comprehensive Cancer Network; BMT, bone marrow transplant.
Reprinted with permission from Gotlib J, Greenberg P. Myelodysplastic syndromes. In Henderson E, Lister TA, Greaves M, eds. Leukemia, 7th Edition. Elsevier Science: Philadelphia, PA; 2002:562.
Proposals for therapeutic options in MDS based on patient’s IPSS category, age, and performance status: NCCN MDS Panel Guidelines.51,52 Treatments are listed in order of preference. The term ‘rAML induction’ indicates use of chemotherapeutic induction regimens planned for ‘resistant-type AML.’
Abbreviations: NCCN, National Comprehensive Cancer Network; BMT, bone marrow transplant.
Reprinted with permission from Gotlib J, Greenberg P. Myelodysplastic syndromes. In Henderson E, Lister TA, Greaves M, eds. Leukemia, 7th Edition. Elsevier Science: Philadelphia, PA; 2002:562.