Abstract
The three presentations in this session encompass clinical, pathophysiological and therapeutic aspects of hematologic diseases which impact most heavily on developing world countries. Dr. Victor Gordeuk discusses new insights regarding the multi-faceted pathogenesis of anemia in the complicated malaria occurring in Africa. He describes recent investigations indicating the possible contribution of immune dysregulation to this serious complication and the implications of these findings for disease management.
Dr. Surapol Issaragrisil and colleagues describe epidemiologic and clinical characteristics of the thalassemic syndromes. In addition to being considered a major health problem in Southeast Asia, the migration throughout the world of people from this region has caused the disease to have global impact. A unique thalassemia variant, Hb Eβ-thalassemia, with distinctive clinical features, has particular relevance for this demographic issue. Special focus will be reported regarding recent prenatal molecular screening methods in Thailand which have proven useful for early disease detection and disease control strategies. Dr. Raul Ribeiro describes a clinical model for providing effective treatment for a complex malignancy (childhood acute lymphoblastic leukemia) in countries with limited resources. With the multidisciplinary approach in Central American of the joint venture between St. Jude Children's Research Hospital International Outreach Program and indigenous health care personnel, major therapeutic advances for this disease have been achieved.
Given the major demographic population shifts occurring worldwide, these illnesses also have important clinical implications globally. These contributions demonstrate that lessons learned within countries of disease prevalence aid our understanding and management of a number of disorders prominently seen in developed countries. They will show how effective partnerships between hematologists in more and less developed nations may work together to produce important advances for treating major hematologic diseases in less developed regions. A major focus relates to the socio-economic and medical burden of these diseases in developing countries with limited resources. As such, these problems provide a challenge and an opportunity for collaborative interaction between hematologists and policy makers worldwide.
I. Mechanisms and Treatment of Malarial Anemia
Victor R. Gordeuk, MD*
Howard University, Center for Sickle Cell Disease, 2121 Georgia Ave., NW, Washington DC 20059
Malaria is a major threat to global health, with about 40% of the world's population exposed to this infection. Every year 300 to 500 million cases of Plasmodium falciparum malaria occur worldwide, and of these 1.5 to 3 million die. Major manifestations of complicated malaria include profound anemia (severe malarial anemia) and coma (cerebral malaria).1 The pathogenic mechanisms of these conditions are not well understood.
Factors Contributing to the Development of Anemia in Acute Falciparum Malaria
Anemia is regularly found in patients with acute falciparum malaria2–,6 and may range from mild to severe (hemoglobin concentration < 5 g/dL in children or < 7 g/dL in adults) in degree. The molecular pathogenesis of P. falciparum malaria anemia is poorly understood. Severe anemia can be observed at low parasitemias, during chronic infection, and even after complete chemotherapeutic elimination of organisms.2,7,8 Several mechanisms have been implicated in the pathogenesis of anemia. These include erythrocyte lysis and phagocytosis, increased sequestration of parasitized red blood cells, and autoimmune erythrocyte destruction, but none of these mechanisms alone can adequately explain the severity and extent of malaria anemia. Hematological studies of patients with severe malaria anemia have demonstrated ineffective erythropoiesis, bone marrow dyserythropoiesis, and lower erythroblast proliferative rates and numbers.2,8,9 Similar observations have been made in murine malaria models.10–,12 Some studies suggest that the suppression of erythropoiesis in malaria occurs despite an adequate production by the host of functional erythropoietin, the growth factor necessary for erythrocyte progenitor development.13,14 Other investigations have reported inadequate erythropoietin production in malarial anemia.15,16 In experimental settings, a vigorous host erythropoietin response has been observed in mice infected with P. berghei, P. vinckei, and P. chabaudi.10,12,14 Of importance, the mechanistic basis for the suppression of erythropoiesis in the presence of erythropoietin is unknown. Potential contributing factors to malarial anemia are summarized in Table 1 .
The degree to which hemolysis of parasitized or non-parasitized RBCs contributes to malarial anemia relative to the contribution of poor marrow erythroid production probably varies according to the immune status and age group of the patients, whether the malaria is acute or chronic, hereditary factors, the geographic location, and other unidentified factors.
Persistent Anemia after Treatment of Malaria
Anemia often persists for weeks following treatment of acute malaria,2,5,20,23,27 and many of the factors listed in Table 1 could potentially contribute to this situation. In some cases, there may be incomplete clearance of the malaria infection, recrudescent malaria, or infection with another plasmodial strain. Even when malaria is cleared and the patient is in an area of non-transmission, anemia may persist for weeks after effective antimalarial treatment and, by day 7 after the acute episode of malaria, the anemia is predominantly hypoproliferative rather than hemolytic.7
In these circumstances, an underlying nutritional deficit or an ongoing inflammatory process other than malaria may explain the anemia. Alternatively, the acute malaria episode may have lingering effects that suppress erythropoiesis. One possible explanation for persistent suppression of erythropoiesis is inadequate production of erythropoietin.15,16 Another possibility is that acute malaria somehow induces a persistent increase in the production of inflammatory cytokines that interfere with erythropoiesis.8
Severe Anemia in Falciparum Malaria
While the condition has not received as much attention from the research community in recent years as cerebral malaria, severe anemia (hemoglobin < 5 g/dL in children or < 7.0 g/dL in adults) is an important complication of falciparum malaria. As a cause of hospitalization, severe malarial anemia seems to be more common than cerebral malaria in Kenya29,30 and Zambia,31 while cerebral malaria seems to be more frequent in the Gambia.1,32 Over a 3-year period, severe malarial anemia was the cause of 10% of pediatric hospital admissions to a rural Zambian hospital, and the case fatality rate was 9%. The development of severe malarial anemia correlated strongly with 1) the degree of parasitemia, 2) the presence of malnutrition as indicated by low weight for age, 3) the absence of fever, and 4) presentation late in the malaria season.31 Thus, preexisting malnutrition and changes in immune response patterns due to prolonged exposure to P. falciparum may contribute to the development of this complication.
The mechanisms that lead to severe malarial anemia are not completely understood.3 Combinations of the factors listed in Table 1, as well as variations in the strains of the infecting plasmodial parasites or combinations of strains, might conceivably lead to the development of severe malarial anemia in a non-specific and sporadic manner. On the other hand, there may be something constitutively distinctive, such as an inherited alteration in immune function. Clark et al proposed that certain pathogenic manifestations of malaria, such as severe anemia and cerebral malaria, may be due to pro-inflammatory mediator release by host cells in response to malaria parasites or their products.33–,35 A soluble mediator released from the bone marrow and spleen cells of P. berghei-, P. chabaudi- or P. vivax-infected (but not uninfected or chemically anemic) mice has been reported to depress in vitro erythropoietin-induced proliferation of erythroid precursors and to be partly responsible for anemia.36 Stevenson and colleagues did not find evidence for tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and interferon-γ (IFN-γ) as the host-derived, soluble inhibitor of erythropoiesis in this model of murine malaria.34,37 However, there is considerable other evidence of immune modulators playing a role, which is discussed below.
The Immune Response and Malaria
Th-1 and Th-2 responses in malaria
Cytokines originating from T-helper cells, natural killer cells and macrophages are major players in the body's response to parasitic infections.38,39 Two CD 4+ T-helper cell subsets exist in mice and probably in man, each of which produces a typical set of cytokines that regulate different immune effector functions and cross-react with each other.38,40,41 T-helper type 1 (Th-1) cells produce IFN-γ, IL-2 and TNF-α. These cytokines activate macrophages, thus contributing to the formation of proinflammatory cytokines such as TNF-α, IL-1 and IL-6, and the induction of cytotoxic immune effector mechanisms of macrophages. By contrast, Th-2 cells produce IL-4, IL-5, IL-10, and IL-13, cytokines that induce a strong antibody response but also inhibit various macrophage functions.38,40,41
The balance between Th-1 and Th-2 cell-mediated immune effector mechanisms is of central importance for the host response to parasitic infections in mice and probably in man.38,39 While Th-1 derived cytokines such as IFN-γ and IL-2 are crucial for effective host defense in the acute phase of certain parasitic infections, increased activity of Th-2 derived cytokines such as IL-4, IL-10 and IL-13 heightens susceptibility to these infections and causes exacerbations. The latter effects may be due to an inhibitory function of IL-4, IL-10 or IL-13 on the production of Th-1 cytokines and on macrophage activation. Th-1 mediated immune effector function appears to be beneficial during early stages of plasmodial infections. In contrast, Th-2 derived cytokines appear to exert a protective role later in chronic infection with plasmodia or in the recovery period.39,42,43
Th-1 cytokines and erythropoiesis
The regulation of erythropoiesis is a complex process that involves not only erythropoietin.44 Bone marrow stromal cells,45 c-kit ligand,46 short range acting hematopoietic growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-3;47 insulin-like growth factor,48 platelet activating factor, prolactin,49 thrombopoietin,50 angiotensin II51 and related receptors52 and transcription factors53 are also important in the regulation of erythropoiesis. Certain cytokines, especially those of the Th-1 lymphocyte-mediated immune pathway, appear to be important in causing the perturbations of erythropoiesis that occur with inflammation and aplastic anemia. IL-1 and TNF-α have been implicated in the pathogenesis of the anemia of inflammation, while IFN-γ has also been implicated in aplastic anemia. IFN-γ is a potent direct inhibitor of erythroid precursor colony formation in vitro.54,55 It leads to apoptosis in human erythroid colony-forming unit cells through an interaction between Fas ligand and Fas.56 TNF-α leads to a reduction in red blood cell mass57 and to a reduction in erythroid precursors58 in mice. This cytokine also inhibits hypoxia-induced erythropoietin production in Hep3B cells.59 Several studies have suggested that increased TNF-α levels may be implicated in the development of ineffective erythropoiesis or dyserythropoiesis in murine malaria.60,61 Th-1 derived cytokines also cause an alteration of iron traffic leading to a diversion of metabolically available iron to the storage compartment in macrophages of the reticuloendothelial system, thus limiting erythropoiesis by reducing iron availability for heme synthesis.2,28,62,63
The immune response and the anemia of malaria
Several lines of evidence indirectly suggest that increased activity of cytokines may have a role in the suppression of erythropoiesis in the setting of acute malaria, contributing to the findings of bone marrow suppression, ineffective erythropoiesis and dyserythropoiesis described in Table 1. IFN-γ, TNF-α, IL-6, and IL-10 are often elevated in acute P. falciparum malaria.64–,67 Some of these cytokines are known to negatively affect erythropoiesis as summarized above. In addition, chronic exposure to IL-6 leads to a dilutional anemia in humans.68,69
Alterations in the immune response could contribute to prolonged anemia after the successful treatment of malaria. Elevated levels of some inflammatory cytokines may persist for one to four weeks after treatment of malaria in certain patients66,67,70 and could therefore potentially contribute to the persistence of anemia in the weeks after successful treatment of malaria. In particular, some evidence suggests that an unusually strong and prolonged Th-1 response71 in conjunction with an inadequately developed Th-2 response72 may contribute to persistent anemia after clearance of parasitemia.
Recent studies also point to the possibility that a dysregulation of the immune response may be involved in the pathogenesis of severe malarial anemia. In Ghanian and Kenyan children, severe malarial anemia was associated with a relative decrease in plasma IL-10 levels compared to TNF-α levels.73,74 Similarly, in Zambian children with cerebral malaria, the odds of severe anemia were significantly greater in children with higher serum concentrations of neopterin, a marker of macrophage activation in response to Th-1 stimulation, and with lower serum concentrations of the Th-2 cytokine, interleukin 4.75 Thus, the development of severe malarial anemia may be directly associated with Th-1 activity and inversely correlated with Th-2 activity.
Not all evidence is consistent with this scenario. In the Gambia, children with severe malarial anemia had lower plasma TNF levels than children with cerebral malaria.76 In murine malaria, deficiency of the Th-1 cytokine, IL-12, was associated with decreased erythropoiesis.77 Also in murine malaria, the administration of IL-12 markedly enhanced erythropoiesis and corrected severe anemia,78 and therapy with IL-12 in addition to chloroquine protected from severe malarial anemia.79
Extensively characterized only in the last few years, the protein known as macrophage migration inhibitory factor (MIF) has been identified to be a macrophage mediator and a T cell mediator that counter-regulates the anti-inflammatory effects of glucocorticoids. MIF is required for T cell activation, antibody production by B cells, and delayed-type hypersensitivity reactions,80 MIF is produced by macrophages upon stimulation by lipopolysaccharide (LPS) or IFN-γ.81 The mediator is also released from T cells, both of the Th-2 and Th-1 subsets,82 and other cells. In recently completed studies, Bucala and co-workers have shown that macrophages release copious amounts of macrophage MIF upon the ingestion of Plasmodium-infected erythrocytes or hemozoin.83 Of importance, MIF has been shown to inhibit erythropoiesis in vitro in the presence of erythropoietin. MIF is produced within the bone marrow and liver, and by spleen cells isolated from P. chabaudi- infected mice with active disease. In this experimental model, serum MIF levels have been found to correlate with disease severity. Taken together, these results suggest that MIF is a likely candidate for a host-derived factor contributing to malarial anemia.
There is evidence that MIF interacts with cytokines related to the Th-1 immune pathway. TNF-α induces the expression of MIF in adipocytes.84,85 Conversely, MIF leads to the killing of Leishmania major by macrophages through the induction of TNF-α and reactive oxygen intermediates.86 This effect of MIF is inhibited by the Th-2 related cytokines, IL-10 and IL-13. MIF-knockout mice show diminished production of TNF-α upon stimulation with LPS and IFN-γ.87
Other Factors Influencing the Development of Severe Malarial Anemia
Pathogenicity of the infecting strain or combination of strains of P. falciparum
Malaria parasites are very diverse genetically, and little is known about how different strains influence the clinical manifestations of the infection in patients. Defined malaria parasite gene sequences can be identified through polymerase chain reaction (PCR) technology. Characterization of the infecting organisms with PCR should help to discern if certain strains of malaria cause particularly severe clinical consequences in the patient, or if infection by multiple strains in a single patient causes more severe clinical complications than infection by a single strain. This is of interest since recent evidence suggests that differences between P. falciparum subtype populations (e.g. polymorphism in the circumsporozoite protein) may affect the immune response pattern and clinical outcome of malaria patients.88,89
The relationship between malaria and the host's immune system is complex. For instance, certain malaria parasite antigens seem to favor the induction of TNF-α production in the host.90 Therefore, certain malaria parasite strains might be more prone to cause severe anemia in the host because of this aspect of their antigenicity.
Genetic polymorphisms
Polymorphisms involving genes for the cytokines discussed above or for other genes could conceivably predispose to severe malarial anemia. Polymorphisms within the TNF-α promoter increase the transcriptional activation of this gene, thus enhancing the TNF-α response following challenge with microorganisms.91 A variant in position 308 of the TNF promoter (TNF2 allele) was reported to be associated with a relative risk of 7 for death or severe neurological sequelae due to cerebral malaria.92 At the same time, higher production of TNF was associated with faster clearance of fever and parasites.93 Nitric oxide is a labile radical that is centrally involved in the body's response towards parasitic infections including malaria.42,43 Polymorphisms in the promoter region for nitric oxide synthase type II, which catalyzes the high output formation of nitric oxide upon cytokine stimulation, have been associated with the clinical course of malaria infection.94 Certain HLA polymorphisms are associated with the degree of IFN-γ production.95
Treatment of Malarial Anemia
The treatment of malarial anemia is fundamentally directed to effective anti-malarial agents to eradicate the underlying infection. In the setting of chloroquine resistance, this implies first-line treatment of a patient with severe malarial anemia with an agent that is reliably effective. In the setting of Africa, this agent is often quinine. In Southeast Asia, quinine or artemesinin derivatives are commonly used. Urgent blood transfusion can be life-saving in the setting of severe malarial anemia and should be strongly considered in children with hemoglobin concentrations < 5 g/dL and in adults with hemoglobin concentrations < 7 g/dL. At the same time, caution is needed because of the risk of HIV and hepatitis virus contaminated blood in areas where it may not be possible to adequately test for these viruses. Additional treatment with folic acid is often given empirically for patients with malarial anemia, but treatment with iron should be reserved for those with documented iron deficiency. Whether any immune modulating agents will find usefulness in the treatment of malarial anemia has not been determined.
Conclusion
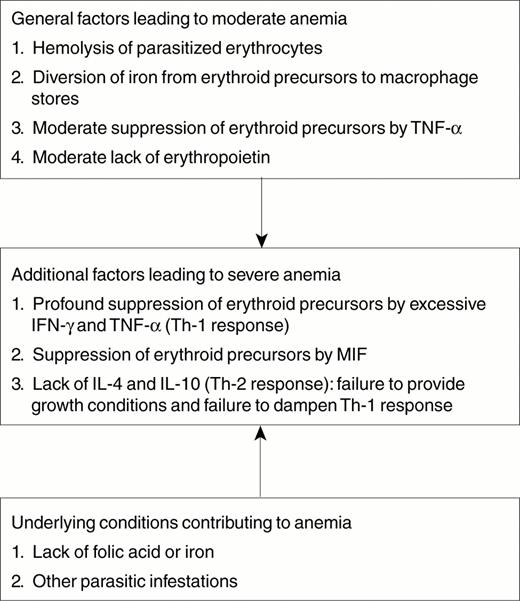
Severe malarial anemia (hemoglobin concentration < 5 g/dL) is an important manifestation of complicated malaria in African children. Factors other than hemolysis of parasitized red blood cells contribute to the development of anemia in patients with malaria, including hemolysis of non-parasitized red blood cells, suppression of the bone marrow, ineffective erythropoiesis and dyserythropoiesis, inadequate erythropoietin production, the general effects of inflammation on erythropoiesis, concommitant parasitic and bacterial infections, and pre-morbid nutritional abnormalities. What specifically leads to the development of severe malarial anemia is not known, but recent investigations point to the possibility that immune dysregulation may be involved. Figure 1 summarizes possible mechanisms contributing to the development of severe malarial anemia. The Th-1 cytokines, IFN-γ and TNF-α, inhibit erythropoiesis, while the Th-2 cytokines, IL-4 and IL-10, down-regulate the production of Th-1 cytokines and may facilitate erythropoiesis in certain circumstances. Studies in Africa have found serum markers of Th-1 immunity to be more markedly elevated in children with severe malarial anemia than in those with milder anemia, and serum levels of Th-2 cytokines to be lower in children with severe malarial anemia compared to those with milder anemia. Emergency blood transfusions can be life saving in children who present with severe malarial anemia, and the anemia eventually is corrected if malaria is treated effectively. Studies to determine if and how dysregulation of immune pathways contributes to the development of severe malarial anemia are needed.
II. Diagnosis and Management of Thalassemia: Thailand as a Model
Surapol Issaragrisil, MD,*
Mahidol University, Siriraj Hospital, Pannok St. 2, Bangkok 10700, Thailand
Thalassemia is the most common genetic disorder in Thailand. With the total population of 60 million, there are approximately 600,000 affected individuals and more than 20 million thalassemia carriers. Thalassemia is therefore one of the major health problems in Thailand. Since the first report of a large series of thalassemia patients in 1954,1 there have been a number of research publications in this field from Thailand. This review aims to address the problems of thalassemia in Thailand and to discuss the diagnosis and management of common thalassemia syndromes as practiced in this country as a model for other developing countries.
Formerly the distribution of thalassemia had been mainly limited to the areas from the Mediterranean across the Middle East through Southern Asia to Southeast Asia in the so called ‘thalassemia belt.’2 However, recent migrations of people have spread thalassemia genes throughout the world. Furthermore, there has been a major transition in the demography of common illnesses over the past years.3 This change includes a remarkable decline in childhood mortality due to infections and malnutrition in some regions. As a result, many infants with serious genetic disorders such as thalassemia can now survive the early months of life and live long enough to require treatment for their hematologic disorders. Given the demographic population shifts, thalassemia is at present considered to be a global health problem.3
In Thailand, both α- and β-thalassemias are prevalent (Table 2 ).4 The frequencies of α-thalassemia are 20% in Bangkok and 30% in northern Thailand. β-thalassemia is detected in 3-9%. Hemoglobinopathies are also common in Thailand.4 Hemoglobin (Hb) E, the hallmark of Southeast Asia, is most frequently found at the border of Thailand, Laos and Cambodia, where the frequencies may reach 50-60%. The average frequency of Hb E is 13%. Hb Constant Spring frequencies vary from 1-9%. There are more than 60 clinical syndromes resulting from various gene interactions. The most common thalassemia syndromes in Thailand include Hb Bart's hydrops fetalis, Hb H disease, homozygous β-thalassemia, and Hb E β-thalassemia. The number of annual births and number of existing patients as calculated according to the number of couples at risk are shown in Table 3 .
Common Thalassemia Syndromes in Thailand
Hemoglobin Bart's hydrops fetalis
Homozygous alpha-thalassemia 1 or Hb Bart's hydrops fetalis is the most severe form of thalassemia syndrome. All of the fetuses die either in utero or soon after birth. Approximately, 75% of the mothers who carry Hb Bart's hydrops fetalis develop toxemia of pregnancy. Hb typing shows these fetuses to have predominant Hb Bart's with minute amount of Hb Portland.
We observed an infant with Hb Bart's hydrops fetalis who survived after exchange blood transfusion and regular blood transfusions thereafter. The patient underwent CD34+ transplantation from her mother. She is at present doing well with evidence of hematopoiesis of mixed chimerism.
Hemoglobin H disease
Hb H disease is the α-thalassemia syndrome characterized by the presence of variable amounts of Hb H that precipitate in the red blood cells as inclusion bodies. Two common genotypes lead to the phenotype of Hb H disease, namely α-thalassemia 1/α-thalassemia 2 and Hb Constant Spring/α-thalassemia 1. Both genotypes are equally common but Hb Constant Spring/α-thalassemia 1 is more severe than α-thalassemia 1/α-thalassemia 2.5
At birth, the babies with Hb H disease are slightly anemic. The cord blood contains about 25% Hb Bart's. When the switch from γ- to β-globin chain production occurs, Hb Bart's (γ4) switches to Hb H (β4) and the typical picture of Hb H disease results.
Hb H disease is usually mild. The patients are mildly anemic and slightly jaundiced. Physical development is usually normal and the facies of thalassemia major is not present. Hepatosplenomegaly may be absent or very mild. Hb phenotype is AH with Hb H comprising from 5 to 15% of the total Hb. Hb Bart's is also frequently detected in minute amounts. Hb H disease can be easily diagnosed by the presence of characteristic multiple intraerythrocytic inclusion bodies in 70-80% of the red cells, detected by mixing with methylene blue or brilliant cresyl blue for an hour.
A striking clinical feature of Hb H disease is the sudden drop in the Hb concentration with associated symptoms of acute anemia during episodes of pyrexia.6 It has been postulated that fever either alone or together with oxidative substances released in the process of infection, induces the unstable Hb H to precipitate in the red cells as inclusion bodies. These red cells then either hemolyze or are rapidly destroyed by the reticuloendothelial cells. Blood transfusions should be given together with treatment for infections. Body temperature should be normalized as quickly as possible in order to reduce induction of Hb H precipitation within the red cells.
Hb Constant Spring is detected in addition to Hb A and H in patients with Hb Constant Spring α-thalassemia 1. They are slightly more severe than classical Hb H disease with lower Hb concentrations, larger spleens, higher levels of Hb H and more red cells containing inclusion bodies.5
Homozygous β-thalassemia
Most of the patients with homozygous β0-thalassemia have typical manifestations of severe thalassemia syndrome (thalassemia major) because there is no Hb A as a consequence of the absence of β-globin chain production. The onset of the disease usually begins in the first year of life. If hypertransfusion and iron chelation are not administered, the patients usually have full blown manifestations and die before the age of 10 years due to heart failure and severe infections.
Patients with detectable Hb A and F may have inherited homozygous β+-thalassemia or compound heterozygosity for β0-thalassemia and β+-thalassemia. Most of these patients present as thalassemia intermedia. The clinical manifestations are milder, reflecting the modest reduction of beta-globin chain synthesis.
Hemoglobin E β-thalassemia
Hb E β-thalassemia is more frequent than homozygous β-thalassemia in Thailand because of the high frequency of Hb E.7,8 It is the most common severe thalassemia syndrome in adults. There are two types of Hb E β-thalassemia, classified based on the presence or absence of Hb A, Hb E β+-thalassemia and Hb E β0-thalassemia. In patients with Hb E β0-thalassemia, only Hb E and Hb F are present without detectable Hb A. Hb E constitutes between 40-60% of the hemoglobin with the rest Hb F. Hb A is present in Hb E β+-thalassemia, resulting in a milder clinical picture than Hb E β0-thalassemia.
Since Hb E β-thalassemia is unique to Southeast Asia in general and to Thailand in particular, details of clinical manifestations as well as variability in disease severity will be discussed. In addition, as population migrations have caused the demography of the disease to shift worldwide, this thalassemia syndrome currently has more global implications.
Clinical manifestations
Most Hb E β-thalassemia patients in Thailand are not transfused or are transfused very little, and iron chelation is rarely administered. The clinical manifestations of this syndrome are heterogeneous ranging from a mild form of thalassemia intermedia to severe transfusion dependency.9,10 About half of the patients have the thalassemia intermedia phenotype whereas the other half have thalassemia major phenotype. In reviewing 803 patients with Hb E βo-thalassemia in the steady state, the hemoglobin concentrations ranged from 3-13 g/dl with the average of 7.7 g/dl.11 This variability in severity is due to the different β-thalassemia mutations present with Hb E and other modulating factors including the co-inheritance of a varying ability to produce Hb F.
At birth, infants with Hb E β-thalassemia are usually asymptomatic because of their high Hb F level. The onset of symptoms may begin at 6-12 months with anemia and abdominal enlargement when Hb F production decreases. In 80% of the patients, the symptoms initially appear during the first decade of life. The initial manifestations vary from patient to patient. The most common symptoms include pallor and enlargement of spleen. Mongoloid or thalassemia facies are observed in 82%, with about half of the patients having obvious changes. Retardation of physical development is present in 75% and absence of secondary sexual development may occur in some patients. Hepatosplenomegaly is detected in 90% of patients. However, there is no correlation between the degree of organ enlargement and the state of the disease. Full blown manifestations develop with time in the absence of blood transfusion. The patients in whom the symptoms initially appear during the first year of life usually have more severe disease.
1. Marrow and extramedullary erythropoiesis.
Erythropoiesis is markedly increased to 10-15 times normal due to high erythropoietin production. Massive erythropoiesis is observed in the bone marrow and extramedullary sites including liver, spleen, lymph node and others. Erythropoietic masses in the chest cavity as visualized by chest x-rays are observed in 8% of the males and 6% of the females.12 Erythropoietic masses in the spinal canal can cause spinal cord compression, or convulsions when they appear intracranially.13,14 These masses respond to local radiation, which can reverse such symptoms. Massive erythropoiesis leads to bone fragility and distortion; bone density is decreased due to osteoporosis and osteomalacia.15
2. Iron overload.
Iron overload occurs as a consequence of multiple blood transfusions and increased gastrointestinal absorption of iron.16,17 Iron deposition is observed in skin, reticuloendothelial organs, heart, liver and endocrine glands. Patients usually have darkened skin, but arrhythmias are infrequently encountered. Liver fibrosis from iron overload is common, but ascites is rare.18 If the patients live long enough, they may develop diabetes mellitus due to iron deposition in the pancreas.19 Some patients who live into their third or fourth decades may develop a terminal wasting syndrome with increased skin hyperpigmentation, poor appetite, weight loss, increasing anemia and eventually death. This syndrome is believed to be due to multiple organ failure caused by uncontrolled tissue oxidation from chronic severe iron overload.
3. Cardiovascular complications.
About half of the patients with Hb E β-thalassemia die of heart failure caused by iron overload and severe anemia. It is of interest to note that iron deposition in the myocardium is not extensive compared to that in the liver and pancreas.20 Cardiomegaly is proportional to the severity of anemia and systolic murmurs are frequently present.21
Pericarditis following upper respiratory tract infection is frequently observed, especially in splenectomized patients. Pericardial effusion may follow, causing cardiac tamponade in some cases. Intractable heart failure caused by constrictive pericarditis may occur in the patients even without severe anemia, and surgical intervention is necessary. Histologic examination of the pericardium reveals nonspecific pericarditis.20
4. Infections.
Infections are a major complication and cause of death of patients with Hb E β-thalassemia, especially splenectomized cases.22,23 Prospective studies indicate increased susceptibility to bacterial, fungal and viral infections.24,25 Post-splenectomy overwhelming infections can be very severe and cause mortality. The causative organisms are mostly gram negative bacteria. Arterial occlusion and gangrene of the legs caused by Pythium insidiosum (a type of water mold which is now classified within the kingdom Chromista together with algae) is observed in Hb E β-thalassemia.26 Although not frequent, this complication is difficult to diagnose and therapy is generally not effective.
The causes of increased susceptibility to infections are unknown, other than the well-appreciated immune defects found in post-splenectomized patients. No specific defect in humoral and cellular immune responses have been reported in thalassemia per se.27–,29 However, the underlying mechanism may involve altered interaction between erythrocytes, cellular and humoral immune responses, complement and the reticuloendothelial system.30
5. Gall stones.
Gall stones are present in 50% of the patients.31 Cholecystitis and ascending cholangitis may occur in some patients, requiring antibiotics and cholecystectomy. However, there are no randomized control trials to determine whether cholecystectomy should be performed in the patients with asymptomatic gall stones.
6. Hypertension, convulsions and cerebral hemorrhage after multiple blood transfusions.
The clinical syndrome of hypertension, convulsion and cerebral hemorrhage following multiple blood transfusions in Hb E β-thalassemia has been described.32 This complication may occur immediately or as late as two weeks after multiple blood transfusions. Monitoring blood pressure during and after blood transfusion is therefore necessary, and prompt therapy with anti-hypertensive drugs will reduce mortality from cerebral hemorrhage.
7. Hypoxemia.
Hypoxemia is observed in a majority of splenectomized patients.33 The underlying mechanism is unknown. Our hypothesis is that platelets, which are increased and may be more associated after splenectomy, aggregate in the circulation and in the pulmonary vasculature.34 This platelet aggregation may release a substance that causes bronchiolar constriction leading to decreased oxygenation. Consistent with this hypothesis are preliminary studies indicating that administration of aspirin can ameliorate the degree of hypoxemia in the majority of cases. Whether prophylaxis with drugs inhibiting platelet aggregation should be administered to splenectomized cases needs to be further investigated.
8. Thromboembolism.
9. Autoimmune hemolytic anemia.
Some patients may develop Coombs' positive hemolytic anemia that worsens their anemia and requires corticosteroid therapy. Recent studies suggest that the thalassemic red cell surface is an active site of complex immune reactions that can be associated with many complications.30
10. Variability in disease severity
. Disease severity of thalassemia syndromes can be assessed based on age at the onset of symptoms, hemoglobin concentration in the steady state, age of first transfusion, frequency of transfusion, presence of hepatosplenomegaly, bone changes and growth retardation. Hb E β-thalassemia is classified as thalassemia intermedia because the patients have inherited a β-thalassemia allele and Hb E, which acts as a mild β+-thalassemia. However, heterogeneity of disease is reflected in the varying hemoglobin concentrations (2.5-11.5 g/dl) found in a series of 803 patients with Hb E βo-thalassemia.11
Possible genetic factors that can influence the severity of Hb E β-thalassemia include β-thalassemia mutations, coinheritance of α-thalassemia, and association with increased Hb F.36
a. β-thalassemia mutations: Some β+-thalassemia mutations can produce only small amounts of β-globin chains and have a phenotype similar to β0-thalassemia, e.g. C→T in IVS-II-654 (the change occurs at nucleotide 654 in IVS-II). Other mutations such as A→G at position -28 and A→G in codon 19 (Hb Malay) have a mild phenotype. Compound heterozygosity between Hb E and mild β+-thalassemia results in a mild disease, whereas Hb E and severe β+ and β0 produce severe disease.36,37
b. Coinheritance of α-thalassemia: Coinheritance of α-thalassemia ameliorates the severity of β-thalassemia disease in patients with at least one allele of mild β− thalassemia genotype. Our studies revealed that Hb E β0-thalassemia patients who have coinherited α-thalassemia-2 have Hb concentrations equal to or above 7.4 g/dL.38 Coinheritance of α-thalassemia-1 with Hb E β0-thalassemia results in very mild disease severity. α-thalassemia gene interaction with β-thalassemia results in a better balanced α/β production ratio and leads to the milder thalassemic disease.
c. Association with increased hemoglobin F: Coinheritance of determinants that increase Hb F expression can ameliorate the severity of β-thalassemia. Inheritance of a β-thalassemia chromosome with the XmnI cleavage site at position -158 of the Gγ-globin gene is associated with increased Hb F and milder anemia.37 Two copies of this allele are necessary to produce a significant effect. Patients with an XmnI +/+ genotype have hemoglobin concentration above 8.5 g/dL whereas those with the XmnI -/- genotype have Hb less than 7 g/dL.
Diagnosis of Thalassemia
Provisional diagnosis of thalassemia syndromes can be made based on characteristic clinical manifestations and morphologic red cell abnormalties.39 Definite diagnosis is confirmed by hemoglobin typing based on the electrophoretic or chromatographic separation of Hb from the blood. Some thalassemia syndromes may be diagnosed with only one laboratory examination. For example, Hb H disease can be diagnosed by detection of 80-90% of intraerythrocytic inclusion bodies. By contrast, certain thalassemia heterozygotes are difficult to diagnose. Several laboratory tests may be necessary for the diagnosis of α-thalassemia 1 and β-thalassemia heterozygote.
Iron deficiency anemia should first be excluded before making the diagnosis of thalassemia because it may complicate the diagnosis. In severe iron deficiency anemia, Hb A2 and Hb E are decreased.40 In β-thalassemia heterozygotes with severe iron deficiency anemia, the Hb A2 level may be in the normal range. In patients with Hb H disease and severe iron deficiency, Hb H and the intraerythrocytic inclusion bodies may be diminished or not detectable.
Thalassemia Treatment and Control
Strategies for thalassemia control consist of offering the best treatment to patients and prevention of the birth of new cases. Treatment with hypertransfusion and iron chelation can prevent pathology and improve the quality of life in many patients and is associated with prolonged life expectancy. Given this approach, the number of thalassemia patients will be increased to a level that burdens the society's economic capability, unless new births of thalassemic patients are prevented. Ultimately, there could be fewer patients with thalassemia in the country. However, there would still be a large number of thalassemia carriers. Prenatal detection programs are ongoing to attempt to prevent new thalassemic births (see below).
Blood transfusion and chelation
Hypertransfusion and iron chelation is at present the standard therapy for thalassemia major. This will provide a marked improvement in survival, growth and sexual development. However, only a minority of the patients in Thailand and other developing countries can afford this type of therapy due to the high cost of the only available iron chelator (desferrioxamine) and the difficulty of its parenteral administration.
A majority of patients with thalassemia major in Thailand receive blood transfusions when they have symptomatic anemia and only a few units of red cell transfusion are given. Routine screening of blood for transfusion transmitted viruses including hepatitis B, hepatitis C, and HIV has improved the safety of blood transfusion in thalassemia patients.
Bone marrow transplantation
Bone marrow transplantation is the only means of curing thalassemia at present and can be considered if a sibling is found to be an HLA-matched donor. The risk is low when transplant is performed at an early age. There is a higher risk as the patient becomes older. Based on the largest experience from Pesaro, Italy where over 1,000 bone marrow transplantations for thalassemia have been performed, patients may be classified into three classes according to the presence of risk factors including hepatomegaly, portal fibrosis and poor quality of iron chelation. Among patients below 16 years, survival and event-free survival, of 95% and 90%, respectively, is reported for Class 1 (without any risk factors), falling to 89% and 64% for Class 3 (with all three risk factors). Those with Class 3 also have a high rate of graft rejection or graft failure with reconstitution of the patient's own bone marrow.41
Our experience has confirmed the finding that bone marrow transplantation performed early when the patients are young has a very high probability of cure. However, those with full blown manifestations and who received inadequate blood transfusion treatment have a higher risk of graft rejection.42 Only a small number of thalassemia patients in Thailand are able to undergo bone marrow transplantation because the procedure is still expensive and there are only 3 or 4 transplant centers in the country.
We first reported that use of umbilical cord blood collected from an unaffected sibling at the time of delivery could cure patients with thalassemia.43 The advantage of this approach is to allow sufficient stem cells to be obtained from sibling donors at birth rather than waiting until the donor is older and ready to be a marrow donor. Transplantation can then be performed earlier with better chance of cure. Furthermore, there is a decreased risk of severe graft-versus-host disease because lymphocytes in cord blood are immature and more immunologically naive.
Up to now, 12 severely thalassemic patients have undergone allogeneic sibling umbilical cord blood transplantation in our institution. The results are favorable although the hematologic recovery is slower than with bone marrow transplantation.
Hydroxyurea treatment
Hydroxyurea can stimulate red cell Hb F production resulting in prolonged life-span and better function in oxygen delivery. Preliminary results in 20 patients with Hb E β-thalassemia who received hydroxyurea 5 mg/kg/day 5 days/week indicated that the Hb F was increased in all patients treated. There was improvement in the sense of well being and exercise intolerance although the Hb concentration was minimally increased (Chancharunee S, personal communication).
Prevention of thalassemia
In Thailand, prevention programs are devoted to controlling three severe thalassemia diseases: Hb Bart's hydrops fetalis, homozygous β-thalassemia and Hb E β-thalassemia. The ultimate objectives of thalassemia prevention are to screen for carriers, to offer prenatal diagnosis of the high risk fetus, and to provide choices for abortion for the couples to consider. Identification of couples at risk is at present performed by pregnancy screening.
Several activities towards prevention and control of thalassemia have been launched by the Thailand Ministry of Public Health in collaboration with major university hospitals and the Thalassemia Foundation. The Department of Medical Science, Ministry of Public Health has developed standards of laboratory investigations for thalassemia screening in hospitals at different levels as follows:
Community hospitals: Complete blood count, osmotic fragility test, and dichlorophenol indophenol (DCIP) precipitation test (Hb E and unstable Hb screening test).
Provincial hospitals: Complete blood count, osmotic fragility test, DCIP precipitation test, and Hb typing by electrophoresis.
Regional hospitals: Complete blood count, osmotic fragility test, DCIP test precipitation test, and Hb typing by electrophoresis or high performance liquid chromatography (HPLC) using automated machines.
These laboratory investigations are essential for diagnosis of thalassemia carriers and Hb E carriers.
Prenatal diagnosis is now available in 17 hospitals under the Department of Maternal and Child Health, Ministry of Public Health.44 Fetal blood is obtained by cordocentesis. Prenatal diagnosis is performed by Hb typing using automated HPLC. Based on the preliminary results, only 40% of pregnant women came to antenatal clinics when the pregnancy was less than 16 weeks and about half of them succeeded in having their husbands tested for being thalassemia carriers. Of 349 pregnant women at risk, the prenatal diagnosis was shown to be normal in 27%, Hb Bart's hydrops fetalis in 12%, homozygous β-thalassemia in 5%, Hb E β-thalassemia in 3% and the rest were heterozygous for thalassemia or Hb E. Termination of pregnancy was chosen by all couples who had fetuses with severe disease.
In university hospitals, prenatal diagnosis is usually performed by analysis of DNA obtained from chorionic villi sampling, amniocentesis or cordocentesis, based on each institutional obstetrician's experiences. A prospective thalassemia prevention and control program was established at Songklanagarind University Hospital in 1992.45 Up to 1998, 7,785 pregnant women entered the screening program. Two hundred and three couples at risk for severe thalassemia were identified (2.6%). Of 184 couples who accepted prenatal diagnosis, 57 cases (31%) had fetuses with severe disease and virtually all elected therapeutic abortion.
Although now a major problem in Thailand, management and control of thalassemia remain unsatisfactory and suboptimal compared to other countries where the disease is prevalent. Some patients, especially in rural areas, are still not diagnosed and receive only symptomatic treatment. They and their families suffer from this disease, which is a critical burden on the society.
Recently, a National Thalassemia Plan has been proposed in Thailand.46 The objectives are to strengthen knowledge in the field of thalassemia and to develop adequate amounts of expert manpower at all levels for thalassemia control. At present, the project has had only limited progress due to the economic crisis within the country. However, we are hopeful that this project will be of interest to international health organizations who help provide support.
III. Management of Childhood Acute Lymphocytic Leukemia in a Developing Region of Central America
Raul C. Ribeiro, MD*
St. Jude Children's Research Hospital, 332 N Lauderdale St., Memphis TN 38105-2729
Over the past 40 years, improvements in therapy and supportive care have increased the cure rates for childhood acute lymphocytic leukemia (ALL) from near zero to about 80% in developed countries.1 In the US and Western Europe, most children are treated on carefully planned clinical trials 2 that promise to further improve cure rates as new compounds and treatment strategies are developed. However, the treatment of ALL is complex and expensive, requiring the commitment of substantial financial and social resources.3 Unfortunately, most children with ALL live in countries that lack the resources to provide adequate treatment, and they continue to experience high rates of mortality.4 The probability of 5-year event-free survival (EFS) for children with ALL in developing countries is estimated to be less than 20%.5 As the control of preventable infectious diseases reduces the overall rate of childhood mortality in many developing countries, the cancer mortality rate becomes an increasingly urgent challenge.6– 8
There is unlikely to be a single effective method for making anticancer treatment for children more widely available. Because developing countries differ economically, culturally, and sociopolitically and have diverse health priorities, more than one model is probably needed for such a process. Research on the development, implementation, and evaluation of such models has implications that go beyond the saving of children's lives in poor countries. The establishment of cancer treatment units in developing countries will also attract more physicians and researchers to this field, and the body of knowledge will be enriched as a result. Moreover, the study of the clinical, biological, and epidemiologic characteristics of childhood cancer across diverse populations will provide a wealth of new data for research. Burkitt lymphoma illustrates such a paradigm: the disease was first observed in Africa,9 Epstein-Barr virus was isolated in Burkitt cells in England,10 effective therapy was developed by French investigators,11 and fundamental molecular changes associated with the disease have been investigated worldwide.12
The development and maintenance of a pediatric hematology-oncology unit in a developing country can be challenging. Local governments are often unsupportive, because pediatric cancer is relatively rare and its treatment is expensive. International agencies recognize the increasing importance of cancer as a cause of childhood mortality in some developing countries but are not yet prepared to devote resources to the establishment of childhood cancer units. In fact, only one article on pediatric cancer has been published during the past 3 years in the Bulletin of the World Health Organization. This article addresses the relationship between electromagnetic fields and childhood leukemia.13
In 1993, physicians at St. Jude Children's Research Hospital, desiring to build on the initial work done in international pediatric hematology-oncology,14–,16 reasoned that ALL could be used as the starting point for establishing a pediatric hematology-oncology treatment unit in a country with limited resources.17 Because the essential components of effective therapy for ALL were well established, the training of health care providers and improvement of overall hospital infrastructure to support treatment of ALL should be relatively straightforward. The establishment of a successful program of ALL therapy would allow the subsequent progressive inclusion of other curable pediatric cancers. Our hope was that this model, if successful, could then be applied to other countries with similar characteristics.
The El Salvador Program
The program in El Salvador began with the vision of a Salvadoran mother whose child, a St. Jude patient, had died of AML in the early 1970s. She and a group of volunteers, after raising funds for 2 years, approached St. Jude for help in establishing a pediatric cancer program in San Salvador, the country's capital. They established the fund-raising foundation Ayudame a Vivir (Helping Me to Live) to provide resources for the purchase of essential anticancer medications, housing and food for patients and their families.
St. Jude investigators visited San Salvador's Hospital Benjamin Bloom, a tertiary pediatric referral center, and assessed what would be needed to establish a modest pediatric hematology-oncology treatment unit. The hospital's infrastructure and general administration were adequate to support such a program. The main problems were those commonly found in countries with limited resources: lack of uniform cancer treatment guidelines, anticancer medications, and trained health care providers. Further, the populace did not view childhood cancer as potentially curable, and frequent abandonment of treatment was an additional obstacle. Because of these factors, the rate of mortality among children with leukemia was very high. Our review found that only 40% of the 401 children with ALL admitted to the hospital between 1987 and 1993 had received treatment, and virtually none were cured.17
The El Salvador I ALL Trial
A Salvadoran pediatric hematologist-oncologist who had been trained in Mexico was chosen as medical director of the program. He was highly motivated and committed his full-time effort to the project. The medical director, nurses, and other health care providers received additional training at St. Jude. A St. Jude pediatric hematologist-oncologist fluent in Spanish was selected to provide telephone and e-mail consultation to the program director during implementation of the treatment protocol.
The diagnosis of ALL was based on the French-American-British classification. Morphologic and cytochemical studies were performed at the Benjamin Bloom Hospital. Bone marrow samples were shipped to St. Jude via 2- to 3-day courier for immunophenotypic, DNA content, and molecular genetic studies. Standard-risk ALL was defined by B-cell immunophenotype, DNA index ≥ 1.16, age > 12 months, white blood cell (WBC) count < 50 x 109/L, and the absence of anterior mediastinal mass, central nervous system (CNS) leukemia at diagnosis, and chromosomal translocations t(4;11), t(1;19), and t(9;22). Patients were treated on the high-risk arm if complete information was not available for risk classification.
The El Salvador I (ELS-I) treatment protocol was based on the St. Jude Total Therapy XI ALL protocol18: uniform induction therapy was followed by risk-stratified consolidation and maintenance therapy. Both risk groups received reinduction therapy (identical to induction therapy) during weeks 17 through 22. CNS-directed intrathecal chemotherapy comprised methotrexate, hydrocortisone, and cytarabine (IT-MHA).
Between January 1994 and December 1996, 153 patients were enrolled on the ELS-I study. The clinical and biological characteristics of these patients appeared to be similar to those observed in more developed countries. Children in El Salvador tended to have higher WBC counts at diagnosis and a lower proportion of T-cell ALL than did children enrolled on Total XI.
Of 151 evaluable patients enrolled on ELS-I, 126 (83.4%) entered complete remission (CR). Two patients with incomplete remission data were excluded. This rate of CR was inferior to that obtained in developed countries, in part because patients whose families refused to continue treatment during the induction phase were considered to have remission failure (11 patients, 7%). Other causes of treatment failure included severe infection, bleeding, metabolic complications and leukostasis.19 All children who completed the planned induction therapy entered CR. Of the 126 patients who entered remission, 28 have experienced relapse (17 isolated marrow relapses, 6 isolated CNS relapses, 1 isolated testicular relapse, 3 combined marrow and CNS relapses, 1 combined marrow and testicular relapse), and 5 have had a diagnosis of secondary AML. Twenty-two patients who entered remission (17.5%) elected to discontinue treatment during maintenance. Figure 2 shows the distribution of estimated event-free survival and survival, respectively. Patients who abandoned treatment after completing remission induction therapy were considered to have treatment failure at the time of last follow-up.
Lessons from the El Salvador I Trial
Analysis of the collaborative efforts of St. Jude, the Foundation Ayudame a Vivir, and Hospital Benjamin Bloom suggested that several factors were crucial to the successful establishment of a pediatric hematology-oncology treatment program in El Salvador. Although developing countries are heterogenous in their medical needs and resources, our experience suggests that some of these principles may have general application. First, the fact that the Salvadoran leadership invited the St. Jude medical team to participate in this endeavor was important in maintaining local ownership of the program and establishing the basis for a genuine partnership. Working closely with the Salvadorian medical team also offered St. Jude personnel an opportunity to appreciate the differences between the two countries' cultures and medical systems.
Second, the establishment of a trained, full-time team of physicians and nurses was essential. The training sessions at St. Jude were usually brief (4 to 8 weeks) but intensive and were focused on issues related to the primary care of children with ALL. The availability of a full-time pediatric hematologist-oncologist and nurse educator in El Salvador allowed uninterrupted assessment of the therapeutic and toxic effects of therapy, resolution of diagnostic difficulties and family social constraints and effective consultation with the St. Jude medical team about these problems. Because salaries provided by Salvadoran public hospitals are very low, St. Jude worked through the foundation to supplement the salaries of key medical personnel. A third related factor was communication with the team. An experienced Spanish-speaking pediatric hematologist-oncologist at St. Jude was available for consultation by phone or e-mail, and St. Jude staff traveled to El Salvador every three months during the first years to assess the progress of the program.
A fourth crucial element was the capability for reliable diagnostic testing. The shipping of bone marrow samples from El Salvador to St. Jude permitted accurate diagnosis and risk classification, but it had several drawbacks. The most important of these was that about 20% of the samples were inadequate for analysis. Further, as the number of samples increased, the process of shipping, testing, and timely reporting of the results proved to be inefficient and costly. To resolve this problem, St. Jude identified a pathology laboratory in Guatemala that could provide immunophenotypic and DNA content studies for the region. An experienced Guatemalan pathologist was trained in flow cytometric analysis at St. Jude, and a Web site was designed to allow him to communicate with St. Jude investigators, who provided quality control and continuing education for his laboratory.20
A fifth key element was the mobilization of local fund-raising and public education efforts. The Foundation Ayudame a Vivir coordinated these activities. The foundation worked to increase awareness of the availability of effective cancer treatment, to provide housing and food to families from rural areas or distant cities, and to raise funds for the hospital for essential medications. Their work not only made treatment possible but also reduced the abandonment of therapy and improved compliance with treatment. The American Lebanese Syrian Associated Charities (ALSAC), St. Jude's fund-raising arm, has worked with the foundation to improve its fund-raising strategies. The foundation provides about 70% of the cost of treatment (average, $10,000 per patient) and has effectively recruited the support of local nongovernmental and governmental agencies.
The El Salvador, Guatemala, Honduras-II (ELSGH-II) Trial
The St. Jude Total XI study,18 which showed an overall 5-year EFS estimate of approximately 70%, formed the basis for the ELS-I trial. However, because Total XI was conducted in the US rather than in El Salvador, the ELS-I protocol was constantly monitored for unexpected complications. The St. Jude Total XI protocol had been very well tolerated, with less than 1% early mortality, and most patients received continuation treatment as outpatients. However, a pilot study of the protocol initiated in El Salvador in 1993 showed an unacceptably high mortality rate during induction: 7 of 25 patients died of treatment-associated complications during the first month of therapy. A combination of factors, including advanced stage-disease, established infections at the time of diagnosis, insufficient supportive care facilities, and treatment intensity, appeared to be responsible. Measures were taken to improve infection control, train physicians and nurses, and streamline communication between Salvadoran and St. Jude physicians, and daunomycin was deleted from induction therapy. These measures were effective in decreasing early mortality. Two of the main causes of treatment failure in the ELS-I trial, abandonment of treatment (33 patients) and toxic death (27 patients), are now rare.
Several other issues of concern that emerged during the El Salvador I study motivated the development of our second protocol, the El Salvador, Guatemala, and Honduras (ELSGH)-II study. First, only 10% of the patients met the criteria for standard-risk ALL, despite reports showing that about 40% of childhood ALL is standard-risk.21 Although the presenting WBC counts of the Salvadoran children appeared to be higher than those of children at St. Jude,6 the stringent risk criteria we used are more likely to have caused this disparity. In fact, under the risk classification criteria proposed by the National Cancer Institute,21 43% of these cases would be classified as standard- or low-risk ALL. The ELSGH-II study (Tables 4, 5, 6Table 4,Table 5,Table 6) uses risk criteria modified to reflect the NCI recommendations and the prognostic implications of the extent and kinetics of leukemic cell clearance.22 Standard-risk ALL is defined by the presence of B lineage ALL, age 1 to 10 years, and WBC count < 50 x 109/L or DNA index ≥ 1.16 and ≤ 1.60. CNS involvement at diagnosis or persistence of lymphoblasts on day 15 or 23 indicate high-risk disease regardless of other features. This risk classification will allow approximately 40% of the children to receive much less complex and intensive therapy, and thus the rate of infectious complications and refusal (abandonment) of therapy are likely to be reduced.
Second, in the ELS-I study it was noted that five patients developed secondary acute myeloid leukemia (AML) as their first adverse event. This rate of secondary AML is higher than that observed in contemporary treatment of childhood ALL. The maintenance arm of the ELS-I protocol was similar to that of the St. Jude Total XI study, which showed a low rate of secondary AML at that time.18 However, recent observations suggest that secondary malignancy is associated with both constitutional determinants of drug metabolism and the administration of specific classes of drugs on certain schedules.23,24 Epipodophyllotoxins were eliminated from the ELSGH-II trial because of their strong association with secondary AML.
Third, during the time of the ELS-1 study, several new treatment strategies proved to be effective in increasing the cure rate of ALL.25–,27 One particularly important strategy was augmentation of the intensity of chemotherapy early in the continuation phase for patients with high-risk leukemia.27 Hence, a double re-induction phase was added to the ELSGH-II trial for patients at high risk of treatment failure.
Finally, because of concerns about neuropsychological and endocrine sequelae and an increased risk of brain tumors after CNS irradiation, we substituted intensive systemic chemotherapy, including dexamethasone, and triple intrathecal chemotherapy for CNS irradiation for most the patients (Tables 4, 5, and 6Table 4,Table 5,Table 6). In ELSGH-II, CNS irradiation is reserved for patients with CNS leukemia at diagnosis (1800 cGy at week 56) and for patients considered to be at high risk of relapse in the CNS. Patients in the latter group (B- lineage ALL with a presenting WBC count > 100 x 109/L or T-lineage ALL with > 50 x 109/L) receive 1200 cGy at week 56.
Although the results of ELSGH-II cannot yet be compared conclusively with those of the ELS-I protocol, the early results are encouraging. Among the first 29 patients enrolled on ELSGH-II (November 2000-June 2001), there have been no toxic deaths, and only two patients abandoned treatment during induction, despite the increased intensity of this protocol.
We are now extending the El Salvador model to the development of pediatric hematology-oncology units in other countries in Central America. The St. Jude International Outreach Program has established partnerships with Guatemala and Honduras, and slightly modified versions of the ELSGH-II protocol are in use in these two countries. These modifications were made to accommodate differences observed in patients' initial responses to treatment. For example, Honduran patients experienced marked toxicity with induction chemotherapy, whereas Salvadoran and Guatemalan patients did not. In Honduras, there have been 16 adverse events (abandonment of treatment, 7; death during induction, 6; death during remission, 3) in a cohort of 42 patients enrolled on the protocol between October 2000 and April 2001. The difficulties faced by the team in Honduras resemble those seen during the beginning of the Salvadoran program, and strategies that proved successful in El Salvador are being implemented in Honduras. In Guatemala, where the hospital infrastructure is better and trained personnel are more readily available, the treatment plan has been well tolerated. However, because the supply of L-asparaginase is limited in Guatemala, only patients with high-risk ALL can be given that agent until the problem is resolved.
Future Directions
The development of pediatric hematology-oncology treatment units and protocols in countries with limited resources can be very challenging. These countries' economies are vulnerable to natural disasters, epidemics, and political turmoil, any of which can affect the availability of hospital beds and essential drugs for patients with cancer. Treatment guidelines must sometimes be abruptly changed to accommodate these realities. Therefore, most developing countries are unlikely to be able to collaborate effectively with institutions in more developed countries to conduct phase III clinical trials. However, effective anticancer programs can be developed and cure rates can be rapidly increased by instituting treatment guidelines based on clinical evidence. Education of the lay and medical communities about the early signs of cancer and appropriate referral will reduce the treatment-associated mortality and increase the likelihood of cure in these countries.
The establishment of partnerships between institutions in developed countries and developing countries and the promotion of alliances among nongovernmental agencies and the private and public sectors have successfully reduced childhood cancer mortality in Central America.28–,30,19 As other countries in the region develop pediatric hematology-oncology programs, there will be opportunities for the development of associations and common programs. Some of these initiatives are already in progress. Pediatric hematologists-oncologists, nurses, and psychologists in Central American countries have created the Association for Pediatric HematologyOncology Central America (AHOPCA) and have worked together to develop guidelines for the management of most childhood cancers. In another effort, representatives of the foundations that support these programs have met to learn from each other how best to acquire anticancer medications and to discuss centralizing the acquisition and distribution of essential anticancer agents. Regional training of health care providers is another initiative. St. Jude, in partnership with the School of Nursing of El Salvador, has developed a 12-week pediatric hematology-oncology course for nurses from Spanish-speaking countries. This program will train 40 to 60 nurses each year. In another project, the St. Jude International Outreach Program has collaborated with the Monza International School of Pediatric Hematology-Oncology (MISPHO) and the University of Guatemala to create a 3-year fellowship program in pediatric hematology-oncology at the Unidad Nacional de Oncologia Pediatrica.
Factors contributing to anemia in patients with P. falciparum malaria.
| Factor . | References . |
|---|---|
| Destruction of parasitized red blood cells. | 2,5,6,17,18,19,20 |
| Destruction of non-parasitized red blood cells by immune and non-immune factors | 21,22,23,24 |
| Splenic sequestration of red blood cells | 21,25,26 |
| Bone marrow suppression | 5,12 |
| Ineffective erythropoiesis and dyserythropoiesis | 4, 8,9,14,19 |
| Inadequate erythropoietin production | 15,16 |
| Pre-morbid nutritional abnormalities such as deficiencies of folic acid or iron | 5 |
| Concomitant parasitic and bacterial infections | 5 |
| Factor . | References . |
|---|---|
| Destruction of parasitized red blood cells. | 2,5,6,17,18,19,20 |
| Destruction of non-parasitized red blood cells by immune and non-immune factors | 21,22,23,24 |
| Splenic sequestration of red blood cells | 21,25,26 |
| Bone marrow suppression | 5,12 |
| Ineffective erythropoiesis and dyserythropoiesis | 4, 8,9,14,19 |
| Inadequate erythropoietin production | 15,16 |
| Pre-morbid nutritional abnormalities such as deficiencies of folic acid or iron | 5 |
| Concomitant parasitic and bacterial infections | 5 |
Common thalassemia syndromes in Thailand.
| . | Genotype . | Phenotype . |
|---|---|---|
| α- thalassemia | ||
| - - / α α | Heterozygous α-thalassemia 1 | Thalassemia minor |
| - α / α α | Heterozygous α-thalassemia 2 | Silent carrier |
| - - / - - | Homozygous α-thalassemia 1 | Hb Bart's hydrops fetalis |
| - α /-α | Homozygous α-thalassemia 2 | Thalassemia minor |
| - - / -α | α-thalassemia 1/α-thalassemia 2 | Hb H disease |
| - - /αcs α | α-thalassemia 1/Hb Constant Spring | Hb H disease |
| β-thalassemia | ||
| Heterozygous β0-thalassemia | Thalassemia minor | |
| Heterozygous β+ -thalassemia | Thalassemia minor | |
| Homozygous β0 -thalassemia | Thalassemia major | |
| β0 -thalassemia /β+-thalassemia | Thalassemia intermedia | |
| HbE β0-thalassemia | Thalassemia intermedia or thalassemia major | |
| HbE β+-thalassemia | Thalassemia intermedia | |
| . | Genotype . | Phenotype . |
|---|---|---|
| α- thalassemia | ||
| - - / α α | Heterozygous α-thalassemia 1 | Thalassemia minor |
| - α / α α | Heterozygous α-thalassemia 2 | Silent carrier |
| - - / - - | Homozygous α-thalassemia 1 | Hb Bart's hydrops fetalis |
| - α /-α | Homozygous α-thalassemia 2 | Thalassemia minor |
| - - / -α | α-thalassemia 1/α-thalassemia 2 | Hb H disease |
| - - /αcs α | α-thalassemia 1/Hb Constant Spring | Hb H disease |
| β-thalassemia | ||
| Heterozygous β0-thalassemia | Thalassemia minor | |
| Heterozygous β+ -thalassemia | Thalassemia minor | |
| Homozygous β0 -thalassemia | Thalassemia major | |
| β0 -thalassemia /β+-thalassemia | Thalassemia intermedia | |
| HbE β0-thalassemia | Thalassemia intermedia or thalassemia major | |
| HbE β+-thalassemia | Thalassemia intermedia | |
Number of annual births and existing patients with thalassemia syndromes.
| Disease . | No. of couples at risk/yr . | No. of births/yr . | No. of existing patients . |
|---|---|---|---|
| Hb Bart's hydrops fetalis | 5,000 | 1,250 | 0 |
| Hb H disease | 28,000 | 7,000 | 420,000 |
| Homozygous β-thalassemia | 2,500 | 625 | 6,250 |
| Hb E β-thalassemia | 13,000 | 3,250 | 97,500 |
| Total | 48,500 | 12,125 | 523,750 |
| Disease . | No. of couples at risk/yr . | No. of births/yr . | No. of existing patients . |
|---|---|---|---|
| Hb Bart's hydrops fetalis | 5,000 | 1,250 | 0 |
| Hb H disease | 28,000 | 7,000 | 420,000 |
| Homozygous β-thalassemia | 2,500 | 625 | 6,250 |
| Hb E β-thalassemia | 13,000 | 3,250 | 97,500 |
| Total | 48,500 | 12,125 | 523,750 |
El Salvador, Guatemala, Honduras II treatment protocol: Induction, intensification, and consolidation.
| Induction | |
| Vincristine | 1.5 mg/m2 IV weekly x 4 |
| Prednisone | 40 mg/m2 PO daily x 28 |
| Asparaginase | 10,000 U/m2 IM days 2, 3, 5, 8, 10, 12 for all patients, plus days15, 17, 19 for patients with high-risk ALL |
| Daunomycin | 25 mg/m2 IV, day 1 for all patients; days 1, 8 for patients with high-risk ALL |
| Intensification | |
| Cyclophosphamide | 1 g/m2 IV day 22 |
| Cytarabine | 75 mg/m2 IV, days 23-26, 30-33 |
| 6-Mercaptopurine | 60 mg/m2 PO days 22-36 |
| Consolidation | |
| Methotrexate | 2 g/m2 (3 g/m2)** IV days 44 and 51 |
| 6-Mercaptopurine | 75 mg/m2 PO days 44-58 |
| Induction | |
| Vincristine | 1.5 mg/m2 IV weekly x 4 |
| Prednisone | 40 mg/m2 PO daily x 28 |
| Asparaginase | 10,000 U/m2 IM days 2, 3, 5, 8, 10, 12 for all patients, plus days15, 17, 19 for patients with high-risk ALL |
| Daunomycin | 25 mg/m2 IV, day 1 for all patients; days 1, 8 for patients with high-risk ALL |
| Intensification | |
| Cyclophosphamide | 1 g/m2 IV day 22 |
| Cytarabine | 75 mg/m2 IV, days 23-26, 30-33 |
| 6-Mercaptopurine | 60 mg/m2 PO days 22-36 |
| Consolidation | |
| Methotrexate | 2 g/m2 (3 g/m2)** IV days 44 and 51 |
| 6-Mercaptopurine | 75 mg/m2 PO days 44-58 |
| * Leucovorin, 15 mg/m2/dose q 6 hours x 8 doses, is begun 24 hours after the start of high-dose methotrexate. Additional doses of leucovorin are given if patients have evidence of methotrexate toxicity. | |||
| ** Patients at high risk only. | |||
| # Days 1, 15, 29 for all patients; days 8, 22 for patients with CNS disease. | |||
| Abbreviations: ALL, acute lymphocytic leukemia; IV, intravenous; IM, intramuscular; IT, intrathecal; CNS, central nervous system. | |||
| CNS-Directed Triple Intrathecal Therapy# | |||
| Triple IT therapy | Days 1, 15, 29 for all patients; add days 8, 22 for patients with CNS disease | ||
| Age < 1 yr | Age 1-3 yr | Age > 3 yr | |
| Methotrexate | 6 mg | 8 mg | 12 mg |
| Hydrocortisone | 12 mg | 16 mg | 24 mg |
| Cytarabine | 18 mg | 24 mg | 36 mg |
| * Leucovorin, 15 mg/m2/dose q 6 hours x 8 doses, is begun 24 hours after the start of high-dose methotrexate. Additional doses of leucovorin are given if patients have evidence of methotrexate toxicity. | |||
| ** Patients at high risk only. | |||
| # Days 1, 15, 29 for all patients; days 8, 22 for patients with CNS disease. | |||
| Abbreviations: ALL, acute lymphocytic leukemia; IV, intravenous; IM, intramuscular; IT, intrathecal; CNS, central nervous system. | |||
| CNS-Directed Triple Intrathecal Therapy# | |||
| Triple IT therapy | Days 1, 15, 29 for all patients; add days 8, 22 for patients with CNS disease | ||
| Age < 1 yr | Age 1-3 yr | Age > 3 yr | |
| Methotrexate | 6 mg | 8 mg | 12 mg |
| Hydrocortisone | 12 mg | 16 mg | 24 mg |
| Cytarabine | 18 mg | 24 mg | 36 mg |
El Salvador, Guatemala, Honduras II treatment protocol: continuation therapy for standard-risk acute lymphocytic leukemia (ALL).
| Weeks 1-3 | Methotrexate | 40 mg/m2 IM weekly |
| Mercaptopurine | 75 mg/m2 PO daily | |
| Week 4 | Dexamethasone | 8 mg/m2 PO daily x 7 |
| Vincristine | 1.5 mg/m2 IV | |
| Week 5 | Methotrexate | 40 mg/m2 IM |
| Mercaptopurine | 75 mg/m2 PO daily | |
| Vincristine | 1.5 mg/m2 IV | |
| Week 6 | Methotrexate | 40 mg/m2 IV/IM |
| Mercaptopurine | 75 mg/m2 PO daily | |
| Weeks 7-11 | Repeat induction and consolidation | |
| Weeks 12-14 | Methotrexate | 40 mg/m2 IV/IM |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 15 | Methotrexate | 2 g/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Weeks 16-18 | Methotrexate | 40 mg/m2 IM weekly |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 19 | Dexamethasone | 8 mg/m2 PO daily x 7 |
| Vincristine | 1.5 mg/m2 IV | |
| Week 20 | Methotrexate | 40 mg/m2 IV/IM weekly |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Vincristine | 1.5 mg/m2 IV | |
| Weeks 21-23 | Methotrexate | 40 mg/m2 IV/IM weekly |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 24 | Methotrexate | 2 g/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Chemotherapy schedule given between weeks 16 and 24 is repeated during the first year (a total of six doses of high-dose methotrexate are administered during continuation) | ||
| Triple IT given on weeks 1, 2, 3, 7, 10, 12, 15, 24, 31, 36, 39, 43, 47, 55 | ||
| Dexamethasone and vincristine stop after week 100 | ||
| Continuation therapy extended 3 years for boys | ||
| Weeks 1-3 | Methotrexate | 40 mg/m2 IM weekly |
| Mercaptopurine | 75 mg/m2 PO daily | |
| Week 4 | Dexamethasone | 8 mg/m2 PO daily x 7 |
| Vincristine | 1.5 mg/m2 IV | |
| Week 5 | Methotrexate | 40 mg/m2 IM |
| Mercaptopurine | 75 mg/m2 PO daily | |
| Vincristine | 1.5 mg/m2 IV | |
| Week 6 | Methotrexate | 40 mg/m2 IV/IM |
| Mercaptopurine | 75 mg/m2 PO daily | |
| Weeks 7-11 | Repeat induction and consolidation | |
| Weeks 12-14 | Methotrexate | 40 mg/m2 IV/IM |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 15 | Methotrexate | 2 g/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Weeks 16-18 | Methotrexate | 40 mg/m2 IM weekly |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 19 | Dexamethasone | 8 mg/m2 PO daily x 7 |
| Vincristine | 1.5 mg/m2 IV | |
| Week 20 | Methotrexate | 40 mg/m2 IV/IM weekly |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Vincristine | 1.5 mg/m2 IV | |
| Weeks 21-23 | Methotrexate | 40 mg/m2 IV/IM weekly |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 24 | Methotrexate | 2 g/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Chemotherapy schedule given between weeks 16 and 24 is repeated during the first year (a total of six doses of high-dose methotrexate are administered during continuation) | ||
| Triple IT given on weeks 1, 2, 3, 7, 10, 12, 15, 24, 31, 36, 39, 43, 47, 55 | ||
| Dexamethasone and vincristine stop after week 100 | ||
| Continuation therapy extended 3 years for boys | ||
El Salvador, Guatemala, Honduras II treatment protocol: continuation therapy for patients with high-risk acute lymphocytic leukemia (ALL).
| Week 1 | Dexamethasone | 12 mg/m2 PO daily x 7 |
| Vincristine | 1.5 mg/m2 IV | |
| Week 2 | Cyclophosphamide | 300 mg/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 3 | Methotrexate | 40 mg/m2 IV/IM |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 4 | Methotrexate | 40 mg/m2 IV/IM |
| Cytarabine | 300 mg/m2 IV | |
| Week 5 | Dexamethasone | 12 mg/m2 PO daily |
| Vincristine | 1.5 mg/m2 IV | |
| Week 6 | Cyclophosphamide | 300 mg/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily | |
| Weeks 7-11 | Repeat induction and consolidation | |
| Week 12 | Dexamethasone | 12 mg/m2 PO daily x 7 |
| Vincristine | 1.5 mg/m2 IV | |
| L-asparaginase | 10,000 I.U./m2 IV | |
| Week 13 | Cyclophosphamide | 300 mg/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 14 | Methotrexate | 40 mg/m2 IV/IM |
| Cytarabine | 300 mg/m2 IV | |
| Week 15 | Dexamethasone | 12 mg/m2 PO daily |
| Vincristine | 1.5 mg/m2 IV | |
| L-asparaginase | 10,000 I.U./m2 IV | |
| Week 16 | Cyclophosphamide | 300 mg/m2 IV weekly |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Weeks 17-20 | Repeat induction and consolidation | |
| Week 21 | Dexamethasone | 12 mg/m2 PO daily |
| Vincristine | 1.5 mg/m2 IV | |
| Week 22 | Mercaptopurine | 75 mg/m2 PO daily x 7 |
| Cyclophosphamide | 300 mg/m2 IV weekly | |
| Week 23 | Mercaptopurine | 75 mg/m2 PO daily x 7 |
| Methotrexate | 40 mg/m2 IV/IM | |
| Week 24 | Mercaptopurine | 75 mg/m2 PO daily x 7 |
| Methotrexate | 3.0 g/m2 | |
| The following drug pairs are given weekly in sequence: dexamethasone plus vincristine; mercaptopurine plus cyclophosphamide; mercaptopurine plus methotrexate; methotrexate plus cytarabine; and mercaptopurine plus methotrexate. | ||
| The high-dose methotrexate plus mercaptopurine course is given every 6 weeks for a total of 6 courses during the first year of continuation therapy. | ||
| Triple IT is given on weeks 1, 2, 3, 7, 10, 12, 20, 24, 28, 31, 36, 39, 43, 47, 51 54, 55, 56 | ||
| Week 1 | Dexamethasone | 12 mg/m2 PO daily x 7 |
| Vincristine | 1.5 mg/m2 IV | |
| Week 2 | Cyclophosphamide | 300 mg/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 3 | Methotrexate | 40 mg/m2 IV/IM |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 4 | Methotrexate | 40 mg/m2 IV/IM |
| Cytarabine | 300 mg/m2 IV | |
| Week 5 | Dexamethasone | 12 mg/m2 PO daily |
| Vincristine | 1.5 mg/m2 IV | |
| Week 6 | Cyclophosphamide | 300 mg/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily | |
| Weeks 7-11 | Repeat induction and consolidation | |
| Week 12 | Dexamethasone | 12 mg/m2 PO daily x 7 |
| Vincristine | 1.5 mg/m2 IV | |
| L-asparaginase | 10,000 I.U./m2 IV | |
| Week 13 | Cyclophosphamide | 300 mg/m2 IV |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Week 14 | Methotrexate | 40 mg/m2 IV/IM |
| Cytarabine | 300 mg/m2 IV | |
| Week 15 | Dexamethasone | 12 mg/m2 PO daily |
| Vincristine | 1.5 mg/m2 IV | |
| L-asparaginase | 10,000 I.U./m2 IV | |
| Week 16 | Cyclophosphamide | 300 mg/m2 IV weekly |
| Mercaptopurine | 75 mg/m2 PO daily x 7 | |
| Weeks 17-20 | Repeat induction and consolidation | |
| Week 21 | Dexamethasone | 12 mg/m2 PO daily |
| Vincristine | 1.5 mg/m2 IV | |
| Week 22 | Mercaptopurine | 75 mg/m2 PO daily x 7 |
| Cyclophosphamide | 300 mg/m2 IV weekly | |
| Week 23 | Mercaptopurine | 75 mg/m2 PO daily x 7 |
| Methotrexate | 40 mg/m2 IV/IM | |
| Week 24 | Mercaptopurine | 75 mg/m2 PO daily x 7 |
| Methotrexate | 3.0 g/m2 | |
| The following drug pairs are given weekly in sequence: dexamethasone plus vincristine; mercaptopurine plus cyclophosphamide; mercaptopurine plus methotrexate; methotrexate plus cytarabine; and mercaptopurine plus methotrexate. | ||
| The high-dose methotrexate plus mercaptopurine course is given every 6 weeks for a total of 6 courses during the first year of continuation therapy. | ||
| Triple IT is given on weeks 1, 2, 3, 7, 10, 12, 20, 24, 28, 31, 36, 39, 43, 47, 51 54, 55, 56 | ||
Possible mechanisms of severe malarial anemia.
Abbreviations: IFN-γ, interferon-gamma; IL, interleukin; MIF, macrophage migration inhibitory factor; TNF-α, tumor necrosis factor-alpha
Possible mechanisms of severe malarial anemia.
Abbreviations: IFN-γ, interferon-gamma; IL, interleukin; MIF, macrophage migration inhibitory factor; TNF-α, tumor necrosis factor-alpha
Kaplan-Meyer estimates of 5-year event-free survival for the 151 evaluable patients enrolled on the El Salvador I study. Patients who discontinued treatment were considered to have treatment failure at the time of the last follow-up.
Kaplan-Meyer estimates of 5-year event-free survival for the 151 evaluable patients enrolled on the El Salvador I study. Patients who discontinued treatment were considered to have treatment failure at the time of the last follow-up.