Abstract
Recent years have witnessed the development of a variety of promising immunotherapies for treating patients with non-Hodgkin's lymphomas. Foremost among these advances is the exciting success of monoclonal antibodies directed against lymphocyte surface antigens. Rituximab is a chimeric (human-mouse) anti-CD20 antibody that induces responses in approximately half of the patients with relapsed indolent lymphomas and a third of patients with relapsed aggressive lymphomas when used as a single agent. Response rates appear even higher (up to 70%) for newly diagnosed patients treated with Rituximab monotherapy. Other promising antibodies for treatment of B cell malignancies include epratuzumab (anti-CD22), CAMPATH-1H (anti-CD52w), and Hu1D10 (anti-class II HLA). Even more exciting than antibody monotherapy is the prospect of combination antibody therapy (e.g. rituximab + epratuzumab) or combination chemotherapy and antibody therapy. In this regard, a recent phase III randomized trial from the GELA group in France demonstrated statistically significantly superior complete and overall response rates and superior event-free and overall survivals for elderly patients with newly diagnosed diffuse aggressive B cell lymphomas treated with CHOP + rituximab compared with CHOP alone. Confirmatory cooperative group trials combining chemotherapy with antibody therapies are currently underway. Another approach to augment the efficacy of antibodies is to deploy them in radiolabeled form. Iodine-131, Yttrium-90, and Copper-67 labeled monoclonal antibodies targeting CD-20, CD-22, HLA class II, and other cell surface antigens have been tested and demonstrate higher overall response rates (50-80%) and complete response rates (20-40%) than unlabeled antibodies. Pilot studies combining radiolabeled antibodies with either standard dose chemotherapy or myeloablative chemoradiotherapy with stem cell transplantation also appear very promising. Lymphoma vaccines have also produced very encouraging results in single institution studies at Stanford and the National Cancer Institute, with responding patients demonstrating superior event-free and overall survival than historical controls. Phase III randomized trials of idiotype vaccines are currently underway and novel new vaccine approaches are also being tested.
I. Monoclonal Antibodies as Single Agents for Non-Hodgkin's Lymphoma
John P. Leonard, MD*
Center for Lymphoma and Myeloma, and Division of Hematology/Oncology, Weill Medical College of Cornell University and New York Presbyterian Hospital, 525 E 68th Street, New York NY 10021
This work was supported by NIH grant 1K23RR16814.
Dr. Leonard receives grant support from IDEC, Immunomedics, Amgen, and Protein Design Labs.
The use of monoclonal antibodies as anti-cancer therapies has been widely explored since their initial development by Kohler and Milstein. In contrast to chemotherapy (and to some degree radiotherapy), with sufficient antigen specificity antibodies can preferentially bind to tumor cells over normal tissues. This specificity may allow for targeted killing of malignant cells and relative sparing of unbound normal tissues. Many investigators have explored immunotoxins or radioimmunoconjugates as methods to deliver a toxic compound or radioactivity to tumor cells. However, unlabeled antibodies may employ a number of mechanisms to cause direct anti-cancer effects or induce a secondary immune response against tumor cells. The relatively vascular nature of most lymphomas, as well as their general responsiveness to immune system manipulation, chemotherapy and radiotherapy, suggest that they may represent a favorable setting for this treatment modality. In fact, the first successful use of antibodies as treatments for cancer was demonstrated in lymphoma, and these agents have now been employed to benefit thousands of patients with non-Hodgkin's lymphoma (NHL). This work has also led the way for the evaluation of this therapeutic modality in other malignancies, including leukemias and solid tumors. The clear activity of monoclonal antibodies as single agents has subsequently led to further evaluation of combinations with chemotherapy and other biologics. This section will review single agent data on several monoclonal antibodies with evidence of therapeutic effects in lymphoma and which are either available for general use or in clinical trials.
Antibody Characteristics
In selecting a target for any anti-tumor agent, one would prefer that tumor cells are preferentially bound while toxicity is minimized by the sparing of normal cells. An initial trial of serotherapy conducted by Nadler et al employed a monoclonal antibody against a lymphoma-associated antigen,1 and evidence of tumor cell killing was observed. Miller, Levy and colleagues developed patient-specific antibodies that were administered therapeutically, and lymphoma regression was also demonstrated.2 The immunoglobulin idiotype molecule expressed on the surface of malignant B-cells is expressed uniquely by the tumor cells of an individual patient and thus serves as an excellent example of a tumor-specific target for this strategy. These trials provided an important proof of concept that monoclonal antibodies could induce anti-tumor effects with acceptable toxicity. There are, however, significant practical issues in the development of a unique monoclonal antibody for treatment of individual tumors (directed against patient-specific idiotypes), particularly with respect to effort and expense.
Investigators in this area have primarily focused on the development of antibodies targeting antigens present on tumors from different patients, allowing more widespread use of an agent. More promiscuous antigens are generally also expressed on normal tissues, which may result in toxicity if normal cells are eliminated or damaged with consequences. Therefore, selection of an appropriate target antigen plays a major role in activity (depending on mechanism of action) and toxicity. Since most lymphomas (about 85-90%) are of B-cell origin, pan-B cell antigens have been extensively evaluated as potential targets for antibody therapy.
Another important issue in therapeutic approaches is the structure of the antibody molecule itself. Most monoclonal antibodies are initially developed in the murine form, with many later re-engineered as either chimeric (roughly 60% human and 40% murine) or humanized (approximately 95% human with the antigen binding site containing the murine component). Antibody structure and subclass may affect penetration into the tumor as well as ability to mediate complement activation and antibody-dependent cellular cytotoxicity. Murine antibodies may be immunogenic (though less so in patients who are immunosuppressed due to malignancy or prior therapy), while this feature is less characteristic of chimeric and humanized antibodies. Formation of a human anti-mouse (HAMA), human anti-chimeric (HACA) or human anti-human (HAHA) antibody could increase clearance from the plasma or might predispose to toxicities such as allergic-type reactions. Therefore, most treatment regimens that have been developed with murine antibodies have been limited to relatively few doses, with repeated dosing schedules more practical with humanized or chimeric agents. Despite these concerns, the full implications of anti-antibody formation are unclear for most settings.
Rituximab
The first monoclonal antibody approved by the Food and Drug Administration (FDA) for the treatment of cancer was rituximab, directed against the CD20 antigen, and it has been administered to over 100,000 patients. Development of this agent was led by Maloney, Levy and Grillo-Lopez, along with many collaborators. This chimeric antibody has been demonstrated to mediate antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity, and to induce apoptosis of lymphoma cells in vitro.3–,5 Phase I/II clinical trials resulted in a standard dosing regimen of 375 mg/m2/week administered intravenously for 4 weeks, and demonstrated an acceptable toxicity profile comprised primarily of infusion reactions (including fever, rigors and myalgias),6– 8 without clear evidence of dose-limiting toxicity. Depletion of circulating B cells occurred rapidly and lasted for approximately 3 to 6 months after the last infusion. Serum levels of normal immunoglobulins remained unchanged. Measurable levels of rituximab remained in the serum for several months after completion of therapy in some patients.
Objective clinical responses were demonstrated in a significant number of patients, prompting the development of a multi-center phase II pivotal trial of rituximab in relapsed indolent NHL.9 One hundred and sixty six patients received the standard dosing regimen, resulting in an overall response rate of 48%, including 6% complete remissions. It is important to keep in mind that time to response may be delayed with biologic agents, and was a median of 50 days in this study. Time to progression was a median of 13 months in those patients who responded, with most assessable patients demonstrating molecular remissions in blood or bone marrow by polymerase chain reaction assays for bcl-2 gene rearrangements. Lower response rates were observed in chemotherapy-resistant patients and in those with small lymphocytic lymphoma (12% overall), as well as in chemotherapy-resistant patients, while patients who had previously undergone high-dose chemotherapy and stem cell transplant demonstrated higher response rates (78% vs 43%). A similar side effect profile to that observed in earlier studies was noted, and infusion reactions were generally adequately managed by premedication with diphenhydramine and acetaminophen in addition to slowing of the intravenous rate as required. An important feature of this therapy for this patient population was that cytopenias were minimal, making this option particularly attractive for individuals with limited bone marrow reserve due to disease or prior treatment. It also suggested that minimal overlapping toxicity would be expected if combinations with chemotherapy were employed. Rituximab was subsequently approved and is widely accepted as a single agent therapy for relapsed and refractory low grade NHL. Subsequent studies have demonstrated efficacy in bulky disease10 and as initial therapy for low grade NHL.11,12 Extended schedules (8 weekly infusions) have been explored with acceptable toxicity, though additional benefit is unclear.13 Patients who initially respond to rituximab and then relapse can safely undergo retreatment, and 40% of this group obtained a second response.14 In some cases, second remissions with rituximab have been observed to have longer durations than those after initial treatment with the agent, though this might be related to disease burden at the time of retreatment. Ongoing studies are evaluating maintenance therapy regimens, as well as employing rituximab as initial therapy in minimally symptomatic or asymptomatic patients with the hope of delaying the initiation of chemotherapy for indolent NHL patients who would otherwise be followed with a “watch and wait” strategy.
While initial studies of rituximab focused on patients with low-grade NHL, this antibody has also been evaluated in more aggressive NHL histologies as a single agent, with significant response rates though less overall effectiveness. Coiffier et al treated 54 patients predominantly with diffuse large cell NHL (many with no previous treatment) with 8 weekly infusions of rituximab. An overall response rate of 31% (9% complete responses) was noted, with median time to progression 246+ days in the responding patients, though shorter overall.15 A group of previously untreated and relapsed mantle cell lymphoma patients demonstrated a response rate of 34% with 14% complete responses and a median time to progression of 7 months16 following rituximab therapy. The limited results observed with rituximab in aggressive NHL have led to its use as a single agent therapy in this setting predominantly as a palliative measure.
The modest response rates in small lymphocytic lymphoma/chronic lymphocytic leukemia from rituximab therapy have been attributed to the significantly diminished (by about 90%) antigen density of CD20 in these histologies. Some investigators have altered rituximab dose and schedule to potentially improve responses in patients with these lymphoma subtypes. Byrd et al have evaluated a thrice weekly schedule,17 while O'Brien augmented antibody doses in another trial.18 These modifications have improved response rates such that one-third of patients with small lymphocyte lymphoma/chronic lymphocytic leukemia (SLL/CLL) can achieve objective tumor reductions, but the practical issues and expense involved with these higher-dose regimens may preclude their use in many settings. Of note, patients with large numbers of circulating tumor cells (such as those with CLL and a high white blood cell count) may be at particular risk for life-threatening infusion-related complications. These reactions have been associated with cytokine release as well as acute tumor lysis, and it is recommended that patients with these features be carefully monitored during treatment.19 Other studies have explored the potential effectiveness of rituximab in other B-cell malignancies such as Waldenström's macroglobulinemia (WM) and multiple myeloma. Treon reviewed a retrospective multicenter experience of rituximab therapy for WM, reporting that approximately one-quarter of patients demonstrated evidence of disease improvement, predominantly through improvement in hematologic parameters and reduction of M protein.20 These findings have led to a prospective multicenter trial that is currently evaluating the potential role for rituximab in WM. Occasional multiple myeloma patients in the subset of those with CD20-expressing plasma cells have also demonstrated response to rituximab therapy,21 and this area is also currently under further examination. Recent reports have also demonstrated that rituximab may be an effective therapy for post-transplant lymphoproliferative disorders, with the majority of treated patients achieving a clinical response.22,23
While most attempts to improve upon results obtained with rituximab therapy have focused on combinations with chemotherapy and as well as other biologic agents, many questions remain under active investigation. A major issue is the elucidation of the dominant mechanisms of action of rituximab. Clynes et al demonstrated that Fc receptor interactions are critical in the therapeutic effects of anti-tumor antibodies, as both activating (FcγRIII) and inhibitory (FcγRIIB) can modulate effects in a murine model.24 Another important issue is why some patients respond to rituximab therapy, while others have tumors that are resistant. As in SLL/CLL, tumor variability in CD20 expression may be an issue; however, with rare exception, CD20 expression generally remains detectable in patients who initially respond to treatment and then become resistant.25 Polymorphisms in Fc receptors may result in variability in antibody-dependent cell-mediated cytotoxicity in different patients, with alterations in therapeutic response as a consequence.26 Antibody engineering to maximize binding to activating Fc receptors might result in enhanced therapeutic effects.27 Other investigators have explored the role of complement inhibitory factors, with findings suggesting that the variability of expression of CD55 and CD59 on tumor cells may account for heterogeneity of tumor responses to rituximab therapy. Further information regarding the mechanisms of the anti-lymphoma activity resulting from rituximab therapy should allow for improved treatment strategies using this and other antibodies both as single agents and in combination, as well as further definition of the optimal settings for their use.
Campath-1H (Alemtuzumab)
Campath-1H is a humanized IgG1 κ monoclonal antibody that targets the CD52 antigen, expressed on normal and malignant B and T lymphocytes, as well as NK cells, monocytes and macrophages.28 Formulations of this agent have been evaluated in a number of clinical settings, including autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, graft-versus-host disease, solid organ transplantation, and in B- and T-cell malignancies. The mechanism of action of this antibody is also unclear, but like rituximab it has been demonstrated in vitro to mediate complement lysis, antibody-dependent cellular cytotoxicity and induction of apoptosis in certain cell lines. CD52 expression has been shown to be greater in some responding patients with lymphoid malignancies relative to non-responders and may account for a higher response rate in T-prolymphocytic leukemia (T-PLL) relative to CLL.29 Cross-linking of CD52 after antibody binding to target cells may be particularly important for tumor cell growth inhibition, while cells surviving in vitro treatment with Campath-1H down-regulate CD52 expression, which may be reversible.30 The correlation of these findings to activity with normal and malignant cells of patients undergoing therapy is under investigation.
Initial trials of intravenous therapy were complicated by infusion reactions that appear to follow ligation of CD16 on NK cells, resulting in release of interleukin-6, tumor necrosis factor-α and interferon-γ.31 As with other monoclonal antibodies, prophylactic therapy with acetaminophen and diphenhydramine can reduce the severity of reactions, in conjunction with use of lower doses in initial infusions. First doses generally consist of 3 mg administered as a 2 hour intravenous infusion on day 1, followed by 10 mg on day 2, with subsequent doses of 30 mg/d administered intravenously three times per week for up to 12 weeks. Common early side effects include fever, rigors, rash, nausea, vomiting, and dyspnea, with hypotension, diarrhea and fatigue occasionally observed as well.32 Subcutaneous injection regimens have also been explored, apparently with fewer acute toxicities.33 Another complicating issue involves prolonged immunosuppression following therapy, due to rapid depletion of CD4-positive and CD8-positive T cells, CD19-positive B cells and CD52-positive cells, which may last for as long as 18 months and can result in opportunistic infections.34 More recent efforts have incorporated prophylaxis with anti-infective agents (including trimethoprim/sulfamethoxazole and famciclovir or the equivalent), which have reduced complications from Pneumocystis carinii and herpes simplex virus in particular. The relative contributions to infection risk of prior immunosuppressive therapy with corticosteroids and purine analogues as well as the underlying disease versus Campath-1H treatment are not entirely clear.
Therapeutic activity with Campath-1H has been most significant in the blood, bone marrow and splenic compartments, with less activity against nodal disease, which is generally more prominent in NHL.35 Chronic lymphocytic leukemia has been most extensively studied with this agent, and a pivotal multicenter trial of 93 fludarabine-refractory patients demonstrated an overall response rate of 33%, virtually all partial responders, with time to progression of greater than 9 months in responding individuals.36 In this trial, infection occurred in 56% of patients, with one-third grade 3-5 despite prophylactic therapy, 25% with opportunistic organisms and about 20% of infections fatal. The recent FDA approval of Campath-1H provides a new treatment option for patients with refractory CLL, though it should be used with care. A number of combination regimens with chemotherapy and other biologics are under evaluation at the present time.
HLL2 (Epratuzumab)
The CD22 antigen is broadly expressed on both normal and malignant B-cells, with a distribution comparable to that of CD20, though antigen density may be more variable. The molecule is internalized and has demonstrated both cytoplasmic and cell surface expression. Epratuzumab is a humanized IgG1 version of the monoclonal antibody LL2, which targets the CD22 antigen.37 This agent was initially evaluated by Goldenberg and colleagues as a radioimmunotherapy for NHL in conjunction with Iodine-131 and Yttrium-90, and has subsequently been studied as an unlabeled antibody. At the New York Weill Cornell Medical Center, we have conducted a phase I/II trial with this agent, and no dose limiting toxicity was observed with doses of 120-1000 mg/m2/weekly for 4 treatments.38,39 Virtually all toxicities were grade 1, consisting primarily of infusion reactions including fevers, rigors, and hypotension. These side effects are infrequent, allowing administration over 30-60 minutes. Partial depletion of B cells was observed in some patients, with no change in hematologic parameters, blood chemistries or serum immunoglobulins. Detectable antibody levels were present in the serum 3-4 months after completion of therapy and immunogenicity appeared to be rare. Significant anti-tumor effects have been detected in patients with follicular NHL and in diffuse large B-cell NHL. Six of 13 follicular patients treated at the optimal dose levels (240 mg/m2/week or greater) demonstrated objective responses, half of which were complete and several of which extended over 1-2 years. In a heavily pretreated group of patients with relapsed diffuse large B cell lymphoma (DLBCL; median age 60, 65% with elevated LDH, median 3 prior regimens), 5 of 22 (23%) achieved objective responses, three of which were complete. One patient with refractory DLBCL remained in an ongoing complete remission 3 years after completion of epratuzumab therapy. Several relapsed patients have undergone retreatment, some with evidence of a second response. Follow-up of additional patients is ongoing. Epratuzumab is currently under evaluation in patients with rituximab-refractory indolent NHL and in combination with rituximab in follicular NHL and diffuse large B-cell NHL. Additional studies are also planned in relapsed aggressive NHL.
Hu1D10
Hu1D10 is a humanized IgG1 monoclonal antibody that binds to a variant (likely post-translational modification) of the HLA-DR B chain.40 Approximately 70% of normal individuals express the target antigen in their peripheral blood (mainly in the B-lymphocyte compartment), while expression has been observed in dendritic cells and peripheral blood monocytes. Hu1D10 has been shown to induce antibody-dependent cellular cytoxicity and complement-mediated lysis as well as signaling via tyrosine phosphorylation in lymphoma cell lines. For these reasons, it is under evaluation as a potential therapy for NHL. By immunohistochemistry, approximately 70% of lymphoma specimens react with the antibody, while comparable binding rates have been observed in CLL and acute lymphoblstic leukemia. Link and colleagues conducted a phase I study of Hu1D10 in NHL under the auspices of the National Cancer Institute. Twenty patients were treated at dose levels ranging from 0.15 mg/kg/week to 5 mg/kg/week x 4, and a 5 consecutive day schedule was also explored.41 Acceptable toxicity (with usual premedications) was observed, consisting frequently of fever, chills, rigors, hypotension, nausea, vomiting, rash, and flushing, as well as other symptoms. Side effects were usually grade 1 or 2 in nature, though occasional grade 3 toxicity was noted as well. In contrast to other antibody agents, a shorter half-life was measured at a median of 11 days. Clear evidence of anti-tumor effects was observed, with 4 of 8 patients with follicular lymphoma treated on a weekly dosing regimen demonstrating an objective response (1 complete response unconfirmed, 3 partial response). Of interest, responses appeared to be delayed, with time to objective response ranging from 105 to 296 days. Known functions of major histocompatibility complex (MHC) class II molecules, the delayed time to response, and demonstrated autologous anti-lymphoma antibodies in one patient (after Hu1D10 clearance) suggest that this agent might induce an active anti-lymphoma response in the host. It is currently under evaluation in a randomized phase II trial in indolent NHL and in CLL. Hu1D10 staining by immunohistochemistry of a number of solid tumor specimens suggest that this antibody may have potential activity in other disease settings as well.42
II. Monoclonal Antibodies Combined with Chemotherapy for the Treatment of Non-Hodgkin's Lymphomas
Bertrand Coiffier, MD, PhD*
Service d'Hematologie, Centre Hospitalier Lyon-Sud, 69495 Pierre Benite, France
Dr. Coiffier receives grants from and is on the speaker's bureau for Roche.
Rituximab (Rituxan, MabThera) was the first recombinant monoclonal antibody (MAb) to be introduced in clinical practice some 7 years ago. Currently, it is the only one licensed for the treatment of lymphoma patients though other MAb are currently being tested. Some of these MAb are attached to a toxin or a radioisotope, and their activity and indications for use are described in another section. Rituximab was first used alone in relapsing patients with diverse types of lymphomas and showed a 10% to 60% response rate with few complete responses and a time to progression of 6 months to > 2 years.1–,4 This activity was most significant in follicular lymphomas and least in small lymphocytic lymphoma, with mantle cell lymphoma and DLBCL being intermediary. The outcome of treatment depended also on the number of previous chemotherapy regimens, the bulkiness of the tumor, and classical prognostic factors such as LDH level. However, because of the high rate of response, although of transient duration, physicians have investigated the utility of rituximab in untreated patients5 and in combination with chemotherapy.6,7
Rationale for Combining Rituximab with Chemotherapy
Immune mechanisms such as complement-dependent cytolysis (CDC) and antibody-dependent cell lysis (ADCC)8–,10 are believed to be the main mechanisms of cell lysis induced by rituximab, though other in vitro mechanisms have been described such as inhibition of cell growth or induction of apoptosis.11–,13 Because those mechanisms of action are not very different from the ones attributed to standard anti-cancer drugs, it was postulated that potentiation might occur when standard chemotherapy drugs and MAb were combined. This putative potentiation was demonstrated in vitro by Demidem et al,14 who showed that cell lysis induced in the DHL-4 cell line by doxorubicin, cisplatin, and etoposide was more than doubled by 2 to 4 days of exposure to rituximab. They concluded that rituximab might sensitize the cells to the activity of standard anti-cancer drugs.
These results were recently confirmed by Ghetie et al.15 They and others had previously shown that MAb may have greater in vitro activity if cross-linked by another antibody. In their recent study they used rituximab, homodimers of rituximab, or tetravalent (Fab')2 parts of rituximab. They tested the ability of these products to exhibit antigrowth activity or to induce apoptosis in different lymphoma cell lines, including cell lines bearing the multidrug-resistant gene. Homodimers of rituximab were more effective in inhibiting cell growth, inducing apoptosis, or inhibiting 3H-thymidine incorporation.15 When rituximab monomers were added to different concentrations of doxorubicin, the growth of DHL-4 or Namalwa/MDR1 (multidrug resistant 1) cells decreased in a dose-dependent manner, suggesting that both the drug-resistant Namalwa/MDR1 cells and the DHL-4 cells became more sensitive to the cytotoxic effect of doxorubicin. When added to the same amount of homodimer (at a non-cytotoxic concentration), doxorubicin became even more toxic for DHL-4 and Namalwa/MDR1 cells. These experiments demonstrated that both monomers and homodimers of rituximab can potentiate the in vitro effect of doxorubicin on partially resistant or MDR1-positive tumor cells, but that homodimers are much more effective. The fact that rituximab can render MDR1-positive cells sensitive to chemotherapy suggested exploring a strategy using rituximab in combination with chemotherapy.
Combinations of Rituximab with Chemotherapy in Indolent Lymphomas
The first study reporting patients treated with chemotherapy and rituximab appeared in 1999.6 Because of the in vitro data, the investigators decided to combine the classical CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) with infusion of rituximab independently from CHOP cycles. Patients received rituximab at the dosage of 375 mg/m² two times before the first cycle of CHOP, two times after the 6th and last cycle, and 2 days before the 3rd and 5th CHOP cycle in an attempt to sensitize the lymphoma cells by a previous exposure to rituximab. Forty patients with intermediate-high grade lymphoma were included, but two did not receive the treatment; 77% had a follicular lymphoma (FL) and 23% had small lymphocytic lymphoma (SLL). One hundred percent of the treated patients responded to the combination, and 58% of them achieved a complete remission (CR). No differences were apparent in subgroups of patients with FL or in the 9 patients who had received prior treatment. At the time of publication, with a median follow-up of 2.5 years, 74% of the patients were continuing in response. These results have been updated with a follow-up now reaching 5 years and more than 60% of the patients have remained in CR.16
Preliminary reports of other types of combination have appeared at different scientific meetings, with fludarabine singly or in combination with mitoxantrone and dexamethasone (FND), DHAP (dexamethasone, cytosine arabinoside, cisplatin) or CVP (cyclophosphamide, vincristine, prednisone) regimens, in naïve or pretreated patients. All these preliminary reports demonstrated good response rates (often better than in other studies with the same regimen but without rituximab), a long duration of response, and the absence of additive toxicity. However, clinical results are difficult to compare in phase II reports and the only conclusions that may be drawn from these studies are (a) rituximab combined with chemotherapy did not add toxicity to the chemotherapy; and (b) the response rate seemed higher than with chemotherapy alone and the duration of response seemed longer. Investigators uniformly concurred that randomized trials will be required to definitively prove the apparent benefit of adding rituximab to chemotherapy regimens.
Several randomized trials are ongoing in naïve or pretreated patients with indolent lymphomas but none has yet reached the target accrual or been presented in a preliminary analysis. The exact place of rituximab in the treatment of these patients is therefore yet undefined, and the best chemotherapy regimen to combine with rituximab is uncertain.
Two studies have reported the effect of combining rituximab with interferon.17,18 Besides the fact that interferon has activity in FL patients, it induces in vitro a greater expression of CD20 antigen on the surface of the cells. However, the results obtained have been disappointing, one study showing a similar response rate to patients treated with rituximab alone but a slightly longer duration of response, the other a slightly higher response rate but a similar duration of response. In both studies, the toxicity of the treatment was substantial because of the specific toxicity of interferon.
Combination of Rituximab with Chemotherapy in Mantle Cell Lymphoma
Only one study has yet been presented in mantle cell lymphoma (MCL) patients.19 Forty newly diagnosed patients received 6 cycles of CHOP with rituximab given 2 days before, every 3 weeks. Nineteen patients achieved a CR (48%) and 19 patients a PR, while 2 had stable disease. Of the 31 patients with bone marrow involvement, 21 (68%) achieved a pathologic CR in the marrow. Of the 23 patients who had a unique Ig or bcl-1 gene rearrangement, 11 (48%) reached a molecular response in the marrow and peripheral blood at the completion of therapy. This high molecular response rate had never been achieved before with chemotherapy or high-dose therapy. However, the median progression-free survival was only 16 months (range 1.7 to 26.8+ months), which is no longer than in patients treated with chemotherapy alone. At time of presentation, 24 patients were alive in best response 4+ to 27+ months after starting therapy. These results suggest that the combination of rituximab with CHOP is associated with a high rate of clinical, bone marrow, and molecular response. However, many of the patients with a molecular response have subsequently relapsed. Nevertheless, in view of the high rate of molecular response in the bone marrow and peripheral blood this combination may provide a purged source of autologous stem cells for additional high-dose therapy. Similar results have been obtained with the combination of rituximab and HyperCVAD/M-A, a regimen of high dose fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate and cytosine arabinoside:20 26 of the 29 evaluable patients reached a CR, and only 2 of them failed with a median follow-up of 8 months.
Combination of Rituximab with Chemotherapy in Diffuse Large B Cell Lymphomas
One important phase II study has been reported for the combination of CHOP and rituximab in aggressive B-cell lymphomas.7 In this study, 33 patients with previously untreated advanced aggressive B-cell NHL received an infusion of rituximab (375 mg/m2) on day -2 of each cycle of CHOP chemotherapy for 6 cycles. The overall response rate was 94% (31 of 33 patients). Twenty patients reached a CR (61%), 11 patients a PR (33%), and 2 patients were classified as having progressive disease. The median duration of response and time to progression had not been reached after a median observation time of 26 months; 29 of the responding patients remained in remission during this period, including 15 of 16 patients with an International Prognostic Index (IPI) score > 2. Bcl-2 gene rearrangement was present in 39% of the patients, indicating either a follicular large cell lymphoma or a transformation of follicular lymphoma, but no difference in response rate was observed for patients with or without a bcl-2 rearrangement. Patients with adverse prognostic parameters had a lower response rate. This combination of CHOP and rituximab did not appear to increase the toxicity compared with either therapy alone. In this first report of the safety and efficacy of rituximab in combination with standard-dose CHOP for the treatment of aggressive B-cell lymphoma, the responses were at least comparable to those that were achieved with CHOP alone with no significant added toxicity.
No study has been presented comparing the efficacy of rituximab alone to chemotherapy. However, the GELA (Groupe d'Etude des Lymphomes de l'Adulte) has recently presented the preliminary results of a study in elderly patients with diffuse large B-cell lymphoma (DLBCL) comparing 8 cycles of CHOP to 8 cycles of CHOP plus rituximab (R-CHOP).21 The classical doses of CHOP were given every 3 weeks for 8 cycles and rituximab was given at the dose of 375 mg/m² the same day of the CHOP. Four hundred newly diagnosed elderly patients were included in this trial, but only 328 were included in the presented interim analysis, 159 in the CHOP arm and 169 in the R-CHOP arm. Elderly (60-80 years old) patients were stratified according to age-adjusted IPI scores (0-1 vs. 2-3), and performance status (PS) less or equal to 2. These patients could not have a cardiac contra-indication to doxorubicin or neurologic contra-indication to vincristine. The primary endpoint was event-free survival (EFS), with events defined as disease progression or relapse, death, or initiation of new alternative treatment. Secondary endpoints were response rate, survival, and safety. The median age of the patients was 69 years old. Adverse prognostic parameters were equally distributed between the two arms: 63% of the patients had stage IV disease, 20% had PS > 1, 38% had B symptoms, 65% had elevated serum lactic dehydrogenase (LDH) levels, 27% had bone marrow involvement, 33% had bulky tumors, 52% had > 1 extranodal disease sites, and 60% had IPI scores of 2 or 3. At the time of the analysis, 86% of the cases were reviewed by an independent panel and DLBCL histology was confirmed in 87%. Sixty-two patients withdrew early, 23 due to treatment failure (18 with CHOP, 5 with R-CHOP), 30 due to adverse events (17 with CHOP, 13 with R-CHOP), and 9 for other reasons. Preliminary analysis revealed no major differences between the two arms in hematological toxicity, or in grade 3 or 4 infection, mucositis, vomiting, liver, cardiac, neurological, renal or lung toxicity. Eighteen patients (11%) had a grade 3 or 4 infusion-related syndrome during the first rituximab infusion, none of them fatal. At the end of treatment, 77% of the patients reached a CR or an undocumented CR (CRu22) in the R-CHOP arm compared to 64% in the CHOP arm, p < 0.01. Twenty-two percent of the patients treated with CHOP had a progression during the treatment compared to 9% in the R-CHOP arm. With a median follow-up of 18 months, 110 events (56% of the patients) were observed in the CHOP arm and 77 (38%) in the R-CHOP arm, most of them due to progression during or after treatment. Interim results based on data from all 399 patients (intent-to-treat analysis) are presented in Table 1 .
This study demonstrated that the addition of rituximab to CHOP chemotherapy led to significant prolongation of EFS and overall survival in elderly patients with DLBCL, without significant additional toxicity. Definitive results on the 400 randomized patients will be available before the end of 2001, but the magnitude of the difference between the two arms is so significant that the probability of a modification of these results is very low. Other randomized studies are ongoing in the same setting (elderly patients) and in younger patients with or without adverse prognostic parameters. If they confirm the GELA results, the combination of CHOP plus rituximab will become the standard therapy for patients with DLBCL and probably other diffuse aggressive B cell lymphomas. As in indolent lymphomas, several other multidrug regimens are tested in combination with rituximab, mainly in phase II trials. Only preliminary results have been presented and few conclusions may be drawn on the benefit observed. If there isn't any theoretical reason for the absence of benefit, it must be demonstrated in a randomized trial for at least another regimen before concluding that the combination of chemotherapy plus rituximab adds to the same chemotherapy alone whatever the situation.
How to Combine Rituximab and Chemotherapy?
In the various reported or ongoing studies, many different methods of combining rituximab with chemotherapy have been explored. The first US study designed according to the in vitro experiments performed by Demidem14 split rituximab and chemotherapy, infusing rituximab 5 and 2 days before the first CHOP cycle, then 2 days before the 3rd and the 5th cycle, then after the chemotherapy. This sequence was designed to allow sensitization of lymphoma cells to chemotherapy and to avoid inhibition of NK cells by corticosteroids. In fact, this last reason may be invoked only for the first cycle because the long half-life of rituximab results in its presence during the whole duration of each cycle of therapy and its action is not limited to a few days following the infusion. Ghetie did not find it necessary to pre-incubate the cells with rituximab to observe a growth inhibition in the presence of doxorubicin.15 Other US studies infused rituximab 2 days before CHOP every cycle. In the GELA study CHOP and rituximab were infused the same day to try to decrease the number of hospital visits for the patient. This was not associated with a greater toxicity than the other methods and has demonstrated a significant benefit for the combination in a randomized setting. Most of the important differences between these studies are due to differences concerning the chemotherapy regimens and patients characteristics, and consequently it is not possible at this time to analyze the effect of the day of rituximab infusion on the efficacy of treatment.
Rituximab as Maintenance Therapy
Because the efficacy of rituximab appears to be greatest when the tumor mass is smaller, its efficacy may theoretically be higher in patients responding to chemotherapy either in first line or in relapse. If studies are ongoing in this setting for patients with follicular lymphoma or DLCL, none has yet been presented.
Rituximab before Harvesting Stem Cells
Rituximab therapy has been shown to be effective for “in vivo purging” of circulating tumor cells from the bloodstream of FL or MCL patients undergoing peripheral blood stem cell collection. Most of the studies reported have demonstrated a disappearance of bcl-2 or bcl-1 rearranged cells in the harvested stem cells. No such marker exists reliably in DLBCL, and the proportion of patients with circulating lymphoma cells is generally less than in indolent lymphoma. Thus, the importance of such in vivo purging for DLBCL may be disputed, and no study has been presented in this setting. Nearly all MCL patients have circulating lymphoma cells or bcl-1 rearranged cells in blood. In vivo purging has been demonstrated with rituximab with the disappearance of the bcl-1 molecular marker but no long-term clinical results have yet been presented.
III. Radioimmunotherapy of Non-Hodgkin's Lymphomas
Oliver W. Press, MD, PhD*
Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview N, D3-190, Seattle WA 98109
Supported by NIH grants R01 CA 76287, and P01 CA44991.
Dr. Press also receives support from Corixa.
The development and validation of monoclonal antibody serotherapy as an effective therapeutic approach for the treatment of NHLs is arguably the most important advance in lymphoma therapy in the past decade. The successes of rituximab and other antibodies make it clear than “unmodified” antibodies will remain critical components of lymphoma regimens for the foreseeable future. Despite their unequivocal success, however, there are limitations to the efficacy of unmodified antibodies. Half the patients with relapsed indolent lymphomas and two-thirds of the patients with relapsed aggressive lymphomas do not obtain objective remissions with rituximab and only a small percentage of responding patients achieve complete remissions.1,2 To enhance the therapeutic potency of antibodies, investigators have conjugated them to cytotoxic radioisotopes to target radiotherapy specifically to tumor sites and improve overall and complete remission rates. This section will review the current status of radioimmunotherapy of NHLs.
Theoretic Advantages of Radioimmunoconjugates
Several advantages support the use of radiolabeled antibodies for treatment of lymphomas. First of all, the exquisite radiosensitivity of lymphomas makes them ideal targets for radioimmunotherapy. Second, radioimmunoconjugates kill tumor cells predominantly by radioactive emissions and consequently can be effective even in cases where unmodified antibodies and immunotoxins are ineffective because of defective host immune function, antigen-negative tumor variants, or tumor penetration problems.3 The β particles emitted by Iodine-131 (I-131) and Yttrium-90 (Y-90) are cytotoxic over many cell diameters, permitting eradication of antigen-negative tumor cells by crossfire from neighboring antibody-coated cells. This feature permits killing antigen-negative mutants and cells deep in tumor nodules that are inaccessible to antibody due to penetration barriers.
I-131 has been the radionuclide employed most often for radioimmunotherapy because it is relatively inexpensive, widely accessible, easily conjugated, can be used for both imaging and therapy, and has demonstrated marked clinical efficacy in treating malignancies such as thyroid cancer and, more recently, B cell lymphomas. However, I-131-conjugates are rapidly degraded after endocytosis into tumor cells, and the resultant small molecular weight I-131-metabolites are rapidly released into the bloodstream.4 In addition, the γ rays emitted by I-131 present a potential radiation hazard for family members and health care providers, thereby necessitating hospitalization for radiation isolation if very large doses are employed.
Y-90 is the second most commonly used radionuclide. It emits β particles that are five times more energetic than those from I-131, emits very few γ rays, has a favorable half-life (2.5 days), is easily employed on an outpatient basis, and is stably retained by tumor cells even after endocytosis.4 However, the absence of γ emissions means that Y-90 cannot be used for radioimmunoscintigraphy by conventional methods. However, Indium-111 is very similar to Y-90 in its radiochemistry and its biodistribution behavior, rendering it suitable for use as a surrogate radioisotope for imaging purposes. Y-90 is generally more expensive and more difficult to obtain than I-131, though the cost differential may be offset by fewer hospitalizations. Y-90 has a tendency to accumulate non-specifically in the liver and bones, though this is less of a problem with newer, more stable chelation methods. Other β emitting isotopes such as Copper-67 also appear very promising and may be superior to I-1315 but their accessibility is currently limited. Astatine-211, Bismuth-212, and Bismuth-213 emit densely ionizing α particles that confer high linear energy transfer to tumor targets, but their short half-lives (1-7 hours), difficult radiochemistry, and extremely limited availability have restricted their clinical use to a few centers. Nevertheless, antibodies labeled with α emitting radionuclides have shown promise for treatment of patients with myeloid leukemia.6
Selection of Target Antigen
It is now clear that radioimmunoconjugates targeting a large variety of antigens on the surface of B cell lymphomas can induce clinical complete remissions. Factors that should be considered in selecting a target antigen for radioimmunotherapy include its density on the B cell surface membrane, the magnitude of antigen shedding into the bloodstream, the rate of internalization into cells after antibody binding, the homogeneity of antigen expression from cell to cell, and the avidity of antibody binding to antigen. Surface idiotypic immunoglobulin is a unique tumor marker that specifically binds anti-idiotypic antibodies and was initially considered by most investigators to be the ideal target for radioimmunotherapy of B cell lymphomas. However, studies revealed several practical difficulties with this antigen for radioimmunotherapeutic applications. Idiotypic immunoglobulin is shed in large amounts into the bloodstream, where it binds circulating radiolabeled anti-idiotypic antibodies, blocking its binding to tumor cells and leading to rapid accumulation of radiolabeled immune complexes in the reticuloendothelial system and the kidney. To ameliorate these problems, plasmapheresis can be performed or “cold” non-radioactive immunoglobulin infused prior to injection of the radiolabeled anti-idiotypic antibody to clear the circulating idiotype protein.7 However, other problems exist. Patient-specific antibodies must be produced for each patient, a laborious and expensive process. Furthermore, idiotypic immunoglobulins undergo rapid somatic mutation, generating variant tumor clones that fail to bind antibody.8 Finally, idiotypic immunoglobulins are rapidly internalized and deliver radioiodinated anti-idiotypic antibodies rapidly in tumor cell lysosomes, where the antibody protein is degraded, permitting radioiodinated small molecular weight metabolites to leave the tumor cells by exocytosis. For this reason, radiometal nuclides are more appropriate for radioimmunotherapy with anti-idiotypic antibodies.7
As a consequence of these adverse features, idiotypic immunoglobulin is not currently favored for radioimmunotherapy despite its exquisite tumor selectivity. Contrary to initial expectations, pan B lymphocyte antigens have proven to be much more effective targets for radioimmunoconjugates than idiotype despite their cross-reactivity with normal B-lymphocytes. Conceptually, the goal of radioimmunotherapy is to focus as much radiation therapy as possible in lymphoma-bearing organs. Therefore, the targeting of normal B cells as well as malignant B cells concentrates more radioactivity in lymph nodes and spleen than would occur with targeting of lymphoma cells alone. On the other hand, the temporary elimination of normal B cells as well as malignant B cells might depress humoral immunity sufficiently to increase the risk of infections. Despite this theoretic concern, infections have not been a major problem with radioimmunotherapy, presumably because of the preservation of plasma cells, which are typically CD20 negative, and the consequent maintenance of serum immunoglobulin levels at baseline levels despite eradication of mature B-lymphocytes for weeks or months.
Of the pan B antigens, CD20 has been the preferred target of most investigators. The CD20 antigen is homogeneously expressed on 90-95% of B cell lymphomas at a density of 50,000-200,000 sites/tumor cell, is minimally shed into the circulation, and therefore there is little free circulating antigen to block delivery of anti-CD20 mAbs to tumor cells, and CD20 is minimally modulated or internalized following antibody binding.9,10 In addition, anti-CD20 mAbs are capable of directly killing B lymphoma cells by both complement-dependent and complement-independent mechanisms11 Furthermore, anti-CD20 mAbs induce apoptosis in B lymphoma cells if cross-linked with Fc-receptor bearing accessory cells.12,13 Although selection of CD20-negative tumor variants has been reported, this appears to occur very rarely with CD20 antibodies, occurring in fewer than 1 in 300 cases.
Alternative antigenic targets on B lymphomas include CD19, CD22, CD37, and HLA class II. CD19 is ubiquitously expressed on B lineage lymphoid cells and has been used extensively as a target for immunotoxins for acute lymphoblastic leukemia and B cell lymphomas because it is rapidly internalized after antibody binding.14–,16 Experience with radiolabeled anti-CD19 antibodies is very limited, however, and the one imaging trial published suggests suboptimal targeting.17 CD22 is expressed on 75-80% of B cell lymphomas, is more variably expressed from cell to cell than CD19 or CD20, and is rapidly internalized after antibody binding.18 Nevertheless, high quality anti-CD22 antibodies have been developed and shown to be effective at targeting B lymphomas and achieving remissions in a substantial fraction of patients.19,20 CD37 is an antigen that is present in high density on most B-lymphocytes and at lower density on T cells and granulocytes. It is internalized to a moderate degree. Two separate groups have studied anti-CD37 radioimmunoconjugates and concluded that they are less desirable than anti-CD20 conjugates.21,22 HLA class II antigens are present on virtually all B-lymphocytes as well as monocytes and dendritic cells. HLA class II variant molecules are reported to be selectively expressed on malignant B cells and the Lym-1 and Hu1D10 antibodies appear to target these lymphoma-related variant HLA molecules.23,24
Clinical Trials of Nonmyeloablative Radioimmunotherapy with Anti-Class II (HLA Antibodies)
Gerald and Sally DeNardo at UC Davis were the first investigators to systemically investigate the potential utility of radioimmunotherapy for the treatment of B cell malignancies.23 They demonstrated the feasibility and tolerability of radioimmunotherapy using an I-131-labeled anti-DR (Lym 1) antibody, obtaining objective remissions in ~50% of patients treated with advanced, relapsed B cell malignancies.25,26 Delayed myelosuppression, especially thrombocytopenia, occurring 4-6 weeks after therapy was the main toxicity. The activity of I-131-Lym 1 has recently been confirmed in a follow-up study in which eleven of 21 patients (52%) with relapsed NHL responded (including 33% complete remissions). This study is noteworthy since the majority of patients (15 of 21) had aggressive lymphomas, which generally respond to antibody-based therapies less well than indolent lymphomas. DeNardo has also compared the biodistribution of Copper-67 (Cu-67) and I-131-labeled Lym 1 antibody, and demonstrated greater tumor uptake and longer tumor retention with Cu-67-Lym-1 than with I-131-Lym-1.5 Dosimetry calculations suggested that twice as much radiation therapy could be delivered to tumors with Cu-67-Lym-1 as with I-131-Lym-1. Unfortunately, the limited availability of Cu-67 currently limits its testing to a few centers.
Clinical Trials of Nonmyeloablative Radioimmunotherapy with Anti-CD20 Antibodies
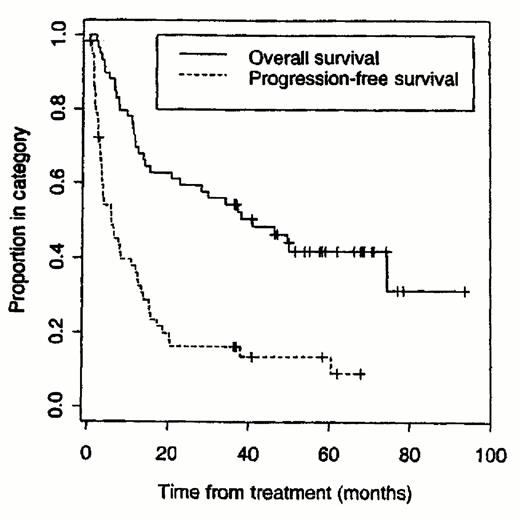
The majority of clinical trials of B cell lymphomas have employed radiolabeled anti-CD20 antibodies. Mark Kaminski has conducted a series of trials at the University of Michigan using the I-131-tositumomab (anti-B1) antibody (Bexxar®).27–,30 Patients initially receive a pre-infusion of unlabeled tositumomab antibody followed by infusion of tositumomab trace-labeled with 5-10 mCi of I-131. Whole body γ imaging is performed three times over the week following the trace-labeled infusion to estimate the whole body half-time and calculate the dose required for the therapeutic infusion to deliver the maximally tolerated dose of 75 cGy of whole body irradiation (usually 90-150 mCi). The therapeutic infusion is administered 7-14 days later. Kaminski has recently published his updated single institution experience with 59 patients treated at the University of Michigan.30 Forty-two patients (71%) responded including 20 (34%) with complete remissions. The median progression-free survival for all responders was 12 months (see Figure 1 ), whereas it was 20.3 months for complete responders. Thirty-five of 42 with low-grade or transformed low-grade lymphomas responded (83%) compared with 7 of 17 (41%) with de novo intermediate grade NHL. Myelosuppression was the dose-limiting toxicity, with 20% of patients experiencing grade IV neutropenia at a median of 43 days after therapy and 20% of patients developing grade IV thrombocytopenia at a median of 35 days after radioimmunotherapy. Nonhematologic toxicities were mild, including low-grade fevers, chills, fatigue, nausea, and elevated thyroid stimulating hormone levels in five patients (8%). Five patients later developed myelodysplasia and three patients developed solid tumors, though the relationship of these neoplasms to the radioimmunotherapy is unclear.
Vose has recently published a multi-center phase II trial confirming Kaminski's results.31 In this trial, 27 of 47 patients responded (57%), including 15 patients (32%) with complete responses. Kaminski has also studied 76 newly diagnosed, previously untreated patients with low-grade lymphomas using this same regimen. Ninety-seven percent of the previously untreated patients achieved objective responses and 63% obtained complete tumor disappearance.32 Most recently, a multicenter pivotal trial using Kaminski's regimen enrolled 60 patients with refractory follicular or transformed lymphomas and observed remissions in 39 patients (65%) including 10 (17%) complete remissions.33 These trials demonstrate that the majority of patients with indolent or transformed lymphomas will respond to I-131-tositumomab, regardless of whether the patients are newly diagnosed, relapsed, or refractory. However, the overall and complete response rates are highest in previously untreated patients, intermediate in patients with relapsed but chemosensitive disease, and lowest in patients with chemotherapy-refractory lymphoma. Therefore, the entry criteria for protocols must be carefully scrutinized when one attempts to compare the response rates observed in different clinical trials.
A similar series of trials have been conducted using the ibritumomab (2B8) antibody conjugated to Y-90 using the tiuxetan chelate (Zevalen®). Knox administered Y-90-labeled anti-CD20 antibodies to 18 patients with relapsed B cell lymphomas (14 with ibritumomab tiuxetan and 4 treated with Y-90-tositumomab antibody) in escalating single doses of 13.5 to 50 mCi.34 Six complete remissions and 7 partial responses were observed (overall response rate, 72%), with a median response duration of six months. Four patients developed human anti-mouse antibodies (HAMA). Grade 4 myelosuppression was seen at doses above 50 mCi of Y-90, but no other serious toxicities were observed. Witzig performed a subsequent multicenter phase I/II trial treating 51 patients with ibritumomab tiuxetan (Y2B8, Zevalen®).35 In the phase I portion of the trial, patients received either 100 or 250 mg/m2 of unlabeled rituximab followed by 0.2, 0.3, or 0.4 mCi/kg of Y2B8. The dose of unlabeled rituximab selected for further study was 250 mg/m2 and the maximally tolerated dose of Y-90 was 0.4 mCi/kg. In an intent-to-treat analysis, the overall response rate was 67%, including 26% CRs and 41% PRs. Eighty-two percent of the indolent lymphoma patients responded (26% CRs, 56% PRs), and 43% of 14 aggressive lymphomas responded. Hematologic toxicity was dose limiting. Only one patient developed an immune response against the chimeric antibody, whereas human anti-mouse antibodies usually develop in 15-35% of lymphoma patients treated with murine antibodies. Toxicities did not correlate with dosimetric estimates of absorbed radiation, prompting the investigators to conclude that trace-labeled infusions and dosimetry are not mandatory prior to radioimmunotherapy with Y-90-ibritumomab tiuxetan.
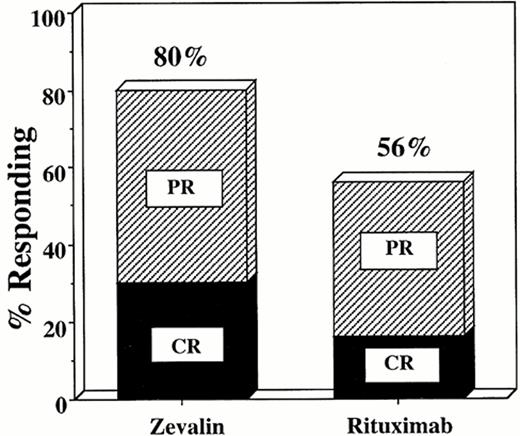
Witzig has recently presented an interim analysis of a randomized clinical trial comparing the remission rates of 143 patients treated with rituximab (375 mg/m2 weekly for 4 weeks) or Y-90-ibritumomab tiuxetan (0.4 mCi/kg).36 The overall response rate was 80% for the Y-90-labeled antibody as compared with 56% for the chimeric unlabeled rituximab antibody (p < 0.001) (Figure 2 ). The complete response rate was also higher in the group receiving radioimmunotherapy compared with the rituximab group (30% vs 16%, respectively). Both regimens were well tolerated, but significantly more myelosuppression occurred in the radiolabeled antibody group compared with the unlabeled chimeric antibody, as expected. This is the first randomized, controlled trial demonstrating unequivocally that a radiolabeled antibody produces higher overall and complete response rates than the corresponding unlabeled antibody. To date, however, the higher response rates with radiolabeled antibodies have not translated into longer event-free or overall survival rates compared with the rituximab-treated patients.
Pretargeted Radioimmunotherapy with Anti-CD20 Antibodies
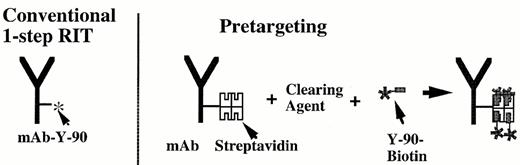
Weiden has recently conducted a pilot study investigating a novel new “pretargeting” strategy of two-step radioimmunotherapy using streptavidin-labeled anti-CD20 antibodies.37 The schema for this approach is diagrammed in Figure 3 . Rituximab was conjugated to the tetravalent streptavidin molecule and infused intravenously, followed 1-2 days later by a proprietary biotinylated, N-acetylgalactosamine-containing “clearing agent” that removes surplus antibody from the bloodstream. Three hours later therapeutic Y-90-biotin was administered. This pilot study enrolled ten patients who had failed standard chemotherapy, and, in some cases, rituximab and I-131-tositumomab as well. The tumor-to-normal organ ratios of absorbed radioactivity that were observed were superior to those reported previously with standard one-step radioimmunotherapy. Four of 7 patients receiving 30-50 mCi of Y-90 biotin achieved objective remissions, including two complete remissions. Weiden and collaborators from NeoRx Corporation are currently testing molecularly engineered anti-CD20-streptavidin fusion proteins that can be produced much more homogeneously than the synthetic chemical conjugates that were initially employed.38
Clinical Trials of Nonmyeloablative Radioimmunotherapy with Anti-CD22 Antibodies
Goldenberg et al have infused multiple doses of an I-131-labeled anti-CD22 antibody LL2 to 21 patients with relapsed B-cell NHL.20,39 Seventeen patients were deemed evaluable for response, and 4 achieved objective remissions (24%), including 1 complete remission. More recently, Juweid and Goldenberg have administered non-myeloablative doses of Y-90-LL2 to 7 patients with B cell lymphomas, with 2 patients achieving PRs.19
Radioimmunotherapy for Chronic Lymphocytic Leukemia, T Cell Lymphomas, and Hodgkin's Disease
Order et al have assessed the utility of radioiodinated polyclonal anti-ferritin antisera for treatment of relapsed Hodgkin's disease.40 These studies demonstrated that radiolabeled anti-ferritin could produce objective remissions in up to 40% of patients with relapsed Hodgkin's disease. Monoclonal anti-ferritin antibodies did not appear to be as effective as the polyclonal antisera, however, and the radiolabeled polycolonal antisera are no longer available for clinical trials. Rosen et al at Northwestern University examined the use of the I-131-labeled anti-CD5 antibody T101 in 6 patients with cutaneous T cell lymphoma and observed temporary partial responses in two patients and minor responses in the other four.41 In a subsequent study by the same group, I-131-T101 produced one minor response among four patients treated with relapsed CLL.42 Waldmann and co-workers have treated 9 patients with adult T cell leukemia with Y-90-anti-Tac (anti-CD25) on a phase I dose-escalation trial and 9 additional patients on a subsequent phase II trial of 10 mCi Y-90-anti-Tac.43 Nine of 16 patients (56%) treated with 5-15 mCi responded, including 2 patients who achieved complete remissions. Partial remissions lasted 1-23 months, whereas the complete remissions lasted 30 and 33+ months. As in other trials, myelosuppression was the major toxicity.
Radioimmunotherapy with Stem Cell Transplantation
Press et al in Seattle have conducted a series of investigations over the past decade investigating the feasibility of replacing external beam total body irradiation (TBI) with targeted anti-CD20 radioimmunotherapy for stem cell transplantation of patients with relapsed lymphomas.21,44,45 Conventional TBI has been an important component of many bone marrow and stem cell transplant regimens for leukemias and lymphomas for many years. However, TBI delivers as much radiation to normal organs as it does to tumor cells. Therefore, in theory at least, it should be preferable to target the radiotherapy selectively to tumor cells using radiolabeled antibodies since more radiation should be delivered to tumor sites and less to normal organs. In initial phase I/II studies, Press administered high doses of I-131-labeled tositumomab antibody to 29 patients with multiply relapsed B cell lymphomas. Biodistribution studies indicated that the tositumomab (anti-CD20) antibody yielded superior targeting compared with other tested antibodies including MB-1 (anti-CD37), and anti-idiotypic antibodies.46 A protein dose of 1.7-2.5 mg/kg of tositumomab achieved optimal biodistributions. The maximally tolerated radiation dose of I-131-tositumomab delivered 27 Gy to dose limiting normal organs with cardiopulmonary toxicity being dose-limiting. Twenty-eight of the 29 patients required autologous bone marrow or peripheral blood stem cell transplantation to reconstitute hematopoietic function. Twenty-five of 29 patients responded to therapy with objective remissions (86%) and 23 had complete responses (79%). Eleven of the 29 patients (39%) have remained alive and free of any recurrences for 5-10 years without any further therapy.
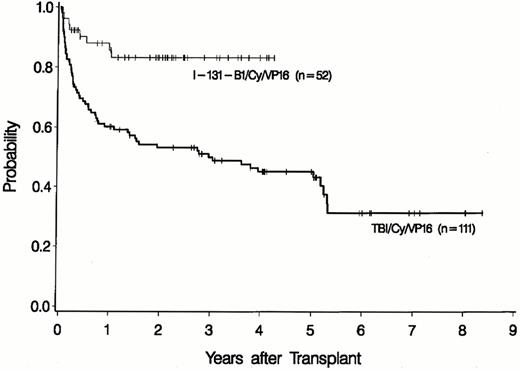
Although the high complete response rates and prolonged remission durations were very encouraging, more than half the patients eventually relapsed with single agent radioimmunotherapy, even when given at myeloablative doses with stem cell transplantation. To increase the percentage of patients with durable complete remissions, Press subsequently conducted a phase I/II study combining I-131-tositumomab therapy with high dose etoposide, cyclophosphamide and hematopoietic stem cell transplantation (HSCT).45 Fifty-two patients were treated on this protocol, which defined the maximally tolerated doses of this combination to be 25 Gy of I-131-tositumomab (1.7 mg/kg), 60 mg/kg etoposide, and 100 mg/kg of cyclophosphamide. Four patients died of opportunistic infections. At the current time the overall survival rate is 85% and the progression-free survival rate is 73% after a median follow-up of two years. These figures were statistically superior in a multivariable analysis to the overall and progression-free survivals (50% [p = 0.01] and 38% [p = 0.006], respectively) of a non-randomized control group of patients treated at our institution with the same doses of etoposide and cyclophosphamide, but who received TBI rather than I-131-B1 (Figure 4 ).
More recently, Vose et al at the University of Nebraska have investigated an alternative stem cell transplantation regimen incorporating I-131-tositumomab. They treated 23 patients with relapsed lymphoma undergoing autologous transplantation using I-131-tositumomab at doses delivering between 30 and 75 cGy to the whole body followed by the standard BEAM conditioning regimen (BCNU, etoposide, cytarabine, and melphalan) on a phase I/II trial.47 They concluded that the addition of I-131-tositumomab at standard doses to the BEAM regimen did not add to the toxicity of the transplant regimen and observed increasing complete remission rates with increasing doses of I-131-tositumomab: 25% CRs at 30 cGy, 50% at 45 cGy, 83% at 60 cGy and 56% at 75 cGy. Vose is currently conducting a phase II trial at the 75 cGy dose level in combination with BEAM.
Summary
Radiolabeled antibodies produce high overall and complete remission rates in patients with B cell lymphomas, and many patients experience long-term disease-free survival particularly when myeloablative doses of radioimmunotherapy are administered in conjunction with stem cell transplantation. Encouraging results have been obtained with radioimmunoconjugates targeting CD20, CD22, HLA class II antigens, and several other targets. Myelosuppression has been the dose limiting toxicity, though delayed myelodysplasia and secondary malignancies are also a concern. A randomized clinical trial has demonstrated convincingly that radiolabeled antibodies can produce substantially higher overall and complete response rates than corresponding unlabeled antibodies, but to date advantages in overall and progression-free survival have not yet been demonstrated. Further studies will be required to determine the optimal settings for radiolabeled and unlabeled antibodies in the armamentarium of lymphoma therapy.
IV. Therapeutic Vaccination for Lymphoid Malignancy
Ronald Levy, MD,*
Stanford University Medical Center, School of Medicine, Division of Oncology, Room M207, Stanford CA 94305-5115
Immunotherapy has now become an important part of our therapeutic armamentarium for lymphoma and leukemia. Several monoclonal antibodies have been approved by the FDA and are in widespread use either alone or in combination with chemotherapy or with other biologic agents. Other passive therapies with various immune cell populations are under investigation. Active immunotherapy, whereby the host is induced to make an immune response against its own tumor cells, has long been a goal of tumor immunologists. At the present time no active immunotherapy maneuver has proven to be routinely effective in the clinic, but intense efforts are underway to develop such an approach. It is important from the outset to define the appropriate clinical setting for vaccination against tumors. Animal experiments have shown repeatedly that active immune responses against tumor cells can be induced in normal individuals and can render those individuals immune to subsequent challenge with the tumor. In the future, as a result of advances in cancer genetics, humans who are at high risk for the development of malignancy will be identifiable. Some day it may be possible to conduct prophylactic immunizations in this high-risk setting. However, for the present, therapeutic vaccination of the tumor-bearing host will be the testing ground for the concept of tumor vaccines. Experiments in animal models have shown that vaccination against actively growing tumors is much more difficult to accomplish. It is therefore not surprising that clinical trials in patients with gross disease will be the most difficult setting in which to demonstrate efficacy. Rather, it is the patient in remission or with minimal residual disease after standard therapy who is more likely to be able to generate an immune response against the tumor and more likely to derive therapeutic benefit.
Tumor vaccine approaches
Strategies of tumor vaccination can be subdivided into two general groups: those in which the tumor antigen is known, where the challenge is to construct a molecularly defined vaccine, and those in which the tumor antigen(s) are uncharacterized but presumed to exist, where the challenge is to imbue the tumor cell (or extracts thereof) with the ability to present its antigens efficiently to the immune system. For presumed tumor antigens, clinical trials are now underway in which tumor cells are being transduced with genes encoding immune stimulatory molecules, such as GM-CSF,1 or with genes encoding molecules that correct the deficiency of the tumor cell to present its antigens to the immune system, such as CD40 ligand or CD80/B7.1.2 These genetically engineered tumor cells are then used as a vaccine. In a different approach, extracts of tumor cells containing immunostimulatory components, such as heat shock proteins, are being tested as vaccines. Tumor cell extracts are also being tested together with dendritic cells,3 which have the ability to take up exogenous proteins and to present them efficiently to T cells. The advantage of these approaches is that the tumor antigen does not need to be identified. However, the potential disadvantage is that autoimmunity may be induced against antigens expressed on tumor cells that are also expressed on normal cells.
It is occasionally possible to identify a molecule that is expressed exclusively by tumor cells, such as a novel protein produced by the fusion of two genes during chromosomal translocations or expressed in greater amounts by tumor cells than by comparable normal cells, such as p53, or expressed in very restricted populations of normal cells, such as MAGE 1, MUC 1 or PSA. In these cases, the tumor specific molecule itself can potentially be the vaccine. The challenge is to find a form of the molecule that will be recognized by the same immune system that had failed to recognize the native molecule. The possibilities for such molecular engineering are numerous and include the construction of small peptides that interact efficiently with major histocompatibility molecules on antigen presenting cells, chemical linkage to other foreign carrier proteins, introduction of antigen molecules into dendritic cells, and construction of recombinant nucleic acid molecules or viruses encoding the tumor antigen together with co-stimulatory molecules. All of these approaches have proven effective in animal models and many of them are being tested in clinical trials.
Tumor-Specific Idiotype Vaccines for Lymphoma
Each B lymphocyte expresses an immunoglobulin molecule that is the product of a unique combination of gene segments. B cell malignancy arises from one original B lymphocyte, and therefore all the members of a given lymphoma tumor population have the same unique immunoglobulin, which can serve as a target for immune therapy. When the idiotype (Id), or unique portion, of each immunoglobulin is used as a vaccine, antibodies and T cells can be induced and each can cause rejection of the tumor by the host. This special opportunity for tumor specificity is accompanied by the challenge of constructing a different vaccine for each patient.
The first clinical trial of Id vaccination for lymphoma was initiated at Stanford University in 1988.4,5 The basic procedure used for vaccine production in this trial is depicted in Figure 5 (see color page 547 ). Tumor cells obtained from lymph node biopsy were fused with a myeloma cell line to generate a “rescue hybridoma” producing large quantities of idiotype protein. Purified Id protein was then chemically coupled to keyhole limpet hemocyanin (KLH) and emulsified in an “oil-in-water” type immunologic adjuvant. The initial trial included patients with low-grade, follicular lymphoma, primarily those in first remission following chemotherapy. Patients were monitored for the development of serum antibodies or T cell proliferative responses to their autologous tumor idiotype protein. Among the first 32 patients in this trial vaccinated while in first remission, roughly half (14/32) mounted anti-Id immune responses to the vaccine.4 These were principally humoral responses rather than cellular responses, and were more common in patients in complete remission than in those with residual tumor at the time of vaccination. Long-term follow-up of these 32 patients has revealed that the development of an immune response is strongly correlated with prolonged freedom from disease progression in comparison to non-responders (Figure 6, see color 547). Overall survival has also been superior in responding patients (Figure 7, see color 547). Prospective, randomized trials have now begun to seek evidence of clinical benefit following Id vaccination.
One approach that has been chosen to improve Id vaccines is the use of dendritic cells (DCs). Dendritic cells are rare, stellate-shaped leukocytes that are the most powerful of all antigen-presenting cells.6 They serve as sentinels for the immune system; they reside in the tissues to trap antigen and, upon activation by local inflammatory stimuli, travel to lymph nodes to efficiently present antigen to lymphocytes to initiate immune responses. These cells can be isolated from the peripheral blood or generated in vitro from monocytes or CD34-positive hematopoietic progenitor cells, co-cultured (“pulsed”) with antigen, and re-infused as a cellular vaccine. B-cell lymphoma Id was the first human tumor antigen to which the technique of DC vaccination was applied.7 Peripheral blood DC precursors are purified from peripheral blood mononuclear cells obtained at leukapheresis by density gradient centrifugation steps and then cultured for 2 days with Id protein to allow the cells to take up the antigen as they undergo maturation and activation. The mature, antigen-loaded DCs are then washed and administered intravenously. This treatment was first applied in a pilot study of ten patients with measurable, relapsed follicular lymphoma.7,8 In these first patients, cellular proliferative responses to Id protein were commonly observed, yet anti-Id antibody responses were notably absent, probably due to the use of native, unmodified Id protein. Most remarkable, however, were the clinical responses in these patients. These included two complete tumor regressions, one lasting 44 months, and another ongoing at 54 months. There was also one partial response lasting 12 months, and one molecular complete response. Given these results, this treatment has now been applied to patients with follicular lymphoma in first remission.8 Id protein coupled to KLH is now being used for DC pulsing based on studies in a mouse model showing improved immunogenicity and tumor protection with this approach.9 In some cases, revaccination with Id-KLH after tumor recurrence has resulted in tumor regression.10
The clinical activity of Id-KLH vaccination has recently been confirmed by investigators at the National Cancer Institute.11 They vaccinated 20 patients with follicular lymphoma in first complete remission following chemotherapy with Id-KLH plus locally administered granulocyte-macrophage colony stimulating factor (GM-CSF) as adjuvant. Following vaccination, most patients' peripheral blood lymphocytes secreted cytokines when co-cultured with tumor cells, and cytotoxicity by CD8-positive T cells against autologous tumor cells was also demonstrated in some cases. Most patients also developed serum anti-Id antibodies. Presence of bcl-2 proto-oncogene product of the t(14;18) translocation characteristic of follicular lymphomas was monitored in the peripheral blood of these patients, and clearance of bcl-2 PCR signal was achieved in 8/11 evaluable cases.
Taken together, the above studies have clearly demonstrated the potential clinical activity of Id vaccination, but how can this customized vaccine approach be applied to large numbers of patients? The ability to rapidly amplify and clone Id genes from B-cell tumor specimens using PCR has opened up a variety of new strategies that are streamlining the production of custom Id vaccines (Figure 8 ). Several alternative sources of recombinant Id proteins are now available for clinical study. These include Id proteins produced in a variety of genetically engineered organisms. The first among these to be clinically evaluated are Id proteins produced in transfected mammalian cells grown in tissue culture.12 Osterroth et al in Germany have recently shown that Id proteins from human lymphoma specimens can be produced in recombinant bacteria, and that DCs pulsed with these proteins (secreted as Fab fragments) can stimulate Id-specific cytotoxic T cells in vitro13 Id proteins can also be made in plants. McCormick et al have exploited the tobacco mosaic virus as a vector for engineering protein production in tobacco plants. In a preclinical study, vaccination of mice with plant-derived Id single-chain variable region (scFv) fragments was able to elicit tumor protection equivalent to that of Id-KLH plus adjuvant,14 and a phase I/II trial is under way.
Conclusions
There is reason for excitement about the prospects for effective vaccine therapies for lymphoma as randomized Id vaccine trials commence and newer cell-based vaccine trials enter the clinic. Id will continue to serve as a highly relevant target for lymphoma vaccine development given its high degree of tumor specificity and the established therapeutic potential and safety of Id vaccines. Patient-specific Id vaccines may also find application in the therapy of other B-cell malignancies such a multiple myeloma,15–,17 and leukemias, as well as T cell malignancies through targeting tumor-specific T cell receptor determinants.18,19 Preliminary clinical studies have demonstrated that Id-specific immunity can be demonstrated in myeloma patients vaccinated with serum-derived myeloma protein coupled to KLH.20,21 As the clinical activity of lymphoma vaccines becomes established, it will be important to determine how to best integrate active vaccination approaches with standard therapeutic approaches.
Results of the analysis of 399 elderly patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) and treated with CHOP or R-CHOP as of April 2001.
| . | CHOP . | R-CHOP . | p value . |
|---|---|---|---|
| Number of patients | 197 | 202 | - |
| CR plus CRu | 126 (64%) | 156 (77%) | < 0.01 |
| Number of events | 110 (56%) | 77 (38%) | = 0.0002 |
| 18-months event-free survival | 43% (± 8%) | 62% (± 8%) | = 0.00012 |
| 2-year overall survival | 61% (± 8%) | 73% (± 8%) | < 0.01 |
| . | CHOP . | R-CHOP . | p value . |
|---|---|---|---|
| Number of patients | 197 | 202 | - |
| CR plus CRu | 126 (64%) | 156 (77%) | < 0.01 |
| Number of events | 110 (56%) | 77 (38%) | = 0.0002 |
| 18-months event-free survival | 43% (± 8%) | 62% (± 8%) | = 0.00012 |
| 2-year overall survival | 61% (± 8%) | 73% (± 8%) | < 0.01 |
Progression-free and overall survival of 59 patients with relapsed or refractory B-cell lymphoma who received dosimetric and/or conventional therapeutic doses of Iodine-131-tositumomab (up to 75cGy whole body dose).
Reproduced with permission in modified form from Kaminski et al.30
Progression-free and overall survival of 59 patients with relapsed or refractory B-cell lymphoma who received dosimetric and/or conventional therapeutic doses of Iodine-131-tositumomab (up to 75cGy whole body dose).
Reproduced with permission in modified form from Kaminski et al.30
Preliminary results of a randomized trial of rituximab (475 mg/m2weekly for 4 weeks) or Y-90 ibritumomab tiuxetan (Zevalen®) for patients with relapsed low grade B cell lymphomas.
Overall and complete response rates were significantly superior in the group receiving the radiolabeled anti-CD20 antibody compared with patients receiving rituximab (see text). Data plotted from the study by Witzig et al.36
Preliminary results of a randomized trial of rituximab (475 mg/m2weekly for 4 weeks) or Y-90 ibritumomab tiuxetan (Zevalen®) for patients with relapsed low grade B cell lymphomas.
Overall and complete response rates were significantly superior in the group receiving the radiolabeled anti-CD20 antibody compared with patients receiving rituximab (see text). Data plotted from the study by Witzig et al.36
Comparison of conventional single step radioimmunotherapy with directly radiolabeled antibodies compared with “two-step” pretargeted radioimmunotherapy using a streptavidin-biotin amplification method (see text).
Comparison of conventional single step radioimmunotherapy with directly radiolabeled antibodies compared with “two-step” pretargeted radioimmunotherapy using a streptavidin-biotin amplification method (see text).
Overall survival of patients with relapsed B-cell lymphomas undergoing high dose chemoradiotherapy with either a standard preparative regimen or one containing myeloablative doses of Iodine-131-tositumomab.
Fifty-two patients were treated with I-131-tositumomab (at doses delivering up to 25 Gy to normal organs), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and autologous stem cell transplantation (upper line). The overall survival is compared to a non-randomized control group of 105 patients transplanted using external beam total body irradiation (TBI 1.5 Gy twice a day for four days), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg) and ASCT (bottom line). Figure reproduced with permission from Press et al.45
Overall survival of patients with relapsed B-cell lymphomas undergoing high dose chemoradiotherapy with either a standard preparative regimen or one containing myeloablative doses of Iodine-131-tositumomab.
Fifty-two patients were treated with I-131-tositumomab (at doses delivering up to 25 Gy to normal organs), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg), and autologous stem cell transplantation (upper line). The overall survival is compared to a non-randomized control group of 105 patients transplanted using external beam total body irradiation (TBI 1.5 Gy twice a day for four days), etoposide (60 mg/kg), cyclophosphamide (100 mg/kg) and ASCT (bottom line). Figure reproduced with permission from Press et al.45
Newer approaches to idiotype vaccine production utilizing recombinant DNA.