Abstract
Thrombocytopenia in the pregnant patient may result from a number of causes, most of which involve either immune-mediated platelet destruction or platelet consumption. Many of these disorders share clinical and laboratory features, making accurate diagnosis difficult. Moreover, uterine evacuation is indicated in the therapy of some disorders, while in others alternative interventions may allow the pregnancy to be carried to term. These and other issues are discussed as part of a comprehensive review of the differential diagnosis and management of thrombocytopenia in pregnancy.
The term “refractory ITP” is used with reference to two distinct groups of patients: 1) patients in whom the platelet count cannot be easily increased, including those who are poorly responsive to initial single agent treatment, and 2) those with persistent thrombocytopenia despite the use of conventional therapies. An approach to management of the former group will be presented, followed by a discussion of patients with chronic refractory ITP. The latter will include presentation of new data on the role of Helicobacter pylori in ITP and whether its treatment ameliorates thrombocytopenia, as well as the use of rituximab and other modalities.
Thrombotic microangiopathies such as thrombotic thrombocytopenic purpura (TTP) are rare, but life threatening causes of thrombocytopenia. Ultra-large multimers of von Willebrand factor (vWF) aggregate platelets intravascularly, and congenital or immune-mediated deficiencies of a metalloprotease that cleaves these ultra-large multimers may cause TTP. However, little information exists concerning the behavior of this protease in other physiological and pathological conditions. Levels of this protease have now been measured in healthy individuals of different ages, full-term newborns, pregnant women and a patients with variety of pathologic conditions, and these data will be reviewed herein.
Heparin-induced thrombocytopenia/thrombosis (HIT/T) remains the most common antibody-mediated, drug-induced thrombocytopenic disorder, and a leading cause of morbidity and mortality. Based on clinical correlations and murine models, there is increasing evidence that antibodies to complexes between platelet factor 4 (PF4) and heparin cause HIT/T, and the molecular composition of the relevant antigen has also become better defined. However, the introduction of sensitive ELISAs to measure anti-PF4/heparin antibodies has complicated diagnosis in some settings in which the incidence of such antibodies in unaffected patients exceeds the incidence of the disease. In addition, the FDA approval of Lepirudin and Argatroban has expanded the repertoire of agents available for therapy of HIT/T and may change the approach to management of asymptomatic patients with thrombocytopenia. However, the optimal use of these drugs in commonly encountered settings remains in evolution, and a need for alternative approaches to prevention and treatment is evident.
I. Pregnancy-Associated Thrombocytopenia: An Update
Keith R. McCrae, MD*
Department of Hematology/Oncology, Case Western Reserve University, School of Medicine, BRB-3, 10900 Euclid Ave., Cleveland OH 44107-4937
Thrombocytopenia affects up to 10% of all pregnancies. Though obstetricians manage most of these cases, more complex thrombocytopenic disorders are often referred for hematologic consultation. Therefore, the consulting hematologist must have a working knowledge of the differential diagnosis and therapy of pregnancy-associated thrombocytopenia. The goals of this section will be to review the differential diagnosis, pathophysiology and management of these disorders.
The Platelet Count in Normal Pregnancy
Before considering pregnancy-associated thrombocytopenia, it is useful to review the effect of normal pregnancy on the platelet count. Though a number of small studies have failed to reach a consensus on this issue, recent studies that assessed platelet counts in more than 4,000 pregnant patients each have demonstrated that the mean and 2.5th percentile platelet count decreases by approximately 10% in pregnant patients, and that the histogram of platelet count distribution at term is normally distributed but shifted to the left.1,2 In most cases, this “physiologic” decrease in platelets occurs in the third trimester. These observations suggest that pregnancy is associated with a mild, and generally unappreciated decrease in the circulating platelet count.
Differential Diagnosis of Pregnancy-Associated Thrombocytopenia
Thrombocytopenia in pregnancy may occur secondary to a variety of causes (Table 1 ), ranging from benign disorders such as gestational thrombocytopenia to syndromes associated with significant morbidity.3– 5 Most of these occur during specific periods of gestation, although these periods may sometimes overlap. On occasion, patients may present with a constellation of symptoms that reflect characteristics of more than one disorder. However, in most cases the underlying cause of thrombocytopenia may be diagnosed when the time of onset, clinical manifestations and laboratory studies are considered together.
Causes of Pregnancy-Associated Thrombocytopenia
Gestational (incidental) thrombocytopenia
Gestational, or incidental, thrombocytopenia is the most common cause of thrombocytopenia in pregnancy. This disorder affects approximately 5% of all pregnant women, and accounts for > 75% of all cases of pregnancy-associated thrombocytopenia.3,5–,7 The pathogenesis of gestational thrombocytopenia is not understood, but may reflect the effects of hemodilution or accelerated platelet clearance through immune or non-immune mechanisms.3,6 Though the majority of patients with gestational thrombocytopenia maintain platelet counts in the range of 110,000-150,000/μL, most authorities consider a platelet count of greater than 70,000/μL in an otherwise healthy pregnant woman with no prior history of immune-mediated thrombocytopenia to be consistent with this disorder. The likelihood of another, more significant cause of thrombocytopenia increases significantly in patients with platelet counts below this value.
Gestational thrombocytopenia is a benign disorder, likely a more pronounced variant of the “physiologic” thrombocytopenia that accompanies pregnancy. Patients with gestational thrombocytopenia do not have an increased incidence of bleeding, nor are they at increased risk for delivery of thrombocytopenic offspring.3,5–,8 Hence, the medical evaluation of an otherwise healthy pregnant woman with a platelet count greater than 70,000/μL and no prior history of thrombocytopenia may be limited to a general physical examination with careful blood pressure measurement and thorough examination of the peripheral blood film.9,10 As it is inevitable that a small number of women meeting these criteria will have mild immune thrombocytopenia purpura (ITP), a thorough review of pre-gestational platelet counts is indicated.
Immune thrombocytopenia purpura. ITP, the most common cause of significant thrombocytopenia in the first trimester, accounts for approximately 1 case of thrombocytopenia per 1,000 pregnancies and 5% of all cases of pregnancy-associated thrombocytopenia.3,5,11,12 The pathogenesis of ITP in pregnant, as in non-pregnant patients, involves the activity of antibodies directed against platelet glycoproteins, primarily GP IIb/IIIa and GP Ib/IX, and clearance of these IgG-coated platelets by the reticuloendothelial system.13
The diagnosis of ITP in pregnancy is not difficult when patients have a prior history of thrombocytopenia or in the setting of moderate to severe thrombocytopenia (i.e. < 50,000/μL). However, it may not be possible to distinguish ITP from gestational thrombocytopenia in a mildly thrombocytopenic patient with no prior history of thrombocytopenia. Levels of platelet-associated IgG are elevated in both groups.14 The utility of MAIPA (monoclonal antibody immobilization of platelet antigens) assays, which are designed to specifically measure antibodies reactive with platelet glycoproteins, is also uncertain in this setting.15 Practically, in the absence of a pre-gestational platelet count, the onset of significant (< 100,000/μL) thrombocytopenia early in gestation, with declining platelet counts as gestation progresses, is most consistent with ITP, while gestational thrombocytopenia appears to develop more commonly in the second or third trimester.11
Though decisions concerning management of ITP during pregnancy affect both mother and fetus, therapy is focused primarily on amelioration of thrombocytopenia in the mother. The need for therapy is based on the severity of thrombocytopenia and whether there is active bleeding. Patients with a platelet count greater than approximately > 30,000/μL and no bleeding generally do not require treatment. However, when the severity of thrombocytopenia increases or bleeding develops, therapy is indicated.9,11,16 Moreover, as pregnancy approaches term, more aggressive measures to raise the platelet count to a safe level (> 50,000/μL) to minimize the hemorrhagic stresses of delivery and allow the administration of epidural anesthesia may be required.9,11,16,17
Management of a pregnant patient with ITP is similar to that of non-pregnant individuals. Due to their efficacy and low cost, many consider corticosteroids to be the first line of therapy.3,9,10,16 However, in addition to their usual side effects such as osteoporosis and weight gain, corticosteroids increase the incidence of pregnancy-induced hypertension and exacerbate gestational diabetes. An alternative to corticosteroids is high-dose (2 gm/kg) intravenous gammaglobulin (IVIG), which some experts consider first-line therapy for pregnancy-associated ITP due to its lower toxicity profile.11 Though efficacious, the effects of IVIG are transient (duration of 3-4 weeks), and the costs of multiple courses exceedingly high. Nevertheless, a regular schedule of IVIG should be considered when more than 10 mg/day of prednisone is required to maintain the platelet count above 20,000-30,000/μL. Due to its relatively rapid onset of action, IVIG may also be useful in raising the platelet count in preparation for delivery.
As in the non-pregnant patient, patients refractory to corticosteroids and IVIG present a difficult management problem. Occasionally, such individuals will respond to combinations of high doses of steroids (methylprednisolone, 1 gm) and IVIG.3 As in non-pregnant individuals, remission of ITP is achieved in approximately 70% of pregnant women who undergo splenectomy, though the long-term efficacy of this modality is debated.18 If splenectomy is required during pregnancy, most experts recommend that it be performed in the second trimester, as it has been associated with an increased incidence of premature labor when performed earlier, and may be technically difficult later in gestation due to obstruction of the surgical field by the gravid uterus.3 Recently, laparoscopic splenectomy has been safely performed in several pregnant patients with ITP.19 Little information is available concerning the safety and efficacy of third and fourth line agents in refractory ITP during pregnancy. Azathioprine is relatively contraindicated, while danazol and vincristine should be avoided,9 though a report on successful use of the latter in a refractory pregnant patient has appeared.20
An area of particular concern in the management of pregnant patients with ITP is the fetal platelet count and its implications for the mode of delivery. Due to transplacental passage of maternal IgG, particularly during the third trimester, the offspring of patients with ITP may also develop thrombocytopenia.3,11 Neonatal platelet counts below 50,000/μL at delivery occur in 10-25% of the offspring of patients with ITP, while counts below 20,000/μL occur in 5% of these patients.21 Moreover, no maternal treatment has been convincingly shown to diminish the incidence of fetal thrombocytopenia.3,11 Bleeding complications may occur in 25-50% of profoundly thrombocytopenic neonates at the time of delivery,3,22 though intracranial hemorrhage is rare.8 Debate has therefore centered on options to minimize this risk, based on the untested hypothesis that neonatal head trauma during passage through the birth canal may precipitate intracranial hemorrhage.
Several studies have attempted to define characteristics of mothers with ITP that predict for delivery of a thrombocytopenic neonate. However, there is no convincing correlation between the fetal platelet count and a number of maternal characteristics including a history of prior splenectomy or an increased level of platelet-bound IgG.3,11 A relationship between the severity of maternal and fetal thrombocytopenia has been observed in occasional studies,23 but not in the majority of them.3,11 The finding of a relationship between levels of circulating maternal anti-platelet IgG and neonatal thrombocytopenia22 has not been widely confirmed. This, as well as a report of widely divergent platelet counts in dizygotic twin offspring24 of a patient with ITP, demonstrates that the pathogenesis of neonatal thrombocytopenia involves not only the level of maternal antibody but the rate of fetal megakaryopoiesis and ability of the fetal reticuloendothelial system to clear antibody-coated platelets.3 Indeed, the most reliable predictor of neonatal thrombocytopenia may be a history of thrombocytopenia in a prior sibling.25
Since maternal clinical or laboratory characteristics predictive of neonatal thrombocytopenia are generally not available, the fetal platelet count may be determined either through fetal scalp sampling during labor or by percutaneous umbilical blood sampling prior to delivery. Of these, the latter method provides a more reliable estimate, although it is associated with a complication rate, primarily bleeding and fetal bradycardia, of 0-1%.26 Since this approaches or exceeds the incidence of complications from severe thrombocytopenia in the offspring of a patient with ITP, two approaches to management of these patients have evolved. One approach, supported by approximately 60% of perinatologists in the US,27 advocates a trial of labor for patients with ITP without prior determination of the fetal platelet count. This approach is based on the belief that severe thrombocytopenia and bleeding in the offspring of these individuals is uncommon and that there is no evidence that the incidence of fetal intracranial hemorrhage is reduced by Cesarean section.9,28 A second approach involves invasive determination of the fetal platelet count, generally by percutaneous umbilical blood sampling, followed by Cesarean section if the platelet count is < 50,000/μL.16
Regardless of the mode of delivery, cord platelet counts should be obtained on all offspring of mothers with ITP9 at the time of delivery. Moreover, since the platelet count may decline for the first 4-5 days after delivery, daily monitoring and institution of appropriate therapy for severe thrombocytopenia, should it develop over this interval, is indicated.
Preeclampsia and the HELLP syndrome
Preeclampsia affects approximately 6% of all pregnancies, most often those of primigravidas.3 The criteria for preeclampsia, though frequently revised, include hypertension and proteinuria (> 300 mg protein/24 hours) developing after 20 weeks of gestation.29 Preeclampsia occurs most commonly in women less than 20 or greater than 30 years old, usually in the third trimester. Preeclampsia in multiparous women may be associated with a change in partners.3 A genetic role in the development of preeclampsia has been suggested but remains poorly defined.30 Recently, an association of preeclampsia with a genetic predisposition to thrombophilia has also been proposed.31
Although the clinical manifestations of preeclampsia are generally not evident until the third trimester, the pathogenesis appears to involve deficient remodeling of the uteroplacental vasculature early in pregnancy, perhaps as a consequence of diminished invasiveness of placental trophoblasts.32 Abnormalities in the expression of cell adhesion molecules33 and activated metalloproteases34 by trophoblasts from preeclamptic women have been described, but whether these are primary or secondary to other pathophysiologic events has not been established. The net result, however, is the formation of an insufficient uteroplacental blood supply, resulting in progressive hypoxia of the fetoplacental unit as pregnancy progresses.35 Resultant imbalances in the release of prostaglandins by placental and extraplacental tissues, enhanced lipid peroxidation, and other undefined factors may contribute to the hypertension, platelet activation and systemic endothelial dysfunction characteristic of this disorder.30
Up to 50% of patients with preeclampsia develop thrombocytopenia, with the severity generally proportional to that of the underlying disease. Thrombocytopenia may be an early manifestation of preeclampsia, even preceding other disease manifestations on occasion.3 The causes of thrombocytopenia are not well defined, though they likely reflect accelerated platelet clearance and/or destruction due adhesion of circulating platelets to damaged or activated endothelium, activation and consumption of platelets as a consequence of activation of the hemostatic system, or clearance of IgG-coated platelets by the reticuloendothelial system.3
Subclinical activation of the coagulation cascade occurs in most patients with preeclampsia. Though routine studies such as the prothrombin time (PT), activated partial thromboplastin time (aPTT) and fibrinogen level are usually normal, more sensitive indices such as fibrinogen D-dimers and thrombin-antithrombin complexes are elevated in many patients who develop thrombocytopenia.3 Activation of hemostasis alone is not likely to be the primary cause of thrombocytopenia in all cases, though markers of intravascular coagulation may be associated with more severe intrauterine growth retardation.36
The HELLP (hemolysis, elevated liver function tests, low platelets) syndrome is a variant of preeclampsia that occurs primarily in white, multiparous women above the age of 25 years.3 Criteria for this disorder include 1) microangiopathic hemolytic anemia (MAHA), 2) levels of serum aspartate aminotransferase (AST, SGOT) > 70 U/L and 3) thrombocytopenia, with a platelet count below 100,000/mL.37 Patients frequently present with epigastric and right upper quadrant pain, often leading to misdiagnosis and referral for evaluation of suspected intraabdominal processes. Approximately 10% of patients with HELLP develop hypertension and proteinuria, and meet criteria for preeclampsia. Despite their similarities, HELLP is associated with a higher rate of maternal and fetal morbidity and mortality than preeclampsia.3
Offspring of mothers with HELLP and preeclampsia may also become thrombocytopenic, though this may not develop until after delivery.3 The pathogenesis of neonatal thrombocytopenia in these infants is not well understood, but in part may represent the effects of prematurity and its complications, such as sepsis and acute respiratory distress.38 Absent umbilical artery end-diastolic velocity, a marker of fetal hypoxemia and acidosis, may be a risk factor for neonatal thrombocytopenia in growth-retarded offspring.39 Though elevated levels of platelet-associated IgG may be present on the platelets of these infants, they do not correlate with the development or severity of thrombocytopenia.3 More recent studies suggest that these infants may manifest impaired responses to thrombopoietin.40
Based on the premise that deficient intravascular production of prostacyclin and excessive platelet activation underlies the pathogenesis of these disorders, the value of prophylactic treatment of unselected and high-risk pregnant women with low dose aspirin in primary prevention of preeclampsia was assessed. This intervention, however, yielded no significant reduction in the incidence of preeclampsia in either group.41,42
Management of preeclampsia and/or HELLP is generally supportive, with the goal of medically stabilizing the patient prior to delivery.4 Some have argued for conservative management of preeclampsia, particularly in milder cases or before 34 weeks gestational age, thereby providing time for further fetal maturation. However, the only indication for this approach in severe preeclampsia or HELLP is to gain additional time (24-48 hours) for fetal lung maturation after administration of betamethasone.4 Platelet transfusions may be given prior to Cesarean section to raise the platelet count above 50,000/μL, with the appreciation that the survival of transfused platelets may be short. Coagulopathy resulting from disseminated intravascular coagulation (DIC) may be managed with fresh frozen plasma, if indicated.3 In almost all cases, manifestations resolve within several days after delivery, although in some patients the platelet count declines for an additional 24-48 hours.4 A subset of patients display prolonged thrombocytopenia, multiorgan dysfunction and an increased LDH following delivery, manifestations that may be reversed or ameliorated in some patients by plasma exchange43 or corticosteroids.44 Hence, these interventions should be considered in patients who remain symptomatic for more than 4-5 days after delivery and in whom other causes of persistent thrombocytopenia are excluded.
Thrombotic thrombocytopenic purpura (TTP) and the hemolytic uremic syndrome (HUS)
TTP and HUS are disorders that share the central features of MAHA and thrombocytopenia. Though neither disease is unique to pregnancy, the incidence of both is increased in this setting.4,45 In some series, up to 10% of all cases of TTP have occurred in pregnant patients.
TTP is characterized by a pentad of findings including MAHA, thrombocytopenia, neurologic abnormalities (confusion, headache and seizures, among others), fever and renal dysfunction, though the classic pentad is present at the time of diagnosis in less than 40% of patients.4 The clinical manifestations of HUS are similar, though neurologic abnormalities are usually prominent in patients with TTP, while renal dysfunction is more severe in patients with HUS. Recently, the potential role of congenital or acquired deficiencies of a specific von Willbrand factor (vWF)-cleaving protease in the pathogenesis of TTP has been evoked,46,47 although involvement of this protease in the pathophysiology of HUS is uncertain. No published information is available concerning the regulation of the vWF-cleaving protease during uncomplicated pregnancy, though this issue is addressed elsewhere in this paper.
TTP and HUS may be difficult to discern from one another, as well as from pregnancy-specific causes of thrombocytopenia such as severe cases of preeclampsia or the HELLP syndrome.48Table 2 lists features that may be of use in differentiating these disorders. First, microangiopathic hemolysis is generally more severe in TTP or HUS than preeclampsia or HELLP. Second, TTP develops relatively early in pregnancy, with a mean onset of approximately 23.5 weeks, while the majority of cases of preeclampsia and HELLP develop in the third trimester.3,4 In contrast, approximately 90% of cases of HUS occur post-partum, with a mean time to development after delivery of approximately 26 days. Third, patients with TTP and HUS generally maintain normal levels of antithrombin, while most of those with preeclampsia and HELLP display modest reductions in antithrombin levels, likely due to consumption.4
Unlike preeclampsia or HELLP, the course of pregnancy-associated thrombotic microangiopathies is not ameliorated by delivery; hence, pregnancy termination is not therapeutic.45 However, TTP in pregnant and non-pregnant patients responds similarly to plasma exchange, with > 75% of patients achieving remission. Chronic relapsing TTP may also be precipitated during pregnancy; such cases have been treated with plasma exchange or infusion, the latter occasionally used, with or without aspirin, on a prophylactic basis.49 In contrast, the prognosis of pregnancy-associated HUS, like that of sporadic HUS occurring in non-pregnant patients, is poor. Although response to plasma exchange occurs infrequently,50 a therapeutic trial is indicated.
Poor fetal outcomes are common in patients with thrombotic microangiopathies, due to placental ischemia and the morbidity associated with prematurity.45 TTP and HUS may recur in subsequent pregnancies, though the frequency with which this occurs is uncertain.4 Pregnancy-associated HUS has also occurred in women with a familial history of this disorder. The long-term morbidity of thrombotic microangiopathies, particularly HUS, is significant, with many patients left with chronic renal insufficiency and hypertension.51
Acute fatty liver of pregnancy
Acute fatty liver of pregnancy (AFLP) affects one of every 5,000 to 10,000 pregnancies, typically developing in the third trimester. Though more common in primiparas, AFLP can initially present after several uncomplicated pregnancies. This disorder may result from a fetal deficiency of long-chain 3-hydroxy-acyl CoA dehydrogenase (LCHAD) or other enzymes involved in mitochondrial fatty acid oxidation.52,53 A glutamic acid to glutamine mutation at position 478 of LCHAD appears to be of particular importance in disease development.53
Clinically, women present with malaise, nausea, epigastric and right upper quadrant pain, dyspnea, mental status changes and cholestatic liver abnormalities. Diabetes insipidus may also occur, and hypoglycemia is common and often severe.4 Levels of fibrinogen and antithrombin are severely depressed, and a prolonged PT accompanied by laboratory evidence of “disseminated intravascular coagulation” is present in 75%, perhaps resulting from impaired synthesis of antithrombin. The severity of microangiopathic hemolysis and thrombocytopenia (if present) is far less than that seen in HELLP, TTP or HUS,54 though up to 50% of patients with AFLP might also meet clinical criteria for preeclampsia. Diagnosis is usually made based on clinical criteria, as hepatic imaging studies are generally not specific. Confirmation may be obtained by staining a liver biopsy specimen with oil red O. Management consists of correction of hypoglycemia and electrolyte imbalances, as well as the underlying coagulopathy. Up to 10 days may be required after delivery for normalization of the coagulation status. Fetal mortality remains at 15%, though maternal mortality occurs in less than 5% of cases.55
Miscellaneous Causes of Pregnancy-Associated Thrombocytopenia
As in the non-pregnant setting, HIV infection should be considered in any thrombocytopenic patient with appropriate risk factors.3 Thrombocytopenia may occur in up to 25% of patients with systemic lupus erythematosus (SLE) secondary to platelet destruction due to antiplatelet antibodies, circulating immune complexes or other causes.56 Antiphospholipid antibodies (APLA) may be associated with an increased risk of preeclampsia in addition to their better-recognized association with thrombosis and recurrent fetal loss.57 Some patients with APLA have been described with syndromes resembling HELLP, HUS or TTP, which may not respond in the usual manner to standard management.4 Drug-induced thrombocytopenia occurs in the pregnant as well as the non-pregnant setting. Unrecognized cocaine use has been associated with the development of a HELLP-like syndrome in pregnant women.58 Disseminated intravascular coagulation, with attendant thrombocytopenia, may complicate several pregnancy-related disorders, including placental abruption, amniotic fluid embolism, uterine rupture and retention of a dead fetus.3 Diagnosis of these obstetrical emergencies is usually straightforward
Finally, congenital platelet disorders, such as May-Hegglin anomaly, may occasionally remain undiagnosed until pregnancy; hence, thorough review of the peripheral blood smear should be an important component of the evaluation of such patients.3 Pseudothrombocytopenia, an in vitro artifact usually caused by EDTA-dependent antiplatelet antibodies, may be transferred from mother to fetus by transplacental passage of the relevant antibody.59 Finally, Type IIb von Willbrand disease may be associated with thrombocytopenia in pregnant women due to increased levels of an abnormal vWF molecule that binds to platelets with high avidity and enhances their clearance.3
II. Novel Approaches to Management of Immune Thrombocytopenic Purpura: Results of Recent Trials
James B. Bussel, MD*
New York Hospital, Division of Pediatrics, 525 E 68th Street, Payson 6, New York NY 10021
Dr. Bussel receives clinical research support from Cangene, Amgen, Genentech, IDEC, Bayer, and Baxter.
Immune (not idiopathic) thrombocytopenic purpura (ITP) was first described as a disease of immune platelet destruction many years ago by William Harrington. This was documented by the thrombocytopenic response in normal volunteers to infusion of plasma from patients with ITP. In certain individuals, however (6 of 17 in one of the early studies), infusion of plasma from ITP patients did not cause thrombocytopenia1 in the recipients. Potential explanations for this included the possibilities that 1) the antiplatelet antibody was entirely bound to the patient's (donor) platelets and therefore was absent from infused plasma; 2) there was a particular characteristic of the recipients that made them not susceptible to the thrombocytopenic effects of the plasma, i.e. they had spherocytosis; or 3) the plasma was obtained from a patient who appeared clinically identical to other patients with ITP but who in reality had another cause of thrombocytopenia, and therefore did not have antiplatelet antibodies at all. This last possibility, the inability to be certain of the diagnosis in individual patients, persists today. The lack of specificity of platelet antibody testing means that it is still not possible to use platelet antibody studies to demonstrate that a given individual either does or does not have ITP. Studies of newer ways of testing for the presence or absence of platelet antibodies as well as additional tests to better define the pathophysiology of the thrombocytopenia and, indirectly, the diagnosis, such as thrombopoietin and glycocalicin levels and platelet reticulocyte counts, are underway.
Even in cases clinically presumed to be ITP the etiology is usually unknown. Table 3 contains a list of immunologic abnormalities or polymorphisms that have been associated with ITP. None of them, however, have a very strong association nor are they present in a majority of patients. This emphasizes the difficulty in understanding what causes this disease. Preliminary data using phage display technology has suggested that antiplatelet antibodies in patients with ITP disproportionately select a limited assortment of VH heavy chains.2 The same study suggested that this phenomenon occurs as a result of antigen driven selection, i.e. persistent antigen exposure. This refers to ongoing exposure to platelets.
Persistent infections are also associated with ITP (Table 4 ). Typically the ITP in these cases would be characterized not only as having a “mixed” pathophysiology, decreased platelet production as well as increased platelet destruction, but also as being “secondary” ITP. Nonetheless, there is an immunologic component to the thrombocytopenia in these cases. Two recent studies have suggested that Helicobacter pylori may promote ITP,3,4 potentially by contributing to either the development and/or the persistence of ITP in infected patients. Therefore, eradication of H. pylori infection may improve the course of ITP or ameliorate the disease. Evidence that this is the case was demonstrated in an initial study in which thrombocytopenia improved in 7 patients with H. pylori whose infection was treated.3 This instigated a second study of 30 chronic ITP patients, 13 of whom were found to be infected with H. pylori. H. pylori was eradicated after treatment in 12 of these 13 infected patients; 6 of these had improvement in their platelet count.4 In our ongoing study, we have tested 16 chronic ITP patients and identified 4 that were infected with H. pylori. There has been no improvement in the platelet count of the 3 who have completed 2 weeks of treatment (prevacid-amoxicillin-biaxin) and an additional 6 weeks of follow-up, and who have had their breath test repeated to demonstrate that they have become negative. In addition we have treated 10 patients with the same antimicrobial combination, the “prevpak,” even though they did not have H. pylori, and this has not improved the platelet count in any of them. Updated results will be reviewed. Of note, IVIG almost certainly has antibodies to H. pylori in it because of the relative ubiquity of this infection. This presence of passive antibody in certain patients is the reason to utilize the breath test to document infectivity. There will likely be an increased incidence of infection in more elderly patients, although it is not known whether this will be related to their ITP.
Table 4 lists other infectious etiologies that have been explored in patients with ITP including cytomegalovirus (CMV), HTLV1, human immunodeficiency virus-1 (HIV1), and hepatitis C. The latter two disorders have been well demonstrated to have an “immune” thrombocytopenia, and their suppression with antiviral therapy is coincident with an improvement in the platelet count in the majority of cases. This has been most clearly proven in multiple studies in HIV-infected patients, suggested in several small series of hepatitis C patients, and is being tested for in those infected with H. pylori.
There are several specific new developments that affect treatment of “acute” ITP. Figure 1 (see color page 549) is a picture of wet purpura in the mouth of a patient with a platelet count of 2,000/μL. Virtually all physicians would agree that such a patient requires a rapid increase in the platelet count and various means of using steroids and/or IVIG have been given to patients such as this one. Recently it has been shown that infusion of IV antiD at a dose of 75 μg/kg, as opposed to 50 μg/kg, typically will result in a substantial overnight platelet increase in the majority of Rh+, non-splenectomized adults and children with ITP.5 This data appears comparable to the platelet increases seen with IVIG, given that the latter is effective in patients who may not respond well to IV anti-D, especially Rh- or splenectomized patients. This is of interest because, although both IVIG and IV anti-D cause “Fc receptor blockade” as an important mechanism of acute platelet increase, they may have additional immunomodulatory effects that are quite different as a result of differences in the way that they cause “Fc receptor blockade.” Two streams of information support important differences between IV anti-D and IVIG.
Recent work in mice has shown that IVIG impacts Fc receptor-mediated clearance of antibody-coated particles specifically by upregulating FcRIIb, the inhibitory Fc receptor.6 This was demonstrated both with an anti-FcRIIb antibody and in an FcRIIb knockout model. In both situations when FcRIIb is rendered inactive, IVIG completely loses its ability to block the thrombocytopenia induced by infusion of an anti-mouse-platelet antibody. In contrast, it is presumed that anti-D directly interacts with and occupies the activating Fc receptor, i.e. FcRIII. Calculations of the number of molecules of anti-D infused as related to the number of D+RBC allow for an average of approximately 500 molecules of anti-D to bind to each red cell. This type of large immune complex would look very different to the FcR system than a doubling or tripling of the serum IgG level in connection with infusion of small quantities of IgG dimers and isohemagglutinins that inconsistently cause a weakly positive direct antiglobulin test.
Clinical studies support these differences in that they provide evidence of important clinical differences between these two seemingly similar treatments that share an acute mechanism of effect: inhibition of destruction of antibody-coated platelets. By 1991, differences were already described between the clinical effects of IVIG and IV anti-D, suggesting that different mechanisms of inhibition of destruction of antibody-coated particles were operative.7 Further differences were subsequently described (Table 5 ) based on different efficacy in HIV-infected patients (anti-D was more effective)8 and the relationship of the acute platelet increase to the duration of response (only for anti-D).8 However despite the accumulation of clinical differences, it was never clear how IVIG was creating its effect since neither tripling the serum IgG concentration nor infusion of a very small amount of IgG dimers would have been expected to inhibit FcRIII or FcRI. Of interest, 5 of 11 refractory ITP patients treated with a monoclonal antibody that inhibited FcRIII responded, while 6 did not. Some of the non-responders were transiently IVIG responsive. In addition, several patients transiently responsive to the monoclonal anti-FcRIII antibody were IVIG non-responsive.9,10 These studies implied that IVIG did not inhibit platelet destruction via interaction with FcRIII.
IV anti-D has been associated with the rare occurrence of severe and/or intravascular hemolysis (IVH), estimated to be at a rate < 1%.11 The higher dose of anti-D, 75 μg/kg, was not associated with a greater development of anemia in the study cited above, though it could possibly be associated with a higher incidence of IVH.5 Only 2 mild cases of transient “pink urine” have been seen at our center, possibly because we do not treat patients with a positive direct antiglobulin test with anti-D and possibly because steroid premedication is routinely used at the 75 μg/kg dose. An ongoing study at our center is exploring the relationship between these issues and other pathophysiologies in connection with hemolysis after anti-D: preliminary results will be presented as available.
Another outgrowth of the studies with IV anti-D and with IVIG has been consideration of combination treatments in especially refractory patients with ITP. This was first considered in 1986 when 2 children with acute ITP who had received, but not responded to, IVIG12 developed intracranial hemorrhages. Combination treatments have been used at our center and the results are being prepared for publication. The preliminary findings support the efficacy of the combination of IVIG, IV methylprednisolone, and the addition of one or both of either IV anti-D and/or vincristine (Table 6 ). This treatment approach is typically restricted to patients proven resistant to IVIG and/or steroids alone, or, rarely, in urgent need of an acute platelet increase (see below). The molecular differences in the effects of IVIG and IV anti-D make their combination more rational. In the future, the addition of thrombopoietin may be important for those patients not producing platelets as well and could be incorporated, if there is demonstration of efficacy, into a combination approach. The bottom line of this summary is that combination therapy is better than individual treatments if the platelet count must be raised urgently or if there is reason to think that the patient will not respond well, e.g. if the patient has not responded to single treatments administered in the past. Urgency typically is associated with a high risk or presence of serious bleeding. Examples include the presence of a very low platelet count and one of the following: 1) wet purpura (see Figure 1, color pages); 2) ongoing serious bleeding, e.g. in the GI tract; 3) head trauma, even if minor; 4) the recent ingestion of an antiplatelet agent; or 5) a co-existing bleeding tendency, e.g. von Willebrand disease or factor XI deficiency. In all of these settings a platelet transfusion could be considered for transient stabilization, but in any event a means of rapidly increasing the patient's own platelet count is mandatory. Emergency splenectomy might also be considered in such settings, but subjecting a severely thrombocytopenic patient to a surgical procedure might be hazardous. Moreover, there is no means of predicting whether splenectomy will be effective, and splenectomy may well be unnecessary if the ITP is an acute and transient event, as for example in childhood ITP, which often resolves spontaneously. Alternatively, the situation may occur in a refractory patients who have already had their spleens removed, as in the patient in Figure 1.
Improved means of defining the “true” pathophysiology of the thrombocytopenia would be useful, particularly in patients with refractory ITP. In addition to a test to confirm the diagnosis of ITP, it might be useful to know whether the primary pathobiology is a very high level of anti-platelet antibodies or a decrease in platelet production. While neither pathophysiology is likely to occur in isolation, one may predominate over the other in individual cases. It is here that the biologic measures discussed in the introductory paragraph (platelet retic count, glycocalicin, or thrombopoietin level) could be very useful if they prove to correlate with clinical events. Preliminary data suggests that the glycocalicin level may correlate with the duration of platelet response to IV anti-D suggesting a better effect for a treatment that inhibits platelet destruction if platelet production is increased.
An emerging debate involves the best management of patients who would “normally” undergo splenectomy after failing an initial course of 1-3 months of prednisone. Data on this topic may include a discussion of the effects of splenectomy, including not only the long-term efficacy but also whether the toxicity has been underestimated. A randomized, multicenter trial has recently been completed comparing IV anti-D to prednisone and splenectomy in newly diagnosed adults with ITP. We have studied IV anti-D treatment in 28 adults who had platelet counts of 30,000/μl or less at study initiation, were not splenectomized, were within 1 year of diagnosis, and were HIV negative. The median follow up is 2 years. Of the 28 initial patients, only 8 have undergone splenectomy. Twelve (42%) have received no therapy for over 6 months while maintaining a platelet count greater than 30,000/μL; 6 maintain platelet counts > 100,000/μL on no treatment, even though some received treatment for > 1 year from diagnosis. The best and highly significant predictor of a good long-term outcome, i.e. no need for any further therapy including splenectomy, was a platelet count > 14,000/μL at study initiation. These results require confirmation, and it must also be determined whether they are in any way specific to anti-D or would be equivalent with any long-term maintenance treatment. For example, certain physicians would use danazol in a similar fashion. Furthermore, the rate of splenectomy success will fall if patients who would have been responders, presumably like the responders to repeated anti-D in this study, are no longer included in the pool of patients undergoing splenectomy.
In current practice, many adults and some physicians choose to delay splenectomy, and it is not yet clear what the outcome of this approach is.13 The most common reason that patients choose to forego splenectomy is the uncertainty of the outcome. At best it appears splenectomy is associated with a 60% rate of continued complete remission after 5-10 years. However, our data suggest that this rate continues to fall as time passes, i.e. no plateau was reached despite 10 years of follow-up. It may be that those with late relapses after splenectomy are those who develop de novo ITP again. Similarly, there are no data to clarify whether those patients who undergo but do not respond to splenectomy will derive a benefit in becoming “more responsive” to other treatments. At the moment all that is known is that the response to IV anti-D is generally lost after splenectomy7 (other than as part of combination treatment).
Patients Who Fail to Respond to Splenectomy
There is no accepted plan of treatment in these patients. Management depends in part upon physician and patient preference, which treatments patients respond to, even if only transiently, and which treatments need to be avoided because their toxicities would become intolerable for the individual patient. Moreover, if single agent treatments are considered based upon the pilot series from which results have been reported, none, with the possible exception of IV cyclophosphamide,14 has a response rate of > 30%. In addition, long-term toxicity of many of the treatments is not well defined, especially in patients with ITP who may be less ill than many patients who have undergone toxic therapies such as autologous marrow or renal transplantation, in whom the long-term outcomes of the treatment have been best described.
Avoidance of toxic therapies, though seemingly straightforward, can be complex. A common set of issues is avoidance of steroids because of diabetes, psychiatric disturbances, an ulcer or gastritis not secondary to H. pylori, hypertension, cataracts or osteoporosis; the latter two issues require specific investigation yearly. Whether there is osteoporosis or cataracts may make the difference between the at least temporary acceptability of a low daily dose of steroids, (5-10 mg/day) versus complete avoidance of steroids. Another example is that of danazol and probably azathioprine, which cannot be used if the transaminases are already abnormal at initiation of treatment. These agents rarely cause a problem if there is no ongoing hepatitis, but are likely to worsen underlying pathology. Another issue regards fertility in women of child-rearing potential, in whom danazol may be considered either useful, because it suppresses menses that may be too heavy, or unacceptable. Cyclophosphamide is more problematic in this regard even though a limited number of infusions may not impair fertility. If there is either pre-existing neurologic impairment or a family history of a hereditary neuropathy, a single dose of vinca alkaloids may result in substantial neuropathy. It does not appear that continuous infusion is markedly superior to bolus administration of vincristine or vinblastine and is more likely to result in vessicant injury. Other infrequently encountered toxicities are worsening of pre-existing mild leukopenia with agents such as azathioprine; increased hemolysis in patients with or without a positive direct antiglobulin test in whom IV anti-D is used; and aseptic meningitis following IVIG infusion.
There appears to be an important clinical distinction, actually a continuum, between two types of patients with refractory ITP. One type is the patient who responds to conventional treatments but cannot discontinue them, while the other type responds poorly, if at all, to any treatment. The approach to all such patients at our center is to begin with combination therapy (see Table 6). This is because of the low rate of success of individual agents as well as the slow onset of the platelet increase in patients treated with single agents even if they do respond.15 In general, combination therapy is highly effective at acutely increasing the platelet count, though its long-term efficacy is less well defined. Our goal is to acutely increase the platelet count, on an as required basis, until the oral medications result in a stable increase in the platelet count. After several months of a stable (? normal) platelet count on full dosage of the oral medications, e.g. danazol and azathioprine, we attempt to taper down to a single tablet of each or to only one agent to maintain the effect. If this approach does not work, or results in unacceptable or undesirable toxicity, there are several possibilities depending upon many factors. Cyclophosphamide based therapy, when given as periodic IV infusions, may be effective in refractory ITP. This treatment mimics that used in lupus nephritis, and cyclophosphamide can be administered either as a single agent or in combination with other agents resembling lymphoma regimens, e.g. vinca alkaloids and steroids with or without additional chemotherapeutic agents.16 An advantage appears to be the duration of response in responders.17 It has been our practice to hold cyclophosphamide in reserve, despite its apparently lower toxicity in patients with ITP, because of its potential for long-term toxicity including not only sterility and interstitial fibrosis of the bladder but also the possible induction of secondary malignancy.
A common approach is for patients to use so-called “alternative” medical therapies. The efficacy of such approaches is uncertain, and data concerning their toxicity is not available. Unsubstantiated claims are abundant. Hopefully more objective information will be accrued with these treatments over the next several years.
Because of the well-described limitations of conventional and alternative therapies, experimental treatments may be enthusiastically approached by clinicians and patients. Table 7 lists a number of treatments that are unproven but have been used in limited numbers of patients or considered for ITP. The primary novel “experimental” therapies to consider at this time are thrombopoietin, anti-CD40 Ligand, rituximab and autologous bone marrow transplant. Each of these has unique advantages and disadvantages. In most cases, the issues are theoretical because there may not be enough experience to quantify benefits or risks. The following is a summary of these newer therapies:
No one of them is the optimal treatment for every patient.
Rituximab has an apparent response rate of up to 50%. The advantages appear to be the lack of toxicity, beyond first infusion reactions, and the durability of the responses (> 6 months and counting).
Anti-CD40 Ligand in current use (IDEC) appears not to have the thromboembolic complications that resulted in another anti-CD40 Ligand (BIOGEN) being withdrawn from trial. Furthermore, some responders have been those unresponsive to all previous treatments who have survived intracranial hemorrhage.
Thrombopoietin has not entered clinical trials in ITP. The excitement regarding its use is centered around the possibility that the patients least responsive to other treatments are those in whom platelet production is particularly poor. However, this remains to be confirmed.
Treatment of patients who fail to respond to splenectomy is very complex and confusing. The best single review that covers the use of specific treatments is that by Dr. McMillian in the Annals of Internal Medicine in 1997.15 Subsequent information has accumulated slowly and has not provided much additional insight. Ideally, these patients should be treated on recognized protocols, whether using combinations of conventional agents or experimental ones. Since there is no consensus as to the optimal approach, the outline below of management is mainly directed at what should be either particularly considered or particularly avoided:
Daily prednisone, even at a very low dose, is problematic and requires careful observance of toxicities (bone density, ophthalmologic examinations, blood glucose, blood pressure, etc.).
The patient population refractory to splenectomy with platelet counts < 20,000/μL is the group of ITP patients with the highest mortality, even though the adverse outcomes here remain poorly defined.
There is a low rate of response to individual agents, and therefore combination treatments appear superior to the use of single conventional agents. The optimal combination to be used remains to be defined and almost certainly varies from patient to patient.
Finally, there is currently no way to define ITP biology that will allow rational selection of treatments according to the “biology of the disease.”
III. von Willebrand Factor Cleaving Protease in Patients with and without Thrombotic Microangiopathies (TTP and HUS)
Pier M. Mannucci, MD,*
The University of Milan, Via Pace 9, 20122 Milano, Italy
Thrombotic thrombocytopenic purpura (TTP) and the hemolytic uremic syndrome (HUS) are diseases with features in common such as thrombocytopenia, hemolytic anemia and thrombotic occlusions in terminal arterioles and capillaries, which form on areas of damaged vascular endothelium and are mainly composed of platelets. The triggers of TTP are varied (including pregnancy, infections, cancer and drugs) but no triggering condition is often apparent. Differentiating clinical features are the presence of focal neurological symptoms in TTP and renal impairment in HUS, the latter often associated with diarrhea and fever in the form that affects children. In practice these differences are seldom clear-cut, and in some instances clinical symptoms may shift from one syndrome to another. Hence, in individual cases the differential diagnosis between TTP and HUS based upon the presence or absence of renal or neurological symptoms is often so uncertain that it has been proposed to group the syndromes under the comprehensive term of thrombotic microangiopathies.
A role for vWF in the pathogenesis of thrombotic microangiopathies has been recognized for many years. vWF is a large multimeric glycoprotein contained in plasma, platelets and vascular endothelial cells that mediates the adhesion of platelets to sites of vascular lesions and, being the carrier protein for coagulation factor VIII, is required for normal factor VIII survival in the circulation. By using immunohistochemical techniques Asada et al showed that in TTP intravascular thrombi and subendothelial hyaline deposits reacted positively for vWF antigen and negatively for fibrin,1 whereas in HUS fibrin is present and vWF is usually absent; Moake et al2 found abnormally large (ultralarge) multimeric forms of vWF in the plasma of patients with chronic relapsing TTP. Ultralarge and large multimers are the most biologically active in platelet-vessel wall interactions and directly induce platelet aggregation in conditions of high shear stress;3 ultralarge multimers were also found in HUS by Rose et al.4 This abnormality of vWF cannot be taken as a diagnostic marker for thrombotic microangiopathies because, particularly in the acute phase of these syndromes, ultralarge and large vWF multimers are sometimes lacking,5 perhaps because they bind avidly to activated platelets and are cleared from plasma.
The mechanism of the relation between ultralarge vWF multimers and thrombotic microangiopathies remained elusive until Furlan et al6 and Tsai7 independently demonstrated that the link is a plasma metalloproteinase that physiologically cleaves vWF at the peptide bond between amino acid residues 842Thr and 843Met in the A2 domain of the subunit.8 The protease degrades ultralarge multimers, normally stored in the Weibel-Palade bodies of vascular endothelial cells from which they are secreted luminally into plasma and abluminally into the subendothelium. Metal ions are needed for full protease activity6,7 and transfusion experiments in TTP patients have shown that the plasma half-life is unusually long (3-4 days).9
In the late 1990s Furlan et al10–,12 and Tsai and Lian13 made the intriguing observation that the protease is deficient in patients with TTP but measurable in normal amounts in those with HUS. The chronic recurrent form of TTP, characterized by recurrent episodes of thrombocytopenia with or without signs of ischemic organ damage, is due to the complete deficiency of functionally active metalloprotease,10 often inherited as an autosomal recessive trait. On the other hand, in the more common acute idiopathic form of TTP, which is usually not familial and occurs sporadically, and in ticlopidineassociated TTP protease activity is low because it is inactivated by specific autoantibodies.11–,14 According to Furlan and Tsai, HUS, the clinical manifestations of which are not easily distinguishable from TTP unless the prototypic renal symptoms are present, can now be clearly distinguished by the presence of normal levels of metalloprotease activity and by the absence of inhibitory antibodies.12,13
These findings are important because they would allow these two disorders to be reliably differentiated for the first time, with potentially important clinical implications. In TTP the therapeutic efficacy of plasma exchange and its slightly greater efficacy over plasma infusion has been demonstrated by a randomized clinical trial.15 However, the possibility of distinguishing cases due to the inherited deficiency of the protease from those due to its inactivation by an autoantibody might help to choose the infusion of plasma only for the inherited cases, leaving the more costly and cumbersome procedure of plasma exchange to immunomediated cases. No randomized clinical trial has yet demonstrated the efficacy of plasma exchange or infusion in HUS, even though this treatment is currently recommended and reported to be effective.16– 18 The availability of protease assays might help to establish these recommendations more objectively, because cases diagnosed as HUS, particularly those occurring in adults, might be misdiagnosed cases of TTP, and vice versa.
How specific is the behavior of the protease in thrombotic microangiopathies? Van der Plas et al19 have shown that in TTP associated with bone marrow transplantation (BMT) plasma levels of the protease are normal. Perhaps BMT-associated TTP and acute sporadic TTP are different disorders with similar clinical manifestations, as also suggested by the fact that there is little clinical benefit for plasma exchange for BMT-associated TTP. However, the protease was unmeasurable also in a typical case of familial HUS reported by te Loo et al.20 In a large series of patients (20 with TTP and 32 with HUS) we obtained additional data challenging the views that low protease means TTP and normal protease means HUS. Although mean protease levels were lower in TTP than in HUS (p < 0.001), 5 HUS patients had unmeasurable protease levels, like many patients with TTP (10 of 22).21 Therefore the deficiency of the activity of the protease that cleaves von Willebrand factor may not be specific for TTP.
Whether or not low plasma levels of the protease are at least a specific beacon of thrombotic microangiopathies or also occur in other physiological and pathological conditions was not known until recently. Using a protease assay different from those used by Furlan and Tsai, based upon the lower binding affinity to human collagen type III of protease-cleaved vWF compared with uncleaved vWF22 and upon a prolonged incubation of the samples with barium chloride and high concentrations of urea to avoid the interference of endogenous vWF in the assay, we have evaluated the plasma changes of the protease in physiological states and in pathological conditions associated with organ failure or acute phase reactions, conditions often confounding the picture of thrombotic microangiopathies.23 The protease, which ranged between 40% and 170% in healthy individuals, was lower in those aged more than 65 years than in those of younger ages, was low in newborns but became normal within 6 months, and decreased in the last two trimesters of pregnancy compared with the first. The protease was also low in cirrhotics and uremics, during acute inflammation, and fell in the post-operative period23 (Table 8 ). Hence, low plasma levels of the protease are not a specific beacon of thrombotic microangiopathies, because the protease is low in several other physiological and pathological conditions.
It is not clear whether low plasma levels of protease activity found in so many physiological and pathological conditions are due to decreased synthesis or increased turnover or to other mechanisms, although mixing experiments have ruled out inactivation by autoantibodies as a mechanism.23 In severe liver failure decreased synthesis is a plausible mechanism, even though there were cases with normal protease levels.23 In uremics the moderately reduced protease levels were not related to serum creatinine taken as an index of renal function. In patients undergoing major abdominal surgery the protease fell consistently in the post-operative period.24 The protein seems to be influenced by high levels of estrogens, because plasma levels decrease progressively from the first trimester of pregnancy, albeit moderately.23 The low values found in full-term newborns are perhaps another phenotypic expression of the immaturity of the newborn liver, as indicated by the return of the protease to normal levels within 6 months of birth.23
In conclusion, we found that low protease values may not be a specific beacon for TTP. There are cases of HUS in which the protease was unmeasurable, although this pattern was seen more frequently in TTP. In both microangiopathies the protease was sometimes measurable, albeit at reduced levels. However, similarly reduced values were found in clinical conditions other than thrombotic microangiopathies, such as liver and renal disease and inflammatory states. Our data challenge the views that low protease levels are highly and consistently specific for a diagnosis of thrombotic microangiopathies. The role of the protease in the pathogenesis of microangiopathies is only partially elucidated and the usefulness of protease assays in diagnosis and management is still uncertain.
Addendum
: Two articles in the September 15, 2001, issue of Blood report on the purification of the von Willebrand factor-cleaving protease, and its identification as a new member of the ADAMTS family of metalloproteinases. The gene encoding this enzyme is located on chromosome 9q34 (Gerritsen et al 2001;98:1654-1661; Fujikawa et al 2001:98:1662-1666). In the same issue two clinical papers provide additional evidence that low levels of protease activity are not specific for TTP (Veyradier et al, 2001;98:1765-1772; Moore et al 2001;98:1842-1846).
IV. Heparin-Induced Thrombocytopenia and Thrombosis
Dept. of Pathology and Lab Medicine, University of Pennsylvania, 513A Stellar-Chance Laboratories, 422 Curie Blvd., Philadelphia PA 19104
Heparin continues to be the most common cause of drug-induced, antibody-mediated thrombocytopenia, and managing its thrombotic sequelae remains a difficult challenge. Over the past five years, new insights into the pathogenesis of heparin-induced thrombocytopenia and thrombosis have emerged, new diagnostic tests have come into clinical use, and new forms of treatment have been introduced. Although these developments provide opportunities for improved management, they also present new challenges. The goal of this session is to critically examine these new developments from the perspective of clinical practice In this review the abbreviation HIT will be used to designate patients with asymptomatic thrombocytopenia, HITT be used to refer to those with thrombosis and HIT/T will used when referring to patients with either or both presentations.
Incidence
Although low molecular weight heparins (LMWH)1 and other recently developed anticoagulants are less likely than unfractionated heparin (UH) to cause thrombocytopenia and thrombosis, HIT/T has not disappeared. Indeed, the incidence of HIT/T is stable or may have increased. This is most likely due to the greater use of heparin prophylaxis, increased numbers of aged individuals with chronic vascular disease receiving medical care, and the lack of alternatives to high dose UH for vascular bypass procedures.
Presentation
The diagnosis must be considered in any patient who develops thrombocytopenia, has an otherwise unexplained fall in the platelet count of at least 30-40%, or develops a new thromboembolic complication (TEC) 5-10 days after ongoing heparin exposure2,3 (Table 9 ). The diagnosis may be difficult in settings where other causes for these findings are more common, e.g. in the intensive care setting or after coronary artery bypass graft (CABG) surgery.4,5 The onset may be rapid (median time 10.5 hrs) in patients exposed to heparin within the preceding three months, whereas the onset is not accelerated when the exposure has been more remote.6–,8 These observations are congruent with the time course of antibody disappearance after heparin withdrawal.6
Pathogenesis
A number of laboratories have recently reported that almost all patients with HIT/T have circulating antibodies to complexes between platelet factor 4 (PF4) and heparin at the time of diagnosis (9,10 among others). It is currently thought that the conformation of PF4 released from activated platelets is altered by UH>LMWH>other glycosaminoglycans, rendering it antigenic.11,12,79 Many HIT/T antibodies are directed towards a limited number of regions in the modified PF4.13,14 Antigen-antibody complexes may form in plasma, but it is likely that the reaction occurs predominantly on the surface of activated platelets, endothelial cells and macrophages,15,16 each of which can bind PF4 or heparin/PF4 (Figure 2 ; see color page 549). Platelets may be activated further when the Fc portion of the antibody occupies the signal transducing receptor FcRγIIA,17,18 releasing procoagulant microparticles19 and causing platelets to aggregate. Tissue factor expression by endothelial cells and macrophages stimulated by HIT/T antibody and products released by activated platelets15,16,20– 22 may also contribute to the prothrombotic tendency.
Although the diagnostic importance and relevance of anti-PF4/heparin antibodies has generated considerable interest, it is more difficult to prove that they actually cause HIT/T. Recently, it has been shown that transgenic mice expressing both human PF4 and human FcRγIIA injected with a murine anti-human PF4/heparin-specific monoclonal antibody develop severe thrombocytopenia and disseminated thrombosis after exposure to heparin.23 Thus, the two major salient features of HIT/T are reproduced in this model, but whether this sequence occurs in vivo is unproven.
Binding of HIT/T antibodies to platelets is optimal when PF4 and heparin are present at approximately equimolar ratios.24 Heparin and PF4 are both cleared rapidly from the circulation. Therefore, for HIT/T to develop PF4 must be released from activated platelets at a time when heparin is also present and these complexes must persist long enough for antibodies to develop. The absence of persistent platelet activation may help to explain why HIT/T does not occur with every exposure to heparin, even in patients with pre-existing anti-PF4/heparin antibodies5,25 or in those with a previous history of the disease,6 and why HITT rarely develops more than 10 days after heparin exposure. It has been proposed that high doses of heparin used during bypass surgery may dissociate PF4/heparin complexes from platelets.24 However, there remains no simple explanation as to why only some patients with HIT develop thrombosis. Although the isotype and functional properties of the antibodies and the genotype of the platelet Fc receptor may contribute,26 it is likely that the health of the vasculature and its response to platelet activation and immune injury are critical factors in the prevalence, timing and localization of thrombosis.
Laboratory testing
Tests in clinical use either rely upon platelet activation or measure binding of antibodies to PF4/heparin. When relating data published by experienced research groups to the practice setting it is important to appreciate that few laboratories provide clinically validated data. Rather, most rely on standards promulgated by others, which in turn may be driven more by statistical considerations than by well-defined clinical criteria validated by experienced physicians. Therefore, it is important that the physician become familiar with the experience of specific laboratory as well as the published operating characteristics of these commonly used tests.
The utility of functional assays is limited by their sensitivity and availability. Failure to adsorb, inactivate, or exclude residual heparin may invalidate results. The 14C-serotonin release assay (SRA), still considered the “gold-standard” in diagnosis, has a positive predictive value approaching 100% and a negative predictive value of approximately 20%.1,27,28 Thus, a negative SRA cannot be used to exclude a diagnosis of HIT/T. The heparin-induced platelet activation test and other tests based on platelet aggregation may be comparable to the SRA in experienced hands, but the test requires careful validation with known positive and negative controls to control for differences in donor platelet responsiveness.29 However, some have found that tests based on platelet aggregation are less sensitive than the SRA (reviewed in 80).
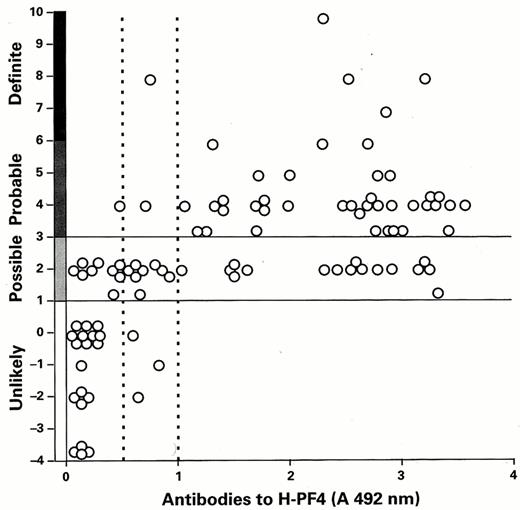
Because of these limitations, increased reliance has been placed on measuring anti-PF4/heparin antibodies, generally by ELISA. FDA-approved diagnostic kits in use employ PF4 bound to heparin or to polyvinyl sulfonate and measure IgG, IgA and IgM antibodies, provide similar, but not identical, results.30 In one recent study based on a comprehensive clinical scoring system to assess the likelihood of HIT/T (Table 9), the sensitivity of the ELISA was estimated to be 97% and 93% using low or high cut-off values in patients with a “definite” or “probable” diagnosis27 (Figure 3 ). Similar findings have been reported by most groups (10,31 among others). The sensitivity of the assay is lower in studies using less rigorously defined pre-analytic clinical inclusion/exclusion criteria.32,33 The incidence of delayed serologic conversion is unknown.34
In this study, the PF4/heparin ELISA was also positive in 23% of patients who received a comparable course of heparin but did not develop HIT/T.27 Thus, the specificity of the ELISA was 86% and 100%, the positive predictive value 93% and 100%, and the negative predictive value 95% and 88%, using the low and high cut-offs, respectively. Even this analysis is an oversimplification. The incidence of “false positive” ELISAs ranges from < 5% in patients on hemodialysis, to ∼10% of medical and surgical patients receiving heparin,35,36 to approximately half of all patients with recent heparin exposure after cardiac bypass surgery.5,25,37 Thus, 25 times as many patients develop anti-PF4/heparin antibodies after CABG as develop HIT/T.38
These findings have several implications. First, it is inappropriate to rely solely on a positive ELISA to make a diagnosis of HIT/T in settings such as CABG, whereas a negative assay may help exclude the diagnosis. Second, research studies and pharmaceutical trials lacking rigorous clinical diagnostic entry criteria and lacking serologic confirmation by more than one technique are likely to include patients with other causes of thrombosis and thrombocytopenia. Third, there is a need to distinguish, if possible, antibodies with “pathogenic” potential from the more prevalent seemingly “non-pathogenic” antibodies in order to develop a more clinically useful assay. Some have suggested that measuring IgG antibodies may represent a first step,81 while others argue that IgM and IgA antibodies may be pathogenic as well.39 It is also unclear whether the epitopes in PF4 recognized by HIT-antibody contribute to outcome.
“Natural History”
Defining the natural history of HIT and HIT/T has become an important issue for two reasons. First, the FDA has relied upon outcomes in historical controls to approve new therapies. Second, physicians must weigh the benefits versus the risks of using these newer treatments in asymptomatic thrombocytopenic patients.
HITT
Historically, mortality rates approach 20%, with ∼10% of patients requiring limb amputations and new TECs developing in ∼30%. The risk can be lessened by managing co-morbid risk factors and by avoiding surreptitious heparin exposure and unwarranted platelet transfusions. It is also important to note that many deaths are due to underlying disease, a distinction that is not addressed adequately in many studies. Nevertheless, it is clear that simply discontinuing heparin is insufficient. Thrombectomy, placement of IVC filters, and the use of fibrinolytic agents or alternative anticoagulant agents are appropriate in individual cases. In one study, 3 of 7 patients treated with heparin withdrawal alone died compared to 2 of 26 patients who were also treated with a variety of anticoagulants (predominantly dextran, ASA and coumadin).40 Newer forms of therapy are discussed below.
HIT
The natural history of HIT is less clear. Prospective studies performed prior to 1990 indicated that the incidence of HITT was approximately 30% that of HIT (reviewed in 41). However, in contemporary practice, HIT is more often considered when there is a substantial fall in the platelet count even in the absence of thrombocytopenia, and it is more generally appreciated that HITT can develop after unintended exposure to seemingly trivial amounts of heparin given “by protocol,” e.g. heparin-bonded central lines, flushing solutions, hyperalimentation solutions. Nevertheless, in some studies 30-50% of patients with HIT treated solely by withdrawing heparin ± coumadin developed TEC within 30 days (80% of which occurred in the first week).40,42,43 On the other hand, in a contemporaneous study of 108 patients with positive heparin-induced platelet aggregation tests conducted between 1991 and 1994, the incidence of death, amputation and new TECs was reduced from 3/7 (42.9%) patients treated by withdrawing heparin ± ASA to 2/25 (8%) patients who received dextran, coumadin and ASA.40 In another study, morbidity in 100 consecutive patients with heparin-dependent antibodies was reduced from 61% to 7.4% and the mortality from 23% to 1.1%, which was attributed to frequent platelet counts, total heparin withdrawal following defined diagnostic criteria, and conventional oral antiplatelet and anticoagulant therapy.8 Similar outcomes have been reported by some groups,44 while others present a far less sanguine picture.45 It is against this variable background that trials showing the efficacy of newer agents have been judged.
New Developments in Management
Danaparoid sodium
The introduction of danaparoid sodium (Orgaran) approximately 10 years ago represented the first significant advance in management. Danaparoid is a low molecular weight glycosaminoglycan composed of heparan sulfate (84%), dermatan sulfate (12%) and chondroitin sulfate. Danaparoid has an anti-Xa/antithrombin activity ratio of > 22. The anti-Xa and antithrombin half-lives are ∼25 hours and 6-9 hours, respectively. The drug is cleared renally. Suggested dosing schedules for various indications are published.46,47 Monitoring with anti-Xa levels is recommended for patients weighing < 55 or > 90 kg, children, those with renal insufficiency, after 2-3 days of use, and during cardiac bypass. Anti-Xa levels between 0.5-0.8 U/mL are appropriate to treat thrombosis and do not affect the INR. There is no antidote for bleeding.
There is considerable experience with the use of danaparoid in HIT/T. Results have been compiled from an “off-label” compassionate use study involving 708 treatment episodes in 666 patients, 65% of whom had HITT or HIT plus a pre-existing thrombosis.48 In 75% of the patients, the diagnosis was confirmed serologically or by inadvertent re-exposure. Diverse dosage schedules were employed. Danaparoid was given for 1 week on average, but the duration of treatment varied widely. Ninety-three percent of 586 evaluable patients had a successful outcome defined as an increase in the platelet count, resolution of DIC, absence of new TECs or stabilization or improvement in ischemia. Danaparoid was considered have caused thrombocytopenia in 12 patients, thrombosis in 9, and death from bleeding or thrombosis in 14.48 All-cause mortality at day 7 in treated patients was 5.1% versus 23.5% in those for whom the drug was requested but not given.47 Five to 15% of HIT/T sera crossreact with danaparoid in vitro, but the drug has been used successfully in some antibody positive patients47,49 and the importance of excluding crossreactivity is uncertain.
In a subsequent uncontrolled series, platelet counts returned to normal in 35 of 37 patients treated with danaparoid.46 A third study involved 42 consecutive patients with HIT/T diagnosed with a carefully constructed scoring system, and serologically confirmed by platelet aggregation or heparin-induced platelet activation tests in 36.50 24/26 patients with HITT had successful outcomes, with 2 deaths from pre-existing thromboses and 4 from the underlying disease, but no new TECs. No TECs developed in the 20 patients with HIT, 5 of whom had a history of HIT, i.e. “latent” disease. Major hemorrhage occurred in 3 patients (6.5%), 2 of whom were also receiving ASA. Of the 42 patients, 6.5% had cross-reactive antibodies, which bore no relationship to outcome. In a recently completed controlled study of patients with new-onset HITT, complete clinical resolution of thromboembolic events was observed in 56% of patients (n = 25) treated with danaparoid and coumadin compared with 14% in those (n = 17) treated with dextran 70 and coumadin.51 Treatment was considered moderately or highly effective (partial or complete response) in 88% of danaparoid-treated patients compared with 47% of the dextran 70-treated patients. No serious adverse events were reported. In a recently reported controlled trial, patients treated with danaparoid (2500 anti-FXa units i.v. bolus followed by 400 anti-FXa units per hour i.v. for 4 hours, 300 anti-FXa units per hour i.v. for 4 hours, followed by 200 anti-FXa units per hour i.v. as maintenance dose) fared comparably to patients treated with lepirudin (see below).82 The use of danaparoid in the US has been largely supplanted by the introduction of two FDA-approved drugs for use in HIT/T (see below), although it still finds use during cardiopulmonary bypass surgery and is still used widely outside of this country.
Recombinant hirudin (lepirudin, Refludin) (r-hirudin)
Lepirudin is the first FDA-approved drug for HIT/T. Lepirudin is a recombinant form of hirudin, a ∼7 kDa peptide that acts directly on circulating and clot-bound thrombin. The anticoagulant effect lasts ∼40 minutes in healthy individuals.52 Lepirudin is cleared by the kidney, necessitating dose adjustments for renal insufficiency. Lepirudin may be given as a slow IV bolus, 0.4 mg/kg body wt (up to 110 kg), followed by a continuous infusion at 0.15 mg/kg body wt (up to 110 kg). Doses are adjusted to maintain the aPTT between 1.5-2.5 times baseline. Anticoagulation can also be monitored using the ecarin clotting time (ECT).52 It is recommended that when patients are to be switched to oral anticoagulation, the dose of lepirudin be reduced to attain an aPTT ratio of 1.5 and discontinued when the INR is > 2.0.
Results from two prospective, historically controlled trials have been reported. In the first, HIT/T was defined by a decrease in platelet count of > 30% or a platelet count < 100,000/μL,53 but the temporal relationship with heparin was unstated. Historical controls consisted of patients with HIT/T identified between 1989 and 1993 meeting the same criteria. All patients had the diagnosis confirmed by heparin-induced platelet activation testing and were treated with the “best available care,” which was not further defined. One of four treatment regimes was employed, but the data were pooled for analysis. Patients undergoing CABG were excluded. The groups were unbalanced with respect to the distribution of surgical versus medical patients, with patients in the treatment group younger on average but more likely to have had multiple TECs. Sixteen patients in the treatment arm had “latent HIT.” The combined endpoints of deaths, new TECs and amputations was reduced from 23% to 9.9% on day 7 and 52.1% to 25.4% on day 35 in the lepirudin treated patients in comparison to the historical control group. The study was not sufficiently powered to demonstrate differences in the individual outcomes. Of the study patients 32.9% experienced at least 1 bleeding event and 11 (13.4%) experienced 15 major bleeding events, but these rates were only slightly higher than in controls.
The entry criteria in the second study were similar except that a > 50% reduction in platelets was required.54 The incidence of death, amputation or new TEC was reduced from 52.1% in historical controls to 30.9% in lepirudin-treated patients at day 35, a difference that did not reach statistical significance (p = 0.12), and more bleeding was seen in the lepirudin-treated patients (44.6% vs 27.2%, p < 0.001). It is important to note, however, that the design of both studies created a window of ∼1.7 days between enrollment and initiation of therapy to allow for serologic confirmation. This is the period when the risk of thrombosis is greatest. Therefore, it is not surprising that ∼45% of TECs developed before lepirudin was started and the average combined adverse rate fell from 5.1% per day in the pre-treatment period to 1.5% per day on therapy.
A subset meta-analysis of patients with HITT from these two studies has been reported.55 Of the patients in the lepirudin arm, 77.3% had HITT compared with 89% of controls, the remainder requiring anticoagulation for pre-existing TECs caused by other conditions. The incidence of the primary endpoints of death, amputation and new TECs was reduced from 52.1% to 22.1% (p = 0.004), due primarily to 17% fewer TECs (10.1% vs 27.1%); there were trends towards a reduction in death (8.9% vs 17.6%) and amputations (6.5% vs 10.4%). The combined event rate per patient day decreased from 6.1% in the pretreatment period to 1.3% during treatment. There was more bleeding requiring transfusion in the lepirudin-treated group, but no fatal bleeding events or intracranial hemorrhage. The study was not powered to show benefit of lepirudin over danaparoid or phenprocoumon.
Antilepirudin antibodies develop commonly.56,57 Some antibodies impair renal clearance of active drug prolonging the aPTT, while others neutralize its activity.58 Re-exposure of antibody positive patients is often successful, but allergic reactions may occur and frequent determinations of the aPTT are indicated. Subcutaneously administered lepirudin (25 mg BID) has been used to manage patients with HITT stabilized by intravenous therapy and to manage patients with HIT.56
Argatroban
Argatroban is a 509 dalton arginine-based direct thrombin inhibitor recently approved by the FDA for use in HIT/T. Argatroban inhibits both soluble and clot-bound thrombin. The therapeutic half-life is 46.2±10.2 min. Steady-state activity is attained within 1-2 hours of continuous infusion. Treatment is initiated at a dose of 2 μg/kg/min. The aPTT is checked 2 hours later, and the dose is adjusted (maximum 10 μg/kg/min) to prolong the aPTT 1.5-3 times the baseline (maximum 100 sec). The liver is the predominant site of metabolism. The half-life is prolonged 2- to 3-fold in patients with hepatic insufficiency, and the starting dose should be reduced by 75%. There is no antidote for bleeding. Conversion to coumadin is accomplished by measuring the INR on combined therapy. When the INR is > 4, argatroban is stopped; the INR measured 4-6 hours later reflects the effect of coumadin.59
Outcomes in 160 patients with HIT who were treated with argatroban have been compared with 147 historical controls, and those in 144 patients with HITT compared with 46 historical controls.60 HIT/T was defined by a platelet count < 100/000/μL or a fall of > 50% in the platelet count on heparin without specific reference of the temporal relationship to drug onset. Serologic confirmation was not required. A positive platelet aggregation test or SRA was documented in 50% and 65% of the argatroban-treated patients in the HIT and HIT/T arm, respectively, compared with 81% and 35% of the historical controls. Eight patients with a history of a positive HIT antibody test (“latent HIT”) were included in the treatment arm. Historical controls were generally managed only with heparin withdrawal and/or oral anticoagulation. Argatroban therapy was continued until resolution, institution of alternative anticoagulation, or for a maximum of 14 days.
Patients with HIT treated with argatroban had a significant reduction in new TECs at 37 days (8.1% vs 22.4%, p < 0.001). The reduction in TECs in patients with HIT/T was more modest (19.4% vs 34.8%, p = 0.044). There was no decrease in death or amputation in HIT or HIT/T, but a significant reduction was seen in death caused by thrombosis (p = 0.005 and < 0.001, respectively). Argatroban was associated with more rapid resolution of thrombocytopenia and no increase in bleeding. Diarrhea (11%) and pain (9%) were the major side effects. Argatroban has also been used successfully in patients with HIT/T undergoing percutaneous angioplasty61 and in several patients undergoing extracorporeal circulation in settings where prolongation of the ACT > 400 seconds is not required,62 but it is not approved for use in coronary bypass surgery.
Others
Bivalirudin (Angiomax), a new direct thrombin inhibitor with distinct biochemical characteristics, has been used successfully in patients with recent-onset or latent HIT undergoing percutaneous coronary interventions and in a limited number of patients with HIT/T to treat thrombosis or for other indications.63 There is anecdotal experience using glycoprotein IIb/IIIa antagonists as adjunctive therapy.64,65
Cardiac bypass surgery
CABG is a hazardous procedure in the setting of HIT/T. Ideally, surgery should be delayed for several months to permit anti-PF4/heparin antibodies to dissipate,6 at which time bypass can often be performed safely using UH, with or without concurrent aspirin.8,66– 69 Indeed, there must be a clear and compelling reason to proceed with surgery more expeditiously, because other approaches to anticoagulation require more complicated monitoring and are associated with significant rates of serious bleeding. However, it is not always possible to delay surgery, and it has been our experience that after an initial fall in titer, the ELISA may remain positive at a low level for many months in some patients, precluding a simple “watch-and-measure-and-wait” approach.
It has been posited that the concentrations of heparin used in bypass may dissociate PF4/heparin complexes, rendering them less antigenic. Residual heparin is rapidly cleared with protamine. Thus, if heparin exposure is strictly limited to the bypass itself by scrupulously avoiding the use of heparin-bonded central lines, heparin flushes, and other surreptitious exposures, antigenic PF4/heparin complexes may remain in the circulation for only a short time. A retrospective analysis of patients undergoing CABG managed in this way showed that none developed HIT/T despite pre-existing anti-PF4/heparin antibodies and clear evidence of antibody production post-procedure.5 Nevertheless, there are no criteria to gauge the safety of exposing patients with known heparin-dependent antibodies in this way, and the risk of complications developing in the perioperative period when platelets have been activated must not be underestimated. Therefore, alternative forms of anticoagulation are recommended in patients with a recent history of HIT/T.
The largest published experience is with danaparoid.48,70–,72 Various dosing regimes have been proposed for the patient and to prime the pump. Current recommendations are that anti-Xa levels should be maintained in the range of 1 U/mL throughout the procedure by intra-operative monitoring73–,75 with dose adjustments for bleeding or thrombosis.76 After surgery, danaparoid is discontinued until hemostasis is attained and then resumed to achieve anti-Xa levels of 0.15-0.8 U/mL. Notwithstanding adherence to these guidelines, the risk of bleeding during CABG remains considerable.
Experience with lepirudin has been limited by the fact that the drug cannot be reliably monitored with the ACT or aPTT at concentrations required for bypass (> 4 μg/mL), although some patients have been managed successfully in this way.77 A number of protocols have been employed. One approach is to give a bolus of additional lepirudin to prime the pump (0.2 mg/kg) and begin a continuous infusion (0.5 mg/min) 5 minutes prior to bypass. Dosing is modified at 5 minute intervals so as to maintain the ECT at ∼400 seconds monitored online with the Thrombolytic Assessment System or monitored manually (albeit at less frequent intervals) based on an in-house standard curve. The initial dose is modified if the patient is already on lepirudin.78 Therapy must be based on clinical observation of hemostasis as much as on laboratory measurement. Adjustments for obesity and impaired renal function may be required.
Causes of thrombocytopenia in pregnancy.
| Isolated thrombocytopenia |
| Gestational |
| Immune (ITP) |
| Drug-induced |
| Type IIb von Willebrand disease |
| Congenital |
| Thrombocytopenia associated with systemic disorders |
| Pregnancy-specific |
| Preeclampsia |
| HELLP (hemolysis, elevated liver function tests, low platelets syndrome) |
| Acute fatty liver |
| Not pregnancy specific |
| Thrombotic microangiopathies |
| Thrombotic thrombocytopenic purpura |
| Hemolytic uremic syndrome |
| Systemic lupus erythematosus |
| Antiphospholipid antibodies |
| Disseminated intravascular coagulation |
| Viral infection (human immunodeficiency virus [HIV],Epstein-Barr virus [EBV], cytomegalovirus [CMV]) |
| Bone marrow dysfunction (primary or secondary) |
| Nutritional deficiency |
| Hypersplenism |
| Isolated thrombocytopenia |
| Gestational |
| Immune (ITP) |
| Drug-induced |
| Type IIb von Willebrand disease |
| Congenital |
| Thrombocytopenia associated with systemic disorders |
| Pregnancy-specific |
| Preeclampsia |
| HELLP (hemolysis, elevated liver function tests, low platelets syndrome) |
| Acute fatty liver |
| Not pregnancy specific |
| Thrombotic microangiopathies |
| Thrombotic thrombocytopenic purpura |
| Hemolytic uremic syndrome |
| Systemic lupus erythematosus |
| Antiphospholipid antibodies |
| Disseminated intravascular coagulation |
| Viral infection (human immunodeficiency virus [HIV],Epstein-Barr virus [EBV], cytomegalovirus [CMV]) |
| Bone marrow dysfunction (primary or secondary) |
| Nutritional deficiency |
| Hypersplenism |
Differentiation of pregnancy-associated microangiopathies.*
| . | MAHA . | Thrombo-cytopenia . | Coagulo-pathy . | Hyper-tension . | Renal Disease . | CNS Disease . | Time of Onset . |
|---|---|---|---|---|---|---|---|
| Abbreviations: MAHA, microangiopathic hemolytic anemia; CNS, central nervous system; HELLP, hemolysis, elevated liver function tests, low platelets syndrome: HUS, hemolytic uremic syndrome: TTP, thrombotic thrombocytopenic purpura; SLE, systemic lupus erythematosus; APS, antiphospholipid syndrome; AFLP, acute fatty liver of pregnancy; ±, variably present; +, mild; ++, moderate; +++, severe | |||||||
| * Modified from McCrae and Cines, Semin Hematol. 1997;34:148 (reference 4) | |||||||
| Preeclampsia | + | + | ± | +++ | + | + | 3rd trimester |
| HELLP | ++ | +++ | + | ± | + | ± | 3rd trimester |
| HUS | ++ | ++ | ± | ± | +++ | ± | Postpartum |
| TTP | +++ | +++ | ± | ± | +/± | +++ | 2nd trimester |
| SLE | ± to +++ | + | ± | ± | +/++ | + | Anytime |
| APS | - to ± | + | ± | ± | ± | + | Anytime |
| AFLP | + | +/± | +++ | ± | ± | + | 3rd trimester |
| . | MAHA . | Thrombo-cytopenia . | Coagulo-pathy . | Hyper-tension . | Renal Disease . | CNS Disease . | Time of Onset . |
|---|---|---|---|---|---|---|---|
| Abbreviations: MAHA, microangiopathic hemolytic anemia; CNS, central nervous system; HELLP, hemolysis, elevated liver function tests, low platelets syndrome: HUS, hemolytic uremic syndrome: TTP, thrombotic thrombocytopenic purpura; SLE, systemic lupus erythematosus; APS, antiphospholipid syndrome; AFLP, acute fatty liver of pregnancy; ±, variably present; +, mild; ++, moderate; +++, severe | |||||||
| * Modified from McCrae and Cines, Semin Hematol. 1997;34:148 (reference 4) | |||||||
| Preeclampsia | + | + | ± | +++ | + | + | 3rd trimester |
| HELLP | ++ | +++ | + | ± | + | ± | 3rd trimester |
| HUS | ++ | ++ | ± | ± | +++ | ± | Postpartum |
| TTP | +++ | +++ | ± | ± | +/± | +++ | 2nd trimester |
| SLE | ± to +++ | + | ± | ± | +/++ | + | Anytime |
| APS | - to ± | + | ± | ± | ± | + | Anytime |
| AFLP | + | +/± | +++ | ± | ± | + | 3rd trimester |
Potential predispositions and etiologies of immune thrombocytopenic purpura (ITP).
| Predispositions . |
|---|
| 1. hypogammaglobulinemia: combined variable immunodeficiency (CVI), severe combined immunodeficiency disease (SCID), IgA and IgG2 deficiency, but not x-linked agammaglobulinemia (XLA) |
| 2. deficiency of classical pathway of complement components, C4, C2 |
| 3. ? certain HLA types, e.g. B8DR3 |
| 4. abnormalities of CD95 (Fas pathway) |
| 5. ? other failure of autoimmune lymphocytes |
| 6. ? certain Fc receptor polymorphisms |
| Predispositions . |
|---|
| 1. hypogammaglobulinemia: combined variable immunodeficiency (CVI), severe combined immunodeficiency disease (SCID), IgA and IgG2 deficiency, but not x-linked agammaglobulinemia (XLA) |
| 2. deficiency of classical pathway of complement components, C4, C2 |
| 3. ? certain HLA types, e.g. B8DR3 |
| 4. abnormalities of CD95 (Fas pathway) |
| 5. ? other failure of autoimmune lymphocytes |
| 6. ? certain Fc receptor polymorphisms |
| Etiologies . |
|---|
| 1. Persistent antigen exposure |
| 2. ? selection of certain heavy chain genes in antibody production |
| 3. unknown tendency to develop autoimmunity: systemic lupus erythematosus (SLE), Evans syndrome |
| 4. abnormal antigen presentation, e.g. in lymphoproliferative states such as chronic lymphocytic leukemia (CLL) |
| 5. persistent infections: see Table 4 |
| Etiologies . |
|---|
| 1. Persistent antigen exposure |
| 2. ? selection of certain heavy chain genes in antibody production |
| 3. unknown tendency to develop autoimmunity: systemic lupus erythematosus (SLE), Evans syndrome |
| 4. abnormal antigen presentation, e.g. in lymphoproliferative states such as chronic lymphocytic leukemia (CLL) |
| 5. persistent infections: see Table 4 |
Persistent infections in patients with immune thrombocytopenic purpura (ITP).
| Associations of ITP . |
|---|
| 1. Human Immunodeficiency Virus (HIV): Multiple studies have documented: |
| a. a high incidence of thrombocytopenia in infected patients |
| b. autoimmune nature of the thrombocytopenia: best evidence: response to treatments (IVIG, IV anti-D) of immune thrombocytopenia |
| c. improvement of the thrombocytopenia with suppression of the virus |
| 2. Hepatitis C: Studies have suggested: |
| a. an incidence of thrombocytopenia in infected patients |
| b. anecdotal evidence of response to treatments of immune thrombocytopenia |
| c. improvement of the thrombocytopenia with suppression of the virus |
| 3.Helicobacter Pylori: Preliminary studies have postulated |
| a. a high incidence of Helicobacter pylori in patients with ITP |
| b. improvement of the thrombocytopenia with eradication of the infection |
| Associations of ITP . |
|---|
| 1. Human Immunodeficiency Virus (HIV): Multiple studies have documented: |
| a. a high incidence of thrombocytopenia in infected patients |
| b. autoimmune nature of the thrombocytopenia: best evidence: response to treatments (IVIG, IV anti-D) of immune thrombocytopenia |
| c. improvement of the thrombocytopenia with suppression of the virus |
| 2. Hepatitis C: Studies have suggested: |
| a. an incidence of thrombocytopenia in infected patients |
| b. anecdotal evidence of response to treatments of immune thrombocytopenia |
| c. improvement of the thrombocytopenia with suppression of the virus |
| 3.Helicobacter Pylori: Preliminary studies have postulated |
| a. a high incidence of Helicobacter pylori in patients with ITP |
| b. improvement of the thrombocytopenia with eradication of the infection |
| Inability to Demonstrate Associations of Infections with ITP . |
|---|
| 1. Cytomegalovirus (CMV): no increased incidence in ITP and no evidence that eradication influences ITP |
| 2. HTLV1: no increased incidence in ITP |
| Inability to Demonstrate Associations of Infections with ITP . |
|---|
| 1. Cytomegalovirus (CMV): no increased incidence in ITP and no evidence that eradication influences ITP |
| 2. HTLV1: no increased incidence in ITP |
Differences between intravenous gammaglobulin (IVIG) and intravenous anti-D (IV anti-D) in immune thrombocytopenic purpura.
| . | IVIG . | IV anti-D . |
|---|---|---|
| Abbreviations: IVIG, intravenous gammaglobulin; HIV, human immundeficiency virus | ||
| 1. platelet responses in Rh- patients: | + | - |
| 2. platelet responses in splenectomized pts: | + | - |
| 3. rate of plt increase: 1 gm/kg & 50 mcg/kg: (24 hr) | fast (72 hr) | slow |
| 4. relative effect in HIV+ patients | - | + |
| 5. duration of plt incr related to acute plt incr | no | yes |
| 6. Fc receptor involved in acute effect | ?II | ?III |
| . | IVIG . | IV anti-D . |
|---|---|---|
| Abbreviations: IVIG, intravenous gammaglobulin; HIV, human immundeficiency virus | ||
| 1. platelet responses in Rh- patients: | + | - |
| 2. platelet responses in splenectomized pts: | + | - |
| 3. rate of plt increase: 1 gm/kg & 50 mcg/kg: (24 hr) | fast (72 hr) | slow |
| 4. relative effect in HIV+ patients | - | + |
| 5. duration of plt incr related to acute plt incr | no | yes |
| 6. Fc receptor involved in acute effect | ?II | ?III |
Combination IV treatment of refractory immune thrombocytopenic purpura.
| Abbreviations: IVIG VCR, intravenous gammaglobulin vincristine |
| 1. MEDS/DOSES: IVIG 1 g/kg, Solumedrol 1 g (30 mg/kg), VCR 0.03 mg/kg (1-1.5 mg IVP), and/or Anti-D 75 μg/kg |
| 2. > 70% acute response rate in largely refractory patients i.e. no response to IVIG alone |
| 3. ORAL MAINTENANCE MEDS/DOSES: Danazol 600-800 mg/kg and Imuran 2 mg/kg (cyclosporin, dapsone, Cellcept, cyclophosphamide can be substituted if needed) |
| 4. 3-4 month response rate > 70% of responders, long-term plan to taper to low dose danazol and/or imuran—long-term response rate uncertain |
| Abbreviations: IVIG VCR, intravenous gammaglobulin vincristine |
| 1. MEDS/DOSES: IVIG 1 g/kg, Solumedrol 1 g (30 mg/kg), VCR 0.03 mg/kg (1-1.5 mg IVP), and/or Anti-D 75 μg/kg |
| 2. > 70% acute response rate in largely refractory patients i.e. no response to IVIG alone |
| 3. ORAL MAINTENANCE MEDS/DOSES: Danazol 600-800 mg/kg and Imuran 2 mg/kg (cyclosporin, dapsone, Cellcept, cyclophosphamide can be substituted if needed) |
| 4. 3-4 month response rate > 70% of responders, long-term plan to taper to low dose danazol and/or imuran—long-term response rate uncertain |
Treatments of refractory immune thrombocytopenic purpura.
| Abbreviations: CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; VCR-IVIG-SM, VCR; intravenous gammaglobulin; SM, Solumedrol |
| I. Promising treatments: |
| a. combination chemotherapy: CHOP-like, VCR-IVIG-SM (Table 6) |
| b. anti-CD40 Ligand |
| c. rituximab |
| d. ? thrombopoietin or thrombopoietin like substances |
| e. autologous bone marrow transplantation |
| II. Treatments that have an apparent low likelihood of success when used alone in refractory patients: |
| a. Interleukin-11 (Neumega) |
| b. Anti-D |
| c. Interferon |
| d. Staph protein A columns |
| e. Plasmapheresis |
| Abbreviations: CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; VCR-IVIG-SM, VCR; intravenous gammaglobulin; SM, Solumedrol |
| I. Promising treatments: |
| a. combination chemotherapy: CHOP-like, VCR-IVIG-SM (Table 6) |
| b. anti-CD40 Ligand |
| c. rituximab |
| d. ? thrombopoietin or thrombopoietin like substances |
| e. autologous bone marrow transplantation |
| II. Treatments that have an apparent low likelihood of success when used alone in refractory patients: |
| a. Interleukin-11 (Neumega) |
| b. Anti-D |
| c. Interferon |
| d. Staph protein A columns |
| e. Plasmapheresis |
Changes of the von Willebrand factor cleaving protease activity in various clinical conditions.
| N denotes normal levels | |
| TTP | |
| Acute sporadic or chronic relapsing ↓↓↓↓ | AGING ↓ |
| Transplantation-associated N | NEWBORNS ↓↓↓ |
| Ticlopidine- or Clopidogrel-associated ↓↓↓ | PREGNANCY ↓↓ |
| Cancer-associated ↓↓↓ | CIRRHOSIS ↓↓↓ |
| HUS | UREMIA ↓↓ |
| Acute sporadic N ↓↓↓ | INFLAMMATION |
| Chronic relapsing ↓↓↓↓ | POST-OPERATIVE PERIOD ↓↓↓ |
| N denotes normal levels | |
| TTP | |
| Acute sporadic or chronic relapsing ↓↓↓↓ | AGING ↓ |
| Transplantation-associated N | NEWBORNS ↓↓↓ |
| Ticlopidine- or Clopidogrel-associated ↓↓↓ | PREGNANCY ↓↓ |
| Cancer-associated ↓↓↓ | CIRRHOSIS ↓↓↓ |
| HUS | UREMIA ↓↓ |
| Acute sporadic N ↓↓↓ | INFLAMMATION |
| Chronic relapsing ↓↓↓↓ | POST-OPERATIVE PERIOD ↓↓↓ |
Clinical criteria for estimating likelihood of diagnosis of heparin-induced thrombocytopenia.
| Reprinted from Am J Clin Pathol. 1999;111:700 with permission. | |
| Platelets | |
| Platelet count > 120 x 103/μL before heparin therapy and < 100 x 103/μL during therapy (or decrease of at least 40%) | |
| with | |
| Thrombocytopenia resolving within 10 days after discontinuation of heparin | +2 |
| or | |
| Platelet count recovered while receiving another kind of heparin | 0 |
| Platelet count recovering while receiving the same kind of heparin | -1 |
| Thrombocytopenia not resolving after discontinuation of heparin | -2 |
| Thrombosis | |
| Arterial thrombosis without predisposing factor | +4 |
| Arterial thrombosis with possible predisposing factor | +2 |
| Venous thrombosis during high-dose heparin therapy | +2 |
| Other thrombosis | +1 |
| Rechallenge | |
| Recurrence of thrombocytopenia after reexposure to heparin | +6 |
| No recurrence of thrombocytopenia after reexposure to heparin | -1 |
| Other causes of thrombocytopenia | |
| Excluded | +2 |
| Another drug possibly responsible | 0 |
| Cause other than drug likely (e.g., sepsis) | -2 |
| Clinical Group | |
| Definite | =6 |
| Probable | 3-5 |
| Possible | 1-2 |
| Unlikely | =0 |
| Reprinted from Am J Clin Pathol. 1999;111:700 with permission. | |
| Platelets | |
| Platelet count > 120 x 103/μL before heparin therapy and < 100 x 103/μL during therapy (or decrease of at least 40%) | |
| with | |
| Thrombocytopenia resolving within 10 days after discontinuation of heparin | +2 |
| or | |
| Platelet count recovered while receiving another kind of heparin | 0 |
| Platelet count recovering while receiving the same kind of heparin | -1 |
| Thrombocytopenia not resolving after discontinuation of heparin | -2 |
| Thrombosis | |
| Arterial thrombosis without predisposing factor | +4 |
| Arterial thrombosis with possible predisposing factor | +2 |
| Venous thrombosis during high-dose heparin therapy | +2 |
| Other thrombosis | +1 |
| Rechallenge | |
| Recurrence of thrombocytopenia after reexposure to heparin | +6 |
| No recurrence of thrombocytopenia after reexposure to heparin | -1 |
| Other causes of thrombocytopenia | |
| Excluded | +2 |
| Another drug possibly responsible | 0 |
| Cause other than drug likely (e.g., sepsis) | -2 |
| Clinical Group | |
| Definite | =6 |
| Probable | 3-5 |
| Possible | 1-2 |
| Unlikely | =0 |
Levels of antibodies to heparin-platelet factor 4 (H-PF4), according to clinical score in patients with suspected heparin-induced thrombocytopenia. Dashed lines represent cutoff values of 0.5 or 1 absorbance unite (A492).
Reprinted with permission from Am J Clin Pathol 1999;14:700.
Levels of antibodies to heparin-platelet factor 4 (H-PF4), according to clinical score in patients with suspected heparin-induced thrombocytopenia. Dashed lines represent cutoff values of 0.5 or 1 absorbance unite (A492).
Reprinted with permission from Am J Clin Pathol 1999;14:700.