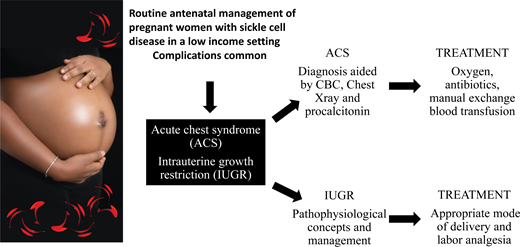
Abstract
Pregnancy in women with sickle cell disease (SCD) is fraught with complications, some of which are life-threatening. Managing pregnancy in these women can be challenging, especially with poor resources, which is often the case in low-income countries. In Nigeria, for instance, up to 90% of patients pay out of pocket for medical care due to the poorly developed health insurance system, and this worsens the morbidity and mortality associated with this condition. We describe a pragmatic approach to routinely managing pregnant women with SCD in the antenatal period, showing the feasibility of effective management of these high-risk pregnancies in limited-resource settings. We also present the case of a pregnant Nigerian woman with SCD who has intrauterine growth restriction (IUGR) and acute chest syndrome (ACS), conditions that are life-threatening for the fetus and the mother, respectively, and require prompt intervention. We highlight how we successfully managed this woman in a cost-effective manner by employing relatively inexpensive tests for diagnosis and treating her effectively with oxygen, appropriate antibiotics and manual exchange blood transfusion for the ACS, and finger pulse oximeters to monitor oxygen saturation. We explore pathophysiological concepts to IUGR in women with SCD and briefly discuss the appropriate mode of delivery, including the options for pain relief in labor.
Learning Objectives
Effectively manage sickle cell pregnancy, with emphasis on acute chest syndrome in pregnancy in resource-poor settings
Review antenatal care in sickle cell pregnancy, the link between sickle cell pregnancy and intrauterine growth restriction, and the appropriate mode of delivery
CLINICAL CASE
A 33-year-old woman with hemoglobin SS (HbSS) in her first pregnancy presented with a cough, severe knee pain, bilateral lower-limb pain, and severe oligohydramnios (reduced amniotic fluid volume) at 34 weeks' gestation. On examination, she was dehydrated. Her temperature was normal, with a pulse rate of 100/min, a respiratory rate of 22/min, and an oxygen saturation (SpO2) level of 88% in room air. She was admitted, received a chest x-ray with abdominal shielding, and administered oxygen with a rebreathable mask. A complete blood count showed an Hb level of 5.5 g/dl and a white cell count of 22 × 106/mL, with neutrophilia of 81%.
Burden of sickle cell disease in pregnancy
Sickle cell disease (SCD) is a hemoglobinopathy caused by a mutation in the sixth codon of the gene encoding β-globulin and is inherited in an autosomal recessive pattern of at least 1 HbS and another abnormal Hb, including S, C, beta thalassemia, D, E, and O Arab. Nigeria is the most populated of the 54 countries in Africa and has the highest number of people living with SCD in the world, with 150,000 born with the condition annually.1 The population of Nigeria is Black African, with a gross domestic product per capita of US $2085, compared to $69 287.5 for the US.2 Nigeria is rated as a lower-middle-income country (LMIC), with approximately 40% of people living under US $2 a day.3
SCD is fraught with many complications, and in a resource-constrained country like Nigeria, where more than 90% of the population pays out of pocket for their health care,4 the attendant mortality from the disease is high. It is estimated that mortality in children under 5 years old with SCD is 4 times greater than in children with HbAA, and this excess mortality among children with SCD contributes 4.2% to the overall national under-5 mortality rate.5 This is particularly the case given that most people are not aware they have it because there is no universal screening for the condition at birth. Of the survivors, many now live long enough to reach their reproductive years. Although they have been reported to have a higher miscarriage rate,6,7 most women with SCD are able to achieve pregnancy. Pregnancy and delivery are often complicated, however, with a higher incidence of vaso-occlusive crises (VOCs), urinary tract infections, acute chest syndrome (ACS), and hypertensive diseases of pregnancy than women who do not have SCD.8 The condition is a known cause of maternal death in pregnancy in most countries, although studies in Nigeria and Ghana have shown the possibility of few or no maternal deaths with individualized and multidisciplinary care.8,9 The babies born to those with SCD are also prone to intrauterine growth restriction (IUGR), prematurity, and an increased incidence of perinatal death.6,8
In LMICs, even people who are aware of their Hb genotype find it difficult to access optimal health care due to the cost of investigations and treatment amid the limited availability of health insurance systems, the long distances to health facilities, the lack of available transport, sociocultural factors such as delays in getting spousal consent to commence antenatal care, and the poor attitudes of health care workers.10,11 The general advice given to pregnant women with SCD is to drink plenty of water, avoid extreme cold or heat, and report to the hospital immediately if they start to feel unwell. However, they often do not report early because of these health care access constraints, and by the time they do present to the hospital, they are often very ill.
Antenatal care
Pregnant women with SCD should have several examinations during pregnancy. A recent UK guideline recommends a routine of up to 6 or more complete blood counts, urine cultures, ultrasound scans, baseline renal and liver function tests, and extended red cell phenotypes for pregnant women with SCD.12 More tests are required when they become unwell, and this equates to even higher costs. To limit the number of tests and control costs while ensuring optimal care, we manage women with SCD as shown in Table 1 below.
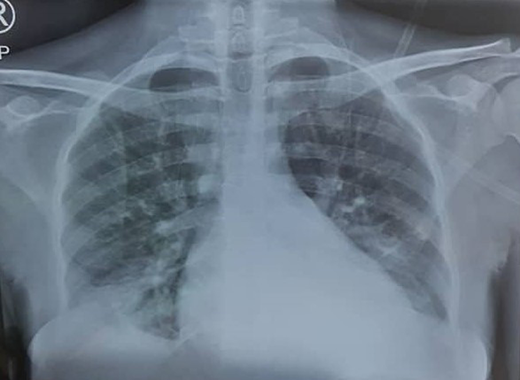
The plan for delivery includes deciding on either an anticipated vaginal delivery or an elective cesarean, a plan for pain relief during labor, preferably epidural analgesia, and the gestational age at which to be electively delivered. Symptoms and signs are evaluated and treated appropriately. Fingertip pulse oximeters are used to determine SpO2, and the woman is watched carefully when the SpO2 falls below 95%. A complete blood count is performed if there is any suspicion of a malarial, bacterial, or viral infection or if there are symptoms of vaso-occlusive disease or any other crisis. White blood cell, neutrophil, and platelet counts have been reported to be higher in pregnant women with SCD than in nonpregnant women and pregnant HbAA women.13,14 Therefore, a white blood cell count elevation after correcting for the nucleated red blood cells (RBCs) seen in an SCD pregnancy has to be interpreted with caution in determining an association with an infection, especially if the woman is also afebrile, as is often the case in lobar pneumonia. We often use more specific tests, such as serum procalcitonin, to determine the likelihood of bacterial infection in such cases.15 Serum procalcitonin has been found useful in distinguishing bacterial infections from viral infections and VOCs and is relatively inexpensive. Values less than 0.5 ng/mL usually rule out an ongoing bacterial infection (Figure 1).16
Chest radiograph (PA view) showing pulmonary infiltrates classic to ACS.
CLINICAL CASE (Continued)
The patient's chest x-ray showed radiopacities (Figure 1). Despite an increase in the oxygen flow rate, the twice-daily administration of 1 g of ceftriaxone, and a simple transfusion of 3 units of packed RBCs (PRBCs) within 24 hours, her Hb level only increased to 6.2 g/d, and she remained oxygen dependent. She subsequently had a manual exchange blood transfusion (EBT) with 6 units of ABO Rh D-compatible HbS-negative PRBCs that were less than 7 days old as per the unit protocol. Her postexchange Hb concentration was 8.6 g/dL, and her HbS fraction was 22.3%.
ACS in pregnant women with SCD
ACS, an important complication of SCD, is characterized by fever and/or respiratory symptoms and a new pulmonary infiltrate on chest x-ray. It is a significant cause of mortality and is frequently seen in pregnant women, with 4 out of 5 deaths in a series of 71 Hb SS women with 177 pregnancies.7 ACS caused nearly 87% of the maternal deaths in a multicenter case series in Ghana.17
The etiology of ACS includes pulmonary vaso-occlusion, pneumonia, marrow fat embolization, and infections. Bone marrow ischemia and necrosis are characteristic of VOCs, which can result in the release of bone marrow and fat into the venous circulation. These particles can then travel to the lungs, triggering ACS.18 While fat embolization is more commonly associated with ACS in the adult SCD patient, infections remain a significant cause, so it is essential to investigate and treat them.19 This should include taking blood and sputum for microscopy and culture.
Clinicians must maintain a high level of vigilance for ACS during a VOC as early treatment lowers mortality, shortens hospital stays, and reduces cost with less chance of recurrence. Once initiated, treatment for ACS must be aggressive since the disease process often escalates quickly.18 This is even more pertinent in pregnant women with SCD. Multidisciplinary care, with hematologists, pulmonologists, microbiologists, and other relevant subspecialists, led by a maternal medicine team, is crucial for the effective management of these high-risk pregnancies.
Essentially, ACS is treated by broad-spectrum antibiotic administration that includes coverage for atypical bacteria, supplemental oxygen administration, pain control, and incentive spirometry. To improve oxygenation, early simple blood transfusion is used in mild cases, and EBT is used in severe hypoxic cases or when there is nonresolution with simple transfusion (Table 2). In conducting simple blood transfusion, care must be taken to prevent circulatory overload, which can predispose the patient to pulmonary edema and worsen symptoms. In our setting we give PRBCs slowly over 2 to 3 hours with the concomitant administration of intravenous frusemide at 20 mg. Due to the high cost of automated red cell exchange, EBT is usually done manually and has been found to be as effective. A recent meta-analysis discovered no evidence of a greater benefit of automated EBT compared to manual, as there was no significant difference in the reduction of HbS percentage or of ferritin levels and no significant increase in adverse event risk.20 Protocols vary for manual EBT, but the unit-based manual RBC EBT method has been shown to achieve a lower HbS percentage target compared to the weight-based method and is shown in Table 3. As individuals who have had an episode of ACS have an increased risk of future episodes,18 hydroxyurea therapy should be considered as soon as possible in the postpartum period along with reassurance of the relative safety of the drug during breastfeeding.21
In situations in which blood is unavailable for transfusion or in which a patient does not give consent for blood transfusion on religious grounds, effective supportive therapy with intravenous hydration to maintain the individual's circulatory volume, oxygen therapy for hypoxia, pain control, and antibiotics to treat any infectious cause should be ensured. Inhaled nitric oxide as an adjunct to these supportive treatments may also be considered in such cases,22 if available. Erythropoietin (EPO) injection can also be considered for treating anemia in SCD, although overcoming the anemia takes some time. Despite the belief that EPO has the potential to cause an increase in blood pressure and clotting events and should therefore be used with caution, especially in SCD pregnancy, its use has demonstrated good results. A retrospective analysis with 19 adults with SCD (HbSS and HbS/beta thalassemia) who had received at least 1 year of EPO therapy showed that 7 patients had a good response to treatment (Hb increment higher than 1 × 5 g/dl), and 9 had a partial response (0.5-1.5 g/dl increment), with no increases in the rates of VOCs or venous thromboembolism compared to the year before the onset of EPO therapy.23 Intravenous iron may be given if there is clear evidence of iron deficiency following laboratory screening. Vitamins and other micronutrients, such as vitamin C, vitamin B12, folic acid, and zinc, which facilitates erythropoiesis, may also be administered as an adjunct to treatment. This approach combined with intensive care has been successfully used to manage a woman with ACS who refused blood on religious grounds.24
It is exceedingly difficult to differentiate ACS from pneumonia. ACS presents with pneumonia-like symptoms, and pneumonia can cause ACS. ACS often follows a VOC, so if a pregnant woman with SCD develops a VOC and new infiltrations appear on a chest radiograph, there should be a high index of suspicion for ACS, and such a woman should be treated appropriately. In rural settings where x-ray facilities may be lacking or where individuals are unable to afford an immediate chest radiograph, reliance on clinical symptoms becomes imperative, and pregnant women with VOCs who develop respiratory symptoms should be started on treatment for ACS. We routinely give antibiotics empirically for treatment and choose from antibiotics that are sensitive to community-acquired pneumonia. Azithromycin and either co-amoxiclav or ceftriaxone are often used in our environment because they are safe in pregnancy, relatively inexpensive, and effective for the treatment of atypical pneumonia.
Ensuring adequate rehydration and fluid balance are key, as improper fluid management leads to significant morbidity and mortality. For those without significant underlying cardiopulmonary and/or renal dysfunction, we assess the patient's fluid status based on physical exam findings and objective data from evaluating vital signs. When dehydration is detected, we administer hypotonic solutions in the form of 4.3% dextrose in 0.18 saline infusion while encouraging the patient to drink as much volume as possible by mouth. Hydration is monitored clinically and by measuring the urinary output and electrolytes. We ensure that a strict intake/output chart is maintained to assess the fluid balance daily and make necessary adjustments to the fluid regimen for sensible and insensible losses as indicated. For patients with underlying cardiac dysfunction or renal failure, we adjust volumes of administration accordingly, as these patients require a lower maintained fluid rate than expected for their body weight.25 We typically give fluid as 500 mL (for insensible loss) plus the previous day's output, and these patients are comanaged with the renal unit and chemical pathologists.
Prompt management of VOCs may help reduce the incidence of ACS in pregnancy by reducing the risk of microvascular occlusions occurring in the lungs. Such a management approach includes recognizing VOC or bone pain crisis early, treating precipitating causes, managing pain adequately and effectively, appropriately rehydrating the patient to maintain euvolemia, and transfusing with HbAA PRBCs as necessary to decrease the HbS fraction in the blood.26
CLINICAL CASE (Continued)
A growth scan showed that the fetus was small for gestational age, and although the fetal umbilical artery Dopplers were normal, a nonstress test revealed recurrent episodes of fetal bradycardia. Dexamethasone was administered for fetal lung maturity, and an emergency cesarean was performed, with the delivery of a live fetus weighing 1.8 kg. The patient recovered well postoperatively and was discharged home after 5 days.
Risk of complications in women with SCD
Pregnant women with SCD have a higher incidence of IUGR, babies with low birth weight, and preterm delivery.27,28 A recent meta-analysis found that women with HbSS had a 2.43-fold increased risk of preeclampsia and a 3.72-fold increase of IUGR compared with non-SCD pregnant women.29 Another meta-analysis found higher incidences of IUGR (pooled overall response, 2.79; 95% CI, 1.85-4.21; P < .001) and preeclampsia (pooled overall response, 2.05; 95% CI, 1.47-2.85; P < .001) in pregnant women with SCD compared with those without; these higher incidences were seen even with stratification into low- and high- income countries.30 In previous studies conducted by our team, we found that pregnant women with SCD did not expand their plasma volume in pregnancy compared with nonpregnant values and had a relatively lower increase in plasma renin concentration in pregnancy, compared with their HbAA counterparts.31,32 We hypothesized this to be a result of vasoconstriction that might be related to inadequate vasodilator activity.
We then conducted a study on prostacyclin, thromboxane, and glomerular filtration rate in pregnancies in women with SCD because of their tendency to develop IUGR and preeclampsia and because the prostacyclin: thromboxane ratio is known to be reversed in these disorders.7,33 We identified a similarly decreased prostacyclin: thromboxane ratio in pregnant women with HbSS, suggesting endothelial damage and an increased tendency to vasoconstriction and clotting.33
IUGR has been associated with the development of preeclampsia but can also occur independently, and women with SCD appear to be more prone to IUGR than preeclampsia, buttressed by the fact that the most recent meta-analyses show higher odds of IUGR than of preeclampsia.29,30 We hypothesized that aspirin would reduce the risk of IUGR and preeclampsia in pregnant women with SCD. We are currently halfway into recruitment to test this hypothesis in a multicenter, randomized, placebo controlled, double blind, parallel trial to evaluate the efficacy, safety, and tolerability of aspirin at a dose of 100 mg/d in reducing the risk of IUGR, miscarriages, and perinatal deaths in pregnant women with SCD (NCT05253781).34 Even though some guidelines already propose the use of aspirin in pregnant women with SCD to prevent preeclampsia, there is no evidence showing a clear benefit in these women, and we believe that their unique genetic pathology, with consequent chronic hemolytic anemia and vasculopathies, warrants a trial to confirm that aspirin reduces their risk of IUGR. This is especially because there is currently no evidence supporting the use of antiplatelet drugs in nonpregnant SCD individuals, because the risk of preeclampsia in women with SCD in studies done in Nigeria has been low,35 and because aspirin is not prescribed routinely to pregnant women with SCD in Nigeria.
Fetal surveillance and the decision to deliver
Fetal surveillance is indicated where the facilities exist. In our center we monitor SCD pregnancies with growth scans biweekly from 28 weeks to detect IUGR early. In women who have IUGR or preeclampsia, we follow up with a biophysical profile and umbilical artery Doppler velocimetry. Delivery is considered regardless of gestational age when the biophysical profile and/or the umbilical artery Doppler is abnormal. For women with ACS, delivery might also be indicated regardless of gestational age if the woman's condition worsens, and there is an urgent need to reduce diaphragmatic compression by the gravid uterus to facilitate resuscitation. Other indications for early delivery include the occurrence of life-threatening obstetric conditions or intrauterine fetal death. However, if the patient's condition improves following treatment for ACS, carrying a pregnancy to term before considering delivery is highly encouraged. As certain complications such as VOCs and ACS predominate more in late pregnancy,7 a recent guideline recommended delivery between 38 and 40 weeks, despite the unavailability of randomized controlled trials to determine the appropriate timing for birth.12
Delivery and postnatal care
Pregnant women with SCD should be delivered vaginally if possible, as this is still the safest route for delivery. A cesarean carries a higher risk of blood loss and wound infection as well as anesthetic complications, especially if general anesthesia is used. Patients should, however, be offered effective pain relief, and this is sometimes difficult in LMIC due to the relative lack of anesthetic personnel for the administration of epidural analgesia and the lack of experience of labor ward staff in monitoring the procedure. The more effective opiates, such as morphine and pethidine, are also often lacking in availability in Nigeria, and pethidine is highly disfavored in SCD because the doses commonly used to manage painful sickle cell crisis may lead to the accumulation of its toxic neuroexcitatory metabolite norpethidine and cause seizures.12 Incentive spirometry should be offered, especially for women who have general anesthesia for a cesarean. Women scheduled for vaginal delivery should be adequately rehydrated during labor to minimize the risk of VOC and given adequate analgesia using strong opiates, such as parenteral morphine, or epidural analgesia if available.
Despite this, pregnant women with SCD are more likely to be delivered by cesarean due to obstetric indications such as severe IUGR or preeclampsia, a previous cesarean, pelvic instability from conditions such as avascular necrosis of the head of the femur, or severe sickle-related illness with a need to deliver urgently or prematurely, such as severe VOC or ACS. The use of antenatal corticosteroids before the delivery of any preterm baby is an established intervention for reducing the risk of respiratory distress syndrome and perinatal death,36 and they should be administered prior to the delivery of any pregnant SCD woman before 36 weeks' gestational age. Although corticosteroids may potentially cause an increase in the incidence of recurrent acute pain episodes,37 the dose for this indication is low and usually innocuous. In our center we give 2 doses of 12 mg of intramuscular dexamethasone at 12-hour intervals for fetal lung maturity, and we have not had any reports of severe adverse events. Routine thromboprophylaxis should be considered during pregnancy and in the puerperium, regardless of the mode of delivery, as pregnant women with SCD have an increased incidence of venous thromboembolism. The Royal College of Obstetricians and Gynaecologists guideline recommends routine thromboprophylaxis with low-molecular-weight heparin from 28 weeks' gestational age until 6 weeks postpartum.38 Due to its cost in our environment, we do not routinely give thromboprophylaxis during pregnancy unless there is a specific indication, but we do prescribe it after delivery. Women with SCD should be observed for longer periods in the hospital following delivery because of the higher risk of complications compared to women without SCD.39
Managing pregnant women with SCD in resource-limited settings can be challenging to both the woman and her care provider. High maternal and perinatal morbidity and mortality can be reduced if we are able to devise strategies to implement health care using available resources in a cost-effective manner, as was done in the Clinical Case presented above.
Conflict-of-interest disclosure
Bosede B. Afolabi: research funding: Tertiary Education Trust Fund (Nigeria).
Ochuwa A. Babah: research funding: Tertiary Education Trust Fund (Nigeria).
Titilope A. Adeyemo: research funding: Tertiary Education Trust Fund (Nigeria).
Off-label drug use
Bosede B. Afolabi: nothing to disclose.
Ochuwa A. Babah: nothing to disclose.
Titilope A. Adeyemo: nothing to disclose.