Abstract
Outcomes for infants diagnosed under 1 year of age with KMT2A-rearranged acute lymphoblastic leukemia (ALL) have remained stagnant over the past 20 years. Successive treatment protocols have previously focused on intensification of conventional chemotherapy, but increased treatment-related toxicity and chemoresistance have led to a plateau in survival. We have now entered an era of immunotherapy with integration of agents, such as blinatumomab or chimeric antigen receptor T-cell therapy, into the standard chemotherapy backbone, showing significant promise for improving the dismal outcomes for this disease. There remains much optimism for the future as a wealth of preclinical studies have identified additional novel targeted agents, such as venetoclax or menin inhibitors, ready for incorporation into treatment, providing further ammunition to combat this aggressive disease. In contrast, infants with KMT2A-germline ALL have demonstrated excellent survival outcomes with current therapy, but there remains a high burden of treatment-related morbidity. Greater understanding of the underlying blast genetics for infants with KMT2A-germline ALL and incorporation of immunotherapeutic approaches may enable a reduction in the intensity of chemotherapy while maintaining the excellent outcomes.
Learning Objectives
Recognize the need for new therapeutic approaches for infants with acute lymphoblastic leukemia (ALL)
Review the current landscape of clinical trials in development for infants with ALL
Identify novel targeted agents that have undergone preclinical assessment and have potential for clinical translation to treat infants with ALL
Introduction
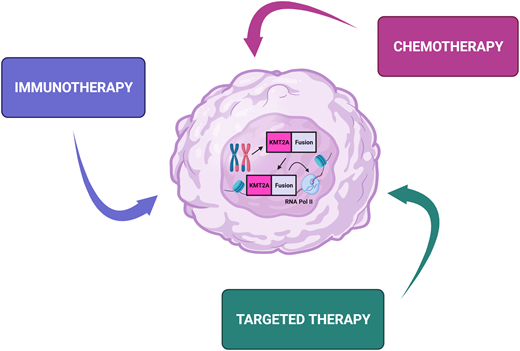
Outcomes for children with acute lymphoblastic leukemia (ALL) have seen remarkable improvements in outcome over the past 70 years. Since the first report of chemotherapy inducing temporary remission in 5 children with acute leukemia in 1948, the 5-year overall survival is now over 90%.1 However, infants diagnosed with ALL before their first birthday constitute a unique subgroup that has proven difficult to treat. Therapeutic protocols have evolved over time, with early studies identifying several independent risk factors associated with an inferior outcome, including presence of a KMT2A rearrangement, hyperleukocytosis at presentation, age less than 6 months at diagnosis, and poor response to initial prednisone therapy.2,3 KMT2A rearrangments are present in ALL cells of up to 80% of infants and are associated with chemoresistance and high rates of relapse. Until now, cooperative group studies have largely focused on intensification of conventional chemotherapy to improve outcome. While this approach has been successful for KMT2A-germline (KMT2A- nonrearranged) infants,4 the outcomes using chemotherapy alone for KMT2A-rearranged infants remain dismal, with 5-year event-free survival (EFS) of less than 40% (Table 1).5,6 The recent MLL-10 study suggests potential benefit of allogeneic hematopoietic stem cell transplantation (HSCT) for high-risk infants, but the indication and benefit of HSCT for infants with KMT2A-rearranged ALL remains contentious.7 The use of intensive treatment modalities for infants with ALL has to be balanced with their increased vulnerability to treatment-related toxicity and risk of short-term sequelae and long-term morbidity in survivors. The development of immunotherapeutic approaches and novel targeted therapies has provided considerable optimism for improving the outcome for infants with ALL (Figures 1 and 2). The following 4 case scenarios are used to highlight the current concepts proposed for the next generation of clinical trials for infant ALL and discuss promising targeted therapies that are currently under preclinical evaluation.
Immunotherapeutic approaches for the treatment of infants with acute lymphoblastic leukemia. mAb, monoclonal antibody.
Immunotherapeutic approaches for the treatment of infants with acute lymphoblastic leukemia. mAb, monoclonal antibody.
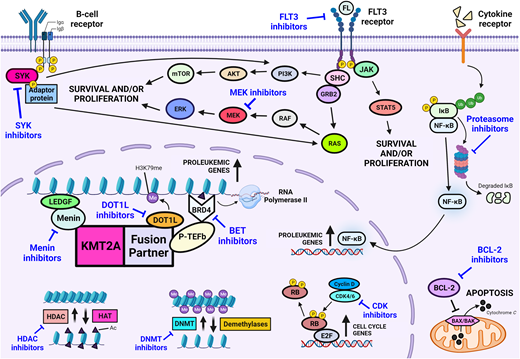
Novel drug classes for the treatment of infants with KMT2A-rearranged acute lymphoblastic leukemia.
Novel drug classes for the treatment of infants with KMT2A-rearranged acute lymphoblastic leukemia.
CLINICAL CASE 1
A 3-month-old boy presenting with irritability, hepatosplenomegaly, and a white blood cell count of 161.7 × 109/L was diagnosed with CD19-positive B-cell ALL (B-ALL). G-banded chromosome analysis and fluorescence in situ hybridization (FISH) studies identified a t(11;19) translocation. A diagnostic lumbar puncture revealed central nervous system (CNS) involvement, CNS2. He was initially treated with Interfant-06 induction, with a good response to the prednisone prephase and clearance of the blasts in his cerebrospinal fluid. Minimal residual disease (MRD), measured by polymerase chain reaction using the KMT2A-MLLT1 marker, was positive at 4 × 10–4 at the end of induction. A 4-week cycle of blinatumomab was administered by continuous infusion at 15 µg/m2/day. An MRD-negative response was achieved following completion of blinatumomab. The patient subsequently proceeded to complete the remainder of Interfant-06 therapy, commencing with Protocol IB, and remains in first complete remission 4 years from diagnosis.
Role of blinatumomab for KMT2A-rearranged infant ALL
Blinatumomab is a bispecific single-chain antibody construct that binds cytotoxic T cells through CD3 receptors and B cells through CD19 receptors, engaging the immune system to eradicate both B-ALL blasts and normal B cells. Clinical trials have demonstrated tolerability and efficacy of blinatumomab for children with relapsed/refractory B-ALL, with favorable outcomes also seen in a series of infants with relapsed/refractory KMT2A-rearranged ALL.8-10 These promising findings led to a phase 2 pilot study that tested the feasibility, safety, and tolerability of the addition of 1 cycle of blinatumomab following induction to the Interfant-06 chemotherapy backbone in 30 infants with KMT2A-rearranged ALL. In addition to confirming the safety and tolerability of blinatumomab in this population, the striking early results of this study identified a 1-year EFS of over 90% compared with 54.8% in the Interfant-06 historical control, where two-thirds of all relapses occurred in the first year from diagnosis.11 Given these outstanding findings, the next generation of clinical trials for infants with ALL will nonrandomly integrate blinatumomab as standard therapy into the Interfant-06 chemotherapy backbone.
CLINICAL CASE 2
A 1-month-old girl presented with a 5-day history of nasal congestion, sneezing, and reduced feeding. Examination revealed marked hepatosplenomegaly and increased respiratory effort. Her white blood cell count was 1115 × 109/L with peripheral blood immunophenotype confirming a diagnosis of B-ALL, expressing both CD19 and CD22. G-banded chromosome analysis and FISH studies identified a 3-way t(4;11;13) translocation, and diagnostic lumbar puncture revealed CNS2 involvement. She had a good response to her prednisone prephase and subsequently received treatment according to the lymphoid-directed arm of Interfant-06 with clearance of blasts in her cerebrospinal fluid during induction. MRD negativity, measured by polymerase chain reaction using the KMT2A-AFF1 marker, was achieved following Protocol IB. However, a rise in MRD to 5 × 10–3 was detected following the OCTADAD block of chemotherapy, with subsequent repeat bone marrow assessment revealing 1.257% MRD by flow cytometry with ongoing CD19 and CD22 positivity in the blast population. A second MRD remission was achieved with chimeric antigen receptor (CAR) T-cell therapy targeting both CD19 and CD22, which was subsequently consolidated with a planned umbilical cord blood HSCT using a treosulfan-, fludarabine-, and thiotepa-based conditioning. She is currently 4.5 years of age and remains in complete remission.
CAR T-cell therapy for KMT2A-rearranged infant ALL
Currently, there is no defined standard for the treatment of infants with relapsed/refractory KMT2A-rearranged ALL. The advent of CAR T-cell therapy over the past decade has revolutionized the care of children and young adults with relapsed/refractory B-ALL. A multitude of products have undergone clinical investigation, producing durable remissions with 1-year relapse-free survival between 50% and 60%.12 CAR T-cell therapy is currently being investigated in the upfront setting for children and young adults with high-risk B-ALL who are MRD positive at the end of consolidation (NCT03876769), and outcomes will be pivotal in determining the utility of CAR T-cell therapy as frontline therapy. However, the ability to draw conclusions for infants will be limited as eligibility for this study has been restricted to over 1 year of age at the time of screening. Exclusion of infants from such trials is in part due to the known challenges faced by infants with regard to collection and manufacture of autologous CAR T-cell products. Infants with KMT2A-rearranged ALL often present with high peripheral blast counts and can be clinically unstable during the intense early phases of therapy, which can also lead to T-cell exhaustion. In addition, infants have a low circulating blood volume, which provides a technical challenge for apheresis. As such, data on the use of CAR T-cells for infants are limited and restricted to relapsed/refractory disease.13-15 Further development and investigation of universal “off-the-shelf” CAR T-cell products may have potential to overcome these challenges.16 The upcoming Interfant-21 study proposes an investigational window permissive for upfront CAR T-cell therapy for high-risk infants with KMT2A-rearranged ALL. Outcomes from such studies, coupled with real-world data, will determine the feasibility of upfront autologous CAR T-cell therapy for infants. This case also highlights CD22 as a therapeutic target, indicating potential for inotuzumab ozogamicin, a CD22-directed humanized monoclonal antibody conjugated to the cytotoxin calicheamicin, as a therapeutic modality worthy of further investigation.17
CLINICAL CASE 3
A 7-month-old boy presented with a 2-week history of increasing pallor. Examination revealed bruising and splenomegaly, with a full blood count revealing marked anemia, thrombocytopenia, and a white blood cell count of 148.2 × 109/L. Flow cytometry of bone marrow revealed a 93% blast population expressing CD10, CD19, and intracellular CD79a. G-banded chromosome analysis and FISH studies identified a t(9;11) translocation. No blasts were present in the cerebrospinal fluid. A diagnosis of B-ALL was made, and the patient was treated according to the lymphoid-directed arm of Interfant-06. MRD negativity, measured by polymerase chain reaction using the KMT2A-MLLT3 marker, was achieved following the MARMA block of chemotherapy, and the patient proceeded to the OCTADAD and maintenance phases of therapy. During maintenance, the patient presented with right testicular swelling. A combined relapse with lineage switch to acute monocytic leukemia was confirmed on bone marrow aspirate and testicular biopsy, which revealed infiltration of blast cells expressing CD15, CD33, CD64, CD123, and intracellular myeloperoxidase and absence of markers for B-ALL and T-cell ALL. Cytogenetic analysis identified the same t(9;11) translocation present at diagnosis. He subsequently achieved second complete remission with myeloid-directed therapy, comprising of 1 cycle of mitoxantrone and cytarabine and a second cycle of fludarabine, cytarabine, and idarubicin. This was consolidated with testicular radiation to 6 Gy and a matched unrelated donor HSCT using a busulfan-, fludarabine-, and thiotepa-based conditioning. He is currently 6.5 years of age and remains in complete remission.
Toward targeted therapy
Although infrequent, this case indicates the potential for lineage switch to acute myeloid leukemia.18 Recent studies have shown that blasts from infants with KMT2A-rearranged ALL display a stem cell signature with increased lineage plasticity.19,20 The selection pressure conferred by lymphoid and/or CD19-directed therapy can further predispose to myeloid escape.21,22 As highlighted by this case, myeloid-directed therapy can be successfully used in patients who undergo lineage switch to achieve a cure.18 Another scenario where myeloid-style consolidation therapy has shown benefit is for infants with high MRD at the end of induction treated on the Interfant-06 protocol.23 A significant association between myeloid antigen coexpression at diagnosis and high MRD at the end of induction was identified in this study.23 Although myeloid therapy has shown benefit for these indications, the increased treatment intensity exacerbates the risk of treatment-related morbidity and mortality, further highlighting the need for novel therapeutic approaches.
Integration of therapies targeting components of the KMT2A fusion complex or signaling pathways implicated in KMT2A-mediated leukemogenesis holds significant promise for the future (Figure 2). Addition of such therapies to a chemoimmunotherapy backbone may serve to improve overall survival outcomes, prevent events such as relapse or lineage switch, and abrogate the need for intensive myeloid-directed therapies and HSCT. Overexpression of FLT3 has been shown in infants with KMT2A-rearranged ALL, and the Children's Oncology Group (COG) AALL0631 study was pivotal in being the first study to demonstrate the safety and feasibility of adding a novel targeted therapy to postinduction chemotherapy for infants with KMT2A-rearranged ALL.6,24 Although addition of the FLT3 inhibitor, lestaurtinib, did not improve overall outcome, benefit was shown for a subset of patients who achieved potent pharmacodynamic inhibition of FLT3 and whose leukemic cells were sensitive to ex vivo FLT3 inhibition.6
The outcomes of studies that have recently completed accrual are eagerly awaited. KMT2A-rearranged infant ALL cells have previously been shown to exhibit promoter hypermethylation, resulting in gene silencing.25,26 This led to COG AALL15P1 (NCT02828358), which investigated the tolerability and safety of adding 5-day cycles of azacitidine, a demethylating agent, prior to each block of postinduction chemotherapy. The TINI study (NCT02553460), conducted by St Jude Children's Research Hospital, tested the safety of bortezomib, a proteasome inhibitor, and vorinostat, a histone deacetylase inhibitor, on a chemotherapy backbone. The rationale for the integration of histone deacetylase inhibitors is supported by several studies that demonstrated preclinical efficacy in this setting.27,28
Preclinical studies have identified several drug classes primed for translation into the next generation of clinical trials for KMT2A-rearranged infant ALL. B-cell lymphoma 2 (BCL-2) family proteins regulate the intrinsic mitochondrial apoptosis pathway with BCL-2 a key antiapoptotic regulator within this pathway. High expression of BCL-2 has been identified in KMT2A-rearranged ALL, and the KMT2A-AFF1 fusion has been shown to upregulate BCL-2 via DOT1L-mediated H3K79 methylation.29 Thus, inhibition of BCL-2 provides an attractive target, with objective responses demonstrated following treatment with venetoclax in infant KMT2A-rearranged patient-derived xenografts.30 Based on this evidence, the COG is currently designing a phase 2 trial to incorporate venetoclax into therapy for infants with KMT2A- rearranged ALL. Menin inhibitors comprise another class of agent for which there is much anticipation. The interaction between menin and the KMT2A fusion is critical for fusion-mediated leukemic transformation, and remarkable in vivo efficacy has been shown for inhibitors of this interaction in KMT2A-rearranged infant ALL.31 This has led to the development of many different menin inhibitors that are currently undergoing early phase investigation, predominantly in adults with relapsed/refractory leukemia. Assessment of menin inhibition in infants will be facilitated through several studies that are currently undergoing rapid development for infants with relapsed/refractory KMT2A-rearranged ALL, in addition to expansion of eligibility criteria permitting inclusion of infants in current studies.
Preclinical investigation of novel agents for KMT2A-rearranged infant ALL remains the subject of intense interrogation. A multitude of drug classes have shown promise for targeted therapy in the future, including but not limited to inhibitors of neuron-glial antigen 2, cyclin-dependent kinase, bromodomain and extra-terminal proteins, spleen tyrosine kinase, the mitogen-activated protein kinase pathway, and DOT1L (Figures 1 and 2).32-36 However, KMT2A-rearranged infant ALL is a rare disease, and the ability to clinically investigate every agent identified from preclinical studies is limited by the number of trials that can be concurrently active and the prolonged periods required to meet target accrual. The development of a single unified global umbrella trial, incorporating lessons learned from the COG AALL0631 study with a personalized approach to drug selection and dosing based on real-time drug sensitivity and pharmacodynamic assessment, has potential to overcome such barriers. Although there remain many practical hurdles to this vision, the recent advent of precision medicine could facilitate such an approach in the future.
CLINICAL CASE 4
A 10-month-old girl presented with a 3-week history of bruising and increased lethargy. Her white blood cell count was 97.5 × 109/L, and a diagnosis of B-ALL with CNS3 involvement was confirmed following a bone marrow aspirate and lumbar puncture. G-banded chromosome analysis revealed a normal karyotype with no evidence for a KMT2A-rearrangement on FISH. She was treated on COG P9407, receiving a cumulative daunorubicin dose of 300 mg/m2. Following completion of the intensive phases of therapy, she presented with respiratory distress, and an echocardiogram revealed left ventricular dilatation with moderately impaired systolic function. Diuretics were instigated in the acute phase with longer-term medical management comprising digoxin and angiotensin-converting enzyme inhibitors. She completed all planned therapy for B-ALL, with serial echocardiograms revealing progressive cardiac dysfunction and development of pulmonary hypertension during follow-up. She successfully underwent an orthotopic heart transplant 8 years following her original diagnosis of B-ALL, for which she continues to remain in first complete remission 18 years later.
Can we de-escalate chemotherapy for infants with KMT2A- germline ALL?
Infants with KMT2A-germline ALL constitute a rare subgroup that has historically been treated according to the same intensive chemotherapy backbone as their KMT2A-rearranged counterparts. Contemporary studies have demonstrated excellent survival outcomes for KMT2A-germline infants with this approach (Table 1).4 Recent genetic characterization has identified an enrichment of NUTM1 rearrangements in this population, with a 3-year EFS of 100% reported for this subgroup.37,38 Despite such advances, this case highlights the risk of treatment-related morbidity and long-term sequelae that can be associated with intensive chemotherapy. This landscape provides the ideal setting to question whether there is an opportunity to de-escalate chemotherapy for infants with KMT2A-germline ALL. However, there is an inherent risk of inferior outcome with de-escalation of therapy, and it should therefore only be considered in the setting of a clinical trial or according to national standards where close monitoring can be performed. As such, a trial that proposes to combine blinatumomab with a less intense high-risk chemotherapy backbone for infants with KMT2A-germline ALL is currently being developed by the COG.
Summary
Infant ALL has been subject to a wealth of basic, translational and clinical research over the past 40 years, leading to greater understanding of the biology and treatment for this disease. Treatment intensification with conventional chemotherapy has led to a plateau in survival outcomes due to the fine balance between relapse and toxicity. The next decade ushers in a new era, with the emergence of immunotherapeutic approaches bringing significant optimism for improving survival outcome and reducing the burden of toxicity for infants with ALL. Ongoing discovery of novel targets and drug development has further added to the repertoire of therapeutic options within the armamentarium, with the next suite of clinical trials primed for incorporation of targeted therapy.
Acknowledgment
Rishi S. Kotecha is supported by the Child Cancer Research Foundation.
Conflict-of-interest disclosure
Rishi S. Kotecha has participated in Scientific Advisory Boards for Amgen, Link Healthcare, and Jazz Pharmaceuticals.
Off-label drug use
Rishi S. Kotecha: nothing to disclose.