Abstract
Multiple myeloma (MM) is a malignancy of the plasma cell characterized by migration and localization to the bone marrow where cells then disseminate and facilitate the formation of bone lesions. Unfortunately, while treatment of this disease is effective in palliating the disease, and even prolonging survival, this disease is generally regarded as incurable. Understanding the basic biology of myeloma cells will ultimately lead to more effective treatments by developing target based therapy.
In Section I, Dr. Bergsagel discusses the molecular pathogenesis of MM and shares insights regarding specific chromosomal translocations and their role in the genesis and progression of MM. New information regarding FGFR3 as an oncogene as well as how activating mutations may contribute to disease evolution and may be an important target for novel therapeutics of MM is presented.
In Section II, Dr. Anderson elaborates on novel therapeutic approaches to MM also targeting fundamental genetic abnormalities in MM cells. Both preclinical and clinical studies of novel agents including PS-341 and IMiDs are highlighted.
In Section III, Dr. Harousseau discusses the role of autologous stem cell transplant in MM. He highlights clinical trials addressing the question of conditioning regimens and the impact of tandem transplants. He also addresses the role of allogeneic BMT and the use of attenuated dose conditioning regimens (so called mini-allogeneic transplants) in the treatment of MM.
In Section IV, Dr. Dalton provides an overview of the current state of myeloma therapy and summarizes the different and exciting approaches being undertaken to cure this disease.
I. Molecular Pathogenesis of Multiple Myeloma
P. Leif Bergsagel, MD,*
New York Hospital, Cornell Medical Center, 525 East 68th Street, Room C609, New York NY 10021
Dr. Bergsagel is on the speakers' bureau for Novartis.
Multiple myeloma (MM) is a malignant plasma cell neoplasm that often is preceded by a common pre-malignant monoclonal expansion of plasma cells called monoclonal gammopathy of undetermined significance (MGUS). MGUS is reported to be present in 1% of the adult population and to progress to MM at a rate of 1% per year. Despite intense efforts over the last 20 years, the 5-year survival rate reported in the SEER database remains unchanged at 28%, and there is no known way to identify those who will progress from MGUS to MM, or to delay or prevent this progression. A better understanding of the molecular pathogenesis of these conditions is fundamental to developing more effective prognostic, treatment and prevention approaches.
Immunoglobulin Gene Translocations Are a Hallmark of B Cell Neoplasms.
Chromosomal translocations into the immunoglobulin heavy chain (IgH) locus on chromosome 14q32—or less often an IgL locus (l on chromosome 22q11 or k on chromosome 2p12)—are the hallmark of many B cell malignancies. In contrast, with the exception of t(11;14)(q13;q32), conventional cytogenetics has largely failed to identify recurrent IgH translocations in MM, mainly due to the low proliferative index of MM cells, the complexity of the karyotypes, and the telomeric location of both the IgH locus and the partner loci involved (4p16, 6p21, 6p25, 16q23). In other B cell malignancies it has been possible to relate the chromosome translocations to errors in the physiological processes that result in double strand DNA breaks of the Ig loci: V(D)J recombination, somatic hypermutation and isotype switch recombination (Figure 1 ). Since MM is almost exclusively a tumor of plasma cells that have undergone the processes of somatic hypermutation and isotype switch recombination in germinal centers, we hypothesized that errors during these physiological processes may occur, leading to chromosome translocations involving the immunoglobulin genes. A Southern blot assay that efficiently discriminates legitimate from illegitimate switch rearrangements (ISR), markers of potential chromosome translocations into the immunoglobulin locus, identified apparent ISR in 15/21(71%) of MM cell lines (HMCL). Although frequently karyotypically silent, IgH translocations could be identified by this assay or conventional cytogenetics in 19/21 (90%) of HMCL.1 Cloning and mapping of translocation breakpoints enabled identification of the loci and of the oncogenes involved. This resulted in a clearer picture of the role of Ig translocations in MM and also development of useful reagents for the analysis of primary patient samples. The application of interphase FISH analysis restricted to plasma cells (identified by cytoplasmic Ig light chain restriction and/or prior CD138 immunoselection) has allowed investigators to examine all patients with MGUS and MM, not just those in whom an abnormal karyotype could be obtained. A comprehensive summary of these studies is provided below.
Incidence of Ig Translocations in MM Cell Lines, Plasma Cell Leukemia, MM, and MGUS
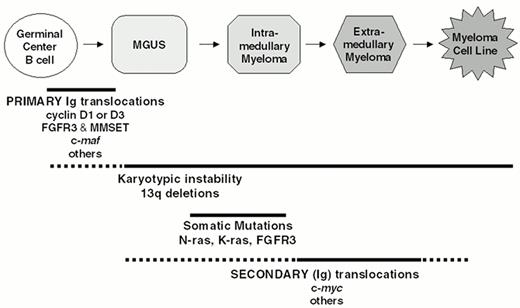
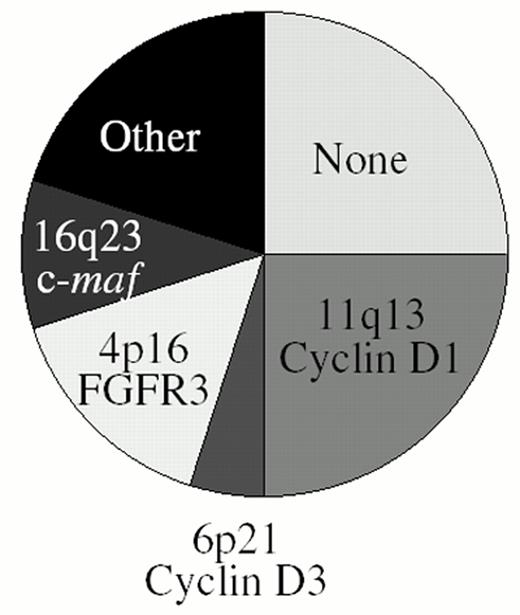
Through a combination of conventional cytogenetics, SKY analyses, metaphase FISH analyses, isolation of molecular clones, and RT-PCR assays1–,6 (and unpublished), we have identified at least one IgH translocation in 36/39 (92%) HMCL examined, with at least 17/39 (44%) HMCL having two or more different IgH translocations. In preliminary metaphase FISH analyses using flanking IgL probes, we identified Igl translocations in 7/30 (23%) HMCL (unpublished). This IgL analysis included the three HMCL lacking an IgH translocation, and all three had Igl translocations, so that all 39 HMCL analyzed have either an IgH or Igl translocation. By interphase FISH analyses, it was reported that IgH translocations are present in about 47% of MGUS tumors, in 60-70% of patients with intramedullary MM, and in > 80% of patients with primary or secondary plasma cell leukemia (PCL).7,8,30 Two different IgH translocations were identified in 3/40 (8%) PCL tumors but in less than 1% of intramedullary MM tumors. Igl translocations are present in approximately 17% of advanced intramedullary tumors, but Igk translocations are rare (unpublished). There are no published data for IgL translocations in MGUS or less aggressive stages of intramedullary MM. Since it is not clear if all MM tumors are derived from MGUS and whether MM cell lines are representative of primary tumors, a definitive interpretation of these results is not possible. However, as a working hypothesis, we suggest that IgH translocations occur as a primary event in approximately 50% of MM tumors, but that secondary IgH or IgL translocations can occur throughout tumor progression from MGUS to intramedullary MM to secondary PCL to MM cell lines (Figure 2 ). These results also suggest that approximately 25% of MM tumors do not have either an IgH or an IgL translocation. The major recurrent loci involved in Ig translocations in MM are 4p16, 6p21, 11q13, and 16q23. Figure 3 provides a pie chart indicating their relative frequency in MM.
11q13 – cyclin D1.
By conventional cytogenetics and interphase FISH analyses the t(11;14)(q13;q32) is present in ∼15-20% of MM.9,10 The breakpoints on 14q32 fall within either the JH region or the switch regions, sites that are compatible with the translocations being mediated by either somatic hypermutation or switch recombination, respectively. In contrast, pre-germinal center mantle cell lymphoma (MCL) tumors have a t(11;14) translocation with the IgH breakpoints occurring at or very near the sites targeted by VDJ recombinase.11 On 11q13, the breakpoints in MM are dispersed over 330kb centromeric to cyclin D1, with no evidence of clustering in the Major Translocation Cluster (MTC) described for MCL.12 Normally B cells express cyclin D2 and cyclin D3, but not cyclin D1. As a result of the translocation cyclin D1 is juxtaposed to the powerful IgH 3′ enhancer(s) on der(14), and its expression is dysregulated.
6p21 – cyclin D3.
Recently we identified a translocation of 6p21 into a switch gamma sequence in a MM cell line.5 The breakpoint is located about 60 kb centromeric to cyclin D3 and is associated with a sixfold increased level of cyclin D3 expression. We identified the t(6;14) in 1/30 MM cell lines and in 6/150 patients with abnormal metaphase karyotypes. By microarray analysis we found 3/53 patients with high levels of cyclin D3 expression and determined that these patients had a juxtaposition of the IgH locus and cyclin D3 by interphase FISH analysis. The finding of dysregulation of cyclin D1 in ∼15-20% and of cyclin D3 in 5% of patients, together with infrequent translocations at 12p13 (?cyclin D2)29, highlights the importance of the D-type cyclins in MM.
4p16.3 – FGFR3 and MMSET.
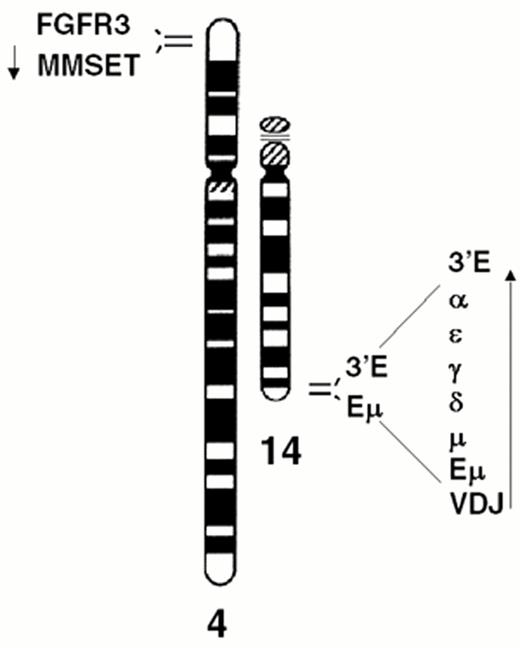
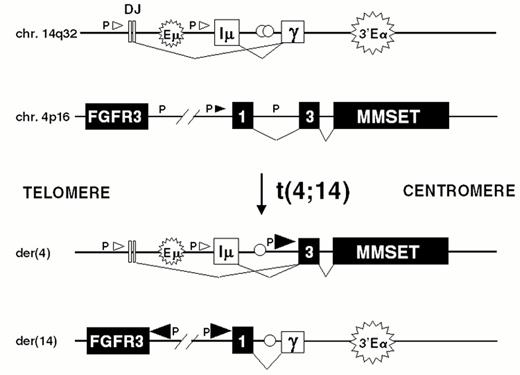
The t(4;14) is identified in approximately 15% of MM tumors.2,3,9 The breakpoints occur in the telomeric region of chromosome 4 and result in a karyotypically silent translocation that cannot be identified by either G-banding or spectral karyotype analysis (Figure 4AF4). In the IgH locus the breakpoints all occur in a switch region and dissociate the intronic enhancer from the 3′ enhancer (Fig. 4B). The 4p16 breakpoints fall 50-100kb centromeric to FGFR3, which becomes associated with the 3′ enhancer(s) on der 14. This region includes the 5′ non-coding (largely) exons of MMSET/WHSC1, so that this gene becomes dysregulated by juxtaposition to the IgH intronic enhancer on der 4, resulting in formation of hybrid mRNA transcripts with the JH and I-mu exons. The hybrid transcripts provide a very specific and sensitive means of detecting this translocation by RT-PCR. We have not identified any variant translocations, nor any translocations into the JH region, suggesting that dysregulation of both MMSET and FGFR3 may be important in the pathogenesis of MM, a hypothesis that is supported by our inability to find HMCL or MM tumors that have lost der 4.
MMSET encodes a nuclear protein with an HMG domain, 4 PHD-type zinc fingers and a SET domain. It thus shares homology to other PHD and SET domain proteins such as ASH1 and trithorax in Drosophila. It also shares these features with MLL1/HRX/ALL1, the gene on 11q23 involved in translocations in acute leukemia, suggesting that it may play a role in the oncogenic transformation of MM cells. This gene falls with the minimally deleted region identified for Wolf-Hirschorn syndrome, a multiple malformation syndrome characterized by mental retardation and developmental defects resulting from partial deletion of the short arm of one chromosome 4.13
FGFR3 is one of four high-affinity tyrosine kinase receptors for the fibroblast growth factor (FGF) family of ligands. It is not normally expressed in plasma cells and is ectopically expressed as a result of the translocation. Activating mutations of the t(4;14) translocated FGFR3 allele are present in a third of the MM cell lines and in some patients, substantiating an oncogenic role for FGFR3 in MM. Interestingly, some of the lines with t(4;14) translocation without FGFR3 mutations contained activating mutations of N or K-ras, but no lines contained mutations of both FGFR3 and ras.14 This suggests that during tumor progression activation of FGFR3 or ras may provide an equivalent stimulus to the cells (Figure 2), a hypothesis that is supported by loss or lack of expression of the dysregulated FGFR3 gene in two HMCL that have ras mutations. Signaling from FGFR3 in MM has been shown to result in phosphorylation of STAT3 and MAPK, and to synergize with IL6 signals in an IL6-dependent plasmacytoma cell line. FGFR3 signaling can substitute for IL6 for the growth and survival of this line, and inhibition of these signals results in apoptosis15. Activated FGFR3 has been shown to be an oncogene that can induce transformation in fibroblasts and that is inhibited by dominant negative inhibitors of the ras/MAPK pathway.14 It has also been shown to be transforming in hematopoeitic cells.16 These experiments validate FGFR3 as a potential target for experimental therapeutics in t(4;14) MM.
16q23 – c-maf.
Translocation t(14;16)(q32;q23) is identified in 5/39 (13%) MM cell lines and in 5-10% of patients with MM.17–,19 The 5 cloned breakpoints on 16q23 occur over a region 550-1350kb centromeric to c-maf, all but one of them within the 800kb intron of an oxidoreductase gene, WWOX/FOR.20,21 This region is a common fragile site, FRA16D, which has been found to undergo frequent homozygous deletion in adenocarcinomas of stomach, lung, colon and ovary. Allelic deletions of this region have not been associated with inactivation of the remaining allele so that a possible role as a tumor suppressor gene is unclear. The identification of t(16;22)(q23;q11) translocations with breakpoints telomeric to c-maf (not involving WWOX) serves to delineate the targeted region of dysregulation and indicates that inactivation of one allele of WWOX by translocation is not required for MM cell transformation by the16q23 translocation.
C-maf is the cellular homologue of v-maf, the transforming gene in the avian maf retrovirus. It is a basic zipper transcription factor that can heterodimerize with jun and fos and small maf proteins. In lymphoid cells, c-maf is a TH2 transcription factor that controls the expression of interleukin-4 (IL-4).22 It is expressed at a high level in MM cells with a 16q23 translocation and at a low to undetectable level in the other MM cell lines.
8q24 (c-myc).
By interphase FISH analyses, it is reported that c-myc is associated with the IgH locus in 3/140 MM tumors representing all stages and in none of 79 MGUS tumors.8 However, three-color FISH analyses of metaphase chromosomes show that 29/33 (88%) HMCL and 17/38 (45%) advanced primary MM tumors have similar, complex karyotypic abnormalities involving L-myc at 1p36 (one HMCL), N-myc at 2p23 (one tumor), or c-myc at 8q246 (and unpublished). Most karyotypic abnormalities are complex translocations and insertions that often are non-reciprocal and frequently involve three different chromosomes. In addition, associated deletions, inversions, duplications, and amplification are sometimes seen. Most of these c-myc karyotypic abnormalities would not be detected by conventional cytogenetics. The karyotypic abnormalities often, but not always, juxtapose c-myc and an IgH or Igl enhancer. Thus translocations of 8q24 in MM are complex and appear to be late progression events.6 Importantly there is expression of only one c-myc allele in all 12 informative HMCL. These secondary translocations of c-myc in MM are in surprising contrast to primary, simple reciprocal Ig translocations that dysregulate c-myc as an early event in Burkitt's lymphoma and mouse plasmacytoma.
6p25 – MUM1/IRF-4.
A t(6;14)(p25;q32) translocation breakpoint was cloned from an HMCL, and found to occur immediately downstream of the IRF-4 gene, a member of the interferon regulatory factor family active in the control of B cell proliferation and differentiation.23 This translocation was identified by metaphase FISH analyses in 3 of 17 (18%) HMCL that were screened.24 Although IRF-4 is normally expressed in most HMCL, the 3 HMCL with the translocation expressed much higher levels. IRF-4 was shown to be transforming in fibroblasts, suggesting that it may contribute to the oncogenesis of the MM tumors.
1q21-24 – IRTA1 and IRTA2.
Unbalanced non-random translocations of 1q and trisomy 1q21-31 were identified in 20-31% of MM.25 A breakpoint from this region was cloned from the FR4 HMCL and resulted in the identification of two novel cell surface receptors (IRTA1 and IRTA2) homologous to the Fc and inhibitor receptor families. IRTA2 is upregulated in most Burkitt's lymphoma cell lines with 1q21 abnormalities. It was also up regulated in one of three HMCL with 1q21 translocations. SKY analyses of 150 tumors showed that the incidence of the t(1;14)(q21;q32) translocation is about 1-2% of advanced intramedullary MM tumors.18,19
20q12 – mafB.
The t(14;20) (q32;q12) translocation has been reported in 1-2% of primary MM tumors, and we found this translocation to be present in 2 of 37 (5%) HMCL (unpublished). In one HMCL, we cloned the t(14;20) breakpoint and found that the breakpoint occurred within the intronic enhancer of the IgH locus. On chromosome 20, the breakpoint is located within a gene sparse area, with the closest oncogene, mafB, located 1100 Kb telomeric to the breakpoint. Northern blot analysis showed mafB to be overexpressed in 5 of 37 (14%) of HMCL, including the 2 HMCL with the t(14;20) translocation. Of the other 3 HMCL that overexpress mafB, 1 has an insertion at 20q12 of the Cl enhancer that was shown by interphase FISH to overlap mafB, whereas the other 2 have complex translocations involving 20q12, with no detectable involvement of Ig sequences. Two other groups have identified 2 additional myeloma cell lines with t(14;20) with breakpoints centromeric to mafB and associated with ectopic expression of mafB.26,27 It appears that these translocations involving 20q12 are secondary translocations that do not involve B cell specific recombination mechanisms (similar to the secondary translocations that dysregulate c-myc during MM progression).
Other partner loci.
A few other loci have been reported as infrequent recurrent 14q32 translocation partners in MM.18,19,28– 30 Mostly these breakpoints have not been cloned, nor the dysregulated genes characterized. These include 1q10-12, 2p23 (?N-myc), 3q21, 4q22-33, 9p13 (?pax-5), 11q23, 12p13 (?cyclin D3), 21q22, and 22q12. Many other partner loci have been identified only once, but breakpoints involving these loci have not been characterized either by FISH mapping or by molecular cloning.
Clinical significance of IgH translocations in MM.
Studies to address the clinical significance of IgH translocations are currently underway, but only very preliminary results are available. In a large cohort of patients enrolled on IFM studies in France, 176/669 (26%) did not have an IgH translocation identified. These patients had a significantly lower frequency of chromosome 13 deletion (22% vs 50%), suggesting that they correspond to a favorable prognostic group. Whereas patients with t(11;14) did not differ from the average, patients with t(4;14) and t(14;16) had a significantly higher frequency of del(13) (87% vs 37%) and elevated β2-microglobulin (90% vs 52%), suggesting that they correspond to a particularly poor prognostic group.7 In a retrospective analysis of patients treated on ECOG 9486/9487, 53/336 (16%) had t(11;14) and appeared to have a slightly better prognosis (50 vs 33 months), although the difference did not reach statistical significance.31 Although the IgH translocations identify distinct, non-overlapping molecular subtypes of MM (Fig. 3), the extent to which these correspond to distinct clinical entities or clinically relevant subtypes remains to be determined.
Karyotypic Instability
By conventional analyses, karyotypic abnormalities are detected at a frequency of 30-50% in large studies of MM tumors.32 The frequency and extent of karyotypic abnormalities correlates with the stage, prognosis, and response to therapy, e.g. approximately 20% abnormal in stage I, 60% in stage III, and > 80% for extramedullary tumor. It appears that virtually all MM tumors would have abnormal karyotypes if an adequate analysis could be done.52 Until late in the disease MM tumors have a very low labelling index (LI), e.g. often < 1% at the earliest stages, whereas contaminating hematopoietic cells in the bone marrow have an LI of about 30%; thus, many normal karyotypes are from the normal cells. By interphase FISH analysis using probes for 5 or 10 different chromosomes, respectively, two studies report that at least one chromosome is trisomic in 96 and 89% of MM tumor samples.33,34 Although conventional karyotypes are not reported for MGUS, it appears that most MGUS plasma cells are aneuploid as well. By FISH analysis using only 4 chromosome probes, the incidence of trisomy for at least one chromosome was 61% in one study of MGUS cells,8 but 100% in a second study that used 6 probes to analyze MGUS cells.53 By FISH analysis, MM cells differ from MGUS cells in that the results for the latter may be somewhat more heterogeneous for a given patient. Despite the limited analyses available for MGUS, it appears that processes leading to karyotypic instability begin in MGUS, but continue—and perhaps progress—throughout the entire course of the disease.
Chromosome 13.
Abnormalities of 13q detected by conventional karyotype are identified in 15-20% of newly diagnosed patient and are associated with a very poor prognosis.37 The use of interphase FISH allows the analysis of patients in whom a metaphase karyotype cannot be obtained and has increased the frequency of detection of 13q deletions to between 38-54%.38–,40 It is detected with a slightly lower frequency in MGUS (4/19 in one study, 13/29 in another); however, in contrast to MM, for MGUS it is restricted primarily to small subpopulations of the clonal cells, indicating that it is a secondary genetic event occurring after clonal expansion.41,42 Although small interstitial deletions of 13q14 occur in some patients, the majority have lost the entire long arm or all of chromosome 13.39,43,44 Despite the fact that it is detected more frequently by interphase FISH, it is still associated with a poor prognosis both for patients treated with conventional chemotherapy (median OS 35 vs 51 months in one study,31 24 vs 60 months in another40) and for those treated with high-dose therapy and stem cell rescue (median OS 27 vs 65 months).38 Although these early results suggest that the superiority of high-dose therapy over conventional chemotherapy may be primarily restricted to the patients without del(13), this needs to be confirmed in subsequent studies.45 Clearly patients with del(13) do poorly with our current treatments, and such patients should be considered for enrollment in clinical protocols testing novel agents and treatment approaches.
N- and K-ras.
Activating mutations of N and K-ras are identified in 40% of patients at diagnosis and in up to 49% at the time of relapse.46 For K-ras, but not for N-ras, there was a significant relation between the presence of a mutation and tumor burden and survival.
p53.
Inactivating mutations of p53 are one of the commonest acquired genetic changes in tumors, particularly solid tumors. These mutations were identified in approximately 4% of intramedullary MM samples and in 20-40% of samples from acute relapsed MM or extramedullary MM, and in nearly 60% of HMCL47 (and unpublished).
PTEN.
PTEN is a recently identified tumor suppressor gene that has a dual phosphatase activity toward both phosphotyrosine and phospholipid substrates. It is considered to be an important negative regulator controlling PI 3′K/Akt activation in vivo. Activated akt phosphorylates several substrates, leading to suppression of apoptosis by several different mechanisms. Loss of PTEN expression has been detected in many cancers, including glioblastomas, breast and prostate carcinomas. Recently, inactivating mutations of PTEN associated with high levels of activated akt were identified in two human HMCL.48 Restoration of PTEN in one HMCL resulted in reduced tumorigenicity in mice.49
p16/INK4a.
p16/INK4a is a negative regulator of the cell cycle that associates with CDK4 and CDK6 and inhibits progression through the G1 phase of the cell cycle. It is inactivated by deletion and promoter methylation in a variety of tumor types. The protein is reported to be expressed in most MM, but is absent from PCL and MM cell lines, correlating with methylation of the promoter in PCL and MM cell lines.50 A recent study analyzing purified plasma cells identified the presence of some methylated p16 promoter DNA in 6/30 MGUS and 4/25 MM.51 This suggests that inactivation of p16 by promoter methylation may occur (at least in a subset of the cells) early in the course of the disease, and that occurrence late in the course of the disease may contribute to extra-medullary dissemination.
A Model for the Multi-step Pathogenesis of MM
The well-defined clinical stages of plasma cell neoplasia can be correlated with a progressive set of genetic alterations. We hypothesize that a primary chromosome translocation at the time of isotype switch recombination (or less frequently during somatic hypermutation) juxtaposes the Ig enhancers to critical oncogenes on the partner loci involved. Dysregulation of these oncogenes is an early—perhaps the first—event leading to immortalization of the plasma cells. As soon as clonal cells can be detected in MGUS there is evidence of a gross karyotypic instability with numeric chromosomal abnormalities. Deletion of chromosome 13 often begins in a subset of MGUS cells, but affects most of the tumor cells in the nearly 50% of MM patients who have this abnormality. Activating mutations of K- or N-ras are rare in MGUS, but at the time of diagnosis occur in about 40% of patients with intra-medullary MM, with some accumulation of additional mutations during tumor progression. Secondary translocations, which apparently do not involve B cell specific DNA modification mechanisms, dysregulate additional oncogenes that contribute to tumor progression. For example, late intramedullary tumor progression and extra-medullary spread, both of which appear to be correlated with an increased PC labelling index, are associated with dysregulation of c-myc (rarely L- or N-myc) as a consequence of secondary translocations. Finally, inactivation of p16, and occasionally p53, occur as very late events, mostly associated with extra-medullary MM and HMCL (which are uniformly derived from extra-medullary MM tumors).
A New Paradigm for the Management of MM
The identification of specific genetic events and the development of molecularly targeted therapy promises to change the way we approach and manage patients with MM. On the basis of IgH translocation and ectopic gene expression, patients can be divided into 5 largely non-overlapping subtypes: 1) No IgH translocation (25%), 2) Cyclin D1 or Cyclin D3 (25%), 3) FGFR3 (15%), 4) c-maf or mafB (?15%), 5) Other IgH translocation (20%) (Fig. 3). Determination of other genetic abnormalities (ras mutation, 13q deletion, c-myc dysregulation) provide further molecular classification of these subtypes. The molecular subtype can be rapidly determined at diagnosis, and appropriate therapy selected on the basis of genotype and gene expression phenotype,. Examples of hypotheses based on this model that can be tested in clinical trials include:
Kinase inhibitors of the cyclin dependent kinases in patients with t(6;14) or t(11;14) and dysregulation of the D-type cyclins or inactivation of p16(INK4a)
Kinase inhibitors of FGFR3 tyrosine kinase in patients with t(4;14)
Ras pathway inhibitors in patients with ras mutations
Chemoprevention with non-toxic ras pathway inhibitors (e.g., lovostatin) in patients without ras mutations (e.g., MGUS, or as maintenance post transplant)
II. Novel Therapies for Multiple Myeloma
Kenneth C. Anderson, MD*
Jerome Lipper Multiple Myeloma Center, Dana-Farber Cancer Institute, Boston MA 02115
Supported by National Institutes of Health Grants CA 50947 and CA 78378; and a Doris Duke Distinguished Clinical Research Scientist Award (KCA)
Therapies Targeting Fundamental Genetic Abnormalities in MM Cells
We have studied expression and function in MM of Ku, a heterodimer of Ku86 and Ku70, which is essential for normal double stranded DNA break repair and normal Ig VDJ recombination, Ig class switching, and B cell development. Our studies have identified a truncated Ku protein in human MM cells with decreased double stranded DNA break repair that correlates with sensitivity to DNA damaging agents; conversely, our studies have shown that normal Ku expression and function in MM cells confers resistance to chemoradiotherapy.3 Moreover, we have delineated the mechanisms whereby Ku is translocated to the cell membrane of MM cells of CD40 activated MM cells, where it mediates heterotypic adhesion to fibronectin and bone marrow stromal cells (BMSCs).4,5 This binding confers protection against apoptosis, and blocking binding confers sensitivity to chemoradiotherapy. Ongoing studies are delineating the role of the truncated Ku variant in Ig rearrangement and Ig class switching in MM versus normal B cells. These studies are directed to defining a potentially common genetic abnormality in MM and determining whether the Ku variant in MM represents a novel therapeutic target.
Therapies Targeting Growth and Apoptotic Signaling Cascades in MM Cells
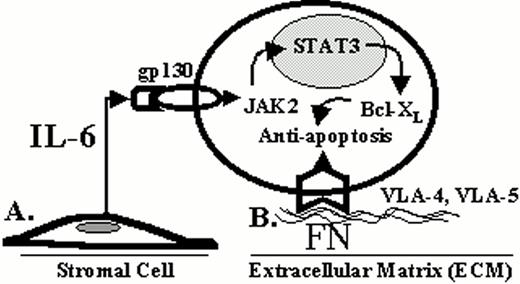
Novel therapies can also be predicated upon interrupting growth or triggering apoptotic signaling in MM cells (Figure 5; see color page 543). We have characterized the growth of MM cells in response to cytokines including interleukin (IL)-6, IL-11, transforming growth factor β, oncostatin M, tumor necrosis factor α (TNFα) and vascular endothelial growth factor (VEGF).6–,12 Proliferation of MM cells is mediated via the MAPK cascade,13–,15 and therapeutic strategies based upon blocking this pathway (i.e., IL-6, IL-6 receptor and kinases) are under evaluation.16 We have also characterized apoptotic signaling in MM cells with the view to trigger death pathways in novel treatments. Apoptosis triggered by γ irradiation, Fas, and Dexamethasone (Dex) is mediated via distinct signaling cascades.17 For example, Dex (but not γ irradiation or Fas) induced apoptosis is mediated via activation of related adhesion focal tyrosine kinase (RAFTK).18 Moreover, Dex mediated MM apoptosis is not associated with mitochondrial cytochrome c release, but is mediated by Second activator of apoptosis (Smac) release from mitochondria, which disrupts the inhibitor of apoptosis XIAP/caspase 9 complex, thereby allowing activation of caspase 9, caspase 3 cleavage, and apoptosis.19,20 Conversely, IL-6 inhibits apoptosis triggered by Dex (but not γ irradiation) by specifically activating SHP2 phosphatase and thereby blocking the activation of RAFTK.21–,23 Further delineation of these pathways, including the use of gene array, will derive therapeutic strategies for overcoming Dex resistance and triggering apoptosis.24,25
Therapies Targeting MM Cell Growth and Survival in the Bone Marrow Microenvironment
Recent reports of increased bone marrow angiogenesis in MM,26 coupled with the known antiangiogenic properties of Thalidomide (Thal),27 provided the rationale for its use to treat MM. Importantly, Thal induced clinical responses in 32% of MM patients whose disease was refractory to conventional and high dose therapy.28 Given its broad spectrum of activities, we speculated that Thal may be acting against MM in several ways.29 We examined Thal and two classes of Thal analogs (Figure 5, color page 543): phosphodiesterase 4 inhibitors that inhibit TNFα but do not enhance T cell activation (selected cytokine inhibitory drugs); and others that are not phosphodiesterase 4 inhibitors, but markedly stimulate T cell proliferation as well as IL-2 and IFN γ production (immunomodulatory drugs, IMiDs).30 Thal and the IMiDs directly induce apoptosis or G1 growth arrest in MM cell lines and patient MM cells that are resistant to melphalan (Mel), doxorubicin (Dox), and dexamethasone (Dex), and enhance the anti-MM activity of Dex.31 As for Dex, and in contrast to γ irradiation and Fas, apoptotic signaling triggered by Thal and the IMiDs is associated with activation of RAFTK. Moreover, Thal and Dex are associated with caspase 8 and caspase 9 activation, respectively. Although Thal and IMiDs do not alter adhesion of MM cells to BMSCs, they inhibit the upregulation of IL-6 and VEGF secretion triggered by MM cell to BMSC binding.32 Importantly, increased natural killer cell mediated killing of autologous MM cells was induced by Thal and IMiD treatment of patient peripheral blood mononuclear cells.33 Finally, Thal and the IMiDs downregulate protectins such as CD59, which are expressed by MM cells and protect them from complement and antibody dependent cellular cytotoxicity.34 Based upon these preclinical studies, we are now undertaking a phase I trial of IMiDs in MM.
Proteasome inhibitors represent another novel potential anticancer therapy targeting the MM cell and its bone marrow microenvironment. These agents inhibit the degradation of multiubiquinated target proteins (i.e. cell cycle regulatory proteins such as cyclins and cyclin-dependent kinase inhibitors) and regulate cell cycle progression.35 We have recently demonstrated that the proteasome inhibitor PS-341 both acts directly on MM cells and inhibits paracrine MM growth mechanisms in the bone marrow microenvironment (Figure 6, see color page 543).11,36 PS 341 directly inhibits proliferation and induces apoptosis in both human MM cell lines and freshly isolated patient MM cells that are resistant to Mel, Dox, and Dex. PS-341 inhibits p44/42 MAPK growth signaling triggered by IL-6 and induces apoptosis, despite induction of p21 and p27, in p53 wild type and p53 mutant MM cells. PS 341 adds to the anti-MM activity of Dex and overcomes IL-6-mediated protection against Dex-induced apoptosis. PS-341 blocks the paracrine growth of human MM cells by decreasing adherence to BMSCs and related NF-κB dependent induction of IL-6 secretion in BMSCs.36,37 Moreover, proliferation and MAPK growth signaling of those residual adherent MM cells is also inhibited. Given the acceptable toxicity profile of PS 341 and evidence of clinical activity in phase I trials, a phase II clinical evaluation of PS-341 is currently underway.
We have recently explored the biological effect of TNFα in MM in order to determine whether TNFα inhibitors or drugs that block NF-κB mediated sequelae of TNFα in MM cells and BMSCs might be useful in MM.11 TNFα, which is produced by some MM cells, induces only low level MM proliferation and MAPK activation in MM cells. However, TNFα markedly upregulates IL-6 secretion from BMSCs. Importantly, TNFα upregulates expression of adhesion molecules (VLA-4 and LFA-1) on MM cells and their receptors (VCAM-1 and ICAM-1) on BMSCs, with resultant increased MM to BMSC binding. Inhibition of TNFα-induced NF-κB activation using PS 341 or Thal/IMiDs inhibits the upregulation of these molecules on MM cells and BMSCs and the resultant increased adhesion. Therefore, inhibiting TNFα and its sequelae may be useful treatment strategies in MM.
Prior studies have shown that VEGF is expressed and secreted by tumor cells as well as BMSCs, and that VEGF produced by MM cells may augment IL-6 production in BMSCs.38,39 We have recently shown that VEGF causes proliferation via MAPK signaling and enhances migration of MM cells in a PKC dependent mechanism.12 These studies are not only of importance in MM pathogenesis but strongly suggest VEGF as a novel therapeutic target in MM. Additional benefits of inhibiting VEGF in MM bone marrow include blocking its stimulation of osteoclastogenesis and angiogenesis, as well as its inhibition of dendritic cell function and host immunity. Already we have shown that a VEGF receptor inhibitor, as well as Thal and IMiDs, can block VEGF triggered growth and migration of MM cells.
Other agents under investigation preclinically and in early clinical trials include 2 methoxy estradiol. 2 methoxy estradiol induces apoptosis of drug resistant MM cell lines and patient cells, adds to Dex, and is not inhibited by IL-6; it increases reactive oxygen species and downstream caspase 9 activation, and it also inhibits bone marrow angiogenesis.40 Arsenic trioxide also induces apoptosis of drug resistant MM cells, adds to Dex, downregulates TNFα induced NF-κB dependent upregulation of MM cell to BMSC binding; and is not blocked by IL-6.41 TNFα-related apoptosis inducing ligand (TRAIL/Apo2L) induces apoptosis of drug resistant MM cell lines and patient cells, and NF-κB inhibitors including SN50 and PS-341 enhance its activity both in vitro and in vivo against plasmacytoma xenografts in nu/xid/bg mice;42,43 phase I trials will begin soon. Finally, AE 491 inhibits angiogenesis, metalloproteinases 2,9, and 12, VEGF signaling, and tumor cell growth;44 a phase II multicenter trial is currently underway in MM.
In conclusion, further defining the mechanisms of MM cell growth and survival in the bone marrow microenvironment provides the basis for a variety of novel treatment approaches to overcome drug resistance and improve outcome for patients with MM.
III. High-Dose Therapy in Multiple Myeloma
J.L. Harousseau, MD*
Hematology Department, otel Dieu, Place Alexis Ricordeau, 44035 Nantes, France
I) Autologous stem cell transplantation.
The role of autologous stem cell transplantation in multiple myeloma.
In the absence of significant improvement of conventional chemotherapy, high dose therapy (HDT) with autologous stem cell transplantation (ASCT) has been increasingly used in the past 15 years in MM.1 Uncontrolled studies have shown that, for patients responding to initial induction chemotherapy, ASCT is a safe (less than 5% toxic deaths) and effective consolidation therapy.2 Most importantly, some of these studies have suggested that 30-50% complete remissions (CR) could be achieved with this approach in newly diagnosed MM and that this tumor burden reduction could be converted into a prolongation of remission and of survival.2 However, these pilot studies are difficult to analyze because the recruitment of patients is subject to selection bias regarding age, performance status, renal function and response to initial chemotherapy. Historical comparisons have suggested that survival of patients less than 65 years of age responding to initial chemotherapy and receiving only conventional chemotherapy was similar to that reported in selected series of patients given early HDT.3 Therefore, prospective randomized trials were needed to compare conventional chemotherapy with HDT. The Intergroupe Français du Myelome (IFM) was the first to conduct a randomized trial showing the superiority of HDT with autologous bone marrow transplantation (ABMT) as compared to conventional chemotherapy.4 In this trial (IFM90), HDT significantly improved the response rate since 38% of patients enrolled in the HDT arm achieved a CR or a very good partial remission (VGPR) versus 14% of patients enrolled in the conventional chemotherapy arm (p < 0.001). An updated analysis of this study confirms that, with a median follow-up of 7 years, HDT significantly improves event free survival (EFS) (median 28 months vs 18 months, 7-year EFS 16% vs 8%, p = 0.01) and overall survival (OS) (median 57 months vs 44 months, 7-year OS 43% vs 25%, p = 0.03) in comparison to conventional chemotherapy. In another randomized French trial 190 patients aged 55 to 65 years were randomized to receive conventional chemotherapy or HDT.5 Although, the results of HDT appeared comparable to those achieved in the IFM90 trial, there was no significant difference in OS between the 2 arms, due to an unexpected survival in the conventional chemotherapy arm (median 55 months with HDT versus 50 months with conventional chemotherapy). It should be noted that in the conventional chemotherapy arm, 17 patients received ASCT at the time of relapse. The results of other randomized studies are pending but the superiority of HDT has also been shown by several historical comparisons6– 8 (Table 1 ). Therefore, HDT represents a significant improvement and is currently proposed as part of front line therapy in younger patients (up to the age of 60-65 years).
How to improve the results of autologous stem cell transplantation?
In the IFM90 trial, the 7-year EFS was only 16% for patients enrolled in the HDT arm and there was no plateau of the survival curves. Therefore strategies to improve these results were clearly warranted. Since in this trial achievement of CR or VGPR was significantly associated with a prolongation of survival, the aim of subsequent studies was to increase the CR rate.
Conditioning Regimen
Improving the conditioning regimen could be one way to attain this objective. The optimal conditioning regimen for ASCT in MM has not yet been determined. Since its introduction in 19879 total body irradiation (TBI) has been used in multiple uncontrolled studies. The combination of TBI plus high-dose melphalan 140 mg/m2 yields CR rates ranging from 20 to 50% according to the disease status at transplantation and to criteria used to define CR. This conditioning regimen was used in the IFM90 trial and could therefore be considered as the standard one. However, in newly diagnosed patients, the Royal Marsden Group has reported an impressive 70% CR rate with high-dose melphalan 200 mg/m2, with a low extramedullary toxicity.10
In 1995 the IFM initiated a randomized study comparing high-dose melphalan 200 mg/m2 and high-dose melphalan 140 mg/m2 plus TBI in 282 patients with newly diagnosed MM.11 In this study, high-dose melphalan 200 mg/m2 was significantly less toxic (shorter duration of neutropenia and thrombocytopenia, lower incidence of grade ≥ 3 mucositis, no toxic death versus 5 in the TBI group). Although the response rate and the EFS were identical, OS was superior in the high-dose melphalan 200 mg/m2, apparently because of a better salvage after relapse. Two other non-randomized failed to show a survival benefit for TBI containing regimens.12–,13 Therefore, high-dose melphalan 200 mg/m2 should be preferred to high-dose melphalan 140 mg/m2 plus TBI as the conditioning regimen for ASCT in MM. Knowing the good tolerance of high-dose melphalan 200 mg/m2, higher doses of melphalan alone or in combination with an anti-IL-6 antibody have been explored with encouraging results.14– 15 In order to further improve the efficacy of conditioning regimens, studies are ongoing with agents that localize preferentially in the bone and that are coupled with radioelements (Holmium, Samarium).
Impact of Tandem Transplants
Another way to increase the CR rate could be to repeat intensive treatments. However, when the first course of HDT was not supported by any hematopoietic support, the hematologic toxicity was severe.6 Thanks to autologous transplantation of peripheral blood progenitor cells (PBPC) and to hematopoietic growth factors, the sequential use of two courses of HDT appears to be well tolerated, to increase the CR rate17–,18 and even to induce molecular remission.19
The largest experience in this setting comes from the Little Rock Group.20 Out of 495 patients enrolled to undergo two transplants including 315 pretreated patients, 95% completed the first course of HDM 200 mg/m2 with PBPC transplantation and 73% completed two transplants. The CR rate increased from 24% after the first transplant to 43% after two transplants. This experience has now been extended to more than 1000 patients.21
However, the actual impact of such an aggressive strategy on EFS and OS needed further evaluation. In 1994, the IFM initiated a randomized trial (IFM94) comparing one versus two transplants.22 From October 1994 to March 1997, 405 untreated patients under the age of 60 years were enrolled by 36 centers. At diagnosis they were randomized to receive either a single ASCT prepared with melphalan (140 mg/m2) and TBI (8 Gy) or a double ASCT: the first one prepared with melphalan (140 mg/m2) and the second one prepared with melphalan (140 mg/m2) and TBI (8 Gy). After initial cytoreduction with VAD, 343 patients eligible for transplantation underwent a second randomization (PBPC versus bone marrow) to support high-dose melphalan 140 mg/m2 plus TBI. Thus four groups were analyzed on an intent to treat basis (Table 2 ). In this trial, double HDT with PBPC autologous transplantation appears to be superior to double HDT with bone marrow autologous transplantation and to single HDT in terms of immediate response, EFS and OS.
Source of Stem Cells
As in other malignancies, PBPC have almost completely replaced bone marrow as the source of stem cells in ASCT for MM. The main reasons for this choice are easier accessibility and availability, faster hematopoietic recovery and possibly lower tumor contamination. However, several issues remain regarding the use of PBPC. Although tumor cell contamination is lower in PBPC harvests than in bone marrow, the superiority of PBPC autologous transplantation as regards the clinical outcome has not yet been demonstrated. Sensitive immunofluorescence studies or PCR based techniques have demonstrated that virtually all PBPC harvests are contaminated with malignant cells. Although the prognostic significance of detecting malignant cells with such sensitive methods is still unknown, attempts to reduce tumor cell contamination of the grafts has been a great concern.
Purging marrow with cyclophosphamide derivatives or with monoclonal antibodies has proven feasible although associated with prolonged myelosuppression after transplantation. Selection of CD 34+ progenitors appears to be a promising alternative, with a 2.5 to 4.5 log-depletion of plasma cells.26–,27 Several pilot studies have confirmed the feasibility of autologous transplants with CD 34+ selected PBPC in MM. In a multicenter phase III trial comparing selected and unselected PBPC in 131 myeloma patients, successful neutrophil engraftment was achieved in all patients by day 15 and there was no significant difference between the two groups as regards platelet engraftment.28 However a recent analysis of this trial failed to show EFS or OS prolongation with CD 34+ cell selection.29 Another randomized study from the EBMT group30 and results of the IFM 94 trial confirm these results. The absence of survival improvement in spite of a lower tumor load in the graft could mean the relapse is also caused by persistence of malignant cells in patients after HDT.
Sensitive PCR techniques using patient-specific oligonucleotide primers show the persistence of myeloma cells in the CD 34+ cell fractions, while highly purified CD 34+ lin- thy 1+ stem cells do not appear to contain clonal myeloma cells.31 Thus, an additional purging step might be necessary to obtain tumor-free grafts. The clinical impact of these cumbersome and expensive procedures is unknown. In a pilot study in 10 patients, neutrophil and platelet engraftment was substantially delayed as compared to unmanipulated PBPC grafts.32 Currently, unselected PBPC appear to be the best source of stem cells for ASCT in MM.
Maintenance Therapy
As there is no plateau of the survival curves in published series with adequate follow-up, some form of maintenance therapy appears necessary. Several randomized studies have shown that, in patients responding to conventional chemotherapy, Interferon-α (IFN-α) maintenance prolongs remission duration by 5 to 12 months as compared to observation. IFN-α a has also been used after HDT with the hypothesis that it could be more effective in patients with minimal residual disease. In a retrospective analysis of the EBMT registry IFN-α was associated with improved progression free survival (PFS) and OS in patients responding to HDT.33 Only one randomized study has been completed up to now.34 This trial compared IFN-α a (3 × 106 U/kg, 3 times weekly) following recovery from HDT and no further therapy. While PFS and OS were significantly better in IFN-α arm with a median follow-up of 52 months, the differences ceased to be significant with median follow-up to 77 months. This means that, although IFN-α a delays relapse, most if not all patients ultimately relapse. However, since this study involved only 85 patients, these results should be interpreted cautiously. Maintenance with IFN-α may have benefit for a selected group of patients. Moreover, some patients in the control arm received Interferon for relapse, which could erase differences in survival curves. Further studies are needed, and a large randomized trial is ongoing in the US. It should be noticed that, in the IFM90 trial, although IFN-α a was to be administered to all patients after HDT, there is no plateau of the EFS curve.4 Therefore, the cure of patients with MM with a single course of HDT followed by IFN-α a is unlikely. New strategies to control minimal residual disease after ASCT are necessary, such as the use of maintenance chemotherapy, thalidomide, diphosphonates and immune therapy (idiotypic or DNA vaccination, vaccination with idiotype pulsed dendritic cells). These strategies are currently being evaluated.
Current Issues in ASCT for MM
Timing of transplantation
Since ASCT is also a useful salvage therapy for primary refractory MM and for chemosensitive relapses, the optimal timing of ASCT is not yet known. Fermand et al published the results of a randomized study showing no significant difference in OS between early ASCT and late ASCT (performed as rescue treatment in case of primary resistance or at relapse).35
Prognostic factors
Barlogie et al recently published the results of tandem transplants in 231 patients with newly diagnosed MM.36 In multivariate analysis, superior EFS and OS were observed in the absence of unfavorable karyotypes (11q breakpoints and/or partial or complete deletion of chromosome 13) and with low β2 microglobulin level at diagnosis (≤ 4 mg/L). When combining these factors, a subgroup of patients with a very poor prognosis was identified: patients with unfavorable cytogenetics and β2 microglobulin level > 4 mg/l had a median survival of only 2.1 years, compared to 7 years for the remaining patients. New therapeutic approaches are clearly needed for these patients.
Using a larger cohort of 1000 consecutive patients (including previously treated patients), the same authors confirmed that independent favorable features were mainly absence of chromosome 13 deletion, low β2-microglobulin level, plus low CRP level and less than 12 months of prior conventional chemotherapy.21 Plateaus of the EFS and OS curves were noted in 45 and 60% of patients with all these favorable characteristics. Thus, durable remissions and possibly cures can be achieved in a high proportion of good risk patients with an intensive strategy including tandem ASCT.
In a recent retrospective analysis of 110 patients treated with HDT, the IFM showed that the detection of chromosome 13 abnormalities (−13, 13q−) by FISH was the most powerful adverse prognostic factor.37 The combination of FISH analysis, β2-microglobulin and IgA isotype produced a very powerful staging system in the context of HDT. Again, patients with a high β2 microglobulin level and chromosome 13 abnormalities had a very poor prognosis.
Selection of patients
Usually, ASCT is limited to patients up to 65 years of age, with a performance status 0-2 and with a normal renal function. The issue of age limits was emphasized by the IFM90 trial, since ASCT was actually performed in 82% of patients 60 years of age or less, versus only 58% in patients aged 60-65.4 As a consequence, in the intention-to-treat analysis, ASCT was significantly superior to conventional chemotherapy only in younger patients. However, the introduction of hematopoietic growth factors has profoundly modified the practice of ASCT. With PBPC collected after priming with G-CSF or GM-CSF, ASCT has become safer and could be offered to older patients. Recently, the Little Rock group has compared the outcome of 49 patients 65 years and older with 49 younger pair mates selected in a cohort of 550 patients treated with HDT.40 The CR rate was higher in younger patients (43% vs 20%, p = 0.02) and the transplant related mortality appeared to be higher in older patients (8% vs 2%). However, since the EFS and OS were comparable, the authors concluded that age is not a biologically adverse parameter for patients treated with HDT and PBPC support and should not constitute an exclusion criteria for participation in what appears to be superior therapy in MM.
In a recent retrospective analysis of 952 patients enrolled in tandem transplant trials, the same group showed that age was not a significant prognostic factor. In three subgroups defined by the β2 microglobulin level and the presence of chromosome 13 abnormalities, survival was identical for patients < 65 years and for patients ≥ 65 years.41
Palumbo et al has shown that 2-3 courses of melphalan 100 mg/m2 supported by PBPC support are feasible on an outpatient basis in patients up to 75 years of age. They compared 71 patients treated with this approach to 71 pair mates treated with conventional chemotherapy and concluded that intensive therapy was superior in terms of CR rate, EFS and OS.8
Allogeneic BMT
Allogeneic BMT is usually performed only in patients aged under 50 years of age and having an HLA identical sibling. Considering the median age of patients with MM, this strategy can be offered only to a small proportion of patients. The procedure-related death rate remains very high, mainly as a consequence of infections and graft-versus-host disease.46–,47 A comparison of data compiled by the European Blood and Marrow Transplantation group has shown no advantage of allogeneic BMT compared with ASCT.48 However, if given early in the course of the disease, allogeneic BMT has shown encouraging results: about one third of patients who have a CR after transplantation remain free of disease 6 years later.46 As a result of the antitumor effect of the graft, allogeneic BMT is possibly the only genuinely curative therapy in MM. Recent reports of CR achieved after the infusion of donor mononuclear cells in patients relapsing after allogeneic BMT, are further evidence of the so-called graft versus myeloma effect.49–,50 Two recent series confirm that donor lymphocyte infusions, alone or following reinduction chemotherapy, may induce remissions in 30-50% of patients, including CR. Some of these remissions may last more than one year. However, this obvious antimyeloma activity is hampered by a high incidence of graft-versus-host disease and by the risk of severe aplasia.51– 52
A recent survey of the EBMT registry shows a significant improvement in survival for transplants performed between 1994 and 1998, compared to previous experience.53 This is the result of a significant reduction in transplant-related mortality, mostly of deaths due to bacterial and fungal infections. This encouraging result corresponds to a better selection of patients with earlier transplantation in patients less heavily pretreated. In this study, the use of PBPC instead of bone marrow was associated with earlier engraftment but yielded no significant survival benefit. Another way of improving results could be the use of attenuated dose conditioning regimens (so-called mini-allotransplants), which are currently being evaluated in MM as well as in other hematopoietic malignancies.
IV. Insights into the Pathogenesis of Myeloma and Potential New Therapeutic Approaches
William S. Dalton, PhD, MD*
Clinical Investigations, H. Lee Moffitt Cancer Center, 12902 Magnolia Drive, MRC 3043, Tampa FL 33612-9497
H. Lee Moffitt Comprehensive Cancer Center Core Support Grant (CA-76292) and NIH/NCI grants (CA-82533) (WSD) and (CA-77859) (WSD)
Effective therapy of myeloma began in the early 1960s with the introduction of the alkylating agents melphalan and cyclophosphamide.1,2 During the past four decades, a number of pharmacological agents have been added to the armamentarium to treat myeloma including other alkylating agents (BCNU), topoisomerase inhibitors (doxorubicin and etoposide), glucocorticoids (prednisone and dexamethasone), and the antitubulin agent vincristine. With the exception of the glucocorticoids, most of the agents are relatively ineffective as single agents and are therefore used in combination. The combination of melphalan and prednisone is considered to be the mainstay of treatment of myeloma, producing responses in approximately 50-60% of patients. Since the introduction of melphalan and prednisone (MP), a number of combinations have been developed, and include various combinations of vincristine (V), carmustine or BCNU (B), Adriamycin (A), melphalan (M), cyclophosphamide (C), prednisone (P), and Dexamethasone (D). A popular example of combination chemotherapy is the M2 protocol consisting of VBMCP.3 Other frequently used combinations include VMCP, VBAP and VAD.4,5 While a higher percentage of patients respond to combination chemotherapy compared to MP, the improvement in overall survival is marginal and appears to occur chiefly for patients with a poor prognosis.6 Generally speaking, melphalan plus prednisone is as effective as combination chemotherapy in patients who are elderly or have a good prognosis, and more aggressive chemotherapy combinations are more effective in patients with a poor prognosis. In all probability, improving myeloma therapy will require the development of strategies that target unique aspects of myeloma cell biology.
Today, at least three different approaches are being investigated to improve the therapeutic outcome for patients with myeloma. These include the following: (1) enhancing the therapeutic benefit of available chemotherapeutic drugs by overcoming drug resistance, (2) identifying new cellular targets that regulate cell survival and growth, and (3) developing means to enhance host immune response against myeloma cells. While the latter approach is of tremendous theoretical interest, the first two mentioned approaches are more developed and this presentation will be limited to these approaches.
During the last decade, substantial progress has been made to identify cellular mechanisms that confer clinical drug resistance. Research chiefly focused on mechanisms that reduce intracellular drug concentration or block the cytotoxic effects of chemotherapy. It was hoped that by identifying individual cellular drug resistance mechanisms, means of overcoming or preventing the emergence of drug resistance would be developed to improve clinical outcome. However, recent evidence has demonstrated that clinical drug resistance is multifactorial and enhancing drug activity of available agents will likely require a multifaceted approach.7 Another approach that to date has proven more effective in enhancing the efficacy of currently available cytotoxic agents is the use of high dose chemotherapy with stem cell rescue. This approach capitalizes on the steep dose response curve of cytotoxic agents and the ability to use hematopoietic stem cells to rescue patients from the major toxicity of myelosuppression (see Section III).
Another approach, which in the long run may be more rewarding than enhancing the efficacy of cytotoxic agents, is the development of molecules that regulate myeloma cell growth and survival. Theoretically, developing small molecules that inhibit or interrupt myeloma cell signaling pathways that control cell proliferation and survival may allow us to specifically target myeloma cells and spare normal cells. Inherent in this approach is the need to understand the biology of the myeloma cell and identify cell signaling pathways that allow for disease progression. An understanding of the genetic abnormalities that contribute to the oncogenesis of myeloma will be important in identifying future targets for drug therapy. Likewise, an appreciation of the tumor microenvironment and its contribution to myeloma growth and survival will also be potentially rewarding for developing novel therapeutic approaches.
New Targets Intrinsic to the Myeloma Cell
As detailed in Section I, modern molecular techniques are identifying genetic abnormalities unique to the myeloma cell. Understanding these genetic alterations and their biological consequence is fundamental to the development of more effective therapies. For example, activating mutations of the t(4;14) translocated FGFR3 allele are present in some myeloma cell lines and patient specimens and likely contribute to the oncogenesis of myeloma.8 These mutation events result in the constitutive activation of the FGFR3 and subsequently activate STAT3, which is known to be involved in abnormal cell growth and survival. Catlett-Falcone et al recently demonstrated that STAT3 was constitutively activated in most patient myeloma cells and demonstrated that activated STAT3 increases the expression of Bcl-Xl and blocked Fas-induced apoptosis (9). These findings illustrate that FGFR3 and the STAT3 signaling pathway may be important targets for novel drug development.
Mutations of the Ras family are among the most common oncogene mutations found in myeloma patients. Neri et al first reported in 1989 that 32% of patients had Ras mutations.10 The most frequent mutation occurred in the N-Ras gene, particularly at codon 61. The second most frequent mutation was found in codon 12 for K-Ras. The frequency for Ras mutations increased from 27% at diagnosis to 46% with advanced disease. Interestingly, these investigators found that patients with Ras mutations were less likely to respond to chemotherapy compared to patients with wild-type Ras. In a more recent study, Liu et al reported a total incidence of 39% for Ras mutations in a large study of 346 newly diagnosed, untreated patients.11 Similar to the study of Neri et al, the most common mutation observed in this population was N-Ras 61 followed by K-Ras 12. Patients with Ras mutations had a median survival of 2.1 years compared to 4.0 years for patients with wild-type Ras. These studies demonstrate the Ras mutations adversely affect patient survival and may reduce response to chemotherapy; therefore, targeting Ras processing may be a novel approach to treatment of myeloma.
Post-translational modification of Ras by adding a farnesyl group has been shown to be essential to its function.12 The enzyme farnesyltransferase is responsible for transferring the farnesyl group from farnesyl diphosphate, a cellular chemical used in the synthesis of cholesterol, to certain proteins such as Ras. This process is important for Ras activation by allowing the protein to attach to the inner plasma cell membrane. Preventing Ras from going to the plasma membrane by blocking its farnesylation short circuits oncogenic growth signals to the nucleus. Inhibition of the farnesylation process is a prime target for drugs aimed at preventing Ras activity. These drugs are known to inhibit Ras farnesylation, and preclinical studies have shown these agents to be effective in tumors with Ras mutations.13 We recently examined the prenylation inhibitors, FTI-277 and GGTI-2166, inhibitors of farnesyltransferase and geranylgeranyl transferase, respectively, for activity in myeloma cell lines.14 We observed that FTI was most effective in cell lines expressing N-61 Ras and wild-type Ras, but was least effective against cells expressing the K12 Ras mutation. We found that cells expressing K12 Ras mutations were capable of overcoming inhibition of farnesylation by alternatively using geranylgeranylation. However, when the FTI and GGTI were combined, processing of Ras was effectively blocked and the cells died of apoptosis.
Although these compounds were originally designed to block the action of the Ras oncogene, it remains unclear whether this is the critical anticancer event of FTIs. Based on these preclinical studies a phase II trial of an FTI drug is now ongoing for patients with drug resistant disease. Studies will measure Ras mutation status and Ras processing to determine the importance of Ras activation status and response to drug.
Other exciting novel agents being investigated clinically for myeloma include derivatives of Thalidomide, proteasome inhibitors (PS 341), arsenic trioxide, and several anti-angiogenic agents. Table 4 lists individual compounds that are currently (or soon will be) investigated in phase II clinical trials. (See Section II.)
The Tumor Microenvironment and Its Influence on Myeloma Cell Survival and Response to Treatment
Host-tumor interactions may contribute to tumor cell survival and progression.15Figure 7 illustrates that this interaction can be classified into two different types of interactions: (1) a soluble form, and (2) a direct cell contact form.16 Examples of the first form include cytokines and growth factors that influence cell growth, migration and survival. Interleukin-6 (IL-6) is the most well characterized soluble factor associated with myeloma and is chiefly produced by bone marrow stromal cells. As mentioned above, IL-6 activates the JAK2/STAT3 pathway in myeloma cells resulting in the overexpression of Bcl-Xl and inhibition of CD95 induced apoptosis.9 In the last 5 years, it has been appreciated that cell-cell or cell-ECM (extracellular matrix) adhesion can also regulate cell survival in a wide variety of tumors.17 The integrin family of cell adhesion molecules is a major class of receptors through which cells interact with ECM components, and recent evidence has implicated the integrins as being involved with the pathogenesis of myeloma. Recently, Shain et al found that myeloma cell adhesion to fibronectin, mediated by the integrins α4β1 and α5β1, blocked CD95 (Fas) induced apoptosis by regulating the cellular availability of FLIP-L, an endogenous inhibitor of CD95 induced death.18 Cells that were adhered to fibronectin became relatively resistant to CD95 induced cell death compared to cells in suspension. Adhesion to fibronectin altered the membrane binding of FLIP-L so that this antagonist was more readily available to bind the DISC complex formed when Fas ligand binds the CD95 receptor. By inhibiting the DISC complex, caspase activation is inhibited and programmed cell death is prevented. Thus, the tumor microenvironment prevents CD95 induced cell death in myeloma cells by at least two different mechanisms: (1) by activating the STAT3 pathway via the IL-6 receptor, and (2) by direct cell contact with fibronectin via β1 integrin receptors. These host-myeloma cell interactions may represent means by which the myeloma cell is able to evade immune surveillance and allow for myeloma cell survival and progression.
In addition to inhibiting CD95 induced cell death, Damiano et al demonstrated that myeloma cell adhesion to fibronectin prevented chemotherapy induced cell death in myeloma cell lines.19,20 Since that report, we have observed that integrin mediated adhesion of other hematopoietic cancer cell lines (U937 and K562 leukemia cell lines) is able to prevent drug induced cell death.21,22 This cell adhesion mediated drug resistance (CAM-DR) phenotype conferred a pluripotent form of drug resistance that included the Bcr-Abl kinase inhibitor, STI-571, in the K562 CML cell line.22 More recently, we have observed the CAM-DR phenotype in patient myeloma cells adhered to fibronectin. In essentially all cases, myeloma cells adhered to fibronectin became relatively resistant to melphalan compared to patient cells exposed to drug in suspension culture.
At least one mechanism related to myeloma cells and CAM-DR is via β1 integrin regulation of p27kip1 levels.23 RPMI 8226 myeloma cells adherent to fibronectin results in a G1 cell cycle arrest associated with p27 protein levels and inhibition of cyclin A and E associated kinase activity. Disruption of cells for fibronectin adhesion resulted in a rapid recruitment of cells into S phase, a decrease in p27 protein levels, and reversion to a drug sensitive phenotype. These studies demonstrated that β1 mediated adhesion of myeloma cells to fibronectin regulates p27 levels and that p27 levels are causally related to CAM-DR. Disruption of β1 integrin mediated fibronectin adhesion may represent a potential target for the potentiation of drug induced apoptosis.
We have also recently observed that adhesion of hematological malignant cells to fibronectin, by means of b1 integrin activation, reduces initial drug induced DNA damage.21 The neutral comet assay was used to determine if initial drug induced DNA damage correlated with CAM-DR. Cellular adhesion resulted in a 40-60% reduction in etoposide and anthracycline induced DNA double strand breaks. We also found that β1 integrin-mediated reduction in drug induced DNA double strand breaks correlated with reduced topoisomerase II activity and alterations in the nuclear pool of topoisomerase IIb (topo II). Confocal studies showed changes in the nuclear localization of topo II; however, alterations in the nuclear:cytoplasmic ratio of topo II in fibronectin adhered cells were not significantly different. Furthermore, high salt extraction of nuclear proteins from adherent versus suspension cells revealed that topo II was more tightly bound to DNA chromatin when cells were adherent. Thus, cellular adhesion via β1 integrins appears to protect cells from initial drug induced DNA damage by reducing topo II activity secondarily to alterations in the nuclear distribution of topo II. Overall, these studies illustrate that cell adhesion to fibronectin protects cells from stress (physiologic and drug induced) by multiple mechanisms (see Figure 6; color page 543). It appears that the bone marrow microenvironment provides a sanctuary for myeloma and other hematological tumors by blocking initial drug induced damage and apoptosis, thereby allowing for tumor progression and the eventual emergence of acquired drug resistance. Understanding the influence of the microenvironment on tumor cell survival and drug response may identify new molecular targets for future therapies of myeloma and other hematological malignancies.
Summary
The treatment of myeloma has not improved substantially in the past three decades, and the mainstay of treatment continues to rely on cytotoxic drugs and glucocorticoids. The use of high dose chemotherapy with stem cell rescue has improved response duration, but even with this treatment, most patients relapse with drug refractory disease. Enhancing the efficacy of these drugs by preventing or overcoming drug resistance may improve the frequency of response and response duration; however, this approach will also likely result in disease relapse. In order to substantially improve the treatment of myeloma, a multifaceted approach capitalizing on newly discovered biological aspects of myeloma is necessary. A wealth of new information now available regarding the pathogenesis of myeloma should provide new therapeutic targets. To date, most of these new targets are most likely to be effective in preventing disease progression or enhancing the efficacy of more traditional treatment approaches. Therefore, a major challenge to improving treatment outcome is not only discovering new therapeutic targets, but also integrating these new approaches with treatments that are known to be effective for most patients. Developing a “total approach” to the treatment of myeloma will likely involve using a treatment that first reduces tumor burden, a second approach that further eradicates residual disease, and a third approach that minimizes the growth and progression of residual myeloma. No one “total approach” is likely to be effective for all patients. Another challenge for effectively treating the majority of patients will be to identify which patients are suitable for a given therapeutic approach. Studies of genomic and proteomic profiles of individual myeloma patients should assist in identifying patients who are suitable for a particular treatment approach. Once again, discovering biological aspects of myeloma and considering unique circumstances of individual patients will assist the physician in the treatment of myeloma patients.
Conventional chemotherapy (CC) versus high-dose therapy (HDT) (median survival in months).
| . | No. of Patients . | Age . | CC . | HDT . | p value . |
|---|---|---|---|---|---|
| Historical Comparisons | |||||
| Barlogie (6) | 246 | < 70 | 48 | 62 + | 0.01 |
| Lenhoff (7) | 548 | < 60 | 44 | NR | 0.001 |
| Palumbo (8) | 144 | > 60 | 48 | 56 + | < 0.01 |
| Randomized Trials | |||||
| IFM (3) | 200 | < 65 | 44 | 57 | 0.03 |
| MAG (5) | 190 | 55–65 | 50 | 55 | NS |
| . | No. of Patients . | Age . | CC . | HDT . | p value . |
|---|---|---|---|---|---|
| Historical Comparisons | |||||
| Barlogie (6) | 246 | < 70 | 48 | 62 + | 0.01 |
| Lenhoff (7) | 548 | < 60 | 44 | NR | 0.001 |
| Palumbo (8) | 144 | > 60 | 48 | 56 + | < 0.01 |
| Randomized Trials | |||||
| IFM (3) | 200 | < 65 | 44 | 57 | 0.03 |
| MAG (5) | 190 | 55–65 | 50 | 55 | NS |
Comparison of single and double autologous transplantation. Results of the Intergroupe Français du Myelome (IFM) 94 (median follow-up 5 years).
| . | Single TX . | Double TX . | . | ||
|---|---|---|---|---|---|
| . | BM . | PBPC . | BM . | PBPC . | p value . |
| Abbreviations: TX, transplantation; BM, bone marrow; PBPC, peripheral blood progenitor cells; CR, complete response; VGPR, very good partial remission; EFS, event-free survival; OS, overall survival | |||||
| No. of patients | 79 | 88 | 85 | 92 | |
| CR+VGPR rate (%) | 43 | 50 | 50 | 61 | < 0.05 |
| 5-year EFS rate (%) | 19 | 20 | 27 | 35 | < 0.01 |
| 5-year OS rate (%) | 35 | 40 | 43 | 60 | < 0.05 |
| . | Single TX . | Double TX . | . | ||
|---|---|---|---|---|---|
| . | BM . | PBPC . | BM . | PBPC . | p value . |
| Abbreviations: TX, transplantation; BM, bone marrow; PBPC, peripheral blood progenitor cells; CR, complete response; VGPR, very good partial remission; EFS, event-free survival; OS, overall survival | |||||
| No. of patients | 79 | 88 | 85 | 92 | |
| CR+VGPR rate (%) | 43 | 50 | 50 | 61 | < 0.05 |
| 5-year EFS rate (%) | 19 | 20 | 27 | 35 | < 0.01 |
| 5-year OS rate (%) | 35 | 40 | 43 | 60 | < 0.05 |
Single versus double intensive remissions therapy: preliminary results of randomized studies.
| . | . | . | EFS . | OS . | ||||
|---|---|---|---|---|---|---|---|---|
| . | No. of Pts . | Median Follow-up . | Single . | Double . | p value . | Single . | Double . | p value . |
| IFM 94 (22) | 403 | 5Y | at 5 yr 17% | 27% | 0.06 | at 5 yr 40% | 48% | NS |
| HOVON (23) | 255 | 29 M | at 3 yr 35% | 36% | NS | at 4 yr 47% | 43% | NS |
| BOLOGNA 96 (24) | 178 | 30 M | (med) 21 m | 29 | NS | at 4 yr 74% | 71% | NS |
| MAG (25) | 193 | 27 M | 41 events | 43 events | NS | 27 deaths | 22 deaths | NS |
| . | . | . | EFS . | OS . | ||||
|---|---|---|---|---|---|---|---|---|
| . | No. of Pts . | Median Follow-up . | Single . | Double . | p value . | Single . | Double . | p value . |
| IFM 94 (22) | 403 | 5Y | at 5 yr 17% | 27% | 0.06 | at 5 yr 40% | 48% | NS |
| HOVON (23) | 255 | 29 M | at 3 yr 35% | 36% | NS | at 4 yr 47% | 43% | NS |
| BOLOGNA 96 (24) | 178 | 30 M | (med) 21 m | 29 | NS | at 4 yr 74% | 71% | NS |
| MAG (25) | 193 | 27 M | 41 events | 43 events | NS | 27 deaths | 22 deaths | NS |
New investigational agents in the treatment of myeloma.
| PS-341 | Millenium Pharm |
| Thalidomide derivatives | Celgene Corp |
| 2ME2 | EntreMed |
| Neovastat | Aeterna Labs |
| R 11 5777 (FTI) | Janssen/CTEP |
| Genasense (Bcl-2) | Genta Inc. |
| Trisenox (arsenic triOx) | CTI |
| SU5416 | CTEP |
| PS-341 | Millenium Pharm |
| Thalidomide derivatives | Celgene Corp |
| 2ME2 | EntreMed |
| Neovastat | Aeterna Labs |
| R 11 5777 (FTI) | Janssen/CTEP |
| Genasense (Bcl-2) | Genta Inc. |
| Trisenox (arsenic triOx) | CTI |
| SU5416 | CTEP |
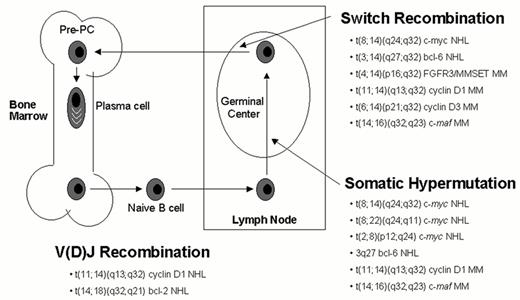
Chromosome translocations mediated by B cell specific mechanisms.
Errors in any of three developmentally regulated B cell specific DNA modification processes can mediate IgH or IgL translocations. V(D)J recombination is involved in receptor formation and editing in early B cells in the bone marrow, whereas most IgH switch recombination and all somatic hypermutation occurs in germinal center B cells. Examples of specific translocations mediated by these three mechanisms are indicated. As indicated by the 3q27 bcl-6 NHL notation in this diagram, the bcl-6 oncogene is subject to somatic hypermutation, which can cause either mutations that dysregulate or structurally alter this gene, or double-stranded breaks that can mediate translocations that do not involve an Ig locus.
Chromosome translocations mediated by B cell specific mechanisms.
Errors in any of three developmentally regulated B cell specific DNA modification processes can mediate IgH or IgL translocations. V(D)J recombination is involved in receptor formation and editing in early B cells in the bone marrow, whereas most IgH switch recombination and all somatic hypermutation occurs in germinal center B cells. Examples of specific translocations mediated by these three mechanisms are indicated. As indicated by the 3q27 bcl-6 NHL notation in this diagram, the bcl-6 oncogene is subject to somatic hypermutation, which can cause either mutations that dysregulate or structurally alter this gene, or double-stranded breaks that can mediate translocations that do not involve an Ig locus.
Multi-step molecular pathogenesis of multiple myeloma. A minimum number of intermediates in the MM pathogenesis pathway are depicted. The approximate timing of some specific oncogenic events is indicated by horizontal lines, with solid lines indicating the most likely time during which these events occur. See text for additional details.
Multi-step molecular pathogenesis of multiple myeloma. A minimum number of intermediates in the MM pathogenesis pathway are depicted. The approximate timing of some specific oncogenic events is indicated by horizontal lines, with solid lines indicating the most likely time during which these events occur. See text for additional details.
Frequency of IgH translocation in multiple myeloma. See text for additional details.
Frequency of IgH translocation in multiple myeloma. See text for additional details.
Dysregulation of FGFR3 and MMSET by t(4;14)(p16.3;q32) in multiple myeloma.
A) The relative positions of the FGFR3 and MMSET genes, both of which are dysregulated by the t(4;14) translocation, are depicted on chromosome 4p16.3, near the telomere. Similarly, the organization of the IgH locus, including the intronic enhancer (Em) and the 3′ enhancer (3′E), are depicted at 14q32 near the telomere. The arrows indicate the transcriptional orientation of the MMSET gene and the IgH locus, both toward the centromere.
B) A translocation into switch regions (a hybrid mu/gamma switch region in this case) separates the two enhancers. The more telomeric Em segregates to der(4), dysregulating MMSET. The telomeric region of chromosome 4, containing FGFR3, segregates to der(14), where it is dysregulated by the powerful influence of the 3′ E. Promoter sites (P) are noted, with the size of the arrowhead indicating the direction and amount of transcription. Exons are represented by boxes, that are joined by RNA splicing as indicated by thin lines. See text for additional details.
Dysregulation of FGFR3 and MMSET by t(4;14)(p16.3;q32) in multiple myeloma.
A) The relative positions of the FGFR3 and MMSET genes, both of which are dysregulated by the t(4;14) translocation, are depicted on chromosome 4p16.3, near the telomere. Similarly, the organization of the IgH locus, including the intronic enhancer (Em) and the 3′ enhancer (3′E), are depicted at 14q32 near the telomere. The arrows indicate the transcriptional orientation of the MMSET gene and the IgH locus, both toward the centromere.
B) A translocation into switch regions (a hybrid mu/gamma switch region in this case) separates the two enhancers. The more telomeric Em segregates to der(4), dysregulating MMSET. The telomeric region of chromosome 4, containing FGFR3, segregates to der(14), where it is dysregulated by the powerful influence of the 3′ E. Promoter sites (P) are noted, with the size of the arrowhead indicating the direction and amount of transcription. Exons are represented by boxes, that are joined by RNA splicing as indicated by thin lines. See text for additional details.
A. Soluble tumor micro-environment interactions (IL-6). B. Direct interaction with tumor micro-environment fibronectin (FN).
A. Soluble tumor micro-environment interactions (IL-6). B. Direct interaction with tumor micro-environment fibronectin (FN).